Efficacy of Propolis Gel on Mature Biofilm Formed by Neocosmospora keratoplastica Isolated from Onychomycosis
Abstract
:1. Introduction
2. Materials and Methods
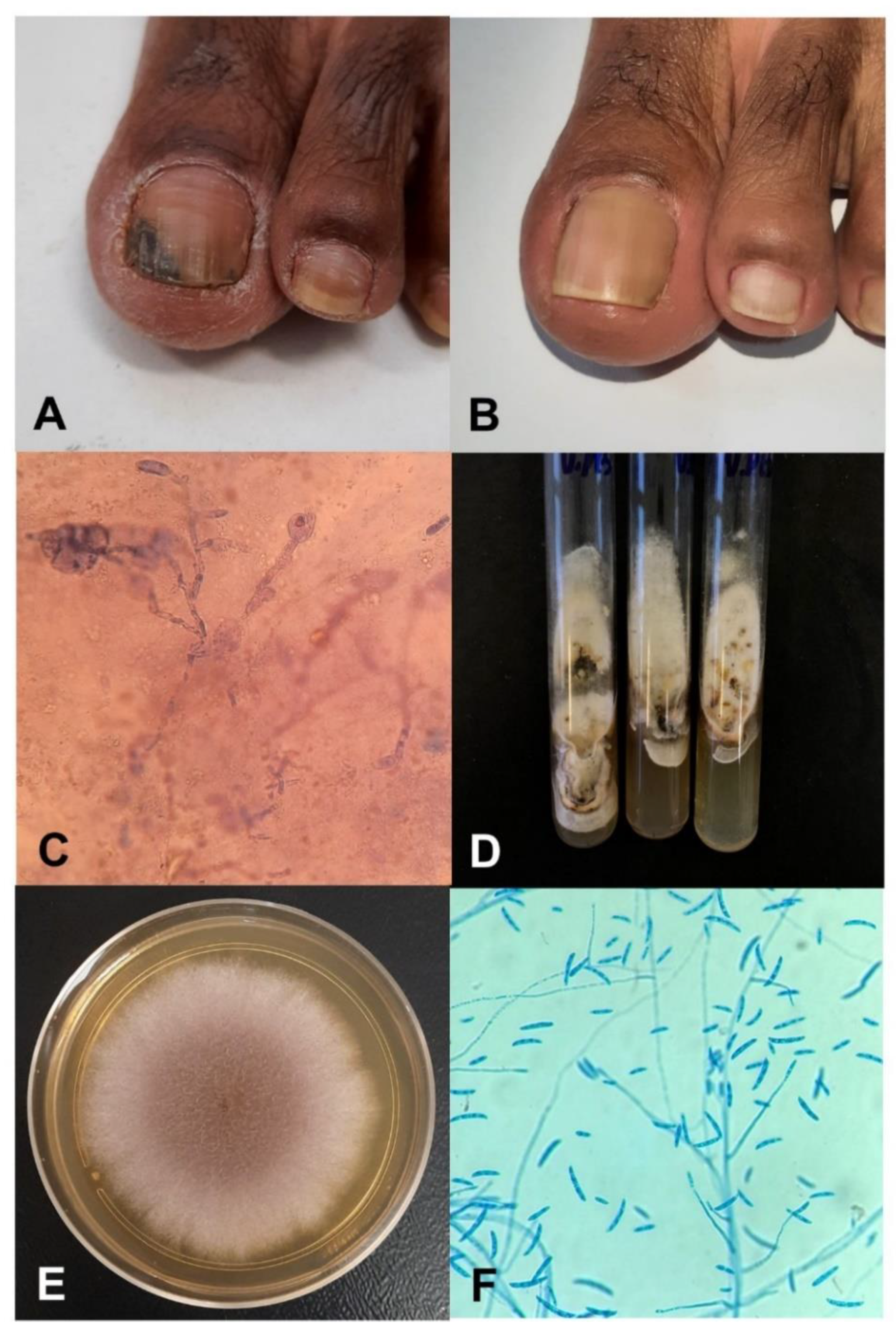
2.1. Fungus Origin and Patient’s History
2.2. Propolis Formulations
2.2.1. Propolis Extract (PE)
2.2.2. Propolis Gel (PG)
2.2.3. Patient Treatment
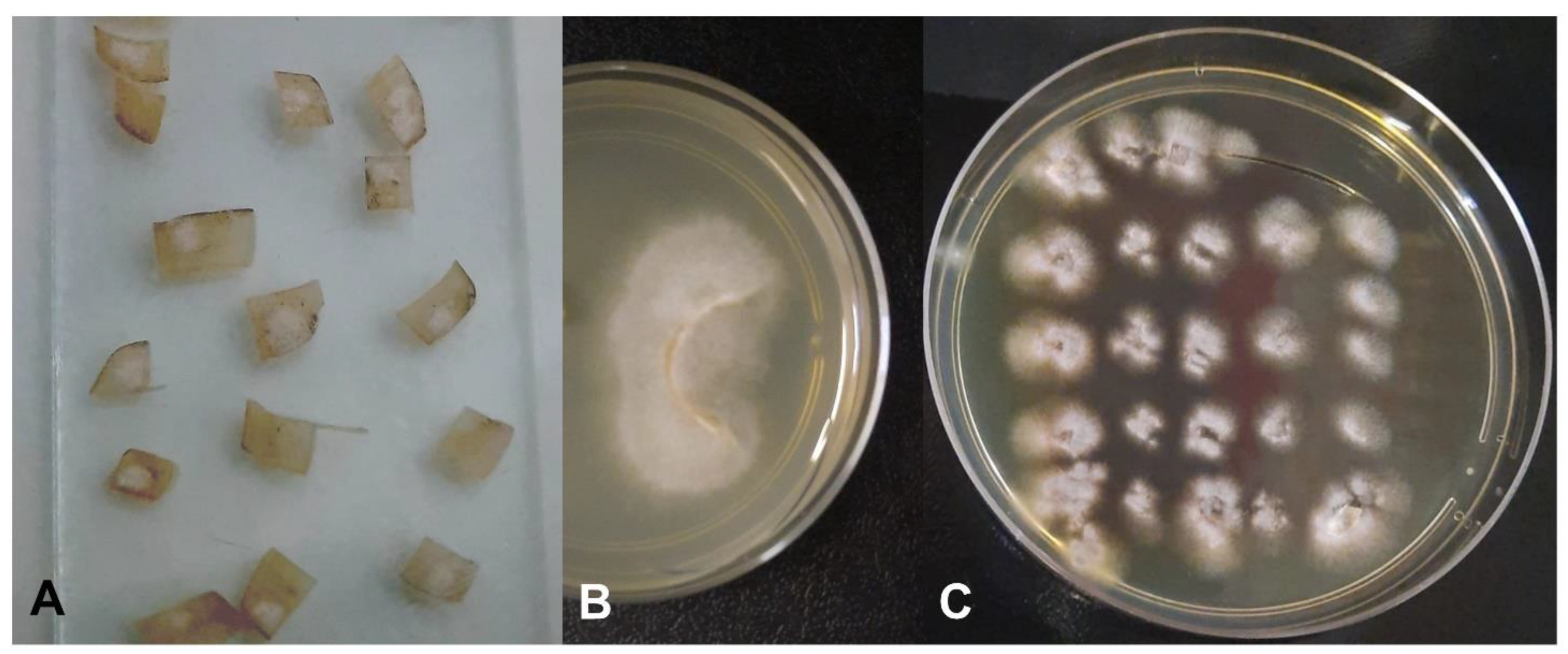
2.3. Characterization of Fungal Growth and Biofilm Formation of the Clinical Isolate in an Ex Vivo Model
2.3.1. Preparation of Inoculum
2.3.2. Pour Plate
2.3.3. Fungal Growth on the Nail Surface
2.4. In Vitro Effects of Propolis on the Isolated Fungus
2.4.1. Antifungal Activity
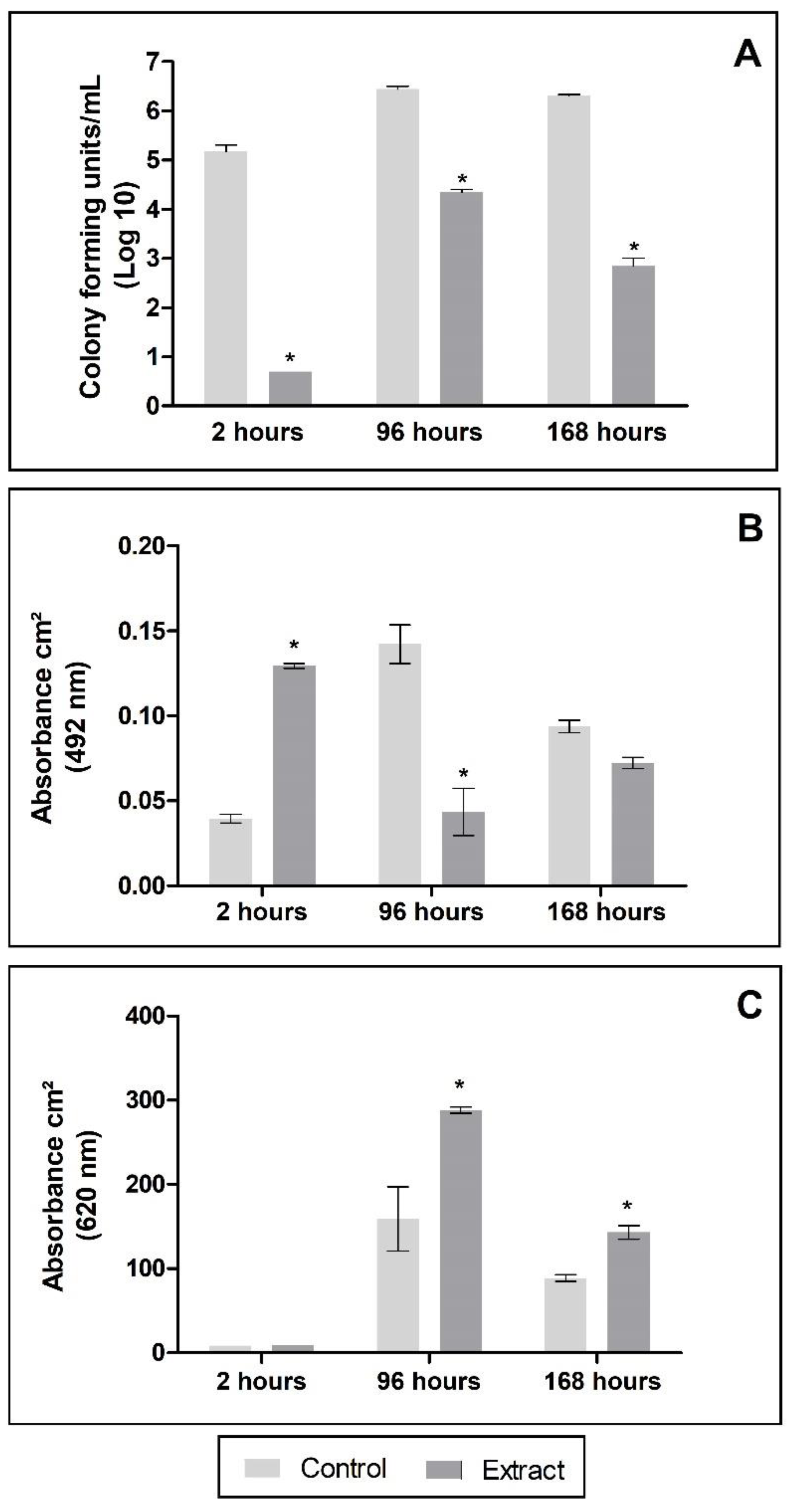
2.4.2. Antibiofilm Activity
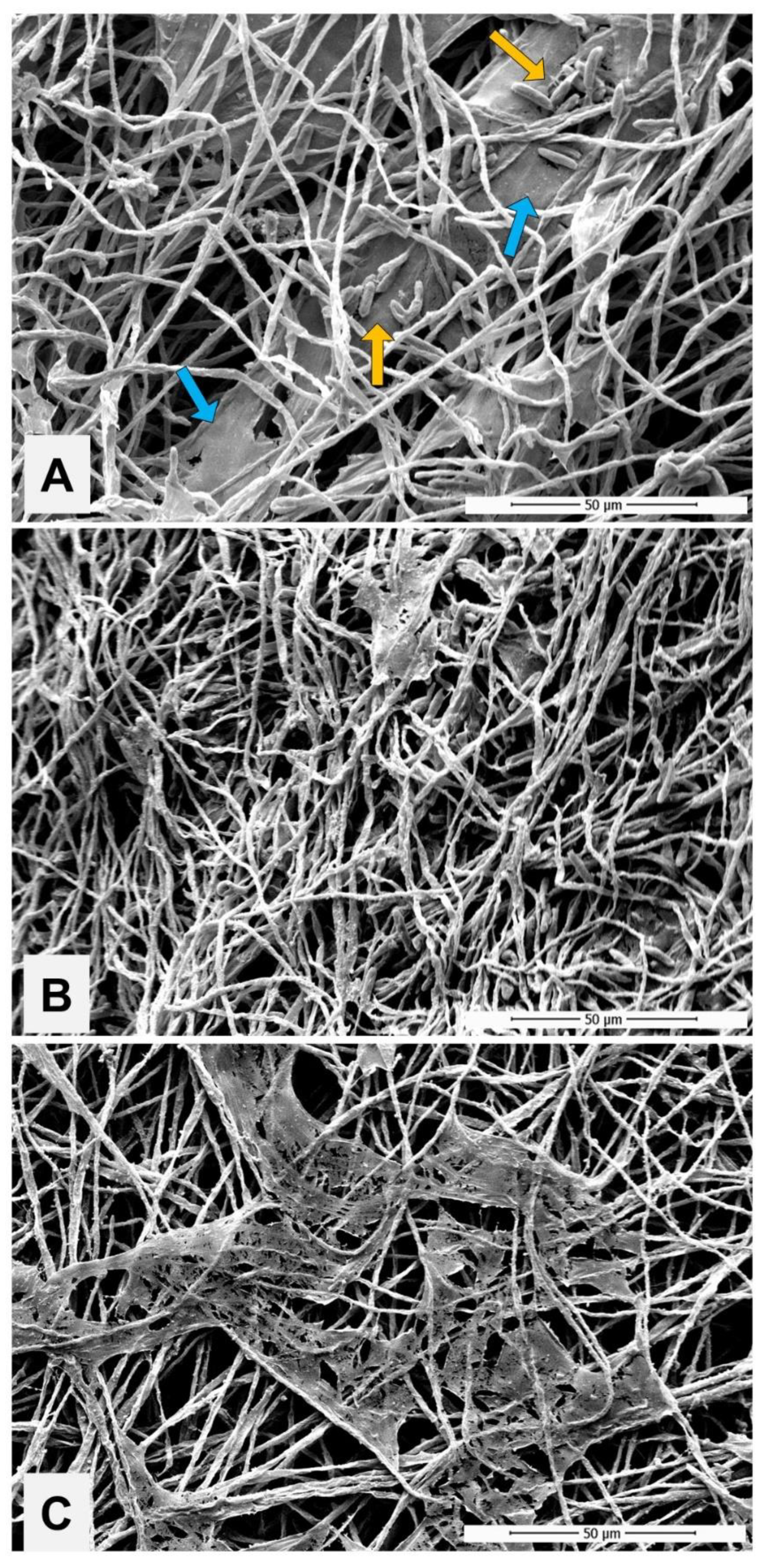
2.4.3. Impact of PE Exposure to the Biofilm
2.4.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Vlahovic, T.C.; Arenas, R. Onychomycosis: An Illustrated Guide to Diagnosis and Treatment, 1st ed.; Springer Nature: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Stewart, C.R.; Algu, L.; Kamran, R.; Leveille, C.F.; Abid, K.; Rae, C.; Lipner, S.R. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: A systematic review. J. Am. Acad. Dermatol. 2021, 85, 1227–1239. [Google Scholar] [PubMed]
- Brasch, J.; Beck-Jendroschek, V.; Wohlfeil, E. Recalcitrant purulent paronychia and onychomycosis caused by Fusarium oxysporum. J. Dtsch. Dermatol. Ges. 2012, 10, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Bombassaro, A.; Vicente, V.A.; Mendes, V.; Baesso, M.L.; Negri, M.; Svidzinski, T.I. Fusarium oxysporum is an onychomycosis etiopathogenic agent. Future Microbiol. 2021, 13, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Pagano, L.; Corvatta, L.; Mele, L.; del Favero, A.; Martino, P. The epidemiology of fusariosis in patients with haematological diseases. Gimema Infection Programme. Br. J. Haematol. 2000, 111, 272–276. [Google Scholar]
- Pérez-Nadales, E.; Alastruey-Izquierdo, A.; Linares-Sicilia, M.J.; Soto-Debrán, J.C.; Abdala, E.; García-Rodríguez, J.; Montejo, M.; Muñoz, P.; Lletí, M.S.; Rezusta, A.; et al. Invasive Fusariosis in Nonneutropenic Patients, Spain, 2000–2015. Emerg. Infect. Dis. 2021, 27, 24–36. [Google Scholar] [CrossRef]
- Tupaki-Sreepurna, A.; Kindo, A.J. Fusarium sp.: The versatile pathogen. Indian J. Med. Microbiol. 2018, 36, 8–17. [Google Scholar] [CrossRef]
- Chacon-Cruz, E.; Male-Valle, F.; Rivas-Landeros, R.M.; Lopatynsky-Reyes, E.Z.; Almada-Salazar, L.A.; Becka, C.M. Severe mycotic keratoconjunctivitis caused by Fusarium sp. in an immunocompetent child successfully treated with intravenous voriconazole and keratoplasty: Case report and short review of the literature. Ther. Adv. Infect. Dis. 2019, 6, 2049936118811213. [Google Scholar] [CrossRef] [Green Version]
- Bissan, A.T.; Iken, M.; Doumbia, M.; Ou-Khedda, N.; El Alaoui, M.; Lmimouni, B. Fusarioses to Fusarium solani in an immunocompetent and immunocompromised diagnosed in military hospital of Rabat. J. Mycol. Med. 2017, 27, 382–386. [Google Scholar] [CrossRef]
- Douglas, A.P.; Chen, S.C.-A.; Slavin, M.A. Emerging infections caused by non-Aspergillus filamentous fungi. Clin. Microbiol. Infect. 2016, 22, 670–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, P.D.; Heidrich, D.; Corrêa, C.; Scroferneker, M.L.; Vettorato, G.; Fuentefria, A.M.; Goldani, L.Z. Genetic diversity and antifungal susceptibility of Fusarium isolates in onychomycosis. Mycoses 2017, 60, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Guilhermetti, E.; Takahachi, G.; Shinobu, C.S.; Svidzinski, T.I.E. Fusarium spp. as agents of onychomycosis in immunocompetent hosts. Int. J. Dermatol. 2007, 46, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Conrado, P.C.; Sakita, K.M.; Arita, G.S.; Gonçalves, R.S.; Cesar, G.B.; Caetano, W.; Hioka, N.; Voidaleski, M.F.; Vicente, V.A.; Svidzinski, T.I.; et al. Hypericin-P123-photodynamic therapy in an ex vivo model as an alternative treatment approach for onychomycosis caused by Fusarium spp. Photodiagnosis Photodyn. Ther. 2021, 35, 102414. [Google Scholar] [CrossRef]
- Shah, S.R.; Dalal, B.D.; Modak, M.S. Nondermatophytic onychomycosis by Fusarium oxysporum in an immunocompetent host. J. Mycol. Med. 2016, 26, e18–e21. [Google Scholar] [CrossRef]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A. Fusarium onychomycosis: Prevalence, clinical presentations, response to itraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int. J. Dermatol. 2015, 54, 1275–1282. [Google Scholar] [CrossRef]
- Nucci, M.; Varon, A.G.; Garnica, M.; Akiti, T.; Barreiros, G.; Trope, B.M.; Nouér, A.S. Increased incidence of invasive fusariosis with cutaneous portal of entry, Brazil. Emerg. Infect. Dis. 2015, 19, 1567–1572. [Google Scholar] [CrossRef]
- Varon, A.G.; Nouer, S.A.; Barreiros, G.; Trope, B.M.; Magalhães, F.; Akiti, T.; Garnica, M.; Nucci, M. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J. Infect. 2014, 68, 85–89. [Google Scholar] [CrossRef]
- Costa, P.S.; Mendes, V.; Veiga, F.F.; Negri, M.; Svidzinski, T.I.E. Relevant insights into onychomycosis’ pathogenesis related to the effectiveness topical treatment. Microb. Pathog. 2022, 169, 105640. [Google Scholar] [CrossRef]
- Hanna, S.; Andriessen, A.; Beecker, J.; Gilbert, M.; Goldstein, E.; Kalia, S.; King, A.; Kraft, J.; Lynde, C.; Singh, D.; et al. Clinical Insights About Onychomycosis and Its Treatment: A Consensus. J. Drugs Dermatol. 2018, 17, 253–262. [Google Scholar]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Effects of Propolis on Infectious Diseases of Medical Relevance. Biology 2021, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Barros, I.L.E.; Corrêa, J.L.; Veiga, F.F.; Bruschi, M.L.; Negri, M.; Svidzinski, T.I.E. Effect of propolis on fungi of human clinical interest. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Elsevier: Amsterdam, The Netherlands, 2022; pp. 173–199. [Google Scholar]
- Khosravi, A.R.; Shokri, H.; Nikaein, D.; Mansouri, P.; Erfanmanesh, A.; Chalangari, R.; Katalin, M. Yeasts as important agents of onychomycosis: In vitro activity of propolis against yeasts isolated from patients with nail infection. J. Altern. Complement. Med. 2013, 19, 57–62. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Shinobu, C.S.; Longhini, R.; Franco, S.L.; Svidzinski, T.I.E. Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Mem. Inst. Oswaldo Cruz. 2006, 101, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalben-Dota, K.F.; Faria, M.G.I.; Bruschi, M.L.; Pelloso, S.M.; Lopes-Consolaro, M.E.; Svidzinski, T.I.E. Antifungal activity of propolis extract against yeasts isolated from vaginal exudates. J. Altern. Complement. Med. 2010, 16, 285–290. [Google Scholar] [CrossRef]
- Corrêa, J.L.; Veiga, F.F.; Jarros, I.C.; Costa, M.I.; Castilho, P.F.; de Oliveira, K.M.P.; Rosseto, H.C.; Bruschi, M.L.; Svidzinski, T.I.E.; Negri, M. Propolis extract has bioactivity on the wall and cell membrane of Candida albicans. J. Ethnopharmacol. 2020, 256, 112791. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos-Pontello, V.; Veiga, F.F.; Gadelha, M.C.; Ribeiro, M.; Negri, M.; Estivalet Svidzinski, T.I. The Success of Topical Treatment of Onychomycosis Seems to Be Influenced by Fungal Features. Evid. Based Complement. Alternat. Med. 2021, 2021, 5553634. [Google Scholar] [CrossRef]
- Galletti, J.; Tobaldini-Valerio, F.K.; Silva, S.; Kioshima, É.S.; Trierveiler-Pereira, L.; Bruschi, M.; Negri, M.; Estivalet Svidzinski, T.I. Antibiofilm activity of propolis extract on Fusarium species from onychomycosis. Future Microbiol. 2017, 12, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Veiga, F.F.; Gadêlha, M.; Da Silva, M.R.T.; Costa, M.I.; Kischkel, B.; De Castro-Hoshino, L.V.; Sato, F.; Baesso, M.L.; Voidaleski, M.F.; Vasconcellos-Pontello, V.; et al. Propolis Extract for Onychomycosis Topical Treatment: From Bench to Clinic. Front. Microbiol. 2018, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- Lipner, S.R.; Joseph, W.S.; Vlahovic, T.C.; Scher, R.K.; Rich, P.; Ghannoum, M.; Daniel, C.R.; Elewski, B. Therapeutic Recommendations for the Treatment of Toenail Onychomycosis in the US. J. Drugs Dermatol. 2021, 20, 1076–1084. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, E.W.; Message, D.; Negri, G.; Salatino, A.; Stringheta, P.C. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid. Based Complement. Alternat. Med. 2010, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. A Paradigm Shift in the Treatment and Management of Onychomycosis. Skin Appendage Disord. 2021, 7, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Baesso, M.L.; Silva, S.; Negri, M.; Svidzinski, T.I.E. Characterization of a biofilm formed by Fusarium oxysporum on the human nails. Int. J. Dermatol. 2022, 61, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Galletti, J.; Negri, M.; Grassi, F.L.; Kioshima-Cotica, É.S.; Svidzinski, T.I.E. Fusarium spp. is able to grow and invade healthy human nails as a single source of nutrients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1767–1772. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Diluition Antifungal Suceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI standart M38; Clinical and Laboratory Standarts Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Barros, I.L.E.; Veiga, F.F.; Corrêa, J.L.; Jarros, I.C.; Negri, M.; Svidzinski, T.I.E. Standardization of resazurin use in susceptibility testing of natural products against yeasts in planktonic cells and in biofilms formation. Acta Sci. Biol. Sci. 2021, 43, e55700. [Google Scholar] [CrossRef]
- Crous, P.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Gupta, C.; Jongman, M.; Das, S.; Snehaa, K.; Bhattacharya, S.N.; Seyedmousavi, S.; van Diepeningen, A.D. Genotyping and In Vitro Antifungal Susceptibility Testing of Fusarium Isolates from Onychomycosis in India. Mycopathologia 2016, 181, 497–504. [Google Scholar] [CrossRef]
- Pakshir, K.; Kamali, M.; Nouraei, H.; Zomorodian, K.; Motamedi, M.; Mahmoodi, M. Molecular characterization and antifungal activity against non-dermatophyte molds causing onychomycosis. Sci. Rep. 2021, 11, 20736. [Google Scholar] [CrossRef]
- Rammlmair, A.; Mühlethaler, K.; Haneke, E. Fusarium onychomycoses in Switzerland-A mycological and histopathological study. Mycoses 2019, 62, 928–931. [Google Scholar] [CrossRef]
- Diongue, K.; Ndiaye, M.; Seck, M.C.; Diallo, M.A.; Badiane, A.S.; Ndiaye, D. Onychomycosis Caused by spp. in Dakar, Senegal: Epidemiological, Clinical, and Mycological Study. Dermatol. Res. Pract. 2017, 2017, 1268130. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H.; Hiruma, M.; Matsumoto, T.; Kano, R.; Ihn, H. Ungual hyalohyphomycosis caused by Fusarium proliferatum in an immunocompetent patient. J. Dermatol. 2017, 44, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Summerbell, R.C.; Venkataraman, M.; Quinlan, E.M. Non-dermatophyte mould onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1628–1641. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Matsumoto, T.; Kimura, U.; Hiruma, M.; Kano, R.; Yaguchi, T.; Ihn, H. Non-dermatophyte Mould Onychomycosis in Japan. Med. Mycol. 2021, 61, 23–31. [Google Scholar] [CrossRef]
- Gupta, A.K.; Daigle, D.; Carviel, J.L. The role of biofilms in onychomycosis. J. Am. Acad. Dermatol. 2016, 74, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Rujitharanawong, C.; Leeyaphan, C.; Bunyaratavej, S.; Prasertworanun, N.; Muanprasart, C.; Matthapan, L. Dermatophytoma: An under-recognized condition. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 188–189. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Muanprasart, C.; Leeyaphan, C.; Matthapan, L.; Prasertworonun, N.; Pattanaprichakul, P. Dermatophytoma: The underrecognized condition by general practitioners and nondermatologist specialists. J. Am. Acad. Dermatol. 2014, 70, AB108. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Galinari, C.B.; Conrado, P.C.; Sakita, K.M.; Arita, G.S.; Melo, R.C.; Capoci, I.R.; Dos Santos, R.S.; Bruschi, M.L.; Kioshima, E.S.; Svidzinski, T.I.; et al. New approach to the use of propolis against dermatomycosis. Nat. Prod. Res. 2021, 36, 4215–4220. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]


| MIC/MBIC | MFC/MBEC | |||
|---|---|---|---|---|
| Extract |  |  | ||
| Gel |  |  | ||
| Extract * | Gel * | Extract * | Gel * | |
| Planktonic | 187.50 | 450 | 375 | 450 |
| 24 h biofilm | 375 | 450 | 375 | 450 |
| 48 h biofilm | 375 | 450 | 375 | 450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.d.S.; Cruz, E.; Veiga, F.; Jarros, I.C.; Negri, M.; Svidzinski, T.I.E. Efficacy of Propolis Gel on Mature Biofilm Formed by Neocosmospora keratoplastica Isolated from Onychomycosis. J. Fungi 2022, 8, 1216. https://doi.org/10.3390/jof8111216
Costa PdS, Cruz E, Veiga F, Jarros IC, Negri M, Svidzinski TIE. Efficacy of Propolis Gel on Mature Biofilm Formed by Neocosmospora keratoplastica Isolated from Onychomycosis. Journal of Fungi. 2022; 8(11):1216. https://doi.org/10.3390/jof8111216
Chicago/Turabian StyleCosta, Polyana de Souza, Elton Cruz, Flávia Veiga, Isabelle Carrilho Jarros, Melyssa Negri, and Terezinha Inez Estivalet Svidzinski. 2022. "Efficacy of Propolis Gel on Mature Biofilm Formed by Neocosmospora keratoplastica Isolated from Onychomycosis" Journal of Fungi 8, no. 11: 1216. https://doi.org/10.3390/jof8111216






