Identification and Characterization of an Intergenic “Safe Haven” Region in Human Fungal Pathogen Cryptococcus gattii
Abstract
:1. Introduction
2. Materials and Methods
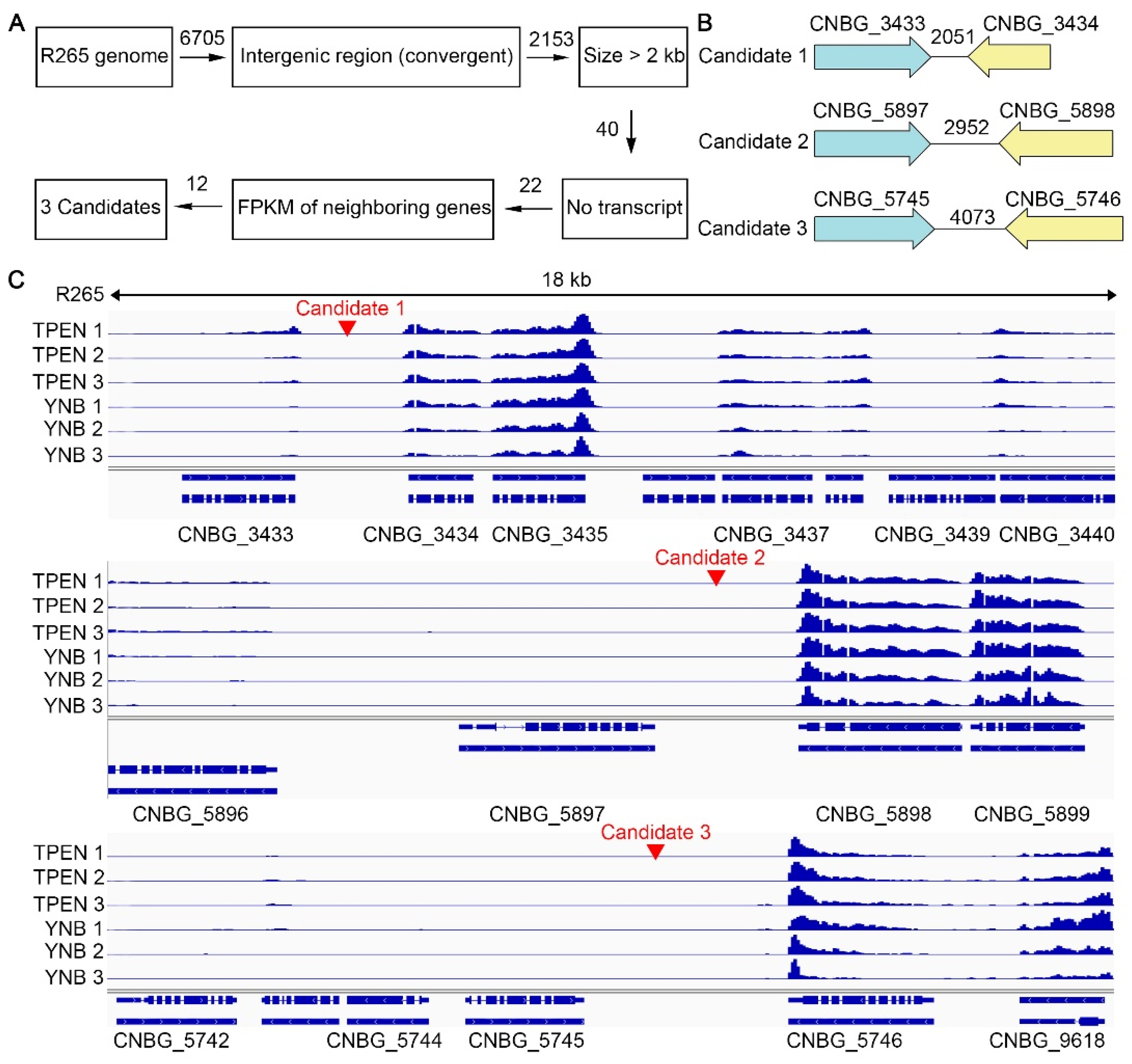
2.1. Procedures to Identify Potential Safe Haven Sites
2.2. Strains and Media
2.3. Generation of mNeonGreen Strains
2.4. Quantitative Real-Time PCR
2.5. Pulsed Field Gel Electrophoresis
2.6. Fluorescence Microscopy
2.7. In Vitro Phenotypic Analysis
2.8. Phagocytosis Assay
3. Results
3.1. Identification of the Putative Safe Haven Regions in the Genome of C. gattii Reference Strain R265
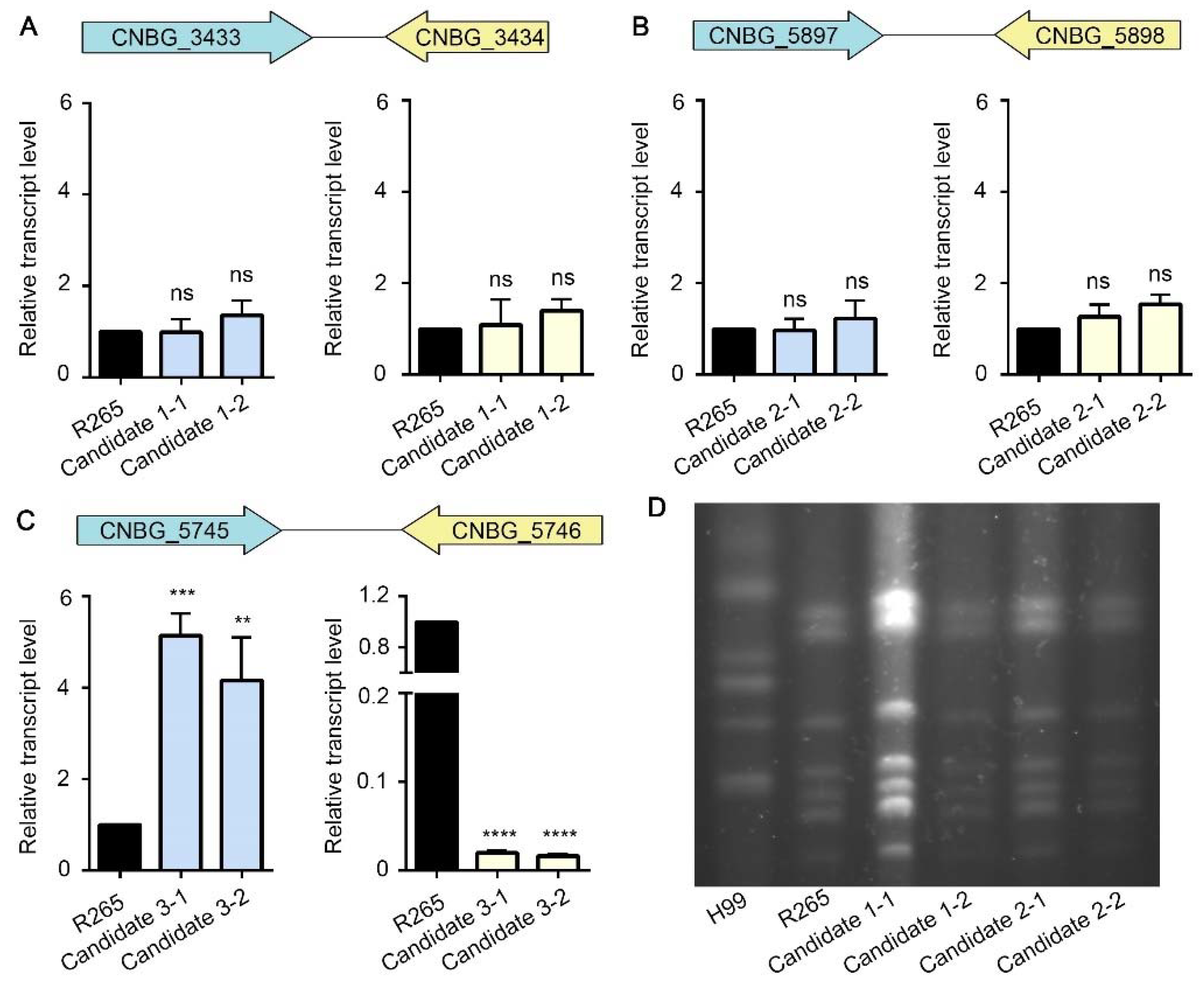
3.2. Insertion of Foreign DNA into the Candidate 1 or the Candidate 2 Site Has No Significant Impact on the Expression of the Neighboring Genes
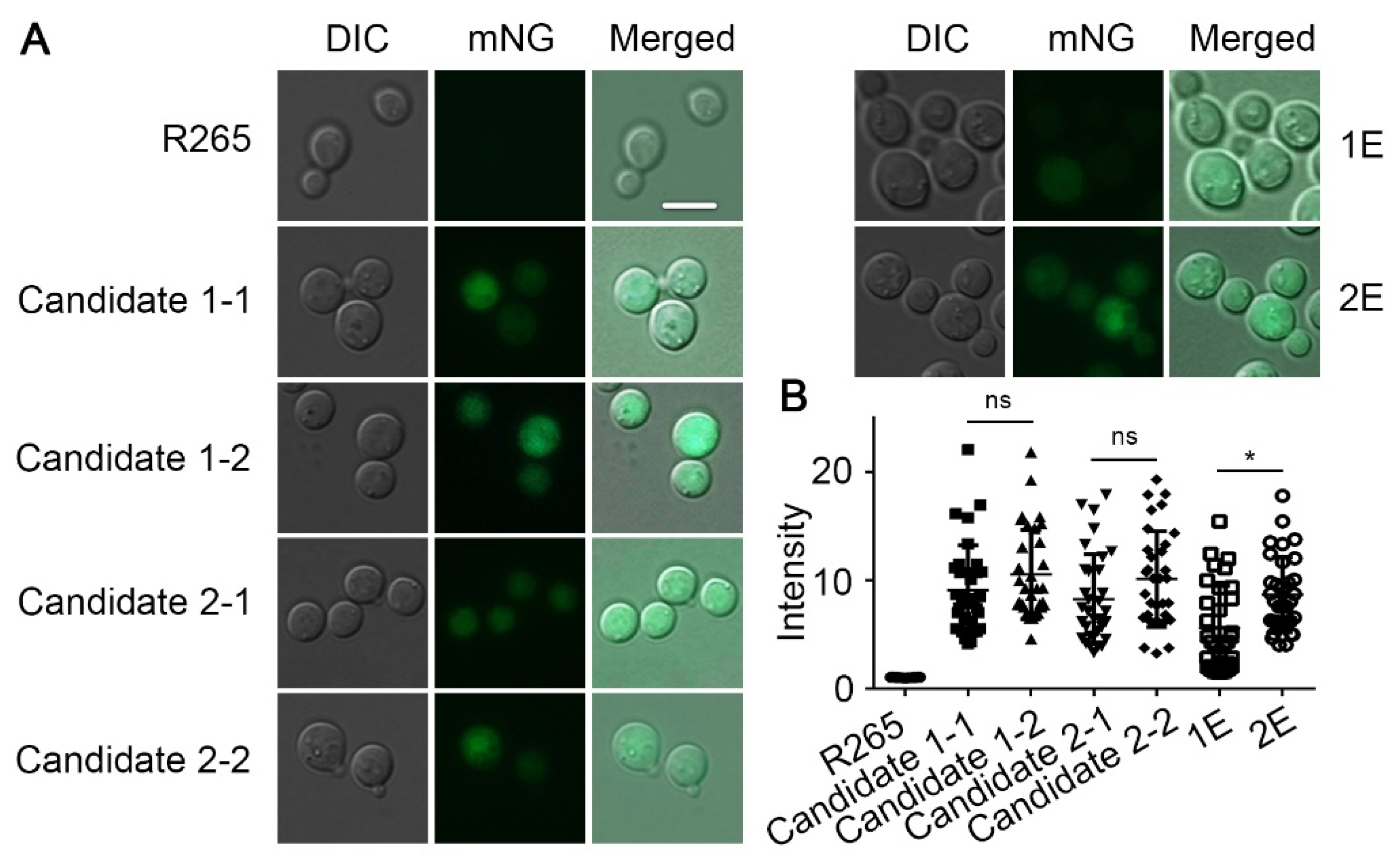
3.3. Genes Inserted in the Candidate 1 and Candidate 2 Sites Are Expressed Irrespective of the Insertion Direction
3.4. Insertion of the Foreign DNA Construct in the Candidate 1 and Candidate 2 Sites Has No Significant Impact on Growth of C. gattii, Its Response to Various Stresses, or Its Ability to Undergo Sexual Reproduction
3.5. A Similar Intergenic Region Exists in Strains of Other Molecular Types within the C. gattii Species Complex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life cycle of Cryptococcus neoformans. Ann. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Ann. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef]
- Casadevall, A.; Perfect, J.R. Cryptococcus neoformans; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Chen, J.; Varma, A.; Diaz, M.R.; Litvintseva, A.P.; Wollenberg, K.K.; Kwon-Chung, K.J. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg. Infect. Dis. 2008, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef] [Green Version]
- Bielska, E.; May, R.C. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016, 16, fov106. [Google Scholar] [CrossRef] [Green Version]
- Stephen, C.; Lester, S.; Black, W.; Fyfe, M.; Raverty, S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 2002, 43, 792–794. [Google Scholar]
- MacDougall, L.; Kidd, S.E.; Galanis, E.; Mak, S.; Leslie, M.J.; Cieslak, P.R.; Kronstad, J.W.; Morshed, M.G.; Bartlett, K.H. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg. Infect. Dis. 2007, 13, 42–50. [Google Scholar] [CrossRef]
- Xu, J.; Vilgalys, R.; Mitchell, T.G. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 2000, 9, 1471–1481. [Google Scholar] [CrossRef] [Green Version]
- Toffaletti, D.L.; Rude, T.H.; Johnston, S.A.; Durack, D.T.; Perfect, J.R. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 1993, 175, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Hettler, E.; Wickes, B.L. Split marker transformation increases homologous integration frequency in Cryptococcus neoformans. Fungal Genet. Biol 2006, 43, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chacko, N.; Wang, L.; Pavuluri, Y. Generation of stable mutants and targeted gene deletion strains in Cryptococcus neoformans through electroporation. Med. Mycol. 2015, 53, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lin, X. Multiple applications of a transient CRISPR-Cas9 coupled with electroporation (TRACE) system in the Cryptococcus neoformans species complex. Genetics 2018, 208, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edman, J.C.; Kwon-Chung, K.J. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol. Cell. Biol. 1990, 10, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Corpina, R.A.; Goldberg, J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 2001, 412, 607–614. [Google Scholar] [CrossRef]
- Tenney, A.E.; Brown, R.H.; Vaske, C.; Lodge, J.K.; Doering, T.L.; Brent, M.R. Gene prediction and verification in a compact genome with numerous small introns. Genome Res. 2004, 14, 2330–2335. [Google Scholar] [CrossRef] [Green Version]
- Arras, S.D.; Chitty, J.L.; Blake, K.L.; Schulz, B.L.; Fraser, J.A. A genomic safe haven for mutant complementation in Cryptococcus neoformans. PLoS ONE 2015, 10, e0122916. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, X. An intergenic “safe haven” region in Cryptococcus neoformans serotype D genomes. Fungal Genet. Biol. 2020, 144, 103464. [Google Scholar] [CrossRef]
- Pham, T.; Xie, X.; Lin, X. An intergenic “safe haven” region in Aspergillus fumigatus. Med. Mycol. 2020, 58, 1178–1186. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Maybruck, B.T.; Donlin, M.J.; Chang, A.L.; Kayode, S.; Ormerod, K.L.; Fraser, J.A.; Doering, T.L.; Lodge, J.K. A fluorogenic C. neoformans reporter strain with a robust expression of m-cherry expressed from a safe haven site in the genome. Fungal Genet. Biol. 2017, 108, 13–25. [Google Scholar] [CrossRef]
- Hu, P.; Ding, H.; Shen, L.; He, G.J.; Liu, H.; Tian, X.; Tao, C.; Bai, X.; Liang, J.; Jin, C.; et al. A unique cell wall synthetic response evoked by glucosamine determines pathogenicity-associated fungal cellular differentiation. PLoS Genet. 2021, 17, e1009817. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Baker, L.G.; Lam, W.C.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Cryptococcus neoformans Cda1 and its chitin deacetylase activity are required for fungal pathogenesis. mBio 2018, 9, e02087-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.; Davy, A.; Holmes, S.; Sun, S.; Yadav, V.; Gusa, A.; Coelho, M.A.; Heitman, J. Dynamic genome plasticity during unisexual reproduction in the human fungal pathogen Cryptococcus deneoformans. PLoS Genet. 2021, 17, e1009935. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, W.; Bruno, V.M.; Phan, Q.T.; Solis, N.V.; Woolford, C.A.; Ehrlich, R.L.; Shetty, A.C.; McCraken, C.; Lin, J.; et al. Determining Aspergillus fumigatus transcription factor expression and function during invasion of the mammalian lung. PLoS Pathog. 2021, 17, e1009235. [Google Scholar] [CrossRef] [PubMed]
- Schneider Rde, O.; Fogaca Nde, S.; Kmetzsch, L.; Schrank, A.; Vainstein, M.H.; Staats, C.C. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS ONE 2012, 7, e43773. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Fan, Y.; Liao, W.; Lin, X. Plant homeodomain genes play important roles in cryptococcal yeast-hypha transition. Appl. Environ. Microbiol. 2018, 84, e01732-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenman, H.C.; Chow, S.K.; Tse, K.K.; McClelland, E.E.; Casadevall, A. The effect of L-DOPA on Cryptococcus neoformans growth and gene expression. Virulence 2011, 2, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Fan, Y.; Lin, X. Transformation of Cryptococcus neoformans by electroporation using a transient CRISPR-Cas9 expression (TRACE) system. Fungal Genet. Biol. 2020, 138, 103364. [Google Scholar] [CrossRef]
- Dickinson, D.J.; Ward, J.D.; Reiner, D.J.; Goldstein, B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 2013, 10, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lin, X. A PAS protein directs metabolic reprogramming during cryptococcal adaptation to hypoxia. mBio 2021, 12, e03602-20. [Google Scholar] [CrossRef]
- Lin, J.; Pham, T.; Hipsher, K.; Glueck, N.; Fan, Y.; Lin, X. Immunoprotection against cryptococcosis offered by Znf2 depends on capsule and the hyphal morphology. mBio 2022, 13, e02785-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, X.; Gyawali, R.; Upadhyay, S.; Foyle, D.; Wang, G.; Cai, J.J.; Lin, X. Morphotype transition and sexual reproduction are genetically associated in a ubiquitous environmental pathogen. PLoS Pathog. 2014, 10, e1004185. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Magee, B.B.; Magee, P.T. Separation of chromosomes of Cryptococcus neoformans by pulsed field gel electrophoresis. Infect. Immun. 1989, 57, 2624–2627. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Billmyre, R.B.; Mieczkowski, P.A.; Heitman, J. Unisexual reproduction drives meiotic recombination and phenotypic and karyotypic plasticity in Cryptococcus neoformans. PLoS Genet. 2014, 10, e1004849. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, P.; Sun, S.; Darwiche, S.; Idnurm, A.; Heitman, J. Transgene induced co-suppression during vegetative growth in Cryptococcus neoformans. PLoS Genet. 2012, 8, e1002885. [Google Scholar] [CrossRef]
- Zhao, Y.; Upadhyay, S.; Lin, X. PAS Domain protein Pas3 interacts with the chromatin modifier bre1 in regulating cryptococcal morphogenesis. mBio 2018, 9, e02135-18. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, V.; Chaturvedi, S. Cryptococcus gattii: A resurgent fungal pathogen. Trends Microbiol. 2011, 19, 564–571. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, C.A.; Kronstad, J.W.; Taylor, G.; Warren, R.; Yuen, M.; Hu, G.; Jung, W.H.; Sham, A.; Kidd, S.E.; Tangen, K.; et al. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2011, 2, e00342-10. [Google Scholar] [CrossRef] [Green Version]
- Farrer, R.A.; Ford, C.B.; Rhodes, J.; Delorey, T.; May, R.C.; Fisher, M.C.; Cloutman-Green, E.; Balloux, F.; Cuomo, C.A. Transcriptional heterogeneity of Cryptococcus gattii VGII compared with non-VGII lineages underpins key pathogenicity pathways. mSphere 2018, 3, e00445-18. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Zhai, B.; Lin, X.; Idnurm, A. Congenic strains for genetic analysis of virulence traits in Cryptococcus gattii. Infect. Immun. 2013, 81, 2616–2625. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, B.J.; Lin, X. On the history and applications of congenic strains in cryptococcus research. Pathogens 2020, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, E.J.; Heitman, J. Cryptococcus gattii outbreak expands into the Northwestern United States with fatal consequences. F1000 Biol. Rep. 2009, 1, 62. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Haase, G.; Chaturvedi, V.; Walsh, T.J.; Meyer, W.; Lackner, M. Taxonomy of medically important fungi in the molecular era. Lancet Infect. Dis. 2013, 13, 385–386. [Google Scholar] [CrossRef]
- Kidd, S.E.; Hagen, F.; Tscharke, R.L.; Huynh, M.; Bartlett, K.H.; Fyfe, M.; Macdougall, L.; Boekhout, T.; Kwon-Chung, K.J.; Meyer, W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 2004, 101, 17258–17263. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Pham, T.; Xie, X.; Lin, X. Identification and Characterization of an Intergenic “Safe Haven” Region in Human Fungal Pathogen Cryptococcus gattii. J. Fungi 2022, 8, 178. https://doi.org/10.3390/jof8020178
Li Y, Pham T, Xie X, Lin X. Identification and Characterization of an Intergenic “Safe Haven” Region in Human Fungal Pathogen Cryptococcus gattii. Journal of Fungi. 2022; 8(2):178. https://doi.org/10.3390/jof8020178
Chicago/Turabian StyleLi, Yeqi, Tuyetnhu Pham, Xiaofeng Xie, and Xiaorong Lin. 2022. "Identification and Characterization of an Intergenic “Safe Haven” Region in Human Fungal Pathogen Cryptococcus gattii" Journal of Fungi 8, no. 2: 178. https://doi.org/10.3390/jof8020178
APA StyleLi, Y., Pham, T., Xie, X., & Lin, X. (2022). Identification and Characterization of an Intergenic “Safe Haven” Region in Human Fungal Pathogen Cryptococcus gattii. Journal of Fungi, 8(2), 178. https://doi.org/10.3390/jof8020178





