Current Insight into Traditional and Modern Methods in Fungal Diversity Estimates
Abstract
:1. Introduction
2. Fungal Diversity: General Outline with Updated Estimates
| Title | Reference |
|---|---|
| Orders of Ascomycetes | [41] |
| Laboulbeniales as a separate class of Ascomycota, Laboulbeniomycetes | [42] |
| One hundred and seventeen clades of euagarics | [43] |
| Toward resolving family-level relationships in rust fungi (Uredinales) | [44] |
| Higher level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences | [45] |
| A phylogenetic overview of the family Pyronemataceae (Ascomycota, Pezizales) | [46] |
| A higher-level phylogenetic classification of the Fungi | [47] |
| Dictionary of the Fungi. (10th edn) | [48] |
| Outline of Ascomycota | [49] |
| Glomeromycota: two new classes and a new order | [50] |
| Entomophthoromycota: a new phylum and reclassification for entomophthoroid fungi | [51] |
| Incorporating anamorphic fungi in a natural classification checklist and notes for 2011 | [52] |
| Taxonomic revision of Ustilago, Sporisorium and Macalpinomyces | [53] |
| Phylogenetic systematics of the Gigasporales | [54] |
| List of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants | [55] |
| A phylogeny of the highly diverse cup fungus family Pyronemataceae (Pezizomycetes, Ascomycota) | [56] |
| Families of Dothideomycetes | [57] |
| Taxonomic revision of the Lyophyllaceae (Basidiomycota, Agaricales) based on a multigene phylogeny | [58] |
| Recommended names for pleomorphic genera in Dothideomycetes | [27] |
| Towards a natural classification and backbone tree for Sordariomycetes | [34] |
| Phylogenetic classification of yeasts and related taxa within Pucciniomycotina | [59] |
| Entomophthoromycota: a new overview of some of the oldest terrestrial fungi | [60] |
| Systematics of Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina | [61] |
| A phylum-level phylogenetic classification of Zygomycete fungi based on genome–scale data | [62] |
| Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota | [63] |
| Sequence–based classification and identification of fungi | [64] |
| Morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria | [65] |
| Families of Sordariomycetes | [35] |
| Proposal to conserve the name Diaporthe eres, with a conserved type, against all other competing names (Ascomycota, Diaporthales, Diaporthaceae) | [66] |
| Taxonomy and phylogeny of dematiaceous Coelomycetes | [67] |
| Multigene phylogeny of Endogonales | [68] |
| Classification of lichenized fungi in the Ascomycota and Basidiomycota-Approaching one thousand genera | [69] |
| Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps | [70] |
| An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence | [71] |
| Families, genera, and species of Botryosphaeriales | [72] |
| Ranking higher taxa using divergence times: a case study in Dothideomycetes | [73] |
| A revised family-level classification of the Polyporales (Basidiomycota) | [74] |
| Notes for genera: Ascomycota | [22] |
| Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016 | [23] |
| Notes for genera: basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota) | [19] |
| Outline of Ascomycota: 2017 | [75] |
| Classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach | [76] |
| A taxonomic summary and revision of Rozella (Cryptomycota) | [77] |
| Sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) | [78] |
| Evolutionary complexity between rust fungi (Pucciniales) and their plant hosts | [79] |
| High-level classification of the Fungi and a tool for evolutionary ecological analyses | [18] |
| Taxonomy and phylogeny of operculate Discomycetes: Pezizomycetes | [33] |
| Molecular phylogeny of the Laboulbeniomycetes (Ascomycota) | [80] |
| Families in Botryosphaeriales | [81] |
| Natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae | [82] |
| Classification of the Dictyostelids | [83] |
| Revisiting Salisapiliaceae | [84] |
| Phylogenetic revision of Savoryellaceae | [85] |
| Notes, outline and divergence times of Basidiomycota | [86] |
| A new phylogenetic classification for the Leotiomycetes | [87] |
| Taxonomy and phylogeny of hyaline-spored Coelomycetes | [88] |
| Refined families of Sordariomycetes | [36] |
| Outline of Fungi and fungus-like taxa | [17] |
| The genera of Coelomycetes | [89] |
| A higher-rank classification for rust fungi, with notes on genera | [90] |
| Indian Pucciniales: taxonomic outline with important descriptive notes | [91] |
| Incorporating asexually reproducing fungi in the natural classification and notes for pleomorphic genera | [92] |
| How to publish a new fungal species, or name, version 3.0 | [93] |
| Phylum | Class | Order | Family | Genera |
|---|---|---|---|---|
| Aphelidiomycota | 1 | 1 | 1 | 4 |
| Ascomycota | 21 | 148 | 624 | 4511 |
| Basidiobolomycota | 1 | 1 | 1 | 2 |
| Basidiomycota | 19 | 69 | 240 | 1521 |
| Blastocladiomycota | 2 | 4 | 8 | 12 |
| Calcarisporiellomycota | 1 | 1 | 1 | 2 |
| Caulochytriomycota | 1 | 1 | 1 | 1 |
| Chytridiomycota | 9 | 13 | 52 | 97 |
| Entomophthoromycota | 2 | 2 | 5 | 20 |
| Entorrhizomycota | 1 | 2 | 2 | 2 |
| Glomeromycota | 3 | 5 | 16 | 49 |
| Kickxellomycota | 6 | 6 | 7 | 61 |
| Monoblepharomycota | 3 | 3 | 7 | 9 |
| Mortierellomycota | 1 | 1 | 1 | 6 |
| Mucoromycota | 3 | 3 | 17 | 62 |
| Neocallimastigomycota | 1 | 1 | 1 | 11 |
| Olpidiomycota | 1 | 1 | 1 | 4 |
| Rozellomycota | 2 | 7 | 41 | 162 |
| Zoopagomycota | 1 | 1 | 5 | 25 |
| Total | 79 | 270 | 1031 | 6561 |
3. General Methods of Fungal Identification
3.1. Classical Methods
3.1.1. Opportunistic Approach
3.1.2. Substrate Based Approach
3.1.3. Moist Chambers Techniques
3.1.4. Culture Media Technique
3.1.5. Advantages and Disadvantages of Truly Classical and Culture Based Methods
Advantages of Truly Classical and Culture Based Methods
- These methods are still considered as the sources which can provide complete information on fungal communities of different areas with variable habitats. Because of the non-availability of DNA-based sequence data of all the fungi, it is the only criteria to determine basic information about individual species, such as geographic range, host relationships and ecological distribution.
- The effects of abiotic variables (pH, soil nutrient content, weather-related variables) and biotic variables on fungi of the variable substrate and environmental conditions can be more easily studied by these methods.
- As compared to an advanced one, these methods are more economical and can be executed with less specialized equipment.
- Overall, the developing nations where adequate research funding is still a big challenge; these methods are important considerations for many investigators.
Disadvantages of Classical and Culture Based Methods
- For the fungi which are unable to grow or produce reproductive structures on culture or hardly reproduce naturally, these methods are not suitable and become a major limitation in identifying, classifying and outlining fungi of a specific area.
- The detailed procedure of sampling, culturing, isolation and identification methods are considerably more time consuming in comparison to more advanced techniques. The confirmation of new genera or species can be predicted more efficiently and accurately from the repeatedly sampled areas [120].
- Due to the above-mentioned disadvantages, classical taxonomists are now considered to be endangered, as the interests of young researchers in classical methods is considerably reducing. If one willing to peruse a career in classical mycology, it takes a long duration of training. Similarly, to identify all of the collections based on the classical approach increases the time duration to find out final results. In molecular methods, technical expertise is quite enough to carry out research which also poses a major limitation to classical methods.
3.1.6. Advantages and Disadvantages of DNA Based Modern Methods
4. Assessment of Fungal Taxonomy and Diversity
5. Polyphasic Identification
| Family | Genus | Genetic Marker for Genus Level | Genetic Markers for Species Level | References |
|---|---|---|---|---|
| Pleosporaceae | Alternaria | LSU and SSU | ITS, GAPDH, rpb2 and tef1-α | [365,366,367,368] |
| Physalacriaceae | Armillaria | ITS | ITS, IGS1 and tef1-α | [369,370] |
| Botryosphaeriaceae | Barriopsis | ITS | tef1-α | [371,372] |
| Didymellaceae | Ascochyta, Boeremia, Didymella, Epicoccum, Phoma | LSU and ITS | rpb2, tub2 and tef1-α | [373,374,375,376] |
| Pleosporaceae | Bipolaris | GPDH | ITS, tef1-α and GPDH | [377] |
| Botryosphaeriaceae | Botryosphaeria | LSU, SSU and ITS | tub and tef1-α | [378,379] |
| Nectriaceae | Calonectria, Cylindrocladium | LSU and ITS | ITS, tub, tef1-α, cmdA, His3 and ACT | [380,381,382,383,384] |
| Mycosphaerellaceae | Cercospora | LSU and ITS | ITS, tef1-α, ACT, CAL, HIS, tub2, rpb2 and GAPDH | [385,386,387,388,389] |
| Cryptobasidiaceae | Clinoconidium | ITS and LSU | ITS and LSU | [390,391,392] |
| Choanephoraceae | Choanephora | ITS | ITS | [393] |
| Glomerellaceae | Colletotrichum | GPDH, tub; ApMat-Intergenic region of apn2 and MAT1-2-1 genes can resolve within the C. gloeosporioides complex | GS-glutamine synthetase-CHS-1, HIS3-Histone3 and ACT-Actin-Placement within the genus and also some species-level delineation | [394,395,396] |
| Schizoparmaceae | Coniella | LSU and ITS | ITS, LSU, tef1-α, rpb2 and His3 | [397,398,399,400,401] |
| Pleosporaceae | Curvularia | LSU | GDPH | [402,403,404] |
| Nectriaceae | Cylindrocladiella | ITS and LSU | HIS, tef1-α and tub2 | [405,406] |
| Cyphellophoraceae | Cyphellophora | LSU and SSU | ITS, LSU, tub2 and rpb1 | [407,408] |
| Botryosphaeriaceae | Diplodia | ITS, tef1-α and tub | LSU and SSU | [378,409] |
| Botryosphaeriaceae | Dothiorella | tub | tef1-α | [378,410] |
| Elsinoaceae | Elsinoe | ITS | rpb2 and tef1-α | [411,412] |
| Xylariaceae | Entoleuca | LSU and ITS | rpb2 and tub2 | [413] |
| Entylomataceae | Entyloma | ITS | ITS | [80,414,415] |
| Corticiaceae | Erythricium | LSU | ITS | [416] |
| Botryosphaeriaceae | Eutiarosporella | LSU and SSU | ITS and LSU | [372,417,418] |
| Hymenochaetaceae | Fomitiporia | ITS | LSU, ITS, tef1-α and rpb2 | [419,420,421,422,423] |
| Hymenochataceae | Fulvifomes | LSU | ITS, tef1-α and rpb2 | [424,425] |
| Nectriaceae | Fusarium | ATP citrate lyase (Acl1), tef1-α and ITS | Calmodulin encoding gene (CmdA), tub2, tef1-α, rpb1 and rpb2 | [426,427,428] |
| Ganodermataceae | Ganoderma | ITS | rpb2 and tef1-α | [429,430,431,432,433,434,435] |
| Erysiphaceae | Golovinomyces | ITS and LSU | ITS and LSU, IGS, rpb2 and CHS | [436,437,438,439,440] |
| Bondarzewiaceae | Heterobasidion | LSU | rpb1 and rpb2 | [441] |
| Nectriaceae | Ilyonectria | ITS, LSU, tef1-α and tub2 | tef1-α, tub2 and His3 | [442,443,444,445,446] |
| Corticiaceae | Laetisaria, Limonomyces | LSU | ITS | [447,448] |
| Botryosphaeriaceae | Lasiodiplodia | SSU and LSU | ITS, tef1-α and tub2 | [378,449] |
| Botryosphaeriaceae | Macrophomina | LSU and SSU | ITS, tef1-α, ACT, CmdA and tub2 | [378,450] |
| Medeolariaceae | Medeolaria | ITS | ITS | [451] |
| Caloscyphaceae | Caloscypha | SSU and LSU | SSU, LSU | [452] |
| Meliolaceae | Meliola | LSU and SSU | ITS | [453,454] |
| Mucoraceae | Mucor | LSU and SSU | ITS and rpb1 | [455,456,457,458,459] |
| Erysiphaceae | Neoerysiphe | ITS and LSU | ITS | [460,461,462] |
| Dermataceae | Neofabraea | LSU | ITS, LSU, rpb2 and tub2 | [463] |
| Botryosphaeriaceae | Neofusicoccum | SSU, LSU | ITS, tef1-α, tub2 and rpb2 | [464] |
| Nectriaceae | Neonectria | LSU, ITS, tef1-α and tub2 | ITS, tef1-α and tub2 | [446] |
| Sporocadaceae | Neopestalotiopsis | LSU | ITS, tub2 and tef1-α | [465,466,467] |
| Didymellaceae | Nothophoma | LSU and ITS | tub2 and rpb2 | [468,469,470,471] |
| Sporocadaceae | Pestalotiopsis | LSU | ITS, tub2 and tef1-α | [472,473] |
| Togninicaceae | Phaeoacremonium | SSU and LSU | ACT and tub2 | [474,475,476] |
| Hymenochataceae | Phellinotus | LSU | ITS, tef1-α and rpb2 | [477] |
| Hymenochaetaceae | Phellinus | LSU | ITS, tef1-α and rpb2 | [478,479,480,481] |
| Phyllostictaceae | Phyllosticta | ITS | ITS, LSU, tef1-α, GAPDH and ACT | [57,482,483] |
| Peronosporacae | Phytophthora | LSU, SSU and COX2 | LSU, tub2 and COX2 | [484,485] |
| Peronosporaceae | Plasmopara | LSU | LSU | [486] |
| Leptosphaeriaceae | Plenodomus | LSU | ITS, tub2 and rpb2 | [487] |
| Sporocadaceae | Pseudopestalotiopsis | LSU | ITS, tub2 and tef1-α | [488,489] |
| Pyriculariaceae | Pseudopyricularia | LSU and rpb1 | ACT, rpb1, ITS and CAL | [490,491] |
| Saccotheciaceae | Pseudoseptoria | LSU | LSU, ITS and rpb2 | [492,493] |
| Rhizopodaceae | Rhizopus | ITS and rpb1 | SSU, LSU and ACT | [494,495,496] |
| Xylariaceae | Rosellinia | LSU and ITS | ITS | [497,498,499,500] |
| Didymellaceae | Stagonosporopsis | ITS | tub2 and rpb2 | [373,501,502] |
| Pleosporaceae | Stemphylium | ITS | CmdA and GAPDH | [503,504,505,506] |
| Dothidotthiaceae | Thyrostroma | LSU | ITS, tef1-α, rpb2 and tub2 | [507,508] |
| Tilletiaceae | Tilletia | LSU | ITS | [509,510,511,512] |
| Ustilaginaceae | Ustilago | LSU | ITS | [53,513] |
| Venturiaceae | Venturia | LSU and SSU | ITS | [514,515] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsioumani, E.; Tsioumanis, A. International Institute for Sustainable Development Photo: NASA (CC0 1.0) STILL ONLY ONE EARTH: Lessons from 50 Years of UN Sustainable Development Policy Biological Diversity: Protecting the Variety of Life on Earth Key Messages. 2020. Available online: https://www.iisd.org/system/files/2020-09/still-one-earth-biodiversity.pdf (accessed on 15 October 2021).
- Schmit, J.P.; Mueller, G.M. An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 2007, 16, 99–111. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, S.; Gupta, G. Fungal Biodiversity: Evolution & Distribution—A Review. Int. J. Appl. Res. Stud. 2012, 1, 1–8. [Google Scholar]
- Cox, R.J. Polyketides, proteins and genes in fungi: Programmed nano-machines begin to reveal their secrets. Org. Biomol. Chem. 2007, 5, 2010–2026. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Sheikh, I.; Yadav, N.; Yadav, A.N.; Kumar, V.; Singh, B.P.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. In Advances in Endophytic Fungal Research; Springer International Publishing: Cham, Switzerland, 2019; pp. 105–144. [Google Scholar]
- Berbee, M.L.; James, T.Y.; Strullu-Derrien, C. Early Diverging Fungi: Diversity and Impact at the Dawn of Terrestrial Life. Annu. Rev. Microbiol. 2017, 71, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Blackwell, M. The fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Peršoh, D. Plant-associated fungal communities in the light of meta’omics. Fungal Divers. 2015, 75, 1–25. [Google Scholar] [CrossRef]
- Bálint, M.; Bahram, M.; Eren, A.M.; Faust, K.; Fuhrman, J.; Lindahl, B.; O’Hara, R.B.; Öpik, M.; Sogin, M.L.; Unterseher, M.; et al. Millions of reads, thousands of taxa: Microbial community structure and associations analyzed via marker genes. FEMS Microbiol. Rev. 2016, 40, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciechowska, I. The Leather Underground: Biofabrication Offers New Sources for Fabrics. AATCC Rev. 2017, 17, 18–23. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, K.K.R.; Wang, C.-H.; John, S. Waste-derived low-cost mycelium composite construction materials with improved fire safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.; Al-Ani, L.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.; Zhao, R.; Aptroot, A.; Leontyev, D.; Saxena, R.K.; et al. Outline of fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef] [Green Version]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for genera: Basal clades of fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers. 2018, 92, 43–129. [Google Scholar]
- James, T.Y.; Stajich, J.E.; Hittinger, C.T.; Rokas, A. Toward a Fully Resolved Fungal Tree of Life. Annu. Rev. Microbiol. 2020, 74, 291–313. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Allen, W.C.; Braun, U.; Castlebury, L.; Chaverri, P.; Crous, P.W.; Hawksworth, D.L.; Hyde, K.D.; Johnston, P.; Lombard, L.; et al. Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 2016, 7, 289–308. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar] [CrossRef] [Green Version]
- Wijayawardene, N.N.; Hyde, K.D.; Tibpromma, S.; Wanasinghe, D.N.; Thambugala, K.M.; Tian, Q.; Wang, Y. Towards incorporating asexual fungi in a natural classification: Checklist and notes 2012–2016. Mycosphere 2017, 8, 1457–1555. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Seifert, K.A.; Samuels, G.J.; Minnis, A.M.; Schroers, H.J.; Lombard, L.; Crous, P.W.; Põldmaa, K.; Cannon, P.F.; Summerbell, R.C.; et al. Genera in Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales) proposed for acceptance or rejection. IMA Fungus 2013, 4, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.E.; Jeewon, R.; Xu, J.-C.; Lumyong, S.; Wanasinghe, D.N. Morpho-Phylo Taxonomy of Novel Dothideomycetous Fungi Associated with Dead Woody Twigs in Yunnan Province, China. Front. Microbiol. 2021, 12, e654683. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Crous, P.W.; Kirk, P.M.; Hawksworth, D.L.; Boonmee, S.; Braun, U.; Dai, D.-Q.; D’Souza, M.J.; Diederich, P.; Dissanayake, A.J.; et al. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers. 2014, 69, 1–55. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Crous, P.W.; Hyde, K.D.; Hawksworth, D.L.; Aptroot, A.; Bezerra, J.L.; Bhat, J.D.; Boehm, E.; Braun, U.; Boonmee, S.; et al. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 2015, 6, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, J.D.; Liu, N.-G.; et al. Refined families of Dothideomycetes: Orders and families incertaesedis in Dothideomycetes. Fungal Divers. 2020, 105, 17–318. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Boonmee, S.; Lücking, R.; Bhat, D.J.; Liu, N.G. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1533–2107. [Google Scholar] [CrossRef]
- Johnston, P.R.; Seifert, K.A.; Stone, J.K.; Rossman, A.Y.; Marvanová, L. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 2014, 5, 91–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Luo, J.; Rossman, A.Y.; Aoki, T.; Chuma, I.; Crous, P.W.; Dean, R.; de Vries, R.; Donofrio, N.; Hyde, K.D.; et al. Generic names in Magnaporthales. IMA Fungus 2016, 7, 155–159. [Google Scholar] [CrossRef]
- Baral, H.-O.; Weber, E.; Gams, W.; Hagedorn, G.; Liu, B.; Liu, X.; Marson, G.; Marvanová, L.; Stadler, M.; Weiß, M. Generic names in the Orbiliaceae (Orbiliomycetes) and recommendations on which names should be protected or suppressed. Mycol. Prog. 2018, 17, 5–31. [Google Scholar] [CrossRef] [Green Version]
- Ekanayaka, A.H.; Hyde, K.D.; Jones, E.B.G.; Zhao, Q. Taxonomy and phylogeny of operculate Discomycetes: Pezizomycetes. Fungal Divers. 2018, 90, 161–243. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Huang, S.-K.; Abdel-Wahab, M.A.; Daranagama, D.A.; Dayarathne, M.; D’Souza, M.J.; Goonasekara, I.D.; et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015, 72, 199–301. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.-K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Réblová, M.; Untereiner, W.A.; Štěpánek, V.; Gams, W. Disentangling Phialophora section Catenulatae: Disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes). Mycol. Prog. 2017, 16, 27–46. [Google Scholar] [CrossRef]
- Smith, G.J.; Liew, E.C.Y.; Hyde, K.D. The Xylariales: A monophyletic order containing 7 families. Fungal Divers. 2003, 13, 185–218. [Google Scholar]
- Senanayake, I.C.; Maharachchikumbura, S.; Hyde, K.D.; Bhat, J.D.; Jones, E.B.G.; McKenzie, E.H.C.; Dai, D.Q.; Daranagama, D.A.; Dayarathne, M.C.; Goonasekara, I.D.; et al. Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers. 2015, 73, 73–144. [Google Scholar] [CrossRef]
- Healy, R.A.; Bonito, G.; Trappe, J.M. Calongea, a new genus of truffles in the Pezizaceae (Pezizales). Anales del JardínBotánico de Madrid 2009, 66, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Hawksworth, D.L.; Eriksson, O.E. The names of accepted orders of ascomycetes. Syst. Ascomycetum 1986, 5, 175–184. [Google Scholar]
- Weir, A.; Blackwell, M. Molecular data support the Laboulbeniales as a separate class of Ascomycota, Laboulbeniomycetes. Mycol. Res. 2001, 105, 1182–1190. [Google Scholar] [CrossRef]
- Moncalvo, J.-M.; Vilgalys, R.; Redhead, S.A.; Johnson, J.E.; James, T.Y.; Aime, M.C.; Hofstetter, V.; Verduin, S.J.; Larsson, E.; Baroni, T.J.; et al. One hundred and seventeen clades of euagarics. Mol. Phylogenet. Evol. 2002, 23, 357–400. [Google Scholar] [CrossRef]
- Aime, M.C. Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience 2006, 47, 112–122. [Google Scholar] [CrossRef]
- Aime, M.C.; Matheny, P.B.; Henk, D.A.; Frieders, E.M.; Nilsson, R.H.; Piepenbring, M.; McLaughlin, D.J.; Szabo, L.J.; Begerow, D.; Sampaio, J.P.; et al. An overview of the higher level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit RDNA sequences. Mycologia 2006, 98, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.A.; Hansen, K.; Pfister, D.H. A phylogenetic overview of the family Pyronemataceae (Ascomycota, Pezizales). Mycol. Res. 2007, 111, 549–571. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI: Wallingford, UK, 2008. [Google Scholar]
- Lumbsch, H.T.; Huhndorf, S.M. Myconet Volume 14. Part One. Outline of Ascomycota—2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Sci. 2010, 1, 1–64. [Google Scholar] [CrossRef]
- Oehl, F.; Alves a Silva, G.; Goto, B.T.; Costa Maia, L.; Sieverding, E. Glomeromycota: Two new classes andaneworder. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Humber, R.A. Entomophthoromycota: A new phylum and reclassification for entomophthoroid fungi. Mycotaxon 2012, 120, 477–492. [Google Scholar] [CrossRef]
- Wijayawardene, D.N.N.; McKenzie, E.H.C.; Hyde, K.D. Towards incorporating anamorphic fungi in a natural classification checklist and notes for 2011. Mycosphere 2012, 3, 157–228. [Google Scholar] [CrossRef]
- McTaggart, A.R.; Shivas, R.G.; Geering, A.D.W.; Vánky, K.; Scharaschkin, T. Taxonomic revision of Ustilago, Sporisorium and Macalpinomyces. Persoonia 2012, 29, 116–132. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.; Maia, L.C.; Oehl, F. Phylogenetic systematics of the Gigasporales. Mycotaxon 2013, 122, 207–220. [Google Scholar] [CrossRef]
- Kirk, P.M.; Stalpers, J.A.; Braun, U.; Crous, P.W.; Hansen, K.; Hawksworth, D.L.; Hyde, K.D.; Lücking, R.; Lumbsch, T.H.; Rossman, A.Y.; et al. A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus 2013, 4, 381–443. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet. Evol. 2013, 67, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.-K.; Ariyawansa, H.; Boehm, E.W.A.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.-Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Hofstetter, V.; Redhead, S.A.; Kauff, F.; Moncalvo, J.-M.; Matheny, P.B.; Vilgalys, R. Taxonomic Revision and Examination of Ecological Transitions of the Lyophyllaceae (Basidiomycota, Agaricales) Based on a Multigene Phylogeny. Cryptogam. Mycol. 2014, 35, 399–425. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Yurkov, A.; Göker, M.; Lumbsch, H.; Leavitt, S.; Groenewald, M.; Theelen, B.; Liu, X.-Z.; Boekhout, T.; Bai, F.-Y. Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Stud. Mycol. 2015, 81, 149–189. [Google Scholar] [CrossRef] [Green Version]
- Humber, R.A. Entomophthoromycota: A new overview of some of the oldest terrestrial fungi. In Biology of Microfungi; Springer International Publishing: Cham, Switzerland, 2016; pp. 127–145. [Google Scholar]
- Benny, G.L.; Smith, M.E.; Kirk, P.M.; Tretter, E.D.; White, M.M. Challenges and future perspectives in the systematics of Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In Biology of Microfungi; Springer International Publishing: Cham, Switzerland, 2016; pp. 65–126. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; James, T.Y.; O’Donnell, K.; Roberson, R.W.; Taylor, T.N.; Uehling, J.; Vilgalys, R.; White, M.M.; Stajich, J.E.; et al. A phylum-level phylogenetic classification of Zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Galindo, L.J.; López-García, P.; Torruella, G.; Karpov, S.; Moreira, D. Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota. Nat. Commun. 2021, 12, 4973. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Abarenkov, K.; Koljalg, U.; Öpik, M.; Chai, B.; Cole, J.R.; Wang, Q.; Crous, P.; Robert, V.A.R.G.; Helgason, T.; et al. Sequence-based classification and identification of Fungi. Mycologia 2016, 108, 1049–1068. [Google Scholar]
- Jaklitsch, W.M.; Gardiennet, A.; Voglmayr, H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 2016, 37, 82–105. [Google Scholar] [CrossRef] [Green Version]
- Rossman, A.Y.; Udayanga, D.; Castlebury, L.A.; Hyde, K. Proposal to conserve the name Diaporthe eres against all other competing names (Ascomycota, Diaporthales, Diaporthaceae). Taxon 2014, 63, 934–935. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Wanasinghe, D.N.; Papizadeh, M.; Goonasekara, I.D.; Camporesi, E.; Bhat, D.J.; McKenzie, E.H.C.; Phillips, A.J.L.; Diederich, P.; et al. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers. 2016, 77, 1–316. [Google Scholar] [CrossRef]
- Desirò, A.; Rimington, W.R.; Jacob, A.; Pol, N.V.; Smith, M.E.; Trappe, J.M.; Bidartondo, M.I.; Bonito, G. Multigene phylogeny of Endogonales, an early diverging lineage of fungi associated with plants. IMA Fungus 2017, 8, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Malysheva, V.; Spirin, V. Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps. Fungal Biol. 2017, 121, 689–715. [Google Scholar] [CrossRef] [Green Version]
- Hongsanan, S.; Maharachchikumbura, S.; Hyde, K.D.; Samarakoon, M.C.; Jeewon, R.; Zhao, Q.; Al-Sadi, A.; Bahkali, A.H. An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers. 2017, 84, 25–41. [Google Scholar] [CrossRef]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, genera, and species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef]
- Liu, J.-K.; Hyde, K.D.; Jeewon, R.; Phillips, A.; Maharachchikumbura, S.; Ryberg, M.; Liu, Z.-Y.; Zhao, Q. Ranking higher taxa using divergence times: A case study in Dothideomycetes. Fungal Divers. 2017, 84, 75–99. [Google Scholar] [CrossRef]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.-H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Lumbsch, T.; Liu, J.-K.; Maharachchikumbura, S.; Ekanayaka, A.H.; Tian, Q.; Phookamsak, R. Outline of Ascomycota: 2017. Fungal Divers. 2018, 88, 167–263. [Google Scholar] [CrossRef]
- Kraichak, E.; Huang, J.-P.; Nelsen, M.; Leavitt, S.D.; Lumbsch, H.T. A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot. J. Linn. Soc. 2018, 188, 233–249. [Google Scholar] [CrossRef]
- Letcher, P.M.; Powell, M.J. A taxonomic summary and revision of Rozella (Cryptomycota). IMA Fungus 2018, 9, 383–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aime, M.C.; Castlebury, L.; Abbasi, M.; Begerow, D.; Berndt, R.; Kirschner, R.; Marvanová, L.; Ono, Y.; Padamsee, M.; Scholler, M.; et al. Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA Fungus 2018, 9, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Aime, M.C.; Bell, C.D.; Wilson, A.W. Deconstructing the evolutionary complexity between rust fungi (Pucciniales) and their plant hosts. Stud. Mycol. 2018, 89, 143–152. [Google Scholar] [CrossRef]
- Goldmann, L.; Weir, A. Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biol. 2018, 122, 87–100. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Hyde, K.D.; Alves, A.; Liu, J.-K. Families in Botryosphaeriales: A phylogenetic, morphological and evolutionary perspective. Fungal Divers. 2019, 94, 1–22. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Tian, Q.; Samarakoon, M.C.; McKenzie, E.H.C.; Jayasiri, S.C.; Tibpromma, S.; Bhat, J.D.; et al. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Sheikh, S.; Thulin, M.; Cavender, J.C.; Escalante, R.; Kawakami, S.-I.; Lado, C.; Landolt, J.C.; Nanjundiah, V.; Queller, D.C.; Strassmann, J.E.; et al. A New Classification of the Dictyostelids. Protist 2018, 169, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.; Thines, M. Revisiting Salisapiliaceae. Fungal Syst. Evol. 2019, 3, 171–185. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; Dong, W.; Devadatha, B.; Yang, J.; Ekanayaka, A.H.; De Silva, W.; Sarma, V.V.; Al-Sadi, A.M.; et al. Phylogenetic Revision of Savoryellaceae and Evidence for Its Ranking as a Subclass. Front. Microbiol. 2019, 10, 840. [Google Scholar] [CrossRef] [Green Version]
- He, M.-Q.; Zhao, R.-L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef] [Green Version]
- Johnston, P.R.; Quijada, L.; Carl, S.; López-Giráldez, F.; Wang, Z.; Townsend, J.P.; Smith, C.; Baral, H.-O.; Hosoya, T.; Baschien, C.; et al. A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; McKenzie, E.H.C.; Liu, J.-K.; Bhat, D.J.; Dai, D.-Q.; Camporesi, E.; Tian, Q.; Maharachchikumbura, S.S.N.; Luo, Z.-L.; Shang, Q.-J.; et al. Taxonomy and phylogeny of hyaline-spored Coelomycetes. Fungal Divers. 2020, 100, 279–801. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Dai, D.Q.; Tang, L.Z.; Fiuza, P.O.; Barbosa, F.R.; Cantillo-Perez, T.; Rajeshkumar, K.C. The genera of Coelomycetes; including genera of lichen forming, sexual morphs and synasexual morphs with coelomycetous morphs (A–C). MycoAsia 2020, 3, 1–92. [Google Scholar]
- Aime, M.; McTaggart, A. A higher-rank classification for rust fungi, with notes on genera. Fungal Syst. Evol. 2021, 7, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K.; Avasthi, S.; Verma, R.K.; Devadatha, B.; Jayawardena, R.S.; Sushma; Ranadive, K.R.; Kashyap, P.L.; Bhadauria, R.; Prasher, I.B.; et al. Indian Pucciniales: Taxonomic outline with important descriptive notes. Mycosphere 2021, 12, 89–162. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Anand, G.; Dissanayake, L.S.; Tang, L.Z.; Dai, D.Q. Towards incorporating asexually reproducing fungi in the natural classification and notes for pleomorphic genera. Mycosphere 2021, 12, 238–405. [Google Scholar] [CrossRef]
- Aime, M.C.; Miller, A.N.; Aoki, T.; Bensch, K.; Cai, L.; Crous, P.W.; Hawksworth, D.L.; Hyde, K.D.; Kirk, P.M.; Lücking, R.; et al. How to publish a new fungal species, or name, version 3.0? IMA Fungus 2021, 12, e11. [Google Scholar] [CrossRef]
- Heitman, J.; Howlett, B.J.; Crous, P.W.; Stukenbrock, E.H.; James, T.Y.; Gow, N.A.R. The Fungal Kingdom; John Wiley & Sons: Hoboken, NJ, USA; American Society for Microbiology: Washington, DC, USA, 2017. [Google Scholar]
- Blackwell, M.; Haelewaters, D.; Pfister, D.H. Laboulbeniomycetes: Evolution, natural history, and Thaxter’s final word. Mycologia 2020, 112, 1048–1059. [Google Scholar] [CrossRef]
- Genevieve, L.; Pierre-Luc, C.; Roxanne, G.-T.; Amélie, M.; Danny, B.; Vincent, M.; Hugo, G. Estimation of fungal diversity and identification of major abiotic drivers influencing fungal richness and communities in northern temperate and Boreal Quebec Forests. Forests 2019, 10, 1096. [Google Scholar] [CrossRef] [Green Version]
- Wijayawardene, N.N.; Bahram, M.; Sánchez-Castro, I.; Dai, D.-Q.; Ariyawansa, K.G.S.U.; Jayalal, U.; Suwannarach, N.; Tedersoo, L. Current Insight into Culture-Dependent and Culture-Independent Methods in Discovering Ascomycetous Taxa. J. Fungi 2021, 7, 703. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.J. State of the World’s Fungi; Report; Kew Publishing: Kew, UK, 2018. [Google Scholar]
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Hibbett, D.S.; Ohman, A.; Glotzer, D.; Nuhn, M.; Kirk, P.; Nilsson, R.H. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol. Rev. 2011, 25, 38–47. [Google Scholar] [CrossRef]
- Dai, Y.-C.; Cui, B.; Si, J.; He, S.-H.; Hyde, K.D.; Yuan, H.-S.; Liu, X.-Y.; Zhou, L.-W. Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol. Prog. 2015, 14, 62. [Google Scholar] [CrossRef]
- Bisby, G.; Ainsworth, G. The numbers of fungi. Trans. Br. Mycol. Soc. 1943, 26, 16–19. [Google Scholar] [CrossRef]
- Martin, G.W. The numbers of fungi. Proc. Iowa Acad. Sci. 1951, 58, 175–178. [Google Scholar]
- Pascoe, I. History of systematic mycology in Australia. In History of Systematic Botany in Australia; Short, P.S., Ed.; Australian Syst. Bot. Soc.: South Yarra, Australia, 1990; pp. 259–264. [Google Scholar]
- Hywel-Jones, N. A systematic survey of insect fungi from natural tropical forest in Thailand. In Aspects of Tropical Mycology; Isaac, S., Frankland, J.C., Watling, R., Whalley, A.J.S., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 300–301. [Google Scholar]
- Hammond, P.M. The current magnitude of biodiversity. In Global Biodiversity Assessment; Heywood, V., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 113–138. [Google Scholar]
- Hammond, P.M. Species inventory. In Global Biodiversity: Status of the Earth‘s Living Resources; Groombridge, B., Ed.; Chapman and Hall: London, UK, 1992; pp. 17–39. [Google Scholar]
- Smith, D.; Waller, J.M. Culture collections of microorganisms: Their importance in tropical plant pathology. Fitopatol. Bras. 1992, 17, 1–8. [Google Scholar]
- Rossman, A.Y. Strategy for an all-taxa inventory of fungal biodiversity. In Biodiversity and Terrestrial Ecosystems; Peng, C.I., Chou, C.H., Eds.; Academia Sinica Monograph Series No. 14; Institute of BotanyAcademia Sinica: Taipei, Taiwan, 1994; pp. 169–194. [Google Scholar]
- Dreyfuss, M.M.; Chapela, I.H. Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. In The Discovery of Natural Products with Therapeutic Potential; Gullo, V., Ed.; Butterworth Heinemann: London, UK, 1994; pp. 49–80. [Google Scholar]
- Shivas, R.G.; Hyde, K.D. Biodiversity of plant pathogenic fungi in the tropics. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 47–56. [Google Scholar]
- May, R.M. The dimensions of life on earth. In Nature and Human Society: The Quest for a Sustainable World; Raven, P.H., Williams, T., Eds.; National Academy Press: Washington, DC, USA, 2000; pp. 30–45. [Google Scholar]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.M.; Vilgalys, R. Fungal Community Analysis by Large-Scale Sequencing of Environmental Samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Norphanphoun, C.; Chen, J.; Dissanayake, A.J.; Doilom, M.; Hongsanan, S.; Jayawardena, R.; Jeewon, R.; Perera, R.H.; Thongbai, B.; et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018, 93, 215–239. [Google Scholar] [CrossRef]
- Lücking, R.; Forno, M.D.; Sikaroodi, M.; Gillevet, P.M.; Bungartz, F.; Moncada, B.; Yánez-Ayabaca, A.; Chaves, J.L.; Coca, L.F.; Lawrey, J.D. A single macrolichen constitutes hundreds of unrecognized species. Proc. Natl. Acad. Sci. USA 2014, 111, 11091–11096. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Johnston, M.K.; Kalb, K.; Mangold, A.; Manoch, L.; Mercado-Diaz, J.A.; Moncada, B.; Mongkolsuk, P.; Papong, K.B.; Parnmen, S.; et al. One hundred and seventy-five new species of Graphidaceae: Closing the gap or a drop in the bucket? Phytotaxa 2014, 189, 7–38. [Google Scholar] [CrossRef]
- Aptroot, A.; Cáceres, M.E.S.; Johnston, M.K.; Lücking, R. How diverse is the lichenized fungal family Trypetheliaceae (Ascomycota: Dothideomycetes)? A quantitative prediction of global species richness. Lichenologist 2016, 48, 983–994. [Google Scholar] [CrossRef] [Green Version]
- Lodge, D.J.; Ammirati, J.; O’Dell, T.E.; Mueller, G.M. Collecting and describing macrofungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: New York, NY, USA, 2004; pp. 123–168. [Google Scholar]
- Straatsma, G.; Ayer, F.; Egli, S. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Schmit, J.P.; Murphy, J.F.; Mueller, G.M. Macrofungal diversity of a temperate oak forest: A test of species richness estimators. Can. J. Bot. 1999, 77, 1014–1027. [Google Scholar]
- Huhndorf, S.M.; Lodge, D.J.; Wang, C.J.K.; Stokland, J. Macrofungi on woody substrata. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: New York, NY, USA, 2004; pp. 123–168. [Google Scholar]
- Cantrell, S.A. Sampling methods to assess discomycete diversity in wet tropical forests. Caribb. J. Sci. 2004, 40, 8–16. [Google Scholar]
- Lodge, D.J.; Cantrell, S. Diversity of litter agarics at Cuyabeno, Ecuador: Calibrating sampling efforts in tropical rainforest. Mycologist 1995, 9, 149–151. [Google Scholar] [CrossRef] [Green Version]
- Polishook, J.D.; Bills, G.; Lodge, D.J. Microfungi from decaying leaves of two rain forest trees in Puerto Rico. J. Ind. Microbiol. Biotechnol. 1996, 17, 284–294. [Google Scholar] [CrossRef]
- Rambelli, A.; Mulas, B.; Pasqualetti, M. Comparative studies on microfungi in tropical ecosystems in Ivory Coast forest litter: Behaviour on different substrata. Mycol. Res. 2004, 108, 325–336. [Google Scholar] [CrossRef]
- Schnittler, M.; Stephenson, S.L. Myxomycete biodiversity in four different forest types in Costa Rica. Mycologia 2000, 92, 626–637. [Google Scholar] [CrossRef]
- Bills, G.F.; Polishook, J.D. Selective isolation of fungi from dung of Odocoileus hemionus (mule deer). Nova Hedwigia 1993, 57, 195–206. [Google Scholar]
- Krug, J.C.; Benny, G.L.; Keller, H.W. Coprophilous fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: New York, NY, USA, 2004; pp. 467–500. [Google Scholar]
- Richardson, M.J. Diversity and occurrence of coprophilous fungi. Mycol. Res. 2001, 105, 387–402. [Google Scholar] [CrossRef]
- Trappe, J.M.; Rossman, A.Y.; Tulloss, R.E.; O’Dell, T.E.; Thorn, R.G. Protocols for an All Taxa Biodiversity Inventory of Fungi in a Costa Rican Conservation Area. Mycologia 1998, 90, e1093. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Soytong, K. The fungal endophyte dialema. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Bélanger, R.; Avis, T. Ecological processes and interactions in leaf surface fungi. In Phyllosphere Microbiology; Lindow, S.E., Hecht-Poinar, E.I., Elliot, V.J., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2002; pp. 193–207. [Google Scholar]
- Lindow, S.E.; Hecht-Poinar, E.I.; Elliot, V.J. Phyllosphere Microbiology; American Phytopathological Society Press: St. Paul, MN, USA, 2002. [Google Scholar]
- Bills, G.F.; Polishook, J.D. Abundance and diversity of microfungi in leaf litter of a lowland rain forest in Costa Rica. Mycologia 1994, 86, 187–198. [Google Scholar] [CrossRef]
- Bills, G.F.; Polishook, J.D. Microfungi from decaying leaves of Heliconia mariae (Heliconiaceae). Brenesia 1994, 41/42, 27–43. [Google Scholar]
- Basu, S.; Bose, C.; Ojha, N.; Das, N.; Das, J.; Pal, M.; Khurana, S. Evolution of bacterial and fungal growth media. Bioinformation 2015, 11, 182–184. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.; Boonmee, S.; Zhao, Q.; et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.; Liu, M.; Wanasinghe, D.; Xu, J.; Zhao, W.; Zhang, W.; Zhou, Y.; Hyde, K.D.; et al. High Genetic Diversity and Species Complexity of Diaporthe Associated with Grapevine Dieback in China. Front. Microbiol. 2019, 10, e1936. [Google Scholar] [CrossRef]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Senanayake, I.; Crous, P.; Groenewald, J.; Maharachchikumbura, S.; Jeewon, R.; Phillips, A.; Bhat, J.; Perera, R.; Li, Q.; Li, W.; et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud. Mycol. 2017, 86, 217–296. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Jeewon, R.; Chomnunti, P.; Wanasinghe, D.N.; Norphanphoun, C.; Karunarathna, A.; Pem, D.; Perera, R.H.; Camporesi, E.; McKenzie, E.H.C.; et al. Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Divers. 2018, 93, 241–443. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Liu, M.; Zhang, W.; Chen, Z.; Udayanga, D.; Chukeatirote, E.; Li, X.H.; Yan, J.Y.; Hyde, K.D. Morphological and molecular characterization of Diaporthe species associated with grapevine trunk disease in China. Fungal Biol. 2015, 119, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Phukhamsakda, C.; McKenzie, E.H.C.; Phillips, A.J.L.; Jones, E.B.G.; Bhat, D.J.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2021, 106, 7–28. [Google Scholar] [CrossRef]
- de Silva, N.I.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Bhat, D.J.; Karunarathna, S.C.; Tennakoon, D.S.; Phookamsak, R.; Jayawardena, R.S.; Lumyong, S.; Hyde, K.D. Morphomolecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere 2021, 11, 163–237. [Google Scholar] [CrossRef]
- Shinohara, N.; Woo, C.; Yamamoto, N.; Hashimoto, K.; Yoshida-Ohuchi, H.; Kawakami, Y. Comparison of DNA sequencing and morphological identification techniques to characterize environmental fungal communities. Sci. Rep. 2021, 11, 2633. [Google Scholar] [CrossRef]
- Walker, A.K.; Robicheau, B.M. Fungal diversity and community structure from coastal and barrier island beaches in the United States Gulf of Mexico. Sci. Rep. 2021, 11, 3889. [Google Scholar] [CrossRef]
- Haelewaters, D.; Gorczak, M.; Kaishian, P.; De Kesel, A.; Blackwell, M. Laboulbeniomycetes, enigmatic fungi with a turbulent taxonomic history. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 263–283. [Google Scholar]
- Hartmann, M.; Howes, C.G.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Nilsson, R.H.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef] [Green Version]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- Davey, M.L.; Heegaard, E.; Halvorsen, R.; Ohlson, M.; Kauserud, H. Seasonal trends in the biomass and structure of bryophyte-associated fungal communities explored by 454 pyrosequencing. New Phytol. 2012, 195, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Baraloto, C.; Fine, P. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.L.; Heegaard, E.; Halvorsen, R.; Kauserud, H.; Ohlson, M. Amplicon-pyrosequencing-based detection of compositional shifts in bryophyte-associated fungal communities along an elevation gradient. Mol. Ecol. 2013, 22, 368–383. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Taylor, J.W.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Liao, H.-L.; Smith, M.E.; et al. Endemism and functional convergence across the North American soil mycobiome. Proc. Natl. Acad. Sci. USA 2014, 111, 6341–6346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, e1256688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, K.; Sato, H.; Yamamoto, S.; Tanabe, A.; Hidaka, A.; Toju, H. Detection of the horizontal spatial structure of soil fungal communities in a natural forest. Popul. Ecol. 2014, 56, 301–310. [Google Scholar] [CrossRef]
- Geml, J.; Gravendeel, B.; Van Der Gaag, K.; Neilen, M.; Lammers, Y.; Raes, N.; Semenova, T.A.; De Knijff, P.; Noordeloos, M.E. The Contribution of DNA Metabarcoding to Fungal Conservation: Diversity Assessment, Habitat Partitioning and Mapping Red-Listed Fungi in Protected Coastal Salix repens Communities in the Netherlands. PLoS ONE 2014, 9, e99852. [Google Scholar] [CrossRef]
- Davey, M.L.; Kauserud, H.; Ohlson, M. Forestry impacts on the hidden fungal biodiversity associated with bryophytes. FEMS Microbiol. Ecol. 2014, 90, 313–325. [Google Scholar] [CrossRef] [Green Version]
- McHugh, T.A.; Schwartz, E. Changes in plant community composition and reduced precipitation have limited effects on the structure of soil bacterial and fungal communities present in a semiarid grassland. Plant Soil 2015, 388, 175–186. [Google Scholar] [CrossRef]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and Validation of Some ITS Primer Pairs Useful for Fungal Metabarcoding Studies. PLoS ONE 2014, 9, e97629. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Kadowaki, K.; Toju, H. Spatial segregation and aggregation of ectomycorrhizal and root-endophytic fungi in the seedlings of two Quercusspecies. PLoS ONE 2014, 9, e96363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.M.; Lawrence, B.R.; Esterline, D.; Graham, S.P.; Edelbrock, M.A.; Wooten, J.A. A metagenomics-based approach to the top-down effect on the detritivore food web: A salamanders influence on fungal communities within a deciduous forest. Ecol. Evol. 2014, 4, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Veach, A.; Dodds, W.K.; Jumpponen, A. Woody plant encroachment, and its removal, impact bacterial and fungal communities across stream and terrestrial habitats in a tallgrass prairie ecosystem. FEMS Microbiol. Ecol. 2015, 91, fiv109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wei, X.-L.; Zhang, Y.-Q.; Liu, H.-Y.; Yu, L.-Y. Diversity and distribution of lichen-associated fungi in the Ny-Ålesund Region (Svalbard, High Arctic) as revealed by 454 pyrosequencing. Sci. Rep. 2015, 5, e14850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.R.; Caporn, S.J.M.; Nwaishi, F.; Nilsson, H.; Sen, R. Bacterial and Fungal Communities in a Degraded Ombrotrophic Peatland Undergoing Natural and Managed Re-Vegetation. PLoS ONE 2015, 10, e0124726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geml, J.; Morgado, L.N.; Semenova, T.A.; Welker, J.M.; Walker, M.D.; Smets, E. Long-term warming alters richness and composition of taxonomic and functional groups of arctic fungi. FEMS Microbiol. Ecol. 2015, 91, fiv095. [Google Scholar] [CrossRef]
- Hoppe, B.; Purahong, W.; Wubet, T.; Kahl, T.; Bauhus, J.; Arnstadt, T.; Hofrichter, M.; Buscot, F.; Krüger, D. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers. 2016, 77, 367–379. [Google Scholar] [CrossRef]
- Jarvis, S.G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Chaput, D.L.; Hansel, C.M.; Burgos, W.D.; Santelli, C.M. Profiling Microbial Communities in Manganese Remediation Systems Treating Coal Mine Drainage. Appl. Environ. Microbiol. 2015, 81, 2189–2198. [Google Scholar] [CrossRef] [Green Version]
- van der Wal, A.; Ottosson, E.; de Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 2015, 96, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, K.E.; Finlay, R.; Dahlberg, A.; Stenlid, J.; Wardle, D.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, Y.; Shi, N.; Zheng, Y.; Chen, L.; Wubet, T.; Bruelheide, H.; Both, S.; Buscot, F.; Ding, Q.; et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical distribution of the soil microbiota along a successional gradient in a glacier forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Durling, M.B.; Clemmensen, K.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Štursová, M.; Bárta, J.; Šantrůčková, H.; Baldrian, P. Small-scale spatial heterogeneity of ecosystem properties, microbial community composition and microbial activities in a temperate mountain forest soil. FEMS Microbiol. Ecol. 2016, 92, fiw185. [Google Scholar] [CrossRef] [Green Version]
- Semenova, T.A.; Morgado, L.N.; Welker, J.M.; Walker, M.D.; Smets, E.; Geml, J. Compositional and functional shifts in arctic fungal communities in response to experimentally increased snow depth. Soil Biol. Biochem. 2016, 100, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J. Vertical and seasonal dynamics of fungal communities in boreal Scots pine forest soil. FEMS Microbiol. Ecol. 2016, 92, fiw170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rime, T.; Hartmann, M.; Frey, B. Potential sources of microbial colonizers in an initial soil ecosystem after retreat of an alpine glacier. ISME J. 2016, 10, 1625–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy-Bolduc, A.; Laliberté, E.; Hijri, M. High richness of ectomycorrhizal fungi and low host specificity in a coastal sand dune ecosystem revealed by network analysis. Ecol. Evol. 2016, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Roy-Bolduc, A.; Laliberté, E.; Boudreau, S.; Hijri, M. Strong linkage between plant and soil fungal communities along a successional coastal dune system. FEMS Microbiol. Ecol. 2016, 92, fiw156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Põlme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Koricheva, J.; et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, R.C.; Cardenas, E.; Leung, H.; Maas, K.; Hartmann, M.; Hahn, A.; Hallam, S.; Mohn, W.W. A metagenomic survey of forest soil microbial communities more than a decade after timber harvesting. Sci. Data 2017, 4, 170092. [Google Scholar] [CrossRef]
- Urbina, H.; Scofield, D.G.; Cafaro, M.; Rosling, A. DNA-metabarcoding uncovers the diversity of soil-inhabiting fungi in the tropical island of Puerto Rico. Mycoscience 2016, 57, 217–227. [Google Scholar] [CrossRef]
- Valverde, A.; De Maayer, P.; Oberholster, T.; Henschel, J.; Louw, M.K.; Cowan, D. Specific microbial communities associate with the rhizosphere of Welwitschia mirabilis, a Living Fossil. PLoS ONE 2016, 11, e0153353. [Google Scholar] [CrossRef]
- Nacke, H.; Goldmann, K.; Schöning, I.; Pfeiffer, B.; Kaiser, K.; Castillo-Villamizar, G.A.; Schrumpf, M.; Buscot, F.; Daniel, R.; Wubet, T. Fine spatial scale variation of soil microbial communities under European Beech and Norway Spruce. Front. Microbiol. 2016, 7, 2067. [Google Scholar] [CrossRef] [Green Version]
- Newsham, K.K.; Hopkins, D.; Carvalhais, L.C.; Fretwell, P.T.; Rushton, S.P.; O’Donnell, A.G.; Dennis, P.G. Relationship between soil fungal diversity and temperature in the maritime Antarctic. Nat. Clim. Chang. 2016, 6, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Boberg, J.; Ihrmark, K.; Stenström, E.; Stenlid, J. Do foliar fungal communities of Norway spruce shift along a tree species diversity gradient in mature European forests? Fungal Ecol. 2016, 23, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, K.; Schröter, K.; Pena, R.; Schöning, I.; Schrumpf, M.; Buscot, F.; Polle, A.; Wubet, T. Divergent habitat filtering of root and soil fungal communities in temperate beech forests. Sci. Rep. 2016, 6, e31439. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Kohout, P.; Anslan, S.; Harend, H.; Abarenkov, K.; Tedersoo, L. Stochastic distribution of small soil eukaryotes resulting from high dispersal and drift in a local environment. ISME J. 2016, 10, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, C.A.; Hayer, M.; Flores-Rentería, L.; Krohn, A.F.; Schwartz, E.; Dijkstra, P. Cheatgrass invasion alters the abundance and composition of dark septate fungal communities in sagebrush steppe. Botany 2016, 94, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Gourmelon, V.; Maggia, L.; Powell, J.; Gigante, S.; Hortal, S.; Gueunier, C.; Letellier, K.; Carriconde, F. Environmental and Geographical Factors Structure Soil Microbial Diversity in New Caledonian Ultramafic Substrates: A Metagenomic Approach. PLoS ONE 2016, 11, e0167405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, A.; Fitzgerald, A.; Meintjes, T.; Mele, P.M.; Reith, F.; Dennis, P.G.; Breed, M.; Brown, B.; Brown, M.V.; Brugger, J.; et al. Introducing BASE: The Biomes of Australian Soil Environments soil microbial diversity database. GigaScience 2016, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Cox, F.; Newsham, K.K.; Bol, R.; Dungait, J.A.J.; Robinson, C.H. Not poles apart: Antarctic soil fungal communities show similarities to those of the distant Arctic. Ecol. Lett. 2016, 19, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-Y.; Fong, J.J.; Park, M.S.; Lim, Y.W. Distinctive Feature of Microbial Communities and Bacterial Functional Profiles in Tricholoma matsutake Dominant Soil. PLoS ONE 2016, 11, e0168573. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef] [Green Version]
- de Gannes, V.; Bekele, I.; Dipchansingh, D.; Wuddivira, M.N.; De Cairies, S.; Boman, M.; Hickey, W.J. Microbial Community Structure and Function of Soil Following Ecosystem Conversion from Native Forests to Teak Plantation Forests. Front. Microbiol. 2016, 7, 1976. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, S.; Jiang, L.; Zhang, L.; Cui, S.; Meng, F.; Wang, Q.; Li, X.; Zhou, Y. Changes of soil microbial community under different degraded gradients of alpine meadow. Agric. Ecosyst. Environ. 2016, 222, 213–222. [Google Scholar] [CrossRef]
- Kielak, A.M.; Scheublin, T.R.; Mendes, L.W.; Van Veen, J.A.; Kuramae, E.E. Bacterial Community Succession in Pine-Wood Decomposition. Front. Microbiol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; van Dorst, J.; Bissett, A.; Brown, M.V.; Palmer, A.S.; Snape, I.; Siciliano, S.D.; Ferrari, B.C. Microbial diversity at Mitchell Peninsula, Eastern Antarctica: A potential biodiversity “hotspot”. Polar Biol. 2016, 39, 237–249. [Google Scholar] [CrossRef]
- Baldrian, P.; Zrůstová, P.; Tláskal, V.; Davidová, A.; Merhautová, V.; Vrška, T. Fungi associated with decomposing deadwood in a natural beech-dominated forest. Fungal Ecol. 2016, 23, 109–122. [Google Scholar] [CrossRef]
- Barnes, C.J.; Maldonado, C.; Frøslev, T.G.; Antonelli, A.; Rønsted, N. Unexpectedly High Beta-Diversity of Root-Associated Fungal Communities in the Bolivian Andes. Front. Microbiol. 2016, 7, 1377. [Google Scholar] [CrossRef]
- Porter, T.M.; Shokralla, S.; Baird, D.; Golding, G.B.; Hajibabaei, M. Ribosomal DNA and Plastid Markers Used to Sample Fungal and Plant Communities from Wetland Soils Reveals Complementary Biotas. PLoS ONE 2016, 11, e0142759. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Tian, L.; Zhang, J.; Ma, L.; Li, X.; Tian, C. Rhizospheric Fungi and Their link with the nitrogen-fixing Frankia harbored in host plant Hippophaerhamnoides L. J. Basic Microbiol. 2017, 57, 1055–1064. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, Y.; Cao, L.; Tan, H.; Zhang, R. Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.). Microbiol. Res. 2016, 188–189, 1–8. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter: Seasonal dynamics of a soil microbial community. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef]
- Van Der Wal, A.; Gunnewiek, P.J.A.K.; Cornelissen, J.H.C.; Crowther, T.W.; De Boer, W. Patterns of natural fungal community assembly during initial decay of coniferous and broadleaf tree logs. Ecosphere 2016, 7, 01393. [Google Scholar] [CrossRef] [Green Version]
- Varenius, K.; Lindahl, B.D.; Dahlberg, A. Retention of seed trees fails to lifeboat ectomycorrhizal fungal diversity in harvested Scots pine forests. FEMS Microbiol. Ecol. 2017, 93, fix105. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, A.; Klein Gunnewiek, P.; de Hollander, M.; de Boer, W. Fungal diversity and potential tree pathogens in decaying logs and stumps. For. Ecol. Manag. 2017, 406, 266–273. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Yang, Z.; Chen, N.; Chi, X.; Pan, L.; Wang, T.; Yu, S.; Guo, X. Influence of Peanut Cultivars and Environmental Conditions on the Diversity and Community Composition of Pod Rot Soil Fungi in China. Mycobiology 2017, 45, 392–400. [Google Scholar] [CrossRef] [Green Version]
- van der Wal, A.; kleinGunnewiek, P.; de Boer, W. Soil-wood interactions: Influence of decaying coniferous and broadleaf logs on composition of soil fungal communities. Fungal Ecol. 2017, 30, 132–134. [Google Scholar] [CrossRef]
- Vasutova, M.; Edwards-Jonášová, M.; Baldrian, P.; Čermák, M.; Cudlín, P. Distinct environmental variables drive the community composition of mycorrhizal and saprotrophic fungi at the alpine treeline ecotone. Fungal Ecol. 2017, 27, 116–124. [Google Scholar] [CrossRef]
- Vaz, A.B.; Fonseca, P.; Góes-Neto, A.; Leite, L.R.; Badotti, F.; Salim, A.C.; Araujo, F.M.; Cuadros-Orellana, S.; Duarte, Â.A.; Rosa, C.A.; et al. Using next-generation sequencing (NGS) to uncover diversity of wood-decaying fungi in neotropical Atlantic forests. Phytotaxa 2017, 295, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Adams, J.; Shi, Y.; He, J.-S.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil fungal diversity in natural grasslands of the Tibetan Plateau: Associations with plant diversity and productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Wicaksono, C.Y.; Gutiérrez, J.A.; Nouhra, E.; Pastor, N.; Raes, N.; Pacheco, S.; Geml, J. Contracting montane cloud forests: A case study of the Andean alder (Alnus acuminata) and associated fungi in the Yungas. Biotropica 2017, 49, 141–152. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.; Shi, Y.; Sun, H.; Cheng, L.; Zhang, Y.; Chu, H. Fungal community assemblages in a high elevation desert environment: Absence of dispersal limitation and edaphic effects in surface soil. Soil Biol. Biochem. 2017, 115, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lu, Z.; Yang, K.; Zhu, J. Impacts of conversion from secondary forests to larch plantations on the structure and function of microbial communities. Appl. Soil Ecol. 2017, 111, 73–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Tian, L.; Ma, L.; Luo, S.; Zhang, J.; Li, X.; Tian, C. Fungal communities in ancient peatlands developed from different periods in the Sanjiang Plain, China. PLoS ONE 2017, 12, e0187575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purahong, W.; Pietsch, K.A.; Lentendu, G.; Schöps, R.; Bruelheide, H.; Wirth, C.; Buscot, F.; Wubet, T. Characterization of Unexplored Deadwood Mycobiome in Highly Diverse Subtropical Forests Using Culture-independent Molecular Technique. Front. Microbiol. 2017, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Poosakkannu, A.; Nissinen, R.; Männistö, M.; Kytöviita, M.-M. Microbial community composition but not diversity changes along succession in arctic sand dunes: Deschampsiaflexuosa associated microbiomes. Environ. Microbiol. 2017, 19, 698–709. [Google Scholar] [CrossRef] [Green Version]
- Bergottini, V.; Hervé, V.; Sosa, D.; Otegui, M.; Zapata, P.D.; Junier, P. Exploring the diversity of the root-associated microbiome of Ilex paraguariensis St. Hil. (Yerba Mate). Appl. Soil Ecol. 2017, 109, 23–31. [Google Scholar] [CrossRef]
- Dean, S.L.; Tobias, T.B.; Phippen, W.B.; Clayton, A.W.; Gruver, J.; Porras-Alfaro, A. A study of Glycine max (soybean) fungal communities under different agricultural practices. Plant Gene 2017, 11, 8–16. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.A.; Pérez-Ortega, S.; Pointing, S.B.; Green, T.G.A.; Pintado, A.; Rozzi, R.; Sancho, L.; Rios, A.D.L. Microbial succession dynamics along glacier forefield chronosequences in Tierra del Fuego (Chile). Polar Biol. 2017, 40, 1939–1957. [Google Scholar] [CrossRef]
- Ge, Z.-W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Gomes, S.I.F.; Aguirre-Gutiérrez, J.; Bidartondo, M.I.; Merckx, V.S.F.T. Arbuscular mycorrhizal interactions of mycoheterotrophic Thismia are more specialized than in autotrophic plants. New Phytol. 2017, 213, 1418–1427. [Google Scholar] [CrossRef] [Green Version]
- Almario, J.; Jeena, G.; Wunder, J.; Langen, G.; Zuccaro, A.; Coupland, G.; Bucher, M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc. Natl. Acad. Sci. USA 2017, 114, E9403–E9412. [Google Scholar] [CrossRef] [Green Version]
- Anthony, M.A.; Frey, S.D.; Stinson, K.A. Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion. Ecosphere 2017, 8, e01951. [Google Scholar] [CrossRef] [Green Version]
- Grau, O.; Geml, J.; Pérez-Haase, A.; Ninot, J.M.; Semenova-Nelsen, T.A.; Peñuelas, J. Abrupt changes in the composition and function of fungal communities along an environmental gradient in the high Arctic. Mol. Ecol. 2017, 26, 4798–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiiesalu, I.; Bahram, M.; Tedersoo, L. Plant species richness and productivity determine the diversity of soil fungal guilds in temperate coniferous forest and bog habitats. Mol. Ecol. 2017, 26, 4846–4858. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Boberg, J.; Cleary, M.; Bruelheide, H.; Hönig, L.; Koricheva, J.; Stenlid, J. Foliar fungi of Betula pendula: Impact of tree species mixtures and assessment methods. Sci. Rep. 2017, 7, 41801. [Google Scholar] [CrossRef] [Green Version]
- Kolaříková, Z.; Kohout, P.; Krüger, C.; Janoušková, M.; Mrnka, L.; Rydlová, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Oja, J.; Vahtra, J.; Bahram, M.; Kohout, P.; Kull, T.; Rannap, R.; Kõljalg, U.; Tedersoo, L. Local-scale spatial structure and community composition of orchid mycorrhizal fungi in semi-natural grasslands. Mycorrhiza 2017, 27, 355–367. [Google Scholar] [CrossRef]
- Miura, T.; Sánchez, R.; Castañeda, L.E.; Godoy, K.; Barbosa, O. Is microbial terroir related to geographic distance between vineyards? Environ. Microbiol. Rep. 2017, 9, 742–749. [Google Scholar] [CrossRef]
- Oono, R.; Rasmussen, A.; Lefèvre, E. distance decay relationships in foliar fungal endophytes are driven by rare taxa: Distance decay in fungal endophytes. Environ. Microbiol. 2017, 19, 2794–2805. [Google Scholar] [CrossRef]
- Kamutando, C.N.; Vikram, S.; Kamgan-Nkuekam, G.; Makhalanyane, T.P.; Greve, M.; Le Roux, J.J.; Richardson, D.; Cowan, D.; Valverde, A. Soil nutritional status and biogeography influence rhizosphere microbial communities associated with the invasive tree Acacia dealbata. Sci. Rep. 2017, 7, 6472. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities under Different Monoculture Spans. Microb. Ecol. 2018, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Henkel, T.W.; Williams, G.C.; Aime, M.C.; Fremier, A.K.; Vilgalys, R. Investigating niche partitioning of ectomycorrhizal fungi in specialized rooting zones of the monodominant leguminous tree Dicymbecorymbosa. New Phytol. 2017, 215, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Wang, H.; Hui, X.; Wang, Z.; Drijber, R.A.; Liu, J. Changes in soil microbial communities after 10 years of winter wheat cultivation versus fallow in an organic-poor soil in the Loess Plateau of China. PLoS ONE 2017, 12, e0184223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, B.; Domene, X.; Yao, M.; Li, C.; Zhang, S.; Kou, Y.; Wang, Y.; Li, X. Microbial diversity in Chinese temperate steppe: Unveiling the most influential environmental drivers. FEMS Microbiol. Ecol. 2017, 93, fix031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma-Poudyal, D.; Schlatter, D.; Yin, C.; Hulbert, S.; Paulitz, T. Long-term no-till: A major driver of fungal communities in dryland wheat cropping systems. PLoS ONE 2017, 12, e0184611. [Google Scholar] [CrossRef] [Green Version]
- Cross, H.; Sønstebø, J.H.; Nagy, N.E.; Timmermann, V.; Solheim, H.; Børja, I.; Kauserud, H.; Carlsen, T.; Rzepka, B.; Wasak, K.; et al. Fungal diversity and seasonal succession in ash leaves infected by the invasive ascomycete Hymenoscyphus fraxineus. New Phytol. 2017, 213, 1405–1417. [Google Scholar] [CrossRef]
- Kazartsev, I.; Shorohova, E.; Kapitsa, E.; Kushnevskaya, H. Decaying Piceaabies log bark hosts diverse fungal communities. Fungal Ecol. 2018, 33, 1–12. [Google Scholar] [CrossRef]
- Bickford, W.A.; Goldberg, D.E.; Kowalski, K.P.; Zak, D.R. Root endophytes and invasiveness: No difference between native and non-native Phragmites in the Great Lakes Region. Ecosphere 2018, 9, 02526. [Google Scholar] [CrossRef] [Green Version]
- Cline, L.C.; Schilling, J.S.; Menke, J.; Groenhof, E.; Kennedy, P.G. Ecological and functional effects of fungal endophytes on wood decomposition. Funct. Ecol. 2018, 32, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Cregger, M.A.; Veach, A.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, e31. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Fusi, M.; Ramond, J.-B.; Merlino, G.; Booth, J.M.; Maggs-Kölling, G.; Cowan, D.A.; Daffonchio, D. Rhizosheath microbial community assembly of sympatric desert speargrasses is independent of the plant host. Microbiome 2018, 6, e215. [Google Scholar] [CrossRef] [PubMed]
- Glynou, K.; Nam, B.; Thines, M.; Maciá-Vicente, J.G. Facultative root-colonizing fungi dominate endophytic assemblages in roots of nonmycorrhizal Microthlaspi species. New Phytol. 2018, 217, 1190–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, M.; Berruti, A.; Bianciotto, V.; Cremonesi, P.; Giannico, R.; Gusmeroli, F.; Lumini, E.; Pierce, S.; Pizzi, F.; Turri, F.; et al. Differential biodiversity responses between kingdoms (plants, fungi, bacteria and metazoa) along an Alpine succession gradient. Mol. Ecol. 2018, 27, 3671–3685. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Queloz, V.; Sieber, T.N. The Endophytic Mycobiome of European Ash and Sycamore Maple Leaves—Geographic Patterns, Host Specificity and Influence of Ash Dieback. Front. Microbiol. 2018, 9, e2345. [Google Scholar] [CrossRef]
- Schneider-Maunoury, L.; Leclercq, S.; Clément, C.; Covès, H.; Lambourdière, J.; Sauve, M.; Richard, F.; Selosse, M.-A.; Taschen, E. Is Tuber melanosporum colonizing the roots of herbaceous, non-ectomycorrhizal plants? Fungal Ecol. 2018, 31, 59–68. [Google Scholar] [CrossRef]
- Schön, M.E.; Nieselt, K.; Garnica, S. Belowground fungal community diversity and composition associated with Norway spruce along an altitudinal gradient. PLoS ONE 2018, 13, e0208493. [Google Scholar] [CrossRef]
- Rasmussen, P.U.; Hugerth, L.W.; Blanchet, F.G.; Andersson, A.; Lindahl, B.D.; Tack, A.J.M. Multiscale patterns and drivers of arbuscular mycorrhizal fungal communities in the roots and root-associated soil of a wild perennial herb. New Phytol. 2018, 220, 1248–1261. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.J.; Leppanen, C.; Brown, V.; Fordyce, J.A.; LeBude, A.; Ranney, T.; Simberloff, D.; Cregger, M.A. Exploring variation in phyllosphere microbial communities across four hemlock species. Ecosphere 2018, 9, e02524. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Wubet, T.; Kahl, T.; Arnstadt, T.; Hoppe, B.; Lentendu, G.; Baber, K.; Rose, T.; Kellner, H.; Hofrichter, M.; et al. Increasing N deposition impacts neither diversity nor functions of deadwood-inhabiting fungal communities, but adaptation and functional redundancy ensure ecosystem function. Environ. Microbiol. 2018, 20, 1693–1710. [Google Scholar] [CrossRef]
- Qian, X.; Chen, L.; Guo, X.; He, D.; Shi, M.; Zhang, D. Shifts in community composition and co-occurrence patterns of phyllosphere fungi inhabiting Mussaenda shikokiana along an elevation gradient. PeerJ 2018, 6, e5767. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Eimes, J.A.; Oh, S.H.; Suh, H.J.; Oh, S.-Y.; Lee, S.; Park, K.H.; Kwon, H.J.; Kim, S.-Y.; Lim, Y.W. Diversity of fungi associated with roots of Calanthe orchid species in Korea. J. Microbiol. 2018, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mirmajlessi, S.M.; Bahram, M.; Mänd, M.; Najdabbasi, N.; Mansouripour, S.; Loit, E. Survey of Soil Fungal Communities in Strawberry Fields by Illumina Amplicon Sequencing. Eurasian Soil Sci. 2018, 51, 682–691. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Hoppe, B.; Jariyavidyanont, K.; Arnstadt, T.; Baber, K.; Otto, P.; Kellner, H.; Hofrichter, M.; et al. Determinants of deadwood-inhabiting fungal communities in temperate forests: Molecular evidence from a large scale deadwood decomposition experiment. Front. Microbiol. 2018, 9, 2120. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Shao, W.; Yu, H.; Yang, X.; Gao, D.; Qiao, X.; Wang, Z.; Wu, G. Rhizosphere Microenvironments of Eight Common Deciduous Fruit Trees Were Shaped by Microbes in Northern China. Front. Microbiol. 2018, 9, e3147. [Google Scholar] [CrossRef]
- Saitta, A.; Anslan, S.; Bahram, M.; Brocca, L.; Tedersoo, L. Tree species identity and diversity drive fungal richness and community composition along an elevational gradient in a Mediterranean ecosystem. Mycorrhiza 2018, 28, 39–47. [Google Scholar] [CrossRef]
- Santalahti, M.; Sun, H.; Sietiö, O.-M.; Köster, K.; Berninger, F.; Laurila, T.; Pumpanen, J.; Heinonsalo, J. Reindeer grazing alter soil fungal community structure and litter decomposition related enzyme activities in boreal coniferous forests in Finnish Lapland. Appl. Soil Ecol. 2018, 132, 74–82. [Google Scholar] [CrossRef]
- Sukdeo, N.; Teen, E.; Rutherford, P.M.; Massicotte, H.B.; Egger, K.N. Selecting fungal disturbance indicators to compare forest soil profile re-construction regimes. Ecol. Indic. 2018, 84, 662–682. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Xu, X.; Liu, T.; Wu, D.; Zheng, X.; Tang, S.; Dai, Q. Potential use of high-throughput sequencing of soil microbial communities for estimating the adverse effects of continuous cropping on ramie (Boehmeria nivea L. Gaud). PLoS ONE 2018, 13, e0197095. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, X.; Zhang, Y. Successional changes of fungal communities along the biocrust development stages. Biol. Fertil. Soils 2018, 54, 285–294. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Weißbecker, C.; Wubet, T.; Lentendu, G.; Kühn, P.; Scholten, T.; Bruelheide, H.; Buscot, F. Experimental evidence of functional group-dependent effects of tree diversity on soil fungi in subtropical forests. Front. Microbiol. 2018, 9, 2312. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Mapook, A.; Wu, Y.T.; Chen, C.T. Characterization of the Castanopsis carlesii deadwood mycobiome by pacbio sequencing of the full-length fungal nuclear ribosomal Internal Transcribed Spacer (ITS). Front. Microbiol. 2019, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frøslev, T.G.; Kjøller, R.; Bruun, H.H.; Ejrnæs, R.; Hansen, A.J.; Læssøe, T.; Heilmann-Clausen, J. Man against machine: Do fungal fruitbodies and eDNA give similar biodiversity assessments across broad environmental gradients? Biol. Conserv. 2019, 233, 201–212. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Takahashi, K.; Dong, K.; Song, H.-K.; Moroenyane, I.; Waldman, B.; Adams, J.M. Fungal elevational rapoport pattern from a high mountain in Japan. Sci. Rep. 2019, 9, 6570. [Google Scholar] [CrossRef] [Green Version]
- Ovaskainen, O.; Abrego, N.; Somervuo, P.; Palorinne, I.; Hardwick, B.; Pitkänen, J.-M.; Andrew, N.R.; Niklaus, P.A.; Schmidt, N.M.; Seibold, S.; et al. Monitoring fungal communities with the global spore sampling project. Front. Ecol. Evol. 2020, 7, 511. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Li, H.; Wang, Y.; Wu, B.; Wu, M.; Chen, L.; Li, X.; Zhang, Y.; Wang, X.; Shi, M.; et al. Leaf and root endospheres harbor lower fungal diversity and less complex fungal co-occurrence patterns than rhizosphere. Front. Microbiol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Snoek, L.B.; Koorem, K.; Geisen, S.; Bloem, L.J.; ten Hooven, F.; Kostenko, O.; Krigas, N.; Manrubia, M.; Caković, D.; et al. Range-expansion effects on the belowground plant microbiome. Nat. Ecol. Evol. 2019, 3, 604–611. [Google Scholar] [CrossRef]
- Pellitier, P.T.; Zak, D.R.; Salley, S. Environmental filtering structures fungal endophyte communities in tree bark. Mol. Ecol. 2019, 28, 5188–5198. [Google Scholar] [CrossRef]
- Semenova-Nelsen, T.A.; Platt, W.J.; Patterson, T.R.; Huffman, J.; Sikes, B.A. Frequent fire reorganizes fungal communities and slows decomposition across a heterogeneous pine savanna landscape. New Phytol. 2019, 224, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Cong, J.; Lu, H.; Yang, L.; Liu, Q.; Li, D.; Zhang, Y. Broad-leaved forest types affect soil fungal community structure and soil organic carbon contents. Microbiology Open 2019, 8, e874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal dynamics of soil fungal and bacterial communities in cool-temperate montane forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröter, K.; Wemheuer, B.; Pena, R.; Schöning, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly processes of trophic guilds in the root mycobiome of temperate forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef]
- Singh, J.; Silva, K.J.P.; Fuchs, M.; Khan, A. Potential role of weather, soil and plant microbial communities in rapid decline of apple trees. PLoS ONE 2019, 14, e0213293. [Google Scholar] [CrossRef]
- Song, H.; Singh, D.; Tomlinson, K.W.; Yang, X.; Ogwu, M.C.; Slik, J.W.F.; Adams, J.M. Tropical forest conversion to rubber plantation in southwest China results in lower fungal beta diversity and reduced network complexity. FEMS Microbiol. Ecol. 2019, 95, fiz092. [Google Scholar] [CrossRef] [Green Version]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Zimmerman, N.B.; Carbone, I.; May, G.; Arnold, A.E. Host availability drives distributions of fungal endophytes in the imperilled boreal realm. Nat. Ecol. Evol. 2019, 3, 1430–1437. [Google Scholar] [CrossRef]
- Unuk, T.; Martinović, T.; Finžgar, D.; Šibanc, N.; Grebenc, T.; Kraigher, H. Root-associated fungal communities from two phenologically contrasting silver fir (Abies alba Mill.) groups of trees. Front. Plant Sci. 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Araya, J.P.; González, M.; Cardinale, M.; Schnell, S.; Stoll, A. Microbiome dynamics associated with the atacama flowering desert. Front. Microbiol. 2020, 10, 3160. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Garrido, L.; Viñegla, B.; Hortal, S.; Powell, J.R.; Carreira, J.A. Distributional shifts in ectomycorrizhal fungal communities lag behind climate-driven tree upward migration in a conifer forest-high elevation shrubland ecotone. Soil Biol. Biochem. 2019, 137, e107545. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, D. Rhizosphere fungal community structure succession of Xinjiang continuously cropped cotton. Fungal Biol. 2018, 123, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, M.; Feng, H.; Wei, M.; Song, F.; Lou, Y.; Cui, X.; Wang, H.; Zhuge, Y. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Tillage Res. 2020, 198, 104540. [Google Scholar] [CrossRef]
- Detheridge, A.P.; Cherrett, S.; Clasen, L.A.; Medcalf, K.; Pike, S.; Griffith, G.W.; Scullion, J. Depauperate soil fungal populations from the St. Helena endemic Commidendrum robustum are dominated by Capnodiales. Fungal Ecol. 2020, 45, e100911. [Google Scholar] [CrossRef]
- Li, S.-P.; Wang, P.; Chen, Y.; Wilson, M.C.; Yang, X.; Ma, C.; Lu, J.; Chen, X.-Y.; Wu, J.; Shu, W.-S.; et al. Island biogeography of soil bacteria and fungi: Similar patterns, but different mechanisms. ISME J. 2020, 14, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Barghoorn, E.S.; Linder, D.H. Marine fungi: Their taxonomy and biology. Farlowia 1944, 1, 395–467. [Google Scholar] [CrossRef]
- Roth, F.J., Jr.; Orpurt, P.A.; Ahearn, D.G. Occurrence and distribution of fungi in a subtropical marine environment. Can. J. Bot. 1964, 42, 375–383. [Google Scholar] [CrossRef]
- Höhnk, W. Über den pilzlichen Befall kalkigerHartteile von Meerestieren. Ber. Dtsch. Wiss. Komm. Meeresforsch. 1969, 20, 129–140. [Google Scholar]
- Gaertner, A.N. mit Pollen koderbare marine Pilzediesseits und jenseits des Island-Faroer-RiickensimOberflachenwasser und im Sediment. Veroeff. Inst. Meeresforsch. Bremerhav. 1968, 11, 65–82. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Illustrated Key to the Filamentous Higher Marine Fungi. Bot. Mar. 1991, 34, 1–61. [Google Scholar] [CrossRef]
- Raghukumar, C.; Raghukumar, S. Barotolerance of fungi isolated from deep-sea sediments of the Indian Ocean. Aquat. Microb. Ecol. 1998, 15, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Nagahama, T.; Hamamoto, M.; Nakase, T.; Horikoshi, K. Rhodotorula lamellibrachii sp. nov., a new yeast species from a tubeworm collected at the deep-sea floor in Sagami bay and its phylogenetic analysis. Antonie Leeuwenhoek 2001, 80, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, V.P.; Kysela, D.T.; Teske, A.; de Vera Gomez, A.; Sogin, M.L. Benthic eukaryotic diversity in the Guaymas Basin hydrothermal vent environment. Proc. Natl. Acad. Sci. USA 2002, 99, 7658–7662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garcia, P.; Philippe, H.; Gail, F.; Moreira, D. Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc. Natl. Acad. Sci. USA 2003, 100, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghukumar, C.; Raghukumar, S.; Sheelu, G.; Gupta, S.; Nagendernath, B.; Rao, B. Buried in time: Culturable fungi in a deep-sea sediment core from the Chagos Trench, Indian Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1759–1768. [Google Scholar] [CrossRef]
- Biddle, J.; House, C.H.; Brenchley, J.E. Microbial stratification in deeply buried marine sediment reflects changes in sulfate/methane profiles. Geobiology 2005, 3, 287–295. [Google Scholar] [CrossRef]
- Damare, S.; Raghukumar, C.; Raghukumar, S. Fungi in deep-sea sediments of the Central Indian Basin. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Takishita, K.; Tsuchiya, M.; Reimer, J.D.; Maruyama, T. Molecular evidence demonstrating the basidiomycetous fungus Cryptococcus curvatus is the dominant microbial eukaryote in sediment at the Kuroshima Knoll methane seep. Extremophiles 2006, 10, 165–169. [Google Scholar] [CrossRef]
- Damare, S.; Raghukumar, C.; Muraleedharan, U.D.; Raghukumar, S. Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzym. Microb. Technol. 2006, 39, 172–181. [Google Scholar] [CrossRef]
- Bass, D.; Howe, A.; Brown, N.; Barton, H.; Demidova, M.; Michelle, H.; Li, L.; Sanders, H.; Watkinson, S.C.; Willcock, S.; et al. Yeast forms dominate fungal diversity in the deep oceans. Proc. R. Soc. B Boil. Sci. 2007, 274, 3069–3077. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Cao, L.; Tan, H.; Fang, S.; Huang, Y.; Zhou, S. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J. 2007, 1, 756–762. [Google Scholar] [CrossRef]
- López-García, P.; Vereshchaka, A.; Moreira, D. Eukaryotic diversity associated with carbonates and fluid-seawater interface in Lost City hydrothermal field. Environ. Microbiol. 2007, 9, 546–554. [Google Scholar] [CrossRef]
- Damare, S.; Raghukumar, C. Fungi and Macroaggregation in Deep-Sea Sediments. Microb. Ecol. 2008, 56, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.; Barrett, A.; Templeton, A.; Staudigel, H. Fungal Diversity Associated with an Active Deep Sea Volcano: Vailulu’u Seamount, Samoa. Geomicrobiol. J. 2009, 26, 597–605. [Google Scholar] [CrossRef]
- Dupont, J.; Magnin, S.; Rousseau, F.; Zbinden, M.; Frebourg, G.; Samadi, S.; de Forges, B.R.; Jones, E.G. Molecular and ultrastructural characterization of two ascomycetes found on sunken wood off Vanuatu Islands in the deep Pacific Ocean. Mycol. Res. 2009, 113, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal Diversity in Deep-Sea Hydrothermal Ecosystems. Appl. Environ. Microbiol. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [Green Version]
- Burgaud, G.; Le Calvez, T.; Arzur, D.; Vandenkoornhuyse, P.; Barbier, G. Diversity of culturable marine filamentous fungi from deep-sea hydrothermal vents. Environ. Microbiol. 2009, 11, 1588–1600. [Google Scholar] [CrossRef]
- Burgaud, G.; Arzur, D.; Durand, L.; Cambon-Bonavita, M.-A.; Barbier, G. Marine culturable yeasts in deep-sea hydrothermal vents: Species richness and association with fauna: Culturable yeasts from hydrothermal vents. FEMS Microbiol. Ecol. 2010, 73, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Nagano, Y.; Nagahama, T.; Hatada, Y.; Nunoura, T.; Takami, H.; Miyazaki, J.; Takai, K.; Horikoshi, K. Fungal diversity in deep-sea sediments—The presence of novel fungal groups. Fungal Ecol. 2010, 3, 316–325. [Google Scholar] [CrossRef]
- Sauvadet, A.-L.; Gobet, A.; Guillou, L. Comparative analysis between protist communities from the deep-sea pelagic ecosystem and specific deep hydrothermal habitats. Environ. Microbiol. 2010, 12, 2946–2964. [Google Scholar] [CrossRef]
- Singh, P.; Raghukumar, C.; Verma, P.; Shouche, Y. Phylogenetic diversity of culturable fungi from the deep-sea sediments of the Central Indian Basin and their growth characteristics. Fungal Divers. 2010, 40, 89–102. [Google Scholar] [CrossRef]
- Bhadury, P.; Bik, H.; Lambshead, J.D.; Austen, M.C.; Smerdon, G.R.; Rogers, A.D. Molecular Diversity of Fungal Phylotypes Co-Amplified Alongside Nematodes from Coastal and Deep-Sea Marine Environments. PLoS ONE 2011, 6, e26445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgcomb, V.P.; Beaudoin, D.; Gast, R.; Biddle, J.F.; Teske, A. Marine subsurface eukaryotes: The fungal majority: Marine subsurface eukaryotes. Environ. Microbiol. 2011, 13, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Eloe, E.A.; Shulse, C.N.; Fadrosh, D.W.; Williamson, S.J.; Allen, E.E.; Bartlett, D.H. Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment: Microbial diversity in the puertorico trench. Environ. Microbiol. Rep. 2011, 3, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, T.; Takahashi, E.; Nagano, Y.; Abdel-Wahab, M.A.; Miyazaki, M. Molecular evidence that deep-branching fungi are major fungal components in deep-sea methane cold-seep sediments. Environ. Microbiol. 2011, 13, 2359–2370. [Google Scholar] [CrossRef]
- Quaiser, A.; Zivanovic, Y.; Moreira, D.; López-García, P. Comparative metagenomics of bathypelagic plankton and bottom sediment from the Sea of Marmara. ISME J. 2010, 5, 285–304. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Raghukumar, C.; Verma, P.; Shouche, Y. Fungal Community Analysis in the Deep-Sea Sediments of the Central Indian Basin by Culture-Independent Approach. Microb. Ecol. 2011, 61, 507–517. [Google Scholar] [CrossRef]
- Singh, P.; Raghukumar, C.; Meena, R.M.; Verma, P.; Shouche, Y. Fungal diversity in deep-sea sediments revealed by culture-dependent and culture-independent approaches. Fungal Ecol. 2012, 5, 543–553. [Google Scholar] [CrossRef]
- Thaler, A.D.; Van Dover, C.L.; Vilgalys, R. Ascomycete phylotypes recovered from a Gulf of Mexico methane seep are identical to an uncultured deep-sea fungal clade from the Pacific. Fungal Ecol. 2012, 5, 270–273. [Google Scholar] [CrossRef]
- Kimes, N.E.; Callaghan, A.V.; Aktas, D.F.; Smith, W.L.; Sunner, J.; Golding, B.T.; Drozdowska, M.; Hazen, T.C.; Suflita, J.M.; Morris, P.J. Metagenomic analysis and metabolite profiling of deep–sea sediments from the Gulf of Mexico following the Deepwater Horizon oil spill. Front. Microbiol. 2013, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Orsi, W.; Biddle, J.; Edgcomb, V. Deep Sequencing of Subseafloor Eukaryotic rRNA Reveals Active Fungi across Marine Subsurface Provinces. PLoS ONE 2013, 8, e56335. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.-Q.; Liu, H.-Y.; Wei, Y.-Z.; Li, H.-L.; Su, J.; Zhao, L.-X.; Yu, L.-Y. Diversity and cold adaptation of culturable endophytic fungi from bryophytes in the Fildes Region, King George Island, maritime Antarctica. FEMS Microbiol. Lett. 2013, 341, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, J.M.; Kormas, K.; Pachiadaki, M.; Rocke, E.; Beaudoin, D.J.; Morrison, C.; Visscher, P.; Cobban, A.; Starczak, V.R.; Edgcomb, V. Benthic protists and fungi of Mediterranean deep hypsersaline anoxic basin redoxcline sediments. Front. Microbiol. 2014, 5, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rédou, V.; Ciobanu, M.C.; Pachiadaki, M.G.; Edgcomb, V.; Alain, K.; Barbier, G.; Burgaud, G. In-depth analyses of deep subsurface sediments using 454-pyrosequencing reveals a reservoir of buried fungal communities at record-breaking depths. FEMS Microbiol. Ecol. 2014, 90, 908–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Otsuka, S.; Senoo, K. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer. Soil Biol. Biochem. 2014, 69, 157–167. [Google Scholar] [CrossRef]
- Zhang, D.; Ge, H.; Zou, J.-H.; Tao, X.; Chen, R.; Dai, J. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014, 16, 1410–1413. [Google Scholar] [CrossRef]
- Rédou, V.; Navarri, M.; Meslet-Cladière, L.; Barbier, G.; Burgaud, G. Species richness and adaptation of marine fungi from deep-subsea floor sediments. Appl. Environ. Microbiol. 2015, 81, 3571–3583. [Google Scholar] [CrossRef] [Green Version]
- Edgcomb, V.P.; Pachiadaki, M.; Mara, P.; Kormas, K.; Leadbetter, E.R.; Bernhard, J. Gene expression profiling of microbial activities and interactions in sediments under haloclines of E. Mediterranean deep hypersaline anoxic basins. ISME J. 2016, 10, 2643–2657. [Google Scholar] [CrossRef] [Green Version]
- Tisthammer, K.H.; Cobian, G.; Amend, A.S. Global biogeography of marine fungi is shaped by the environment. Fungal Ecol. 2016, 19, 39–46. [Google Scholar] [CrossRef]
- Boraks, A.; Amend, A.S. Fungi in soil and understory have coupled distribution patterns. PeerJ 2021, 9, e11915. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.-F.; Liu, H.-Y.; Zhang, Y.-Q.; Yu, L.-Y. Soil pH is a Key Determinant of Soil Fungal Community Composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Ali, S.H.; Alias, S.A.; Siang, H.Y.; Smykla, J.; Pang, K.L.; Guo, S.Y.; Peter, C. Studies on diversity of soil microbfungi in the Hornsund area, Spitsbergen. Pol. Polar Res. 2013, 34, 39–54. [Google Scholar]
- Pachiadaki, M.; Rédou, V.; Beaudoin, D.J.; Burgaud, G.; Edgcomb, V.P. Fungal and Prokaryotic Activities in the Marine Subsurface Biosphere at Peru Margin and Canterbury Basin Inferred from RNA-Based Analyses and Microscopy. Front. Microbiol. 2016, 7, 846. [Google Scholar] [CrossRef] [PubMed]
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Gutiérrez, M.H.; Palfner, G.; Pantoja, S. Diversity of culturable filamentous Ascomycetes in the eastern South Pacific Ocean off Chile. World J. Microbiol. Biotechnol. 2017, 33, 157. [Google Scholar] [CrossRef]
- Nagano, Y.; Miura, T.; Nishi, S.; Lima, A.; Nakayama, C.R.; Pellizari, V.; Fujikura, K. Fungal diversity in deep-sea sediments associated with asphalt seeps at the Sao Paulo Plateau. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 146, 59–67. [Google Scholar] [CrossRef]
- Orsi, W.D.; Richards, T.A.; Francis, W.R. Predicted microbial secretomes and their target substrates in marine sediment. Nat. Microbiol. 2018, 3, 32–37. [Google Scholar] [CrossRef]
- Wei, X.; Guo, S.; Gong, L.-F.; He, G.; Pang, K.-L.; Luo, Z.-H. Cultivable Fungal Diversity in Deep-Sea Sediment of the East Pacific Ocean. Geomicrobiol. J. 2018, 35, 790–797. [Google Scholar] [CrossRef]
- Barone, G.; Rastelli, E.; Corinaldesi, C.; Tangherlini, M.; Danovaro, R.; Dell’Anno, A. Benthic deep-sea fungi in submarine canyons of the Mediterranean Sea. Prog. Oceanogr. 2018, 168, 57–64. [Google Scholar] [CrossRef]
- Xu, W.; Gong, L.F.; Pang, K.L.; Luo, Z.H. Fungal diversity in deep-sea sediments of a hydrothermal vent system in the Southwest Indian Ridge. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 16–26. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Gastélum, L.; Chong-Robles, J.; Lago-Lestón, A.; Darcy, J.L.; Amend, A.S.; Riquelme, M. Targeted ITS1 sequencing unravels the mycodiversity of deep-sea sediments from the Gulf of Mexico. Environ. Microbiol. 2019, 21, 4046–4061. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhao, L.; Xu, X.; Feng, H.; Shi, Y.; Deakin, G.; Feng, Z.; Zhu, H. Cultivar-Dependent Variation of the Cotton Rhizosphere and Endosphere Microbiome Under Field Conditions. Front. Plant Sci. 2019, 10, 1659. [Google Scholar] [CrossRef]
- Velez, P.; Gasca-Pineda, J.; Riquelme, M. Cultivable fungi from deep-sea oil reserves in the Gulf of Mexico: Genetic signatures in response to hydrocarbons. Mar. Environ. Res. 2020, 153, 104816. [Google Scholar] [CrossRef] [PubMed]
- Wieser, A.; Schneider, L.; Jung, J.; Schubert, S. MALDI-TOF MS in microbiological diagnostics—Identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 2012, 93, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.Y.; Samuels, G.J. Towards a single scientific name for species of fungi. Inoculum 2005, 56, 3–6. [Google Scholar]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Persoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [Green Version]
- Bhunjun, C.S.; Dong, Y.; Jayawardena, R.; Jeewon, R.; Phukhamsakda, C.; Bundhun, D.; Hyde, K.D.; Sheng, J. A polyphasic approach to delineate species in Bipolaris. Fungal Divers. 2020, 102, 225–256. [Google Scholar] [CrossRef]
- Crous, P.W.; Hawksworth, D.L.; Wingfield, M.J. Identifying and Naming Plant-Pathogenic Fungi: Past, Present, and Future. Annu. Rev. Phytopathol. 2015, 53, 247–267. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Boil. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Aime, M.C.; Hawksworth, D.L.; Hyde, K.D.; Irinyi, L.; Jeewon, R.; Johnston, P.R.; Kirk, P.M.; Malosso, E.; May, T.W.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, J.F.; Grace, D. The consequences of human actions on risks for infectious diseases: A review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M. Phylogenetic Species Recognition and Species Concepts in Fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.T.; Dai, D.Q.; Dai, Y.C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [Green Version]
- Woudenberg, J.; Groenewald, J.; Binder, M.; Crous, P. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [Green Version]
- Woudenberg, J.H.C.; van der Merwe, N.A.; Jurjević, Ž.; Groenewald, J.Z.; Crous, P.W. Diversity and movement of indoor Alternaria alternata across the mainland USA. Fungal Genet. Biol. 2015, 81, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Klopfenstein, N.B.; Hanna, J.W.; McDonald, G.I. Characterization of North American Armillaria species: Genetic relationships determined by ribosomal DNA sequences and AFLP markers. For. Pathol. 2006, 36, 145–164. [Google Scholar] [CrossRef]
- Coetzee, M.P.A.; Wingfeld, B.D.; Wingfeld, M.J. Armillaria root-rot pathogens: Species boundaries and global distribution. Pathogens 2018, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.J.; Phillips, A.J.L.; Li, X.H.; Hyde, K.D. Botryosphaeriaceae: Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef] [PubMed]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.; Jeewon, R.; Ariyawansa, H.A.; Bhat, J.D.; Camporesi, E.; Kang, J.C. Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae). Mycosphere 2017, 8, 1080–1101. [Google Scholar] [CrossRef]
- Berner, D.; Cavin, C.; Woudenberg, J.H.; Tunali, B.; Büyük, O.; Kansu, B. Assessment of Boeremiaexigua var. rhapontica, as a biological control agent of Russian knapweed (Rhaponticum repens). Biol. Control 2015, 81, 65–75. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Cai, L.; McKenzie, E.H.C.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Shivas, R.G.; Tan, Y.P.; Hyde, K.D. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia Complex. Fungal Divers. 2012, 56, 131–144. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; De Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I. Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Kang, J.C.; Schoch, C.L.; Mchua, G.R.A. Phylogenetic relationships of Cylindrocladium pseudogracile and Cylindrocladium rumohrae with morphologically similar taxa, based on morphology and DNA sequences of internal transcribed spacers and β-tubulin. Can. J. Bot. 1999, 77, 1813–1820. [Google Scholar] [CrossRef]
- Schoch, C.L.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Phylogeny of Calonectria based on comparisons of β-tubulin DNA sequences. Mycol. Res. 2001, 105, 1045–1052. [Google Scholar] [CrossRef]
- Lombard, L.; Crous, P.; Wingfield, B.; Wingfield, M. Species concepts in Calonectria (Cylindrocladium). Stud. Mycol. 2010, 66, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombard, L.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Phylogeny and systematics of the genus Calonectria. Stud. Mycol. 2010, 66, 31–69. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Wingfield, M.; Alfenas, A.; Crous, P. The forgotten Calonectria collection: Pouring old wine into new bags. Stud. Mycol. 2016, 85, 159–198. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, J.; Nakashima, C.; Nishikawa, J.; Shin, H.-D.; Park, J.-H.; Jama, A.; Groenewald, M.; Braun, U.; Crous, P. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albu, S.; Sharma, S.; Bluhm, B.H.; Price, P.P.; Schneider, R.W.; Doyle, V.P. Draft Genome Sequence of Cercospora cf. sigesbeckiae, a Causal Agent of Cercospora Leaf Blight on Soybean. Genome Announc. 2017, 5, e00708-17. [Google Scholar] [CrossRef] [Green Version]
- Guatimosim, E.; Schwartsburd, P.; Barreto, R.; Crous, P. Novel fungi from an ancient niche: Cercosporoid and related sexual morphs on ferns. Persoonia 2016, 37, 106–141. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, M.; Arzanlou, M.; Babai-Ahari, A.; Groenewald, J.Z.; Braun, U.; Crous, P.W. Application of the consolidated species concept to Cercospora spp. from Iran. Persoonia 2015, 34, 65–86. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, M.; Arzanlou, M.; Babai-Ahari, A.; Groenewald, J.Z.; Crous, P.W. Novel primers improve species delimitation in Cercospora. IMA Fungus 2018, 9, 299–332. [Google Scholar] [CrossRef]
- Jiang, M.; Kirschner, R. Unraveling two East Asian species of Clinoconidium (Cryptobasidiaceae). Mycoscience 2016, 57, 440–447. [Google Scholar] [CrossRef]
- Kakishima, M.; Ji, J.-X.; Nagao, H.; Wang, Q.; Denchev, C.M. Clinoconidium globosum, nom. nov. (Cryptobasidiaceae) producing galls on fruits of Cinnamomum daphnoides in Japan. Phytotaxa 2017, 299, 267–272. [Google Scholar] [CrossRef]
- Kakishima, M.; Nagao, H.; Ji, J.-X.; Sun, Y.; Denchev, C.M. Clinoconidium onumae, comb. nov. (Cryptobasidiaceae) producing galls on shoot buds of Cinnamomum tenuifolium in Japan. Phytotaxa 2017, 313, 175–184. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, V.P.; Oudemans, P.; Rehner, S.A.; Litt, A. Habitat and Host Indicate Lineage Identity in Colletotrichum gloeosporioides s.l. from Wild and Agricultural Landscapes in North America. PLoS ONE 2013, 8, e62394. [Google Scholar] [CrossRef] [Green Version]
- Gunjan, S.; Navinder, K.; Weir, B.S.; Hyde, K.D.; Shenoy, B.D. Apmat gene can resolve Colletotrichum species: A case study with Mangifera indica. Fungal Divers. 2013, 61, 117–138. [Google Scholar]
- Silva, D.N.; Talhinas, P.; Várzea, V.; Cai, L.; Paulo, O.S.; Batista, D. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: An example from coffee (Coffea spp.) hosts. Mycologia 2012, 104, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Castlebury, L.A.; Rossman, A.Y.; Jaklitsch, W.J.; Vasilyeva, L.N. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 2002, 94, 1017–1031. [Google Scholar] [CrossRef]
- Van Niekerk, J.M.; Groenewald, J.Z.E.; Verkley, G.J.; Fourie, P.H.; Wingfield, M.J.; Crous, P.W. Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycol. Res. 2004, 108, 283–303. [Google Scholar] [CrossRef]
- Phookamsak, R.; Liu, J.-K.; McKenzie, E.H.C.; Manamgoda, D.S.; Ariyawansa, H.; Thambugala, K.M.; Dai, D.-Q.; Camporesi, E.; Chukeatirote, E.; Wijayawardene, N.N.; et al. Revision of Phaeosphaeriaceae. Fungal Divers. 2014, 68, 159–238. [Google Scholar] [CrossRef]
- Alvarez, L.; Groenewald, J.; Crous, P. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Stud. Mycol. 2016, 85, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Chethana, K.W.T.; Zhou, Y.; Zhang, W.; Liu, M.; Xing, Q.K.; Li, X.H.; Yan, J.Y.; Hyde, K.D. Coniellavitis sp. nov. Is the Common Pathogen of White Rot in Chinese Vineyards. Plant Dis. 2017, 101, 2123–2136. [Google Scholar] [CrossRef]
- Manamgoda, D.; Rossman, A.; Castlebury, L.; Crous, P.; Madrid, H.; Chukeatirote, E.; Hyde, K. The genus Bipolaris. Stud. Mycol. 2014, 79, 221–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Norphanphoun, C.; Abreu, V.P.; Bazzicalupo, A.; Chethana, K.W.T.; Clericuzio, M.; Dayarathne, M.C.; Dissanayake, A.J.; Ekanayaka, A.H.; He, M.-Q.; et al. Fungal diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017, 87, 1–235. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombard, L.; Cheewangkoon, R.; Crous, P.W. New Cylindrocladiella spp. from Thailand soils. Mycosphere 2017, 8, 1088–1104. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.; Akulov, A.; Carnegie, A.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.; Guarnaccia, V.; Halleen, F.; et al. Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef] [PubMed]
- Réblová, M.; Untereiner, W.A.; Réblová, K. Novel evolutionary lineagesrevealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS ONE 2013, 8, e63547. [Google Scholar] [CrossRef]
- Feng, P.; Lu, Q.; Najafzadeh, M.J.; van den Ende, A.G.; Sun, J.; Li, R.; Xi, L.; Vicente, V.A.; Lai, W.; Lu, C.; et al. Cyphellophora and its relatives in Phialophora: Biodiversity and possible role in human infection. Fungal Divers. 2014, 65, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Phillips, A.J.L. The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Zare, R.; Phillips, A. A phylogenetic study of Dothiorella and Spencermartinsia species associated with woody plants in Iran, New Zealand, Portugal and Spain. Persoonia 2014, 32, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, R.; Ariyawansa, H.; Singtripop, C.; Li, Y.M.; Yan, J.; Li, X.; Nilthong, S.; Hyde, K.D. A re-assessment of Elsinoaceae (Myriangiales, Dothideomycetes). Phytotaxa 2014, 176, 120–138. [Google Scholar] [CrossRef]
- Fan, X.; Barreto, R.; Groenewald, J.; Bezerra, J.; Pereira, O.; Cheewangkoon, R.; Mostert, L.; Tian, C.; Crous, P. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoe (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef]
- Da Silva, C.S.; Pereira, M.B.; Pereira, J. New accounts on Hypoxylaceae and Xylariaceae from Brazil. Rodriguésia 2020, 71, 03012018. [Google Scholar] [CrossRef]
- Kruse, J.; Piątek, M.; Lutz, M.; Thines, M. Broad host range species in specialised pathogen groups should be treated with suspicion—A case study on Entyloma infecting Ranunculus. Persoonia 2018, 41, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Rooney-Latham, S.; Lutz, M.; Blomquist, C.L.; Romberg, M.K.; Scheck, H.J.; Piątek, M. Entyloma helianthi: Identification and characterization of the casual agent of sunflower white leaf smut. Mycologia 2017, 109, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ghobad-Nejhad, M.; Hallenberg, N. Erythricium atropatanum sp. nov. (Corticiales) from Iran, based on morphological and molecular data. Mycol. Prog. 2011, 10, 61–66. [Google Scholar] [CrossRef]
- Thynne, E.; McDonald, M.C.; Evans, M.; Wallwork, H.; Neate, S.; Solomon, P.S. Re-classification of the causal agent of white grain disorder on wheat as three separate species of Eutiarosporella. Australas. Plant Pathol. 2015, 44, 527–539. [Google Scholar] [CrossRef]
- Burgess, T.I.; Tan, Y.P.; Garnas, J.; Edwards, J.; Scarlett, K.A.; Shuttleworth, L.A.; Daniel, R.; Dann, E.K.; Parkinson, L.; Dinh, Q.; et al. Current status of the Botryosphaeriaceae in Australia. Australas. Plant Pathol. 2019, 48, 35–44. [Google Scholar] [CrossRef]
- Santana, M.D.C.; Amalfi, M.; Robledo, G.; da Silveira, R.M.B.; Decock, C. Fomitiporia neotropica, a new species from South America evidenced by multilocus phylogenetic analyses. Mycol. Prog. 2014, 13, 601–615. [Google Scholar] [CrossRef]
- Chen, H.; Cui, B.-K. Multi-locus phylogeny and morphology reveal five new species of Fomitiporia (Hymenochaetaceae) from China. Mycol. Prog. 2017, 16, 687–701. [Google Scholar] [CrossRef]
- Morera, G.; Robledo, G.; Ferreira-Lopes, V.; Urcelay, C. South American Fomitiporia (Hymenochaetaceae, Basidiomycota) ‘jump on’exotic living trees revealed by multi-gene phylogenetic analysis. Phytotaxa 2017, 321, 277–286. [Google Scholar] [CrossRef]
- Zhou, L.W. Fulvifomes hainanensis sp. nov. and F. indicus comb. nov. (Hymenochaetales, Basidiomycota) evidenced by a combination of morphology and phylogeny. Mycoscience 2014, 55, 70–77. [Google Scholar] [CrossRef]
- Zhou, L.-W. Fulvifomes imbricatus and F. thailandicus (Hymenochaetales, Basidiomycota): Two new species from Thailand based on morphological and molecular evidence. Mycol. Prog. 2015, 14, 89. [Google Scholar] [CrossRef]
- Ji, X.H.; Wu, F.; Dai, Y.C.; Vlasák, J. Two new species of Fulvifomes (Hymenochaetales, Basidiomycota) from America. MycoKeys 2017, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Salvador-Montoya, C.A.; Popoff, O.F.; Reck, M.; Drechsler-Santos, E.R. Taxonomic delimitation of Fulvifomes robiniae (Hymenochaetales, Basidiomycota) and related species in America: F. squamosus sp. nov. Plant Syst. Evol. 2018, 304, 445–459. [Google Scholar] [CrossRef]
- Laurence, M.H.; Summerell, B.A.; Burgess, L.W.; Liew, E.C.Y. Fusarium burgessii sp. nov. representing a novel lineage in the genus Fusarium. Fungal Divers. 2011, 49, 101–112. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. RPB1 and RPB2 phylogeny supports an early Cretaceous origin and a strongly supported clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular Phylogenetic Diversity, Multilocus Haplotype Nomenclature, and In Vitro Antifungal Resistance within the Fusarium solani Species Complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, M.P.A.; Marincowitz, S.; Muthelo, V.G.; Wingfield, M.J. Ganoderma species, including new taxa associated with root rot of the iconic Jacaranda mimosifolia in Pretoria, South Africa. IMA Fungus 2015, 6, 249–256. [Google Scholar] [CrossRef]
- Xing, J.-H.; Song, J.; Decock, C.; Cui, B. Morphological characters and phylogenetic analysis reveal a new species within the Ganoderma lucidum complex from South Africa. Phytotaxa 2016, 266, 115–124. [Google Scholar] [CrossRef]
- Xing, J.H.; Sun, Y.F.; Han, Y.L.; Cui, B.K.; Dai, Y.C. Morphological and molecular identifcation of two new Ganoderma species on Casuarina equisetifolia from China. MycoKeys 2018, 34, 93–108. [Google Scholar] [CrossRef]
- Tchoumi, J.M.T.; Coetzee, M.P.A.; Rajchenberg, M.; Roux, J. Taxonomy and species diversity of Ganoderma species in the Garden Route National Park of South Africa inferred from morphology and multilocus phylogenies. Mycologia 2019, 111, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Luangharn, T.; Karunarathna, S.C.; Mortimer, P.E.; Hyde, K.D.; Xu, J. Additions to the knowledge of Ganoderma in Thailand: Ganoderma casuarinicola, a new record; and Ganoderma thailandicum sp. nov. MycoKeys 2019, 59, 47–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Karunarathna, S.C.; Mortimer, P.E.; Li, H.; Qiu, M.-H.; Peng, X.-R.; Luangharn, T.; Li, Y.-J.; Promputtha, I.; Hyde, K.D.; et al. Ganoderma weixiensis (Polyporaceae, Basidiomycota), a new member of the G. lucidum complex from Yunnan Province, China. Phytotaxa 2019, 423, 75–86. [Google Scholar] [CrossRef]
- Cabarroi-Hernández, M.; Villalobos-Arámbula, A.R.; Torres-Torres, M.G.; Decock, C.; Guzmán-Dávalos, L. The Ganoderma weberianum-resinaceum lineage: Multilocus phylogenetic analysis and morphology confrm G. mexicanum and G. parvulum in the Neotropics. MycoKeys 2019, 59, 95–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, U.; Bradshaw, M.; Zhao, T.-T.; Cho, S.-E.; Shin, H.-D. Taxonomy of the Golovinomyces cynoglossi complex (Erysiphales, Ascomycota) disentangled by phylogenetic analyses and reassessments of morphological traits. Mycobiology 2018, 46, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, U.; Shin, H.; Takamatsu, S.; Meeboon, J.; Kiss, L.; Lebeda, A.; Kitner, M.; Götz, M. Phylogeny and taxonomy of Golovinomyces orontii revisited. Mycol. Prog. 2019, 18, 335–357. [Google Scholar] [CrossRef]
- Takamatsu, S.; Matsuda, S.; Grigaliunaite, B. Comprehensive phylogenetic analysis of the genus Golovinomyces (Ascomycota: Erysiphales) reveals close evolutionary relationships with its host plants. Mycologia 2013, 105, 1135–1152. [Google Scholar] [CrossRef]
- Qiu, P.-L.; Liu, S.-Y.; Li, Y.; Wang, L.-L.; Braun, U.; Bradshaw, M.; Rooney-Latham, S.; Takamatsu, S.; Bulgakov, T.; Tang, S.-R.; et al. Multi-locus phylogeny and taxonomy of an unresolved, heterogeneous species complex within the genus Golovinomyces (Ascomycota, Erysiphales), including G. ambrosiae, G. circumfusus and G. spadiceus. BMC Microbiol. 2020, 20, e51. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.L.; Qi, X.F.; Li, Y.; Braun, U.; Liu, S.Y. Epitypifcation and molecular confrmation of Erysiphe cucurbitacearum as a synonym of Golovinomyces tabaci. Mycoscience 2020, 61, 30–36. [Google Scholar] [CrossRef]
- Chen, J.-J.; Cui, B.; Zhou, L.-W.; Korhonen, K.T.; Dai, Y.-C. Phylogeny, divergence time estimation, and biogeography of the genus Heterobasidion (Basidiomycota, Russulales). Fungal Divers. 2015, 71, 185–200. [Google Scholar] [CrossRef]
- Cabral, A.; Groenewald, J.Z.; Rego, M.C.N.F.; Oliveira, H.; Crous, P.W. Cylindrocarpon root rot: Multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.; Rego, C.; Crous, P.W.; Oliveira, H. Virulence and cross-infection potential of Ilyonectria spp. to grapevine. Phytopathol. Mediterr. 2012, 52, 340–354. [Google Scholar]
- Cabral, A.; Rego, C.; Nascimento, T.; Oliveira, H.; Groenewald, J.Z.; Crous, P.W. Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biol. 2012, 116, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Bezuidenhout, C.M.; Crous, P.W. Ilyonectria black foot rot associated with Proteaceae. Australas. Plant Pathol. 2013, 42, 337–349. [Google Scholar] [CrossRef]
- Lombard, L.; Van Der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar]
- Diederich, P.; Lawrey, J.D.; Sikaroodi, M.; Gillevet, P.M. A new lichenicolous teleomorph is related to plant pathogens in Laetisaria and Limonomyces (Basidiomycota, Corticiales). Mycologia 2011, 103, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Nan, Z.B.; Liu, G.D. First Report of Limonomyces roseipellis causing pink patch on bermudagrass in South China. Plant Dis. 2013, 97, 561. [Google Scholar] [CrossRef]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.O.; Philips, A.J.L.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Sarr, M.P.; Ndiaye, M.; Groenewald, J.Z.; Crous, P.W. Genetic diversity in Macrophomina phaseolina, the causal agent of charcoal rot. Phytopathol. Mediterr. 2014, 53, 250–268. [Google Scholar]
- LoBuglio, K.F.; Pfister, D.H. Placement of Medeolaria farlowii in the Leotiomycetes, and comments on sampling within the class. Mycol. Prog. 2010, 9, 361–368. [Google Scholar] [CrossRef]
- Pfister, D.H.; Agnello, C.; Lantieri, A.; LoBuglio, K.F. The Caloscyphaceae (Pezizomycetes, Ascomycota), with a new genus. Mycol. Prog. 2013, 12, 667–674. [Google Scholar] [CrossRef]
- Hongsanan, S.; Tian, Q.; Persoh, D.; Zeng, X.-Y.; Hyde, K.D.; Chomnunti, P.; Boonmee, S.; Bahkali, A.H.; Wen, T.-C. Meliolales. Fungal Divers. 2015, 74, 91–141. [Google Scholar] [CrossRef]
- Justavino, D.R.; Kirschner, R.; Piepenbring, M. New species and new records of Meliolaceae from Panama. Fungal Divers. 2015, 70, 73–84. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Duong, T.; Lee, H. Characterization of two new records of Mucoralean species isolated from gut of soldier fy larva in Korea. Mycobiology 2016, 44, 310–313. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Jung, H.Y.; Lee, Y.S.; Voigt, K.; Lee, H.B. Phylogenetic status of two undescribed zygomycete species from Korea: Actinomucor elegans and Mucor minutus. Mycobiology 2017, 45, e34. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G. DNA barcoding in Mucorales: An inventory of biodiversity. Persoonia 2013, 30, 11–47. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.; Stielow, J.; De Hoog, G.; Bensch, K.; Schwartze, V.U.; Voigt, K.; Alastruey-Izquierdo, A.; Kurzai, O.; Walther, G. A new species concept for the clinically relevant Mucor circinelloides complex. Persoonia 2020, 44, 67–97. [Google Scholar] [CrossRef]
- Heluta, V.; Takamatsu, S.; Harada, M.; Voytyuk, S. Molecular phylogeny and taxonomy of Eurasian Neoerysiphe species infecting Asteraceae and Geranium. Persoonia 2010, 24, 81–92. [Google Scholar] [CrossRef]
- Gregorio-Cipriano, R.; González, D.; Félix-Gastélum, R.; Chacón, S. Neoerysiphesechii (Ascomycota: Erysiphales): A new species of powdery mildew found on Sechium edule and Sechium mexicanum (Cucurbitaceae) in Mexico. Botany 2020, 98, 185–195. [Google Scholar] [CrossRef]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS Biodiversity Series No. 11; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2012. [Google Scholar]
- Chen, C.; Verkley, G.J.; Sun, G.; Groenewald, J.Z.; Crous, P. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biol. 2016, 120, 1291–1322. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Paul, N.; Lee, K.-H.; Kim, H.-J.; Sang, H. Morphology, Molecular Phylogeny, and Pathogenicity of Neofusicoccum parvum, Associated with Leaf Spot Disease of a New Host, the Japanese Bay Tree (Machilus thunbergii). Forests 2021, 12, 440. [Google Scholar] [CrossRef]
- Prasannath, K.; Shivas, R.G.; Galea, V.J.; Akinsanmi, O.A. Neopestalotiopsis Species Associated with Flower Diseases of Macadamia integrifolia in Australia. J. Fungi 2021, 7, 771. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.; Larignon, P.; Al-Sadi, A.M.; Zuo-Yi, L.I.U. Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol. Mediterr. 2017, 55, 380–390. [Google Scholar]
- Jayawardena, R.; Liu, M.; Maharachchikumbura, S.S.N.; Zhang, W.; Xing, Q.; Hyde, K.D.; Nilthong, S.; Li, X.; Yan, J. Neopestalotiopsis vitis sp. nov. causing grapevine leaf spot in China. Phytotaxa 2016, 258, 63–74. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.A.; Bahkali, A.H.; El-Gorban, A.M.; Hodhod, M.S. Natural products of Nothophoma multilocularis sp. nov. an endophyte of the medicinal plant Rhazya stricta. Mycosphere 2017, 8, 1185–1200. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Jayawardene, R.S.; Zhang, W.; Zhou, Y.Y.; Liu, M.; Hyde, K.D.; Li, X.H.; Wang, J.; Zhang, K.C.; Yan, J.Y. Molecular characterization and pathogenicity of fungal taxa associated with cherry leaf spot disease. Mycosphere 2019, 10, 490–530. [Google Scholar] [CrossRef]
- Zhang, L.X.; Yin, T.; Pan, M.; Tian, C.M.; Fan, X.L. Occurrence and identifcation of Nothophoma spiraeae sp. nov. in China. Phytotaxa 2020, 430, 147–156. [Google Scholar]
- Maharachchikumbura, S.; Guo, L.-D.; Chukeatirote, E.; Bahkali, A.; Hyde, K.D. Pestalotiopsis—Morphology, phylogeny, biochemistry and diversity. Fungal Divers. 2011, 50, 167–187. [Google Scholar] [CrossRef]
- Keith, L.M.; Velasquez, M.E.; Zee, F.T. Identification and Characterization of Pestalotiopsis spp. Causing Scab Disease of Guava, Psidium guajava, in Hawaii. Plant Dis. 2006, 90, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; León, M.; Pérez-Sierra, A.; Burgess, T.; Armengol, J. New Phaeoacremonium species isolated from sandalwood trees in Western Australia. IMA Fungus 2014, 5, 67–77. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.; Correia, K.C.; Barbosa, M.A.G.; Câmara, M.P.S.; Gramaje, D.; Michereff, S.J. Characterization of Phaeoacremonium isolates associated with Petri disease of table grape in Northeastern Brazil, with description of Phaeoacremonium nordesticola sp. nov. Eur. J. Plant Pathol. 2017, 149, 695–709. [Google Scholar] [CrossRef]
- Spies, C.; Moyo, P.; Halleen, F.; Mostert, L. Phaeoacremonium species diversity on woody hosts in the Western Cape Province of South Africa. Persoonia 2018, 40, 26–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsler-Santos, E.R.; Robledo, G.L.; Lima-Júnior, N.C.; Malosso, E.; Reck, M.A.; Gibertoni, T.B.; Cavalcanti, M.A.D.Q.; Rajchenberg, M. Phellinotus, a new neotropical genus in the Hymenochaetaceae (Basidiomycota, Hymenochaetales). Phytotaxa 2016, 261, 218–239. [Google Scholar] [CrossRef]
- Tomšovský, M.; Vampola, P.; Sedlák, P.; Byrtusová, Z.; Jankovský, L. Delimitation of central and northern European species of the Phellinus igniarius group (Basidiomycota, Hymenochaetales) based on analysis of ITS and translation elongation factor 1 alpha DNA sequences. Mycol. Prog. 2010, 9, 431–445. [Google Scholar] [CrossRef]
- Brazee, N.J. Phylogenetic Relationships among Species of Phellinus sensu stricto, Cause of White Trunk Rot of Hardwoods, from Northern North America. Forests 2015, 6, 4191–4211. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.-W.; Vlasák, J.; Qin, W.-M.; Dai, Y.-C. Global diversity and phylogeny of the Phellinus igniarius complex (Hymenochaetales, Basidiomycota) with the description of five new species. Mycologia 2016, 108, 192–204. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, X.; Si, J.; Cui, B.-K. Morphological characters and phylogenetic analysis reveal a new species of Phellinus with hooked hymenial setae from Vietnam. Phytotaxa 2018, 356, 91–99. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.; Groenewald, J.; Lombard, L.; Wingfield, M.; Postma, A.; Burgess, T.; Crous, P. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-P.; Liu, Y.-X.; Yuan, J.; Wang, Y.; Hyde, K.D.; Liu, Z.-Y. Phyllosticta species from banana (Musa sp.) in Chongqing and Guizhou Provinces, China. Phytotaxa 2014, 188, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.E.; Coffey, M.D.; Park, S.-Y.; Geiser, D.M.; Kang, S. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008, 45, 266–277. [Google Scholar] [CrossRef]
- Martin, F.N.; Blair, J.E.; Coffey, M.D. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 2014, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Manawasinghe, I.S.; Zhao, W.; Xu, J.; Brooks, S.; Zhao, X.; Xu, J.P.; Brooks, S.; Zhao, X.; Hyde, K.D.; et al. Multiple gene genealogy reveals high genetic diversity and evidence for multiple origins of Chinese Plasmopara viticola population. Sci. Rep. 2017, 7, 17304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennakoon, D.S.; Phookamsak, R.; Wanasinghe, D.N.; Yang, J.B.; Lumyong, S.; Hyde, K.D. Morphological and phylogentic insights resolve Plenodomus sinensis (Leptosphariaceae) as a new species. Phytotaxa 2017, 324, 73–82. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.; Guo, L.-D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef] [Green Version]
- Maharachchikumbura, S.S.; Guo, L.-D.; Liu, Z.-Y.; Hyde, K.D. Pseudopestalotiopsis ignota and Ps. camelliae spp. nov. associated with grey blight disease of tea in China. Mycol. Prog. 2016, 15, 22. [Google Scholar] [CrossRef]
- Pordel, A.; Khodaparast, S.A.; McKenzie, E.H.C.; Javan-Nikkhah, M. Two new species of Pseudopyricularia from Iran. Mycol. Prog. 2017, 16, 729–736. [Google Scholar] [CrossRef]
- Klaubauf, S.; Tharreau, D.; Fournier, E.; Groenewald, J.; Crous, P.; de Vries, R.; Lebrun, M.-H. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Stud. Mycol. 2014, 79, 85–120. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfeld, M.J.; Burgess, T.I.; Hardy, G.E.S.T.J.; Barber, P.A.; Alvarado, P.; Barnes, C.W.; Buchanan, P.K.; Heykoop, M.; Moreno, G. Fungal planet description sheets: 558–624. Persoonia 2017, 38, 240–384. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Verkley, G.; Shin, H.-D.; Barreto, R.; Alfenas, A.; Swart, W.; Groenewald, J.; Crous, P. Sizing up Septoria. Stud. Mycol. 2013, 75, 307–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, A.; Asano, K.; Sone, T. A Molecular Phylogeny-Based Taxonomy of the Genus Rhizopus. Biosci. Biotechnol. Biochem. 2010, 74, 1325–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryganskyi, A.P.; Golan, J.; Dolatabadi, S.; Mondo, S.; Robb, S.; Idnurm, A.; Muszewska, A.; Steczkiewicz, K.; Masonjones, S.; Liao, H.-L.; et al. Phylogenetic and Phylogenomic Definition of Rhizopus Species. G3 Genes|Genom.|Genet. 2018, 8, 2007–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vebliza, Y.; Sjamsuridzal, W.; Oetari, A.; Santoso, I.; Roosheroe, I.G. Re-identifcation of five strains of Rhizopus arrhizus from tempeh based on ITS regions of rDNA sequence data. AIP Conf. Proc. 2018, 2023, e02016. [Google Scholar]
- Hsieh, H.-M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.-M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenet. Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Bahl, J.; Jeewon, R.; Hyde, K.D. Phylogeny of Rosellinia capetribulensis sp. nov. and its allies (Xylariaiceae). Mycologia 2005, 97, 1102–1110. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkamper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef] [Green Version]
- Keirnan, E.C.; Tan, Y.P.; Laurence, M.H.; Mertin, A.A.; Liew, E.C.Y.; Summerell, B.A.; Shivas, R.G. Cryptic diversity found in Didymellaceae from Australian native legumes. MycoKeys 2021, 78, 1–20. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Hou, L.W.; Hernández-Restrepo, M.; Groenewald, J.; Cai, L.; Crous, P.W. Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 2020, 65, 49–99. [Google Scholar] [CrossRef]
- Moslemi, A.; Ades, P.K.; Groom, T.; Nicolas, M.E.; Taylor, P.W. Alternaria infectoria and Stemphylium herbarum, two new pathogens of pyrethrum (Tanacetum cinerariifolium) in Australia. Australas. Plant Pathol. 2017, 46, 91–101. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Hanse, B.; van Leeuwen, G.C.M.; Groenewald, J.Z.; Crous, P.W. Stemphylium revisited. Stud. Mycol. 2017, 87, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Brahmanage, R.S.; Hyde, K.D.; Li, X.H.; Jayawardena, R.S.; McKenzie, E.H.C.; Yan, J.Y. Are pathogenic isolates of Stemphylium host specifc and cosmopolitan? Plant Pathol. Quarant. 2018, 8, 153–164. [Google Scholar] [CrossRef]
- Senwanna, C.; Wanasinghe, D.N.; Bulgakov, T.S.; Wang, Y.; Bhat, D.J.; Tang, A.M.C.; Mortimer, P.E.; Xu, J.; Hyde, K.D.; Phookamsak, R. Towards a natural classification of Dothidotthia and Thyrostroma in Dothidotthiaceae (Pleosporineae, Pleosporales). Mycosphere 2019, 10, 701–738. [Google Scholar] [CrossRef]
- Crous, P.W.; Schumache, R.K.; Akulov, A.; Thangavel, R.; Hernández Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfeld, M.J.; Summerel, B.A.; Quaedvlieg, W.; et al. New and interesting fungi. 2. Fungal Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef]
- Shivas, R.G.; Barrett, M.D.; Barrett, R.L.; McTaggart, A.R. Tilletia micrairae. Persoonia 2009, 22, 171–172. [Google Scholar]
- Shivas, R.G.; Beasley, D.R.; McTaggart, A.R. Online identification guides for Australian smut fungi (Ustilaginomycotina) and rust fungi (Pucciniales). IMA Fungus 2014, 5, 195–202. [Google Scholar] [CrossRef]
- McTaggart, A.R.; Shivas, R.G. Tilletia challinorae McTaggart & RG Shivas, sp. nov. Persoonia 2009, 23, 36. [Google Scholar]
- Li, Y.M.; Shivas, R.G.; Cai, L. Three new species of Tilletia on Eriachne from north-western Australia. Mycoscience 2014, 55, 361–366. [Google Scholar] [CrossRef]
- Vánky, K.; Lutz, M. Tubisorus, a new genus of smut fungi (Ustilaginomycetes) for Sporisorium pachycarpum. Mycol. Balc. 2011, 8, 129–135. [Google Scholar]
- Zhang, J.; Dou, Z.; Zhou, Y.; He, W.; Zhang, X.; Zhang, Y. Venturia sinensis sp. nov. a new ventuarialean ascomycete from Khingan Mountains. SaudiJ. Biol. Sci. 2016, 23, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Crous, P.; Schoch, C.; Bahkali, A.; Guo, L.; Hyde, K.D. A molecular, morphological and ecological re-appraisal of Venturiales—A new order of Dothideomycetes. Fungal Divers. 2011, 51, 249–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]


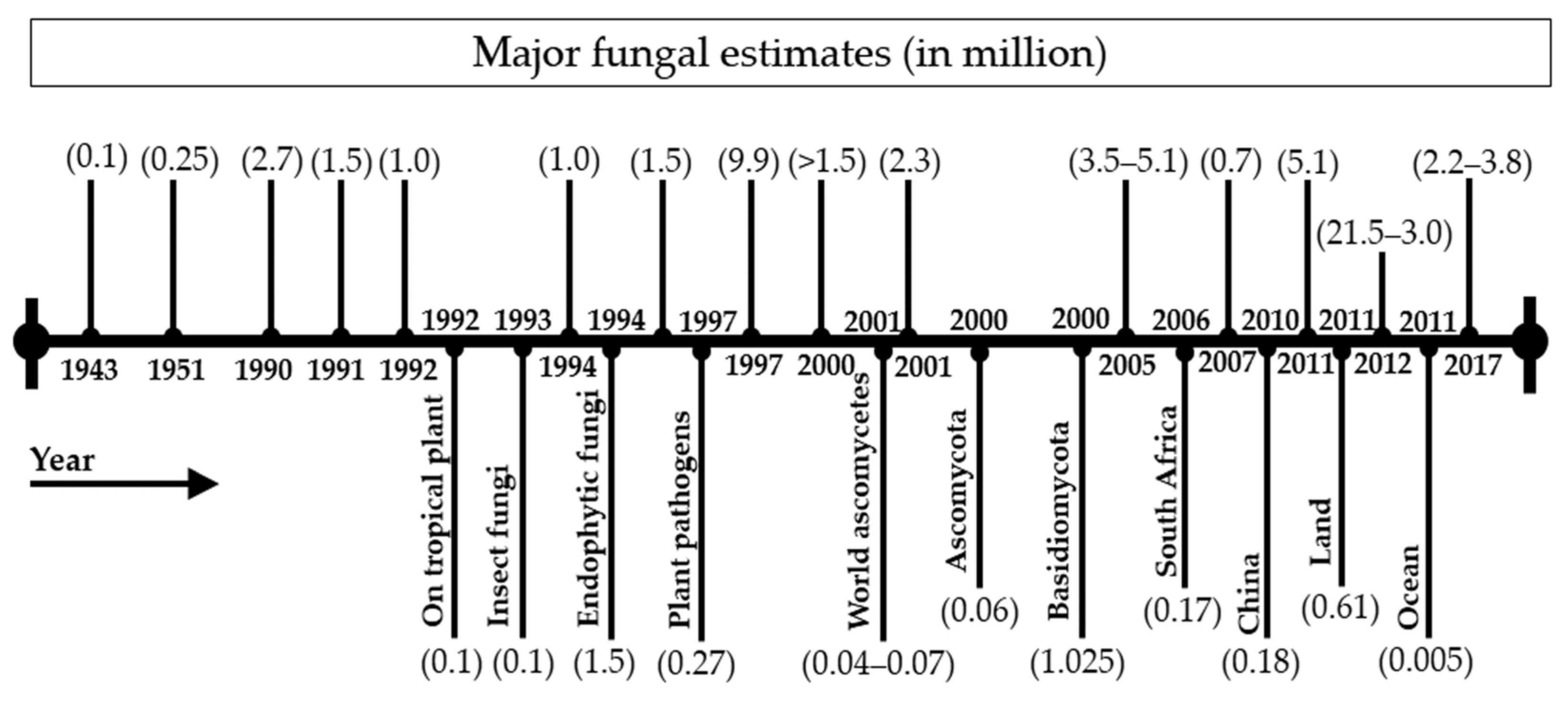
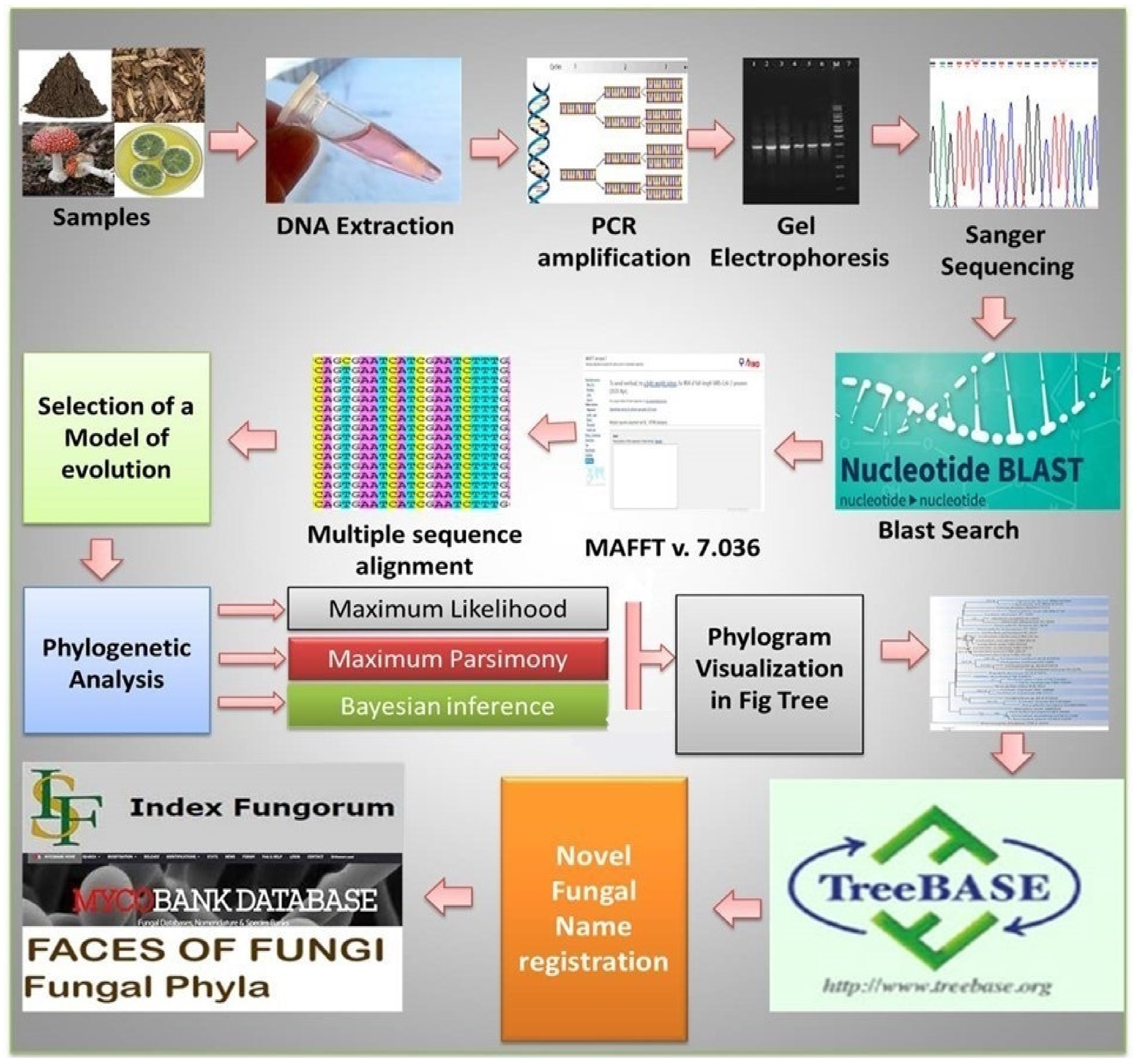


| Global Fungi Study ID | Substrate | Samples | Method | Sequencing Platform | ITS2 Sequences | Reference |
|---|---|---|---|---|---|---|
| Hartmann_2012_B1A3 | – | 6 | Culture independent | 454-pyrosequencing | 2155088 | [150] |
| Ihrmark_2012_3AE5 | Soil, wood, wheat roots and hay | 36 | Culture independent | 454-pyrosequencing | 414896 | [151] |
| Davey_2012_6F6A | Shoots of Hylocomium splendens, Pleurozium schreberi, and Polytrichum commune | 301 | Culture independent | 454-pyrosequencing | 296964 | [152] |
| Peay_2013_74BB | Soil | 36 | Culture independent | 454-pyrosequencing | 86677 | [153] |
| Davey_2013_7683 | Shoots of Dicranum scoparium, Hylocomium splendens, Pleurozium schreberi and Polytrichum commune | 454-pyrosequencing | Culture independent | 454-pyrosequencing | 313084 | [154] |
| Talbot_2014_A187 | Soil | 555 | Culture independent | 454-pyrosequencing | 16977 | [155] |
| Tedersoo_2014_B9DD | Soil | 360 | Culture independent | 454-pyrosequencing | 1979803 | [156] |
| Kadowaki_2014_B85B | Soil | 46 | Culture independent | 454-pyrosequencing | 66067 | [157] |
| Geml_2014_2936 | Soil | 10 | Culture independent | 454-pyrosequencing | 285031 | [158] |
| Davey_2014_2252 | Shoots of Hylocomium splendens | 251 | Culture independent | 454-pyrosequencing | 639746 | [159] |
| McHugh_2015_CAE1 | Soil | 20 | Culture independent | 454-pyrosequencing | 594424 | [160] |
| DeBeeck_2014_14DC | Soil | 20 | Culture independent | 454-pyrosequencing | 32778 | [161] |
| Yamamoto_2014_C3F7 | Seedlings of Quercus sp. | 431 | Culture independent | 454-pyrosequencing | 59021 | [162] |
| Walker_2014_22C1 | Soil | 24 | Culture independent | 454-pyrosequencing | 34267 | [163] |
| Veach_2015_7FDE | Soil | 91 | Culture independent | Illumina MiSeq | 579967 | [164] |
| Zhang_2015_A52F | Seven lichens speciesViz. Cetrariella delisei, Cladonia borealis, C. arbuscula, C. pocillum, Flavocetraria nivalis, Ochrolechia frigida and Peltigera canina | 22 | Culture independent | 454-pyrosequencing | 11087 | [165] |
| Elliott_2015_7CC2 | Soil | 16 | Culture independent | 454-pyrosequencing | 3896 | [166] |
| Geml_2015_1A45 | Soil | 10 | Culture independent | Ion Torrent | 1098472 | [167] |
| Hoppe_2015_BE27 | Wood | 48 | Culture independent | 454-pyrosequencing | 121459 | [168] |
| Jarvis_2015_B613 | Roots of Pinus sylvestris | 32 | Culture independent | 454-pyrosequencing | 112333 | [169] |
| Chaput_2015_41F7 | Soil | 4 | Culture independent | Tag-encoded FLX amplicon pyrosequencing | 1197 | [170] |
| van_der_Wal_2015_1114 | Sawdust from sapwood and heartwood of Quercus robur, Rubus fruticosus, Sorbus aucuparia, Betula pendula, Pteridium aquilinum and Amelanchier lamarckii | 42 | Culture independent | 454-pyrosequencing | 543801 | [171] |
| Clemmensen_2015_B0AE | Soil | 466 | Culture independent | 454-pyrosequencing GL FLX Titanium system | 592836 | [172] |
| Gao_2015_1CEF | Soil | 24 | Culture independent | 454-pyrosequencing GL FLX Titanium system | 93683 | [173] |
| Liu_2015_6174 | Soil | 26 | Culture independent | Roche FLX 454- pyrosequencing | 53978 | [174] |
| Oja_2015_88D4 | Cypripedium calceolus (subfamily Cypripedioideae), Neottia ovata (Epidendroideae) and Orchis militaris (Orchidoideae) and Soil | 158 | Culture independent | 454-pyrosequencing | 63045 | [175] |
| Goldmann_2015_EA26 | Soil | 48 | Culture independent | 454-pyrosequencer | 140966 | [176] |
| Tedersoo_2015_ED81 | Soil | 11 | Culture independent | Illumina MiSeq | 261751 | [177] |
| Rime_2015_89DE | Soil | 36 | Culture independent | 454-pyrosequencing GL FLX Titanium system | 227118 | [178] |
| Sterkenburg_2015_5E14 | Soil | 56 | Culture independent | 454-pyrosequencing | 350560 | [179] |
| Stursova_2016_D385 | Soil | 96 | Culture independent | Illumina MiSeq | 452546 | [180] |
| Semenova_2016_576B | Soil | 10 | Culture independent | Ion Torrent sequencing | 1007509 | [181] |
| Santalahti_2016_74FC | Soil | 117 | Culture independent | 454-pyrosequencing | 739877 | [182] |
| Rime_2016_E0E4 | Soils and sediments | 2 | Culture independent | 454-pyrosequencing | 35937 | [183] |
| RoyBolduc_2016_E50C | Root and soil | 63 | Culture independent | 454-pyrosequencing | 248325 | [184] |
| RoyBolduc_2016_F11B | Soil | 77 | Culture independent | 454-pyrosequencing | 280272 | [185] |
| Tedersoo_2016_TDEF | Soil | 136 | Culture independent | 454-pyrosequencing | 788372 | [186] |
| UOBC_2016_5CA6 | Soil | 655 | Culture independent | Illumina HiSeq | 7138323 | [187] |
| Urbina_2016_CE8E | Soil | 21 | Culture independent | Ion Torrent sequencing | 564332 | [188] |
| Valverde_2016_5E5C | Soil from the rhizosphere of Welwitschia mirabilis | 8 | Culture independent | 454-pyrosequencing | 2677 | [189] |
| Nacke_2016_8F49 | Soil from the rhizosphere Fagus sylvatica and Picea abies | 160 | Culture independent | 454-pyrosequencing | 386432 | [190] |
| Newsham_2016_191B | Soil | 29 | Culture independent | 454-pyrosequencing | 509483 | [191] |
| Nguyen_2016_D8E8 | Shoots of Picea abies, Abies alba, Fagus sylvatica, Acer pseudoplatanus, Fraxinus excelsior, Quercus robur, Pinus sylvestris, Betula pendula, Carpinus betulus and Quercus robur | 221 | Culture independent | 454-pyrosequencing | 63853 | [192] |
| Goldmann_2016_0757 | Root and soil samples from beech-dominated plots | 29 | Culture independent | 454-pyrosequencing | 85867 | [193] |
| Bahram_2016_7246 | Soil | 123 | Culture independent | 454-pyrosequencing | 213249 | [194] |
| Gehring_2016_E395 | Roots and root-associated (rhizosphere) soil of sagebrush, cheatgrass, and rice grass plants | 60 | - | - | 1161117 | [195] |
| Gourmelon_2016_9281 | Soil | 32 | Culture independent | Illumina MiSeq | 91814 | [196] |
| Bissett_AAAA_2016 | Soil | 2061 | Culture independent | Illumina MiSeq | 50810033 | [197] |
| Cox_2016_EDC5 | Soil | 135 | Culture independent | 454-pyrosequencing | 886200 | [198] |
| Oh_2016_DEBA | Soil | 12 | Culture independent | 454-pyrosequencing | 98376 | [199] |
| Frey_2016_5D5C | Soil | 12 | Culture independent | Illumina MiSeq v3 | 500999 | [200] |
| Gannes_2016_5E98 | Soil | 23 | Culture independent | Illumina MiSeq system | 218946 | [201] |
| Li_2016_1EBC | Soil | 21 | Culture independent | Illumina MiSeq system | 129184 | [202] |
| Kielak_2016_1110 | Wood of Pinus sylvestris | 75 | Culture independent | 454-pyrosequencing | 1281356 | [203] |
| Ji_2016_C06E | Soil | 13 | Culture independent | 454-pyrosequencing | 277 | [204] |
| Baldrian_2016_DE02 | Sawdust | 118 | Culture independent | llumina MiSeq | 1205580 | [205] |
| Barnes_2016_0042 | Roots of Cinchona calisaya | 21 | Culture independent | llumina MiSeq | 239387 | [206] |
| Porter_2016_CD8D | Soil | 2 | Culture independent | 454-pyrosequencing | 20123 | [207] |
| Zhou_2016_A8F1 | Soil | 126 | Culture independent | Illumina MiSeq | 3542416 | [208] |
| Zhang_2016_1DA0 | Soil | 13 | Culture independent | 454-pyrosequencing | 2362 | [209] |
| Wang_2016_6223 | Roots, stems, and sprouts of rice plant | 1 | Culture independent | Illumina MiSeq | 1850 | [210] |
| Zifcakova_2016_4C03 | Soil | 24 | Culture independent | ILLUMINA HISEQ2000 | 123869 | [211] |
| VanDerWal_2016_4C9C | Sawdust from sapwood and heartwood | 130 | Culture independent | 454-pyrosequencing | 1215932 | [212] |
| Varenius_2017_BCFB | Soil | 517 | Culture independent | PacBio RSII platform by SciLifeLab | 186474 | [213] |
| van_der_Wal_2017_2D0D | Sawdust samples of Larix stumps, and Quercus stumps | 88 | Culture independent | Illumina MiSeq | 877425 | [214] |
| Wang_2017_7E18 | Soil | 6 | Culture independent | 454-pyrosequencing | 53737 | [215] |
| van_der_Wal_2017_3070 | Soil | 135 | Culture independent | Illumina MiSeq | 1572834 | [216] |
| Vasutova_2017_3070 | Soil | 28 | Culture independent | GS Junior sequencer | 9370 | [217] |
| Vaz_2017_C16E | Woody debris | 2 | Culture independent | Personal Genome Machine | 11817 | [218] |
| Yang_2017_2AFC | Soil | 180 | Culture independent | llumina MiSeq platform PE250 | 12688168 | [219] |
| Wicaksono_2017_3B9E | Root samples of Alnus acuminata | 24 | Culture independent | Ion Torrent | 3596531 | [220] |
| Yang_2017_EB1D | Soil | 26 | Culture independent | Illumina MiSeq platform PE250 | 1450233 | [221] |
| Zhang_2017_02C2 | Plant litter and soil | 54 | Culture independent | Illumina MiSeq | 2904476 | [222] |
| Zhang_2017_F933 | Peat soil | 9 | Culture independent | Illumina HiSeq2000 | 320199 | [223] |
| Purahong_2017_8EFD | Wood sample | 116 | Culture independent | Genome Sequencer 454-FLX System | 299831 | [224] |
| Poosakkannu_2017_B342 | Bulk soil, rhizosphere soil, and D. flexuosa Leaf | 43 | Culture independent | IonTorrent | 259743 | [225] |
| Bergottini_2017_02C2 | Roots of Ilex paraguariensis | 11 | Culture independent | 454-pyrosequencing | 189048 | [226] |
| Dean_2017_F5A5 | Roots of Glycine max (soybean) and Thlaspi arvense | 12 | Culture independent | 454-FLX titanium | 12596 | [227] |
| Fernandez_Martinez_2017_14C3 | Soil | 11 | Culture independent | 454-pyrosequencing | 138524 | [228] |
| Ge_2017_4DC8 | Roots of Quercus nigra, Q. virginiana, Q. laevis, Carya cf. glabra, Carya cf. tomentosa as well as several Carya and Quercus spp. | 9 | Culture independent | 454-pyrosequencing | 44 | [229] |
| Gomes_2017_2AFC | Roots of Thismia sp. | 61 | Culture independent | Ion Torrent | 4067438 | [230] |
| Almario_2017_2082 | Root and rhizosphere of Arabis alpina | 26 | Culture independent | Illumina Miseq | 805679 | [231] |
| Anthony_2017_647F | Soil | 142 | Culture independent | Illumina Miseq | 12453259 | [232] |
| Grau_2017_E29A | Soil | 27 | Culture independent | Ion Torrent | 960177 | [233] |
| Hiiesalu_2017_E29A | Soil | 1 | Culture independent | 454-pyrosequencing | 4616 | [234] |
| Nguyen_2017_6F2C | Leaf samples of Betula pendula | 20 | Culture independent | 454-pyrosequencing | 1318 | [235] |
| Kolarikova_2017_EB1D | Roots of Salix caprea and Betula pendula | 24 | Culture independent | 454-pyrosequencing | 47543 | [236] |
| Kyaschenko_2017_89D4 | Soil | 30 | Culture independent | PacBio sequencing | 64010 | [237] |
| Oja_2017_AD29 | Roots and rhizosphere soil of | 333 | Culture independent | 454-pyrosequencing | 446296 | [238] |
| Miura_2017_2BE5 | Leaves and berries of grapes | 36 | Culture independent | Illumina MiSeq | 2250530 | [239] |
| Oono_2017_B342 | Needles of Pinus taeda | 143 | Culture independent | Illumina MiSeq | 9755183 | [240] |
| Kamutando_2017_6F2C | Soil | 3 | Culture independent | Illumina MiSeq | 4 | [241] |
| Shen_2017_C7F4 | Soil | 1 | Culture independent | Illumina MiSeq | 1 | [242] |
| Smith_2017_2AFC | Root of Dicymbe corymbosa | 8 | Culture independent | 454-pyrosequencing | 94 | [243] |
| Tian_2017_F933 | Soil | 3 | Culture independent | 454-GS FLX+pyrosequencing machine | 25001 | [244] |
| Tu_2017_BCFB | Soil | 60 | Culture independent | Illumina MiSeq | 696557 | [245] |
| Sharma_Poudyal_2017_F933 | Soil | 53 | Culture independent | 454-FLX titanium | 7680 | [246] |
| Cross_2017_2AFC | Leaflet, petiole upper and petiole base tissues of ash leaves of Fraxinus excelsior (common ash) | 27 | Culture independent | 454-pyrosequencing | 171094 | [247] |
| Kazartsev_2018_1115 | Bark of Picea abies | 20 | Culture independent | 454-pyrosequencing | 22918 | [248] |
| Bickford_2018_2EE0 | Roots of Phragmites spp. | 3 | Culture independent | PacBio-RS II | 66439 | [249] |
| Cline_2018_0BCC | Wood of Betula papyrifera | 15 | Culture independent | 454-FLX titanium | 660 | [250] |
| Cregger_2018_added | Roots, stems, and leaves of Populus deltoides and the Populus trichocarpa × deltoides hybrid | 290 | Culture independent | Illumina MiSeq | 14767409 | [251] |
| Marasco_2018_DBE1 | Rhizosheath-root system of Stipagrostis sabulicola, S. seelyae and Cladoraphis spinosa | 49 | Culture independent | Illumina MiSeq | 4694085 | [252] |
| Glynou_2018_445A | Roots of nonmycorrhizal Microthlaspi spp. | 5 | Culture independent | Illumina Miseq | 7 | [253] |
| Montagna_2018_E316 | Soil | 24 | Culture independent | Illumina Miseq | 2475767 | [254] |
| Schlegel_2018_A231 | Leaves of Fraxinus spp. and Acer pseudoplatanus | 353 | Culture independent | Illumina MiSeq | 24198214 | [255] |
| SchneiderMaunoury_2018_51AB | Different plant species | 78 | Culture independent | Ion Torrent | 352332 | [256] |
| Schon_2018_01F4 | Soil | 18 | Culture independent | Illumina MiSeq | 235709 | [257] |
| Rasmussen_2018_C8E6 | Root samples | 228 | Culture independent | Illumina MiSeq | 428044 | [258] |
| Rogers_2018_147F | Hemlock stems | 6 | Culture independent | Illumina MiSeq | 675067 | [259] |
| Purahong_2018_14C0 | Deadwood logs | 297 | Culture independent | 454-pyrosequencing | 2034928 | [260] |
| Qian_2018_2B1E | Leaves of Mussaenda shikokiana | 20 | Culture independent | Illumina MiSeq | 449179 | [261] |
| Park_2018_569C | Calanthe species: C. aristulifera, C. bicolor, C. discolor, C. insularis and C. striata | 12 | Culture independent | 454-GS FLX +System | 65867 | [262] |
| Mirmajlessi_2018_765D | Soil | 40 | Culture independent | Illumina MiSeq | 1077125 | [263] |
| Purahong_2018_9F2E | Wood samples | 96 | Culture independent | 454-pyrosequencing | 656682 | [264] |
| Si_2018_53B6 | Soil | 27 | Culture independent | Illumina MiSeq | 692169 | [265] |
| Saitta_2018_51C8 | Soil | 16 | Culture independent | Illumina MiSeq | 4923667 | [266] |
| Santalahti_2018_3794 | Soil | 38 | Culture independent | 454-pyrosequencing | 218387 | [267] |
| Sukdeo_2018_1DF4 | Soil | 126 | Culture independent | Illumina MiSeq | 32336646 | [268] |
| Zhu_2018_1E38 | Soil | 12 | Culture independent | Illumina MiSeq | 1031479 | [269] |
| Zhang_2018_F81F | Soil | 106 | Culture independent | Illumina HiSeq | 1673070 | [270] |
| Zhang_2018_491A | Bare sand, algal crusts, lichen crusts, and moss crusts | 17 | Culture independent | Illumina Miseq | 442056 | [271] |
| Sun_2018_1B01 | Soil | 36 | Culture independent | Illumina Miseq | 1188520 | [272] |
| Weissbecker_2019_6A75 | Soil | 394 | Culture independent | GS-FLX + 454 pyrosequencer | 1109208 | [273] |
| Purahong_AD_2019 | Wood chips of rotted heartwood deadwood from C. carlesii | 3 | Culture independent | PacBio RS II system | 22886 | [274] |
| Egidi_AD_2019 | Soil | 161 | Culture independent | Illumina MiSeq | 14131987 | [275] |
| Froeslev_2019_CA74 | Soil | 276 | Culture independent | Illumina MiSeq | 6114124 | [276] |
| Ogwu_2019_38FE | Soil | 13 | Culture independent | Illumina Miseq | 724483 | [277] |
| Ovaskainen_2019_air | Soil particles, spores, pollen, bacteria, and small insects | 75 | Culture independent | Illumina Miseq | 935812 | [278] |
| Qian_2019_9691 | Leaves and soil | 30 | Culture independent | Illumina HiSeq | 2133292 | [279] |
| Ramirez_2019_D0B2 | Soil | 810 | Culture independent | Illumina Miseq | 6555903 | [280] |
| Pellitier_2019_82BC | Bark of black oak (Quercus velutina), white oak (Q. alba), red pine (Pinus resinosa), eastern white pine (P. strobus) and red maple (Acer rubrum) | 15 | Culture independent | Illumina MiSeq | 10649956 | [281] |
| Semenova-Nelsen_2019_add | Litter and the uppermost soil | 121 | Culture independent | Illumina MiSeq | 3205748 | [282] |
| Sheng_2019_66AC | Soil | 16 | Culture independent | Illumina MiSeq | 447840 | [283] |
| Shigyo_2019_5B19 | Soil | 144 | Culture independent | Illumina MiSeq | 4353704 | [284] |
| Schroter_2019_1B64 | Fine roots and soil | 3 | Culture independent | Roche GS-FLX+ pyrosequencer | 144 | [285] |
| Singh_2019_EA7F | Fine roots and soil | 96 | Culture independent | Illumina MiSeq | 3138303 | [286] |
| Song_2019_ad2 | Soil | 46 | Culture independent | Illumina MiSeq | 920391 | [287] |
| U’Ren_2019_add | Fresh, photosynthetic tissues of a diverse range of plants and lichens | 486 | Culture-based sampling and culture-independent | Illumina MiSeq | 5671834 | [288] |
| Unuk_2019_567A | Fine roots and soil | 30 | Culture independent | Ilumina MiSeq | 470786 | [289] |
| Araya_2019_add | Soil | 36 | Culture independent | Illumina MiSeq | 8083471 | [290] |
| Alvarez-Garrido_2019_add | Root tips from A. pinsapo trees following the trunk to the superficial secondary roots | 76 | Culture independent | Illumina MiSeq | 1795423 | [291] |
| Wei_2019_3796 | Soil | 1 | Culture independent | Illumina HiSeq | 18 | [292] |
| Pan_2020_addZ | Soil from the rhizosphere of potato | 1 | Culture independent | Illumina MiSeq | 2 | [293] |
| Detheridge_2020_Z | Soil | 70 | Culture independent | 1832454 | [294] | |
| Li_2020_AS | Soil | 19 | Culture independent | Illumina MiSeq | 116660 | [295] |
| Location | Source | Sequencing | Method | Target Gene | Reference | ||
|---|---|---|---|---|---|---|---|
| Woods Hole Harbor Massachusetts | Wood | – | Culture dependent | Direct observation | – | – | [296] |
| Atlantic Ocean | Water | – | Culture dependent | Incubation of sample and direct observation | – | – | [297] |
| Rumanian coast of the Black Sea | Calcareous substances | – | Culture dependent | Incubation of sample and direct observation | – | – | [298] |
| Iceland-Faroe ridge | Water | – | Culture dependent | Incubation of sample and direct observation | – | – | [299] |
| Bahamas | Wood | – | Culture dependent | Incubation of sample and direct observation | – | – | [300] |
| Bay of Bengal and Arabian Sea | Sediment | Culture dependent | Culture media | – | – | [301] | |
| Northwest Pacific Ocean (Sagami Bay and Suruga Bay; Palau-Yap Trench and Mariana Trench) | Sediments | Sanger | Culture dependent | Culture media | – | ITS and 5.8S | [302] |
| Guaymas Basin hydrothermal vent | Sediment | Sanger | Culture independent | – | Clone library | SSU | [303] |
| Mid-Atlantic Ridge hydrothermal area | Sediment | Sanger | Culture independent | – | Clone library | SSU | [304] |
| Chagos Trench, Indian Ocean | Sediment | – | Culture dependent/Direct detection | Culture media | – | – | [305] |
| Peru Margin | Sediment | Sanger | Culture dependent | Culture media | – | SSU | [306] |
| Central Indian Basin | Sediment | – | Culture dependent | Culture media | – | – | [307] |
| Kuroshima Knoll in Okinawa | Sediment | Sanger | Culture dependent | Clone library | SSU | [308] | |
| Central Indian Basin | Sediment | Sanger | Culture dependent | Culture media | – | – | [309] |
| Different locations | Water and sediment | Sanger | Culture dependent | Clone library | SSU | [310] | |
| South China Sea | Sediment | Sanger | Culture dependent | – | Clone library | ITS | [311] |
| Lost City | Water | Sanger | Culture dependent | – | Clone library | SSU | [312] |
| Central Indian Basin | Sediment | Direct detection | – | – | – | [313] | |
| Vailulu’u is an active submarine volcano at the eastern end of the Samoan volcanic chain | Water | Sanger | Culture dependent | Culture media | – | ITS | [314] |
| Vanuatu archipelago | Deepsea water, wood and debris | Sanger | Culture dependent | Culture media | – | SSU and LSU | [315] |
| East Pacific Rise, Mid-Atlantic Ridge and Lucky Strike | Deepsea hydrothermal ecosystem | Sanger | Culture dependent/Cultureindependent | Culture media | Clone library | SSU | [316] |
| Southwest Pacific | Deepsea hydrothermal ecosystems | Sanger | Culture dependent | Culture media | – | SSU | [317] |
| Different locations | Deep-sea hydrothermal ecosystems | Sanger | Culture dependent | Culture media | – | LSU | [318] |
| Japanese islands, including a sample from the deepest ocean depth, the Mariana Trench | Sediment | Sanger | Culture independent | – | Clone library | SSU, ITS and LSU | [319] |
| Southern East Pacific Rise | Water and bivalves | Sanger | Culture independent | – | Clone library | SSU | [320] |
| Central Indian Basin | Sediment | Sanger | Culture dependent | Culture media | Full ITS and SSU | [321] | |
| Southern Indian Ocean | Sediment | Sanger | Culture independent | – | Clone library | SSU | [322] |
| Peru Margin and the Peru Trench | Sediment | Sanger | Culture independent | – | Clone library | SSU | [323] |
| Puerto Rico Trench | Water | Sanger | Culture independent | – | Clone library | SSU | [324] |
| Sagami-Bay | Deep-sea methane cold-seep sediments | Sanger | Culture independent | – | Clone library | SSU | [325] |
| Marmara Sea | Sediment | Sanger and 454-pyrosequencing | Culture independent | – | Clone library | SSU | [326] |
| Central Indian Basin - Several stations | Sediment | Sanger | Culture independent | – | Clone library | Full ITS and SSU | [327] |
| Central Indian Basin - Several stations | Sediment | Sanger | Culture dependent/Culture independent | Culture media | Clone library | SSU (Fungal isolates)/ITS (DNA sediment) | [328] |
| Central Indian Basin - Several stations | Sediment | Sanger | Culture independent cloning | – | Clone library | Full ITS and SSU | [328] |
| Alaminos Canyon 601 methane seep in the Gulf of Mexico | Methane seeps sediment | Sanger | Culture independent | – | Clone library | ITS and LSU | [329] |
| The area surrounding the DWH oil spill in the Gulf of Mexico | Deep-sea samples from the area surrounding the Deepwater Horizon oil spill | 454-pyrosequencing | Culture independent | – | Shotgun | assA and bssA | [330] |
| Hydrate Ridge, Peru Margin, Eastern Equatorial Pacific | Sediment | Sanger and 454-pyrosequencing | Culture independent | – | TRFLP/Metatranscriptomics | SSU | [331] |
| Peru Margin | Sediment | Illumina | Culture independent | Metatranscriptomics | – | [331] | |
| South China Sea | Sediment | Sanger | Culture dependent | Culture media | – | Full ITS | [332] |
| Mediterranean Sea | Hypsersaline anoxic basin | 454-pyrosequencing | Culture independent | – | – | SSU | [333] |
| Canterbury basin, on the eastern margin of the South Island of New Zealand | Sediment Ocean Drilling Program | 454-pyrosequencing | Culture independent | – | Metatranscriptomics | ITS and SSU | [334] |
| The Pacific Ocean and MarianaTrench | Sediment | Sanger | Culture independent | – | Clone library | ITS | [335] |
| East Indian Ocean | Sediment | Sanger | Culture dependent/Culture independent | Culture media | Clone library | ITS | [336] |
| Canterbury basin, on the eastern margin of the South Island of New Zealand | Sediment | Sanger | Culture dependent | Culture media | – | SSU, ITS and LSU | [337] |
| Urania, Discovery and L’Atalante basins | Hypsersaline anoxic basin | Illumina | Culture independent | – | Metatranscriptomics | – | [338] |
| Several locations around the world/The ICoMM data set | Pelagic and benthic samples | 454-pyrosequencing | Culture independent | – | – | SSU | [339] |
| The Pacific Ocean and MarianaTrench | Sediment | Sanger | Culture independent | – | Clone library | ITS, SSU and LSU | [340] |
| Okinawa | Sediment | Illumina | Culture independent | – | – | ITS | [341] |
| Southwest Indian Ridge (SWIR) | Sediment and Deepsea hydrothermal ecosystems | Sanger and Illumina | Culture dependent/Culture independent | With and without Culture media | – | ITS | [342] |
| Continental margin of Peru | Sediment | Illumina | Culture independent | – | – | SSU | [343] |
| North Atlantic and Arctic Basin | Marine snow | Culture independent | – | CARD-FISH | – | [344] | |
| Northern Chile | Water | Sanger | Culture dependent | – | – | Full ITS | [345] |
| The Sao Paulo Plateau | Asphalt seeps | Ion Torrent | Culture independent | – | – | ITS | [346] |
| Peru Margin | Sediment | Illumina | Culture independent | – | Metatranscriptomics | [347] | |
| East Pacific | Sediment | Sanger | Culturedependent | Culture media | Full ITS | [348] | |
| The Ionian Sea (Central Mediterranean Sea) | Sediment | Illumina | Culture independent | – | FISH | ITS | [349] |
| South-central western Pacific Ocean | Water | Illumina | Culture independent | – | – | SSU | [350] |
| Challenger deep | Water | Illumina | Culture independent | – | – | ITS | [351] |
| Mexican Exclusive Economic Zone-Gulf of Mexico | Sediment | Illumina | Culture independent | – | – | ITS | [352] |
| Yap Trench | Sediment | Sanger and Illumina | Culture dependent/Culture independent | – | – | ITS | [353] |
| Mexican Exclusive Economic Zone-Gulf of Mexico | Sediment | Sanger | Culture dependent | Culture media | – | Full ITS and tub | [354] |
| General Identification Tools and Data Repositories | |
|---|---|
| BOLD | http://www.boldsystems.org/ (accessed on 6 November 2021) |
| Westerdijk Fungal BiodiversityInstitute | https://wi.knaw.nl/page/Collection (accessed on 6 November 2021) |
| CIPRES | https://www.phylo.org/ (accessed on 6 November 2021) |
| Dryad | http://datadryad.org/ (accessed on 6 November 2021) |
| FUSARIUM-ID | http://isolate.fusariumdb.org/ (accessed on 6 November 2021) |
| One Stop Shop Fungi | http://onestopshopfungi.org/ (accessed on 6 November 2021) |
| GreenGenes | http://greengenes.lbl.gov/cgi-bin/nph-index.cgi (accessed on 6 November 2021) |
| MaarjAM | http://maarjam.botany.ut.ee/ (accessed on 6 November 2021) |
| Mothur | http://www.mothur.org/ (accessed on 6 November 2021) |
| Naïve Bayesian Classifier | http://aem.asm.org/content/73/16/5261.short?rss=1&ssource=mfc (accessed on 6 November 2021) |
| Open Tree of Life | http://www.opentreeoflife.org/ QIIME http://qiime.org/ (accessed on 6 November 2021) |
| PHYMYCO database | http://phymycodb.genouest.org/ (accessed on 6 November 2021) |
| RefSeq Targeted Loci | http://www.ncbi.nlm.nih.gov/refseq/targetedloci/ (accessed on 6 November 2021) |
| Ribosomal Database Project (RDP) | http://rdp.cme.msu.edu/ (accessed on 6 November 2021) |
| Silva | http://www.arb-silva.de/ (accessed on 6 November 2021) |
| TreeBASE | http://treebase.org/ (accessed on 6 November 2021) |
| TrichoBLAST | http://www.isth.info/tools/blast/ (accessed on 6 November 2021) |
| UNITE | http://unite.ut.ee/ (accessed on 6 November 2021) |
| United Kingdom National Culture Collection | http://www.ukncc.co.uk/ (accessed on 6 November 2021) |
| Data standards | |
| BIOM | http://biom-format.org/ (accessed on 6 November 2021) |
| MIMARKS | http://www.nature.com/nbt/journal/v29/n5/full/nbt/1823.html (accessed on 6 November 2021) |
| Darwin | Core http://rs.tdwg.org/dwc/ (accessed on 6 November 2021) |
| Genomics databases and tools | |
| AFTOL | http://aftol.umn.edu/ (accessed on 6 November 2021) |
| 1000 Fungal Genomes Project (1KFG) | http://1000.fungalgenomes.org/home/ (accessed on 6 November 2021) |
| FungiDB | http://fungidb.org/fungidb/ (accessed on 6 November 2021) |
| GEBA | http://jgi.doe.gov/our-science/science-programs/microbial-genomics/phylogenetic-diversity/ (accessed on 6 November 2021) |
| MycoCosm | http://genome.jgi.doe.gov/programs/fungi/index.jsf (accessed on 6 November 2021) |
| Functional database | |
| FUNGuild | http://github.com/UMNFuN/FUNGuild (accessed on 6 November 2021) |
| Nomenclature and nomenclatural databases and organizations | |
| Catalogue of Life (COL) | http://www.catalogueoflife.org/ (accessed on 6 November 2021) |
| EPPO-Q-bank | http://qbank.eppo.int/ (accessed on 6 November 2021) |
| Faces of Fungi | http://www.facesoffungi.org/ (accessed on 6 November 2021) |
| Index Fungorum | http://www.indexfungorum.org/ (accessed on 6 November 2021) |
| International code of nomenclature for algae, fungi, and plants (ICNAFP) | http://www.iapt-taxon.org/nomen/main.php (accessed on 6 November 2021) |
| International Commission on the Taxonomy of Fungi (ICTF) | http://www.fungaltaxonomy.org/ (accessed on 6 November 2021) |
| List of prokaryotic names with standing in nomenclature (LPSN) | http://www.bacterio.net/ (accessed on 6 November 2021) |
| MycoBank | http://www.mycobank.org/ (accessed on 6 November 2021) |
| Outline of fungi | http://www.outlineoffungi.org/ (accessed on 6 November 2021) |
| Biodiversity collections databases | |
| Global Biodiversity Information Facility (GBIF) | http://www.gbif.org/ (accessed on 6 November 2021) |
| iDigBio | http://www.idigbio.org/ (accessed on 6 November 2021) |
| MycoPortal | http://mycoportal.org/portal/index.php (accessed on 6 November 2021) |
| World Federation of Culture Collections (WFCC) | http://www.wfcc.info/ (accessed on 6 November 2021) |
| Sequencing Independent Methods | High-Throughput Sequencing Platforms |
|---|---|
| ARDRA (Amplified Ribosomal DNA Restriction Analysis) | 454 Pyrosequencing (second-generation platform) |
| ARISA (Amplified Intergeneric Spacer Analysis) | Illumina MiSeq sequencing (second-generation) |
| DGGE (Denaturing Gradient Gel Electrophoresis) | Ion Torrent PGM and GeneStudio |
| FISH (Fluorescence in Situ Hybridization) | PacBio RSII and Sequel (This third-generation HTS platform) |
| LAMP (Loop-Mediated Isothermal Amplification) | Oxford Nanopore MinION, GridION and PrometION (third-generation) |
| MT-PCR (Multiplexed tandem PCR) | – |
| RCA (Rolling Circle Amplification) | – |
| RDBH (Reverse Dot Blot Hybridization) | – |
| RFLP (Restriction Fragment Length Polymorphism) | – |
| SSCP (Single-Strand Conformation Polymorphism) | – |
| TGGE (Thermal Gradient Gel Electrophoresis) | – |
| TRFLP (Terminal Restriction Fragment Length Polymorphism) | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, A.K.; Verma, R.K.; Avasthi, S.; Sushma; Bohra, Y.; Devadatha, B.; Niranjan, M.; Suwannarach, N. Current Insight into Traditional and Modern Methods in Fungal Diversity Estimates. J. Fungi 2022, 8, 226. https://doi.org/10.3390/jof8030226
Gautam AK, Verma RK, Avasthi S, Sushma, Bohra Y, Devadatha B, Niranjan M, Suwannarach N. Current Insight into Traditional and Modern Methods in Fungal Diversity Estimates. Journal of Fungi. 2022; 8(3):226. https://doi.org/10.3390/jof8030226
Chicago/Turabian StyleGautam, Ajay Kumar, Rajnish Kumar Verma, Shubhi Avasthi, Sushma, Yogita Bohra, Bandarupalli Devadatha, Mekala Niranjan, and Nakarin Suwannarach. 2022. "Current Insight into Traditional and Modern Methods in Fungal Diversity Estimates" Journal of Fungi 8, no. 3: 226. https://doi.org/10.3390/jof8030226
APA StyleGautam, A. K., Verma, R. K., Avasthi, S., Sushma, Bohra, Y., Devadatha, B., Niranjan, M., & Suwannarach, N. (2022). Current Insight into Traditional and Modern Methods in Fungal Diversity Estimates. Journal of Fungi, 8(3), 226. https://doi.org/10.3390/jof8030226








