Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mycorrhizal Inoculums
2.2. Plant Culture and Salt and AMF Treatments
2.3. Variable Determinations
2.4. Data Analysis
3. Results and Discussion
3.1. Effects of NaCl on Root Colonization of Tomato by P. occultum
3.2. Effects of P. occultum on Plant Growth of Tomato Exposed to NaCl Stress
3.3. Effects of P. occultum on Index of N Balance, Chlorophyll, and Flavonoids of Tomato Exposed to NaCl Stress
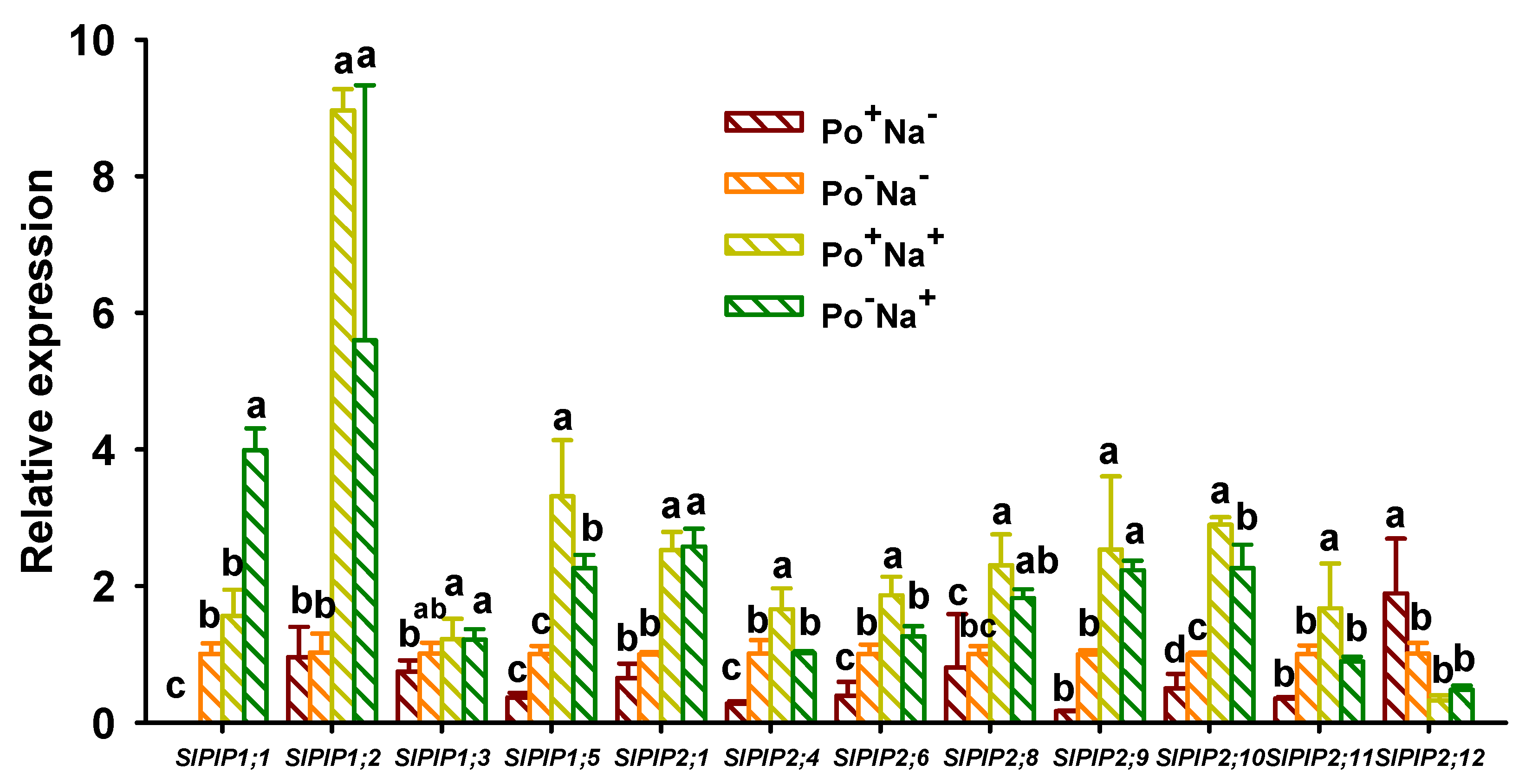
3.4. Effects of AMF on PIPs’ Expressions in Leaves of Tomato under NaCl Stress
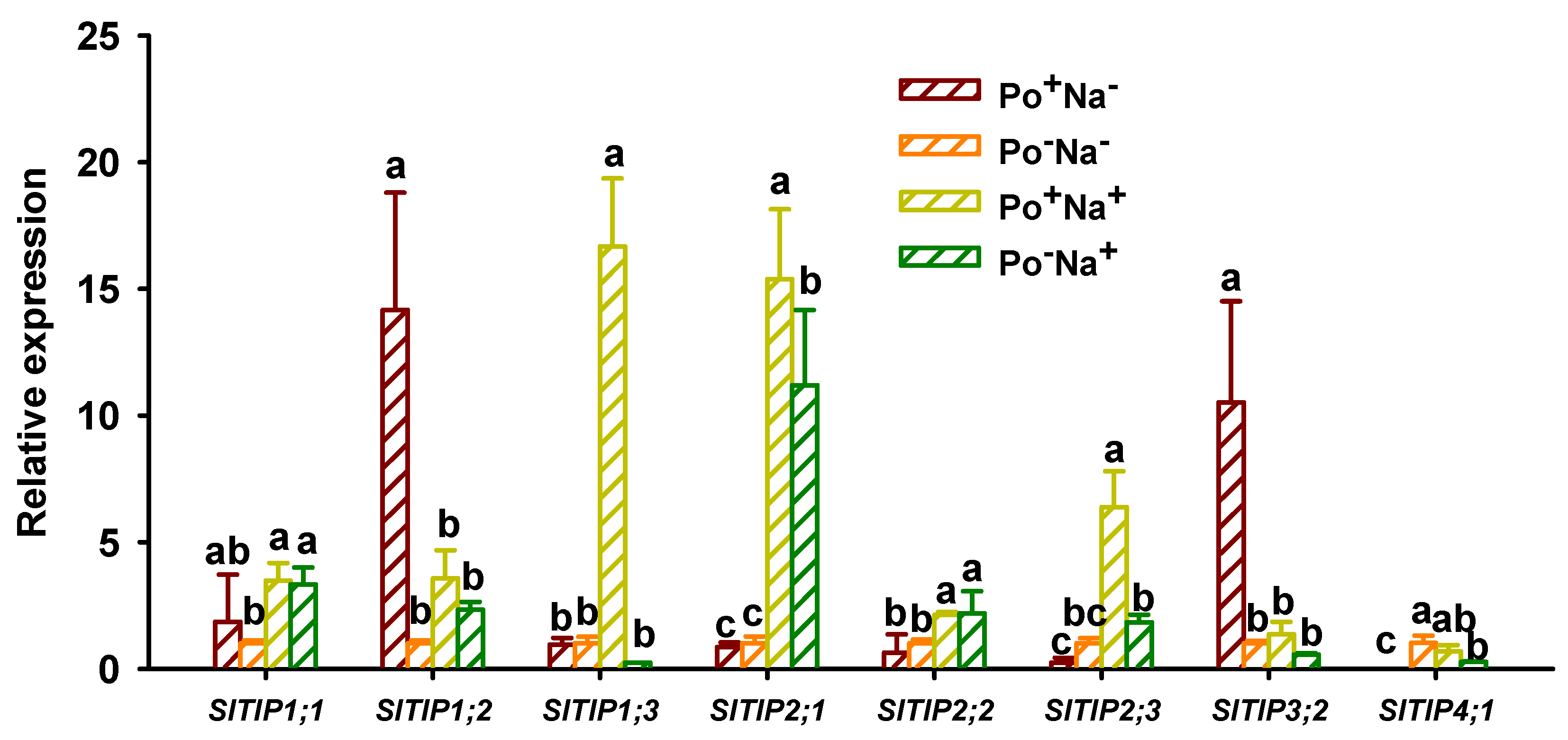
3.5. Effects of AMF on TIPs’ Expressions in Leaves of Tomato under NaCl Stress
3.6. Effects of AMF on SOS Expressions in Leaves of Tomato under NaCl Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.Y.; Cheng, D.H.; Yan, X.; Zhao, J.; Yuan, F.; Xu, H.; Zhang, Y. Effect of exogenous SNP on the growth and antioxidant enzyme activities in melon seedlings under salt stress. Acta. Bot. Boreal-Occident. Sin. 2022, 42, 994–1002. [Google Scholar]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, J.T.; Dong, X.L.; Tian, L.; Zhang, X.J.; Liu, X.J.; Sun, H.Y. Regulation of fulvic acid on tomato yield and quality under saline water irrigation. Chin. J. Eco-Agric. 2022, 30, 1–11. [Google Scholar]
- Coban, A.; Akhoundnejad, Y.; Dere, S.; Dasgan, H.Y. Impact of salt-tolerant rootstock on the enhancement of sensitive tomato plant responses to salinity. HortScience 2020, 55, 35–39. [Google Scholar] [CrossRef]
- Higo, M.; Azuma, M.; Kamiyoshihara, Y.; Kanda, A.; Tatewaki, Y.; Isobe, K. Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Saia, S.; Aissa, E.; Luziatelli, F.; Ruzzi, M.; Colla, G.; Ficca, A.G.; Cardarelli, M.; Rouphael, Y. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 2019, 30, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Soldo, B.; Ban, S.G.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Bubola, K.B.; Colla, G.; Rouphael, Y.; et al. Fruit quality and volatile compound composition of processing tomato as affected by fertilisation practices and arbuscular mycorrhizal fungi application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hort. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Serio, C.D.; Maserti, B.; Guerrieri, E.; et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Aliasgharzad, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2009, 331, 313–327. [Google Scholar] [CrossRef]
- Latef, A.A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Huertas, R.; Olías, R.; Eljakaoui, Z.; Gálvez, F.J.; Li, J.; De Morales, P.A.; Belver, A.; Rodríguez-Rosales, M.P. Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ. 2012, 35, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; He, C.X.; Yan, Y.; Zhang, Z.B.; Wang, H.S.; Li, H.X.; Tang, H.R. Regulative effect of arbuscular mycorrhizal fungi on water absorption and expression of aquaporin genes in tomato under salt stress. Acta. Hortic. Sin. 2011, 38, 273–280. [Google Scholar]
- Cheng, X.-F.; Wu, H.-H.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Thakral, V.; Padalkar, G.; Rajora, N.; Dhiman, P.; Raturi, G.; Sharma, Y.; Tripathi, D.K.; Deshmukh, R.; Sharma, T.R.; et al. Significance of solute specificity, expression, and gating mechanism of tonoplast intrinsic protein during development and stress response in plants. Physiol. Plant 2021, 172, 258–274. [Google Scholar] [CrossRef]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 2013, 8, e79052. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Wang, Y.S. Seven species of VA mycorrhizal fungi from northern China. Acta. Mycol. Sin. 1991, 10, 13–21. [Google Scholar]
- Wang, Y.S.; Zhang, S.B.; Zhang, M.Q. Resources and Germplasm of Arbuscular Mycorrhizal Fungi in China; China Agriculture Press: Beijing, China, 2012; pp. 1–264. [Google Scholar]
- Amacher, M.C.; Henderson, R.E.; Breithaupt, M.D.; Seale, C.L.; LaBauve, J.M. Unbuffered and buffered salt methods for exchangeable cations and effective cation-exchange capacity. Soil. Sci. Soc. Am. J. 1990, 54, 1036–1042. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Ait-El-Mokhtar, M.; Ben Laouane, R.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil. Sci. Plant. Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Chen, G.; Ji, R.T.; Shi, W.M. Effects of nitrogen fertilizer levels on nitrogen balance index and yield of hybrid super rice. Soils 2021, 53, 700–706. [Google Scholar]
- Dai, F.; Rong, Z.; Wu, Q.; Abd-Allah, E.F.; Liu, C.; Liu, S. Mycorrhiza improves plant growth and photosynthetic characteristics of tea plants in response to drought stress. Biocell 2022, 46, 1339–1346. [Google Scholar] [CrossRef]
- Fattahi, M.; Mohammadkhani, A.; Shiran, B.; Baninasab, B.; Ravash, R.; Gogorcena, Y. Beneficial effect of mycorrhiza on nutritional uptake and oxidative balance in pistachio (Pistacia spp.) rootstocks submitted to drought and salinity stress. Sci. Hortic. 2021, 281, 109937. [Google Scholar] [CrossRef]
- Fayaz, F.; Zahedi, M. Beneficial effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) nutritional status and tolerance indices under soil salinity stress. J. Plant Nutr. 2022, 45, 185–201. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; He, X.-H. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta. Physiol. Plant 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Kumar, K.; Mosa, K.A.; Meselhy, A.G.; Dhankher, O.P. Molecular insights into the plasma membrane intrinsic proteins roles for abiotic stress and metalloids tolerance and transport in plants. Indian J. Plant Physiol. 2018, 23, 721–730. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.-N.; Shu, B.; Wu, Q.-S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through im-proved photosynthesis, water status, and K+/Na+ Homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Janah, I.; Meddich, A.; Elhasnaoui, A.; Khayat, S.; Anli, M.; Boutasknit, A.; Aissam, S.; Loutfi, K. Arbuscular mycorrhizal fungi mitigates salt stress toxicity in Stevia rebaudiana Bertoni through the modulation of physiological and biochemical responses. J. Soil. Sci. Plant Nutr. 2021. [Google Scholar] [CrossRef]
- He, J.D.; Dong, T.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar]
- Bienert, G.P.; Møller, A.L.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Ding, Y.-E.; Fan, Q.-F.; He, J.-D.; Wu, H.-H.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Effects of mycorrhizas on physiological performance and root TIPs expression in trifoliate orange under salt stress. Arch. Agron. Soil. Sci. 2020, 66, 182–192. [Google Scholar] [CrossRef]
- Belver, A.; Olías, R.; Huertas, R.; Rodríguez-Rosales, M.P. Involvement of SlSOS2 in tomato salt tolerance. Bioengineered 2012, 3, 298–302. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Diao, F.; Dang, Z.; Xu, J.; Ding, S.; Hao, B.; Zhang, Z.; Zhang, J.; Wang, L.; Guo, W. Effect of arbuscular mycorrhizal symbiosis on ion homeostasis and salt tolerance-related gene expression in halophyte Suaeda salsa under salt treatments. Microbiol. Res. 2021, 245, 126688. [Google Scholar] [CrossRef]
- Olias, R.; Eljakaoui, Z.; Pardo, J.M.; Belver, A. The Na+/H+ exchanger SOS1 controls extrusion and distribution of Na+ in tomato plants under salinity conditions. Plant Signal. Behav. 2009, 4, 973–976. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Root Mycorrhizal Colonization (%) | Plant Height (cm) | Stem Diameter (cm) | Total Plant Biomass (g/plant) |
|---|---|---|---|---|
| Po+Na− | 49.1 ± 7.6 a | 50.6 ± 5.2 a | 2.4 ± 0.2 a | 18.52 ± 1.49 a |
| Po−Na− | 0 c | 47.0 ± 4.1 a | 2.0 ± 0.3 b | 12.51 ± 1.39 b |
| Po+Na+ | 38.6 ± 7.1 b | 38.5 ± 4.0 b | 2.6 ± 0.3 a | 12.47 ± 1.13 b |
| Po−Na+ | 0 c | 27.4 ± 2.7 c | 1.9 ± 0.1 b | 8.70 ± 0.71 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.-M.; Li, Q.-S.; Liu, M.-Y.; Hashem, A.; Al-Arjani, A.-B.F.; Alenazi, M.M.; Abd_Allah, E.F.; Muthuramalingam, P.; Wu, Q.-S. Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress. J. Fungi 2022, 8, 1305. https://doi.org/10.3390/jof8121305
Liang S-M, Li Q-S, Liu M-Y, Hashem A, Al-Arjani A-BF, Alenazi MM, Abd_Allah EF, Muthuramalingam P, Wu Q-S. Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress. Journal of Fungi. 2022; 8(12):1305. https://doi.org/10.3390/jof8121305
Chicago/Turabian StyleLiang, Sheng-Min, Qiu-Shuang Li, Ming-Yang Liu, Abeer Hashem, Al-Bandari Fahad Al-Arjani, Mekhled M. Alenazi, Elsayed Fathi Abd_Allah, Pandiyan Muthuramalingam, and Qiang-Sheng Wu. 2022. "Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress" Journal of Fungi 8, no. 12: 1305. https://doi.org/10.3390/jof8121305
APA StyleLiang, S.-M., Li, Q.-S., Liu, M.-Y., Hashem, A., Al-Arjani, A.-B. F., Alenazi, M. M., Abd_Allah, E. F., Muthuramalingam, P., & Wu, Q.-S. (2022). Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress. Journal of Fungi, 8(12), 1305. https://doi.org/10.3390/jof8121305









