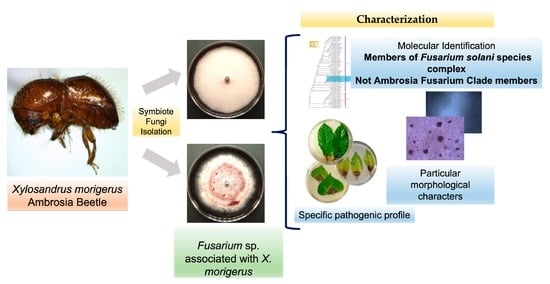
Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. Culture Conditions
2.3. Molecular Identification
2.4. Molecular Phylogenetic Analysis
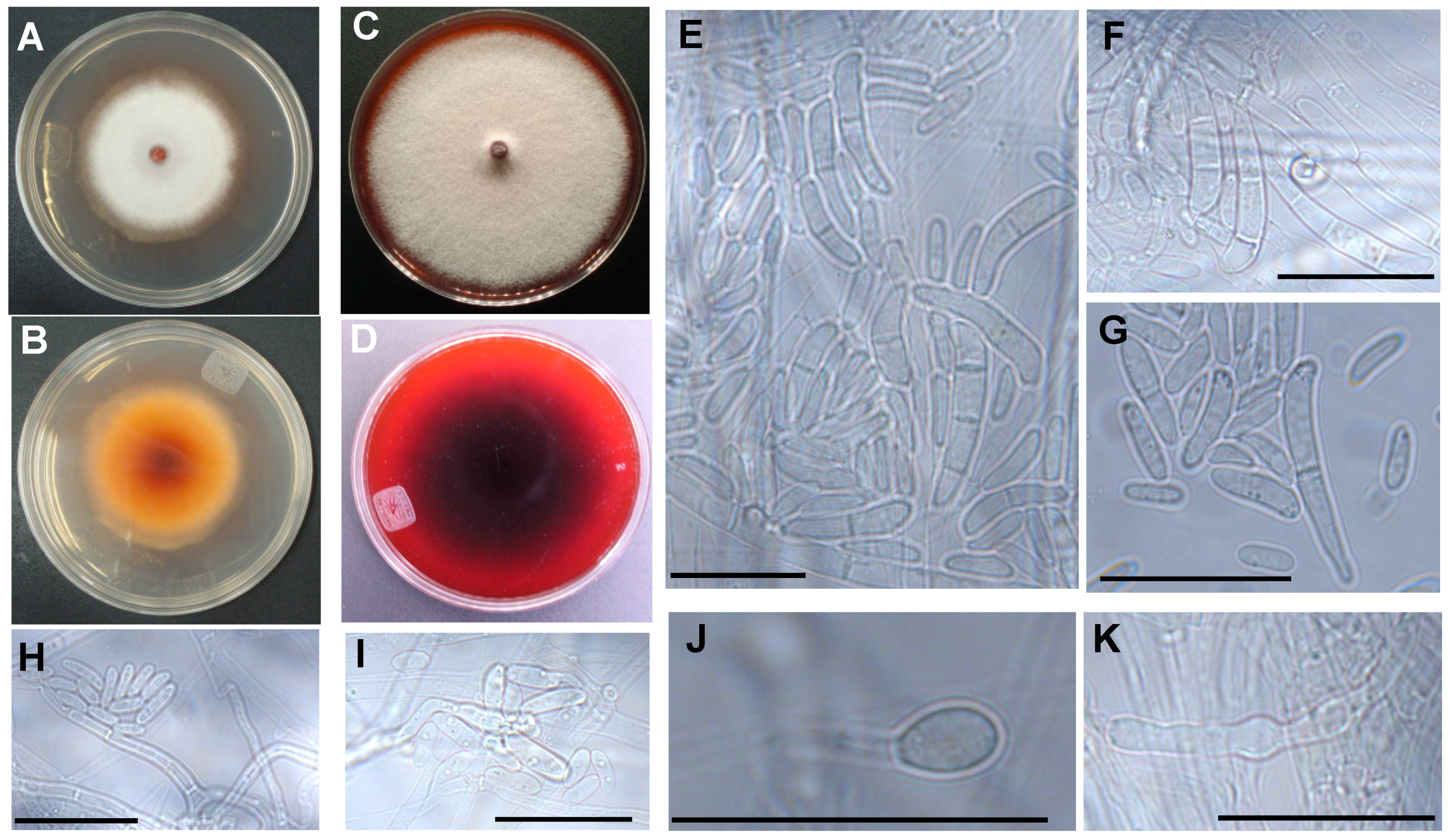
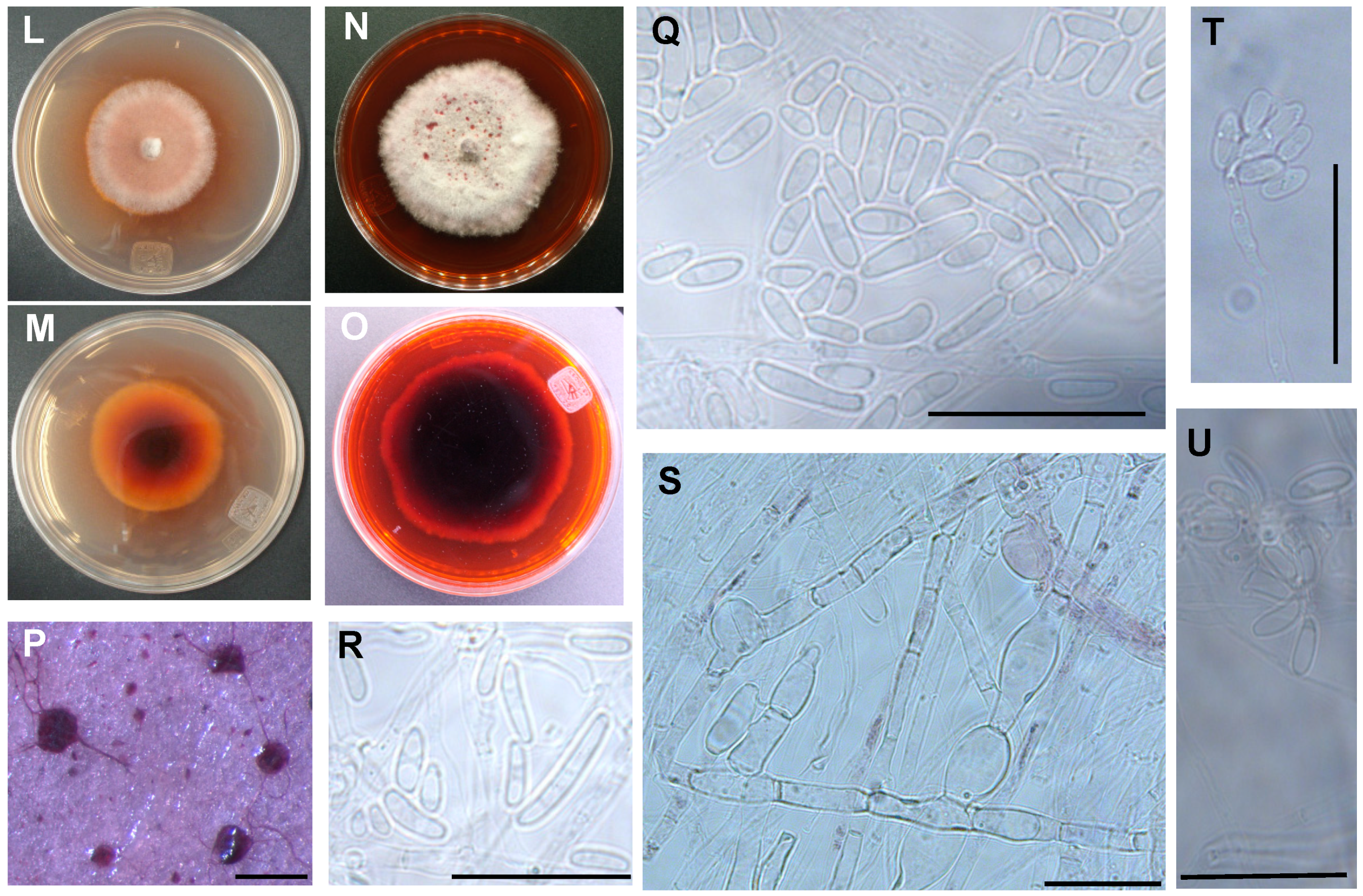
2.5. Macroscopic Morphology Examination
2.6. Colony Radial Growth
2.7. Microscopic Analyses
2.8. Pathogenesis Assay in Coffea arabica, Salix lasiolepis, Populus nigra, Citrus sinensis and Citrus latifolia
2.9. Extracellular Protease Activity Assay
3. Results
3.1. Fungal Isolates INECOL_BM-04 and INECOL BM-06 Belong to the FSSC but Not to the AFC
3.2. The Two Closely Related Fusarium sp. INECOL_BM-04 and INECOL_BM-06 Isolated from X. morigerus Are Phenotypically Different
3.3. Phytopathogenicity Screening Shows Differences in Virulence among Fusarium spp. Associated with Xylosandrus morigerus
3.3.1. Pathogenesis Assays in Coffea arabica
3.3.2. Pathogenesis Assays in Important Citrus Species, Citrus latifolia and Citrus sinensis
3.3.3. Pathogenesis Assays of Salix lasiolepis and Populus nigra
3.4. Extracellular Protease Activity Is Slightly Different among Fusarium spp. Associated with Xylosandrus morigerus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.E.; Biedermann, P.H.W. On Interactions, Associations, Mycetangia, Mutualists and Symbiotes in Insect-Fungus Symbioses. Fungal Ecol. 2020, 44, 100909. [Google Scholar] [CrossRef]
- Lange, L.; Grell, M.N. The Prominent Role of Fungi and Fungal Enzymes in the Ant–Fungus Biomass Conversion Symbiosis. Appl. Microbiol. Biotechnol. 2014, 98, 4839–4851. [Google Scholar] [CrossRef]
- Da Costa, R.; Hu, H.; Li, H.; Poulsen, M. Symbiotic Plant Biomass Decomposition in Fungus-Growing Termites. Insects 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, C.; Vollet-Neto, A.; Marsaioli, A.J.; Zampieri, D.; Fontoura, I.C.; Luchessi, A.D.; Imperatriz-Fonseca, V.L. A Brazilian Social Bee Must Cultivate Fungus to Survive. Curr. Biol. 2015, 25, 2851–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paludo, C.R.; Menezes, C.; Silva-Junior, E.A.; Vollet-Neto, A.; Andrade-Dominguez, A.; Pishchany, G.; Khadempour, L.; do Nascimento, F.S.; Currie, C.R.; Kolter, R.; et al. Stingless Bee Larvae Require Fungal Steroid to Pupate. Sci. Rep. 2018, 8, 1122. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia Symbiosis: From Evolutionary Ecology to Practical Management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, P.H.W.; Vega, F.E. Ecology and Evolution of Insect–Fungus Mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.J.; McKenna, D.D.; Jordal, B.H.; Cognato, A.I.; Smith, S.M.; Lemmon, A.R.; Lemmon, E.M.; Hulcr, J. Phylogenomics Clarifies Repeated Evolutionary Origins of Inbreeding and Fungus Farming in Bark Beetles (Curculionidae, Scolytinae). Mol. Phylogenet. Evol. 2018, 127, 229–238. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and Diversity of Bark and Ambrosia Beetles. In Bark Beetles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–156. [Google Scholar]
- Huang, Y.-T.; Skelton, J.; Hulcr, J. Lipids and Small Metabolites Provisioned by Ambrosia Fungi to Symbiotic Beetles Are Phylogeny-Dependent, Not Convergent. ISME J. 2020, 14, 1089–1099. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle—Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [Green Version]
- Skelton, J.; Johnson, A.J.; Jusino, M.A.; Bateman, C.C.; Li, Y.; Hulcr, J. A Selective Fungal Transport Organ (Mycangium) Maintains Coarse Phylogenetic Congruence between Fungus-Farming Ambrosia Beetles and Their Symbionts. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182127. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J. The F Usarium Solani Species Complex: Ubiquitous Pathogens of Agricultural Importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Liyanage, P.N.H.; Konkol, J.L.; Ploetz, R.C.; Smith, J.A.; Kasson, M.T.; Freeman, S.; Geiser, D.M.; O’Donnell, K. Three Novel Ambrosia Fusarium Clade Species Producing Multiseptate “Dolphin-Shaped” Conidia, and an Augmented Description of Fusarium Kuroshium. Mycologia 2021, 113, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Smith, J.A.; Kasson, M.T.; Freeman, S.; Geiser, D.M.; Geering, A.D.W.; O’Donnell, K. Three novel Ambrosia Fusarium Clade species producing clavate macroconidia known (F. floridanum and F. obliquiseptatum) or predicted (F. tuaranense) to be farmed by Euwallacea spp. (Coleoptera: Scolytinae) on woody hosts. Mycologia 2019, 111, 919–935. [Google Scholar] [CrossRef]
- Aoki, T.; Kasson, M.T.; Berger, M.C.; Freeman, S.; Geiser, D.M.; O’Donnell, K. Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern U.S. and typification of F. ambrosium. Fungal Syst. Evol. 2018, 1, 23–39. [Google Scholar] [CrossRef]
- Kasson, M.T.; O’Donnell, K.; Rooney, A.P.; Sink, S.; Ploetz, R.C.; Ploetz, J.N.; Konkol, J.L.; Carrillo, D.; Freeman, S.; Mendel, Z.; et al. An Inordinate Fondness for Fusarium: Phylogenetic Diversity of Fusaria Cultivated by Ambrosia Beetles in the Genus Euwallacea on Avocado and Other Plant Hosts. Fungal Genet. Biol. 2013, 56, 147–157. [Google Scholar] [CrossRef]
- Lynn, K.M.T.; Wingfield, M.J.; Durán, A.; Oliveira, L.S.S.; de Beer, Z.W.; Barnes, I. Novel Fusarium mutualists of two Euwallacea species infesting Acacia crassicarpa in Indonesia. Mycologia 2021, 113, 536–558. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Libeskind-Hadas, R.; Hulcr, J.; Kasson, M.T.; Ploetz, R.C.; Konkol, J.L.; Ploetz, J.N.; Carrillo, D.; Campbell, A.; et al. Discordant Phylogenies Suggest Repeated Host Shifts in the Fusarium–Euwallacea Ambrosia Beetle Mutualism. Fungal Genet. Biol. 2015, 82, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Malacrinò, A.; Rassati, D.; Schena, L.; Mehzabin, R.; Battisti, A.; Palmeri, V. Fungal Communities Associated with Bark and Ambrosia Beetles Trapped at International Harbours. Fungal Ecol. 2017, 28, 44–52. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Sferrazza, I.; Aleandri, M.P.; Dalla Valle, M.; Speranza, S.; Contarini, M.; Vannini, A. The Fungal Community Associated with the Ambrosia Beetle Xylosandrus Compactus Invading the Mediterranean Maquis in Central Italy Reveals High Biodiversity and Suggests Environmental Acquisitions. Fungal Biol. 2021, 125, 12–24. [Google Scholar] [CrossRef]
- Skelton, J.; Jusino, M.A.; Li, Y.; Bateman, C.; Thai, P.H.; Wu, C.; Lindner, D.L.; Hulcr, J. Detecting Symbioses in Complex Communities: The Fungal Symbionts of Bark and Ambrosia Beetles Within Asian Pines. Microb. Ecol. 2018, 76, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Agnello, A.M.; Breth, D.I.; Tee, E.M.; Cox, K.D.; Villani, S.M.; Ayer, K.M.; Wallis, A.E.; Donahue, D.J.; Combs, D.B.; Davis, A.E.; et al. Xylosandrus Germanus (Coleoptera: Curculionidae: Scolytinae) Occurrence, Fungal Associations, and Management Trials in New York Apple Orchards. J. Econ. Entomol. 2017, 110, 2149–2164. [Google Scholar] [CrossRef]
- Francardi, V.; Noal, A.; Francescato, S.; Pinto, R.; Bruni, A.; Loffredi, L.; Bucini, D.; Guarnieri, D.; Bellantuono, M.; Esposito, N.; et al. Coexistence of Xylosandrus Crassiusculus (Motschulsky) and X. Compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae) in the National Park of Circeo (Lazio, Italy). Redia-G. Zool. 2017, 100, 149–155. [Google Scholar] [CrossRef]
- Dixon, W.; Woodruff, R.; Foltz, J. Black Twig Borer, Xylosandrus Compactus (Eichhoff) (Insecta: Coleoptera: Curculionidae: Scolytinae). Fla. Coop. Ext. Serv. IFAS Univ. Fla. 2003, EENY 311, 1–5. [Google Scholar]
- Hara, A.; Beardsley, J. The Biology of the Black Twig Borer, Xylosandrus Compactus (Eichhoff), in Hawaii. Proc. Hawaii Ent. Soc. 1979, 1, 55–70. [Google Scholar]
- Dole, S.A.; Beaver, R.A. A Review of the Australian Species of Xylosandrus Reitter (Coleoptera: Curculionidae: Scolytinae). Coleopt. Bull. 2008, 62, 481–492. [Google Scholar] [CrossRef]
- Stephen, L.W.; Bright, D.E. A Catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic Index. Volume B. Gt. Basin Nat. Mem. 1992, 13, 835–1557. [Google Scholar]
- Andersen, H.F.; Jordal, B.H.; Kambestad, M.; Kirkendall, L.R. Improbable but True: The Invasive Inbreeding Ambrosia Beetle Xylosandrus Morigerus Has Generalist Genotypes. Ecol. Evol. 2012, 2, 247–257. [Google Scholar] [CrossRef]
- Ranger, C.M.; Schultz, P.B.; Frank, S.D.; Chong, J.H.; Reding, M.E. Non-Native Ambrosia Beetles as Opportunistic Exploiters of Living but Weakened Trees. PLoS ONE 2015, 10, e0131496. [Google Scholar] [CrossRef]
- Hulcr, J.; Dunn, R.R. The Sudden Emergence of Pathogenicity in Insect–Fungus Symbioses Threatens Naive Forest Ecosystems. Proc. R. Soc. B Biol. Sci. 2011, 278, 2866–2873. [Google Scholar] [CrossRef] [Green Version]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive Tree Diseases Associated with Ambrosia and Bark Beetles: Black Swan Events in Tree Pathology? Plant Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, T.C.; McNew, D.; Mayers, C.; Fraedrich, S.W.; Reed, S.E. Ambrosiella Roeperi Sp. Nov. Is the Mycangial Symbiont of the Granulate Ambrosia Beetle, Xylosandrus Crassiusculus. Mycologia 2014, 106, 835–845. [Google Scholar] [CrossRef]
- Mayers, C.G.; McNew, D.L.; Harrington, T.C.; Roeper, R.A.; Fraedrich, S.W.; Biedermann, P.H.W.; Castrillo, L.A.; Reed, S.E. Three Genera in the Ceratocystidaceae Are the Respective Symbionts of Three Independent Lineages of Ambrosia Beetles with Large, Complex Mycangia. Fungal Biol. 2015, 119, 1075–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassati, D.; Marini, L.; Malacrinò, A. Acquisition of Fungi from the Environment Modifies Ambrosia Beetle Mycobiome during Invasion. PeerJ 2019, 7, e8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Bateman, C.; Skelton, J.; Wang, B.; Black, A.; Huang, Y.-T.; Gonzalez, A.; Jusino, M.A.; Nolen, Z.J.; Freeman, S.; et al. Pre-Invasion Assessment of Exotic Bark Beetle-Vectored Fungi to Detect Tree-Killing Pathogens. Phytopathology 2021, 112, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.F.; Villacorta, A.; Herrera, J. Fluctuación Estacional de Las Capturas de La “Broca Del Café” (Hypothenemus Hampei) Con Trampas de Etanol—Metanol e Implicaciones Sobre El Número de Trampas. Entomol. Mex. 2004, 3, 540–544. [Google Scholar]
- Biedermann, P.H.W.; Klepzig, K.D.; Taborsky, M. Fungus Cultivation by Ambrosia Beetles: Behavior and Laboratory Breeding Success in Three Xyleborine Species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef] [Green Version]
- Stephen, L.W. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph. Gt. Basin Nat. Mem. 1982, 6, 1–1359. [Google Scholar]
- Tapia-Tussell, R.; Lappe, P.; Ulloa, M.; Quijano-Ramayo, A.; Cáceres-Farfán, M.; Larqué-Saavedra, A.; Perez-Brito, D. A Rapid and Simple Method for DNA Extraction from Yeasts and Fungi Isolated from Agave Fourcroydes. Mol. Biotechnol. 2006, 33, 67–70. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322. ISBN 0-12-372180-6. [Google Scholar]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a Node or a Foot-Shaped Basal Cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Geiser, D.M.; Al-Hatmi, A.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.K.; Blomquist, C.L.; Bowden, R.; et al. Phylogenomic Analysis of a 55.1 Kb 19-Gene Dataset Resolves a Monophyletic Fusarium That Includes the Fusarium Solani Species Complex. Phytopathology 2021, 111, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Lombard, L.; Crous, P.W. Back to the Roots: A Reappraisal of Neocosmospora. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 90–185. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. TrimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Lam, T.T.-Y.; Zhu, H.; Guan, Y. Two Methods for Mapping and Visualizing Associated Data on Phylogeny Using Ggtree. Mol. Biol. Evol. 2018, 35, 3041–3043. [Google Scholar] [CrossRef]
- Wang, L.-G.; Lam, T.T.-Y.; Xu, S.; Dai, Z.; Zhou, L.; Feng, T.; Guo, P.; Dunn, C.W.; Jones, B.R.; Bradley, T.; et al. Treeio: An R Package for Phylogenetic Tree Input and Output with Richly Annotated and Associated Data. Mol. Biol. Evol. 2020, 37, 599–603. [Google Scholar] [CrossRef]
- Blackwell Publishing. The Fusarium Laboratory Manual; Leslie, J.F., Summerell, B.A., Eds.; Blackwell Publishing: Ames, IW, USA, 2006; ISBN 9780470278376. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones and Bartlett Series in Biology; Jones and Bartlett: Location, UK, 1999; ISBN 9780763701925. [Google Scholar]
- O’Donnell, K. Molecular Phylogeny of the Nectria Haematococca-Fusarium Solani Species Complex. Mycologia 2000, 92, 919. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Criscione, G.; Tropea Garzia, G. Unusual Behavior of Xylosandrus Compactus (Coleoptera: Scolytinae) on Carob Trees in a Mediterranean Environment. Insects 2019, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- CABI Xylosandrus Morigerus. In Invasive Species Compedium; CAB International: Wallingford, UK, 2022.
- Summerell, B.A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Chen, Y.; Ariyawansa, H.A.; Hyde, K.D.; Haelewaters, D.; Perera, R.H.; Samarakoon, M.C.; Wanasinghe, D.N.; Bustamante, D.E.; Liu, J.-K.; et al. Integrative Approaches for Species Delimitation in Ascomycota. Fungal Divers. 2021, 109, 155–179. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Crous, P.W. Removing Chaos from Confusion: Assigning Names to Common Human and Animal Pathogens in Neocosmospora. Persoonia Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Daehler, C.C.; Dudley, N. Impact of the Black Twig Borer, an Introduced Insect Pest, on Acacia Koa in the Hawaiian Islands. Micronesica Suppl. 2002, 6, 35–53. [Google Scholar]
- Dudley, N.; James, R.; Sniezko, R.; Yeh, A. Pathogenicity of Four Fusarium Species on Acacia Koa Seedlings. Forest Health Protection. Numbered Report 07-04. 2007. Available online: https://www.researchgate.net/profile/Richard-Sniezko/publication/228784173_Pathogenicity_of_four_Fusarium_species_on_Acacia_koa_seedlings/links/02e7e52c737caeb912000000/Pathogenicity-of-four-Fusarium-species-on-Acacia-koa-seedlings.pdf (accessed on 28 January 2022).
- Dudley, N.; James, R.L.; Sniezko, R.; Yeh, A. Investigating Koa Wilt and Dieback in Hawai’i: Pathogenicity of Fusarium species on Acacia koa seedlings. Native Plants J. 2007, 8, 259–266. [Google Scholar] [CrossRef]
- Bosso, L.; Senatore, M.; Varlese, R.; Ruocco, M.; Garonna, A.; Bonanomi, G.; Mazzoleni, S.; Cristinzio, G. Severe Outbreak of Fusarium Solani on Quercus Ilex Vectored by Xylosandrus Compactus. J. Plant Pathol. 2012, 94, 99. [Google Scholar] [CrossRef]
- Vannini, A.; Contarini, M.; Faccoli, M.; Valle, M.D.; Rodriguez, C.M.; Mazzetto, T.; Guarneri, D.; Vettraino, A.M.; Speranza, S. First Report of the Ambrosia Beetle Xylosandrus Compactus and Associated Fungi in the Mediterranean Maquis in Italy, and New Host–Pest Associations. EPPO Bull. 2017, 47, 100–103. [Google Scholar] [CrossRef]
- Gazengel, K.; Lebreton, L.; Lapalu, N.; Amselem, J.; Guillerm-Erckelboudt, A.-Y.; Tagu, D.; Daval, S. PH Effect on Strain-Specific Transcriptomes of the Take-All Fungus. PLoS ONE 2020, 15, e0236429. [Google Scholar] [CrossRef]
- Rowe, H.C.; Kliebenstein, D.J. All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens. PLoS Pathog. 2010, 6, e1000759. [Google Scholar] [CrossRef]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.; Wang, Z.; Lu, G. Carbon Catabolite Repression in Filamentous Fungi. Int. J. Mol. Sci. 2017, 19, 48. [Google Scholar] [CrossRef] [Green Version]
- Ries, L.N.A.; Beattie, S.; Cramer, R.A.; Goldman, G.H. Overview of Carbon and Nitrogen Catabolite Metabolism in the Virulence of Human Pathogenic Fungi. Mol. Microbiol. 2018, 107, 277–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ries, L.N.A.; Steenwyk, J.L.; de Castro, P.A.; de Lima, P.B.A.; Almeida, F.; de Assis, L.J.; Manfiolli, A.O.; Takahashi-Nakaguchi, A.; Kusuya, Y.; Hagiwara, D.; et al. Nutritional Heterogeneity Among Aspergillus Fumigatus Strains Has Consequences for Virulence in a Strain- and Host-Dependent Manner. Front. Microbiol. 2019, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H.W.; Klepzig, K.D.; Taborsky, M.; Six, D.L. Abundance and Dynamics of Filamentous Fungi in the Complex Ambrosia Gardens of the Primitively Eusocial Beetle Xyleborinus Saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae). FEMS Microbiol. Ecol. 2013, 83, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.F.; Ploetz, R.C.; Peña, J.E. Lateral Transfer of a Phytopathogenic Symbiont among Native and Exotic Ambrosia Beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Alvidrez-Villarreal, R.; Hernández-Castillo, F.D.; Garcia-Martínez, O.; Mendoza-Villarreal, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Isolation and pathogenicity of fungi associated to ambrosia borer (Euplatypus segnis) found injuring pecan (Carya illinoensis) wood. Agric. Sci. 2012, 3, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Gil, Z.; Bustillo, A.; Gomez, D.; Marin, P. Corthylus n. sp. (Coleoptera: Scolytidae), pest of alder in Rio Blanco basin of Colombia. Rev. Colomb. Entomol. 2004, 30, 171–178. [Google Scholar]
- Kostovcik, M. The Ambrosia Symbiosis Is Specific in Some Species and Promiscuous in Others: Evidence from Community Pyrosequencing. ISME J. 2015, 9, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.E.; Inward, D.J.G.; Gomez-Rodriguez, C.; Baselga, A.; Vogler, A.P. Predicting the Unpredictable: How Host Specific Is the Mycobiota of Bark and Ambrosia Beetles? Fungal Ecol. 2019, 42, 100854. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Corley, J.C.; Liebhold, A.M. Drivers of Global Scolytinae Invasion Patterns. Ecol. Appl. 2020, 30, e02103. [Google Scholar] [CrossRef]
- Williams, G.M.; Ginzel, M.D. Spatial and Climatic Factors Influence Ambrosia Beetle (Coleoptera: Curculionidae) Abundance in Intensively Managed Plantations of Eastern Black Walnut. Environ. Entomol. 2020, 49, 49–58. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreras-Villaseñor, N.; Rodríguez-Haas, J.B.; Martínez-Rodríguez, L.A.; Pérez-Lira, A.J.; Ibarra-Laclette, E.; Villafán, E.; Castillo-Díaz, A.P.; Ibarra-Juárez, L.A.; Carrillo-Hernández, E.D.; Sánchez-Rangel, D. Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus. J. Fungi 2022, 8, 231. https://doi.org/10.3390/jof8030231
Carreras-Villaseñor N, Rodríguez-Haas JB, Martínez-Rodríguez LA, Pérez-Lira AJ, Ibarra-Laclette E, Villafán E, Castillo-Díaz AP, Ibarra-Juárez LA, Carrillo-Hernández ED, Sánchez-Rangel D. Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus. Journal of Fungi. 2022; 8(3):231. https://doi.org/10.3390/jof8030231
Chicago/Turabian StyleCarreras-Villaseñor, Nohemí, José B. Rodríguez-Haas, Luis A. Martínez-Rodríguez, Alan J. Pérez-Lira, Enrique Ibarra-Laclette, Emanuel Villafán, Ana P. Castillo-Díaz, Luis A. Ibarra-Juárez, Edgar D. Carrillo-Hernández, and Diana Sánchez-Rangel. 2022. "Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus" Journal of Fungi 8, no. 3: 231. https://doi.org/10.3390/jof8030231
APA StyleCarreras-Villaseñor, N., Rodríguez-Haas, J. B., Martínez-Rodríguez, L. A., Pérez-Lira, A. J., Ibarra-Laclette, E., Villafán, E., Castillo-Díaz, A. P., Ibarra-Juárez, L. A., Carrillo-Hernández, E. D., & Sánchez-Rangel, D. (2022). Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus. Journal of Fungi, 8(3), 231. https://doi.org/10.3390/jof8030231