Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains Applied in the Study
2.2. Culture Media and Growth Optimization
2.3. Mycelial Linear Growth Rate
2.4. Culture Media and Spawn Production
2.5. Box Cultivation and Substrate Preparation
2.6. Mycelial Harvesting and Mycofabrication
2.7. Specimen Preparation and Analysis
2.8. Physical and Mechanical Properties Analysis
2.9. Chemical Properties Analysis
3. Results
3.1. Culture Media and Growth Optimization of Polyporales Species
3.2. Linear Growth Measurement
3.3. Spawn Production from Polyporales Species
3.4. Box Cultivation and Mycelial Mat Harvesting
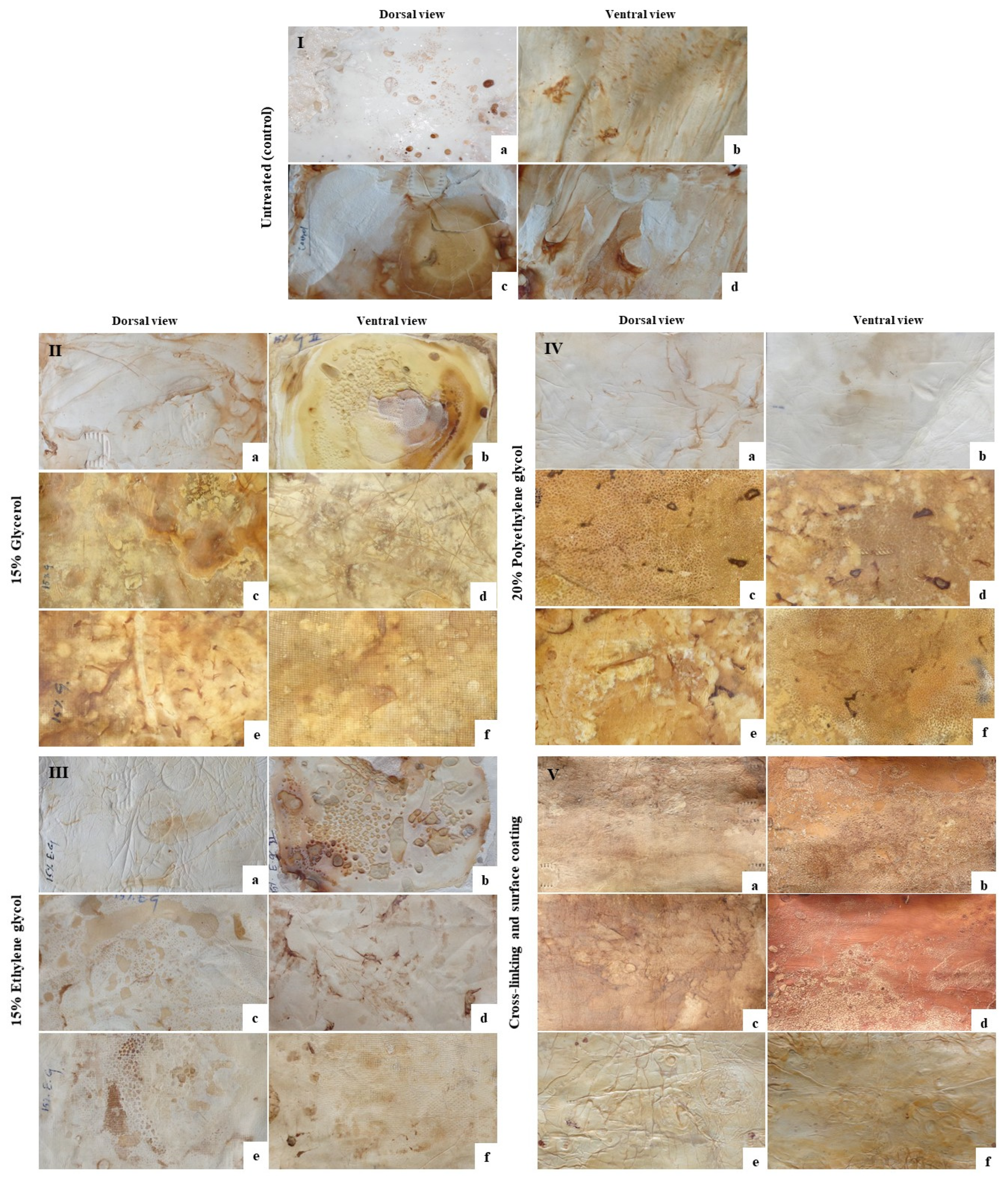
3.5. Mycofabrication and Mycelium-Based Leather (MBL) Production from F. Fraxinea
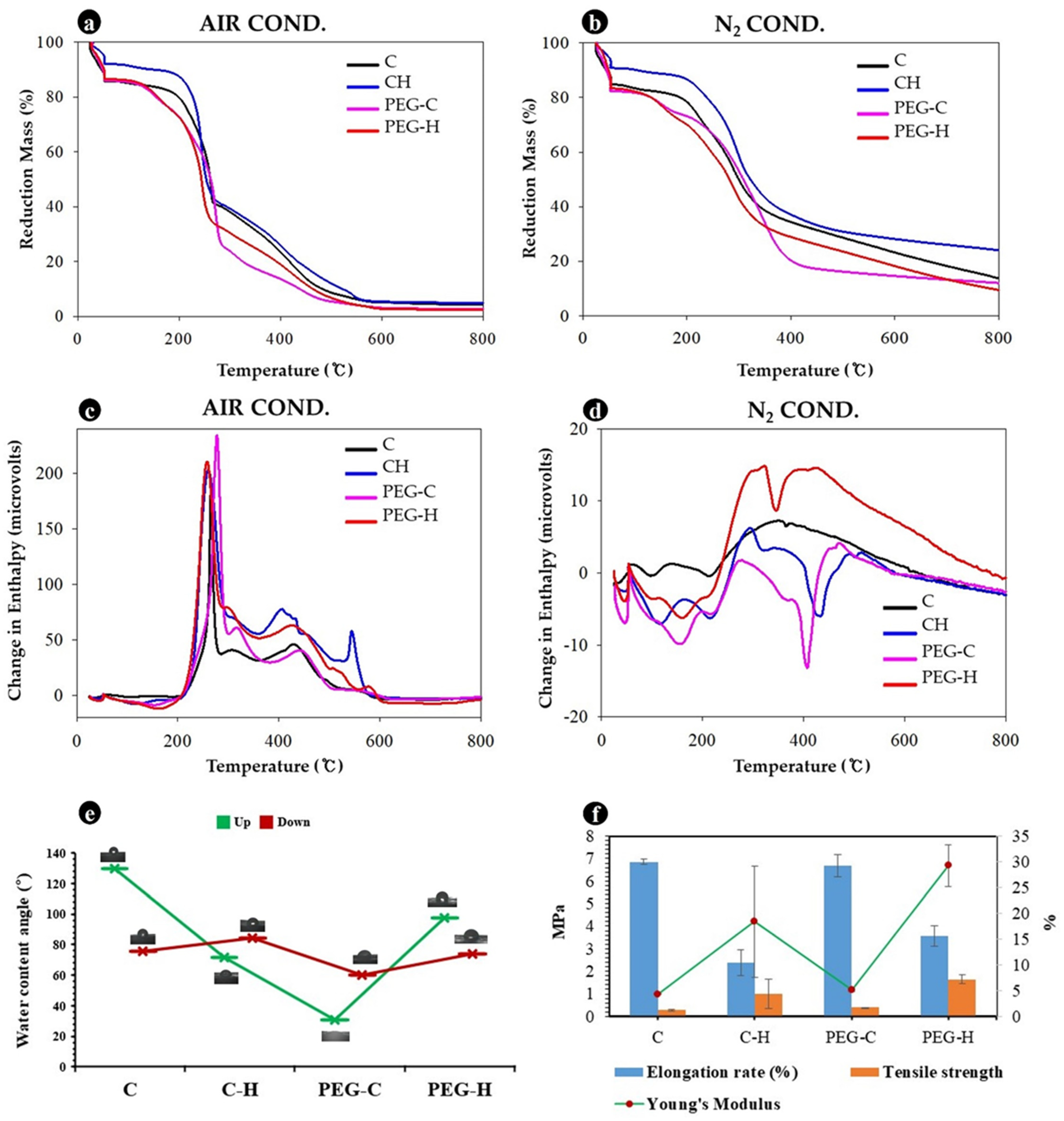
3.6. Physical and Mechanical Properties of MBLs
3.7. Micromorphology of MBLs from F. Fraxinea
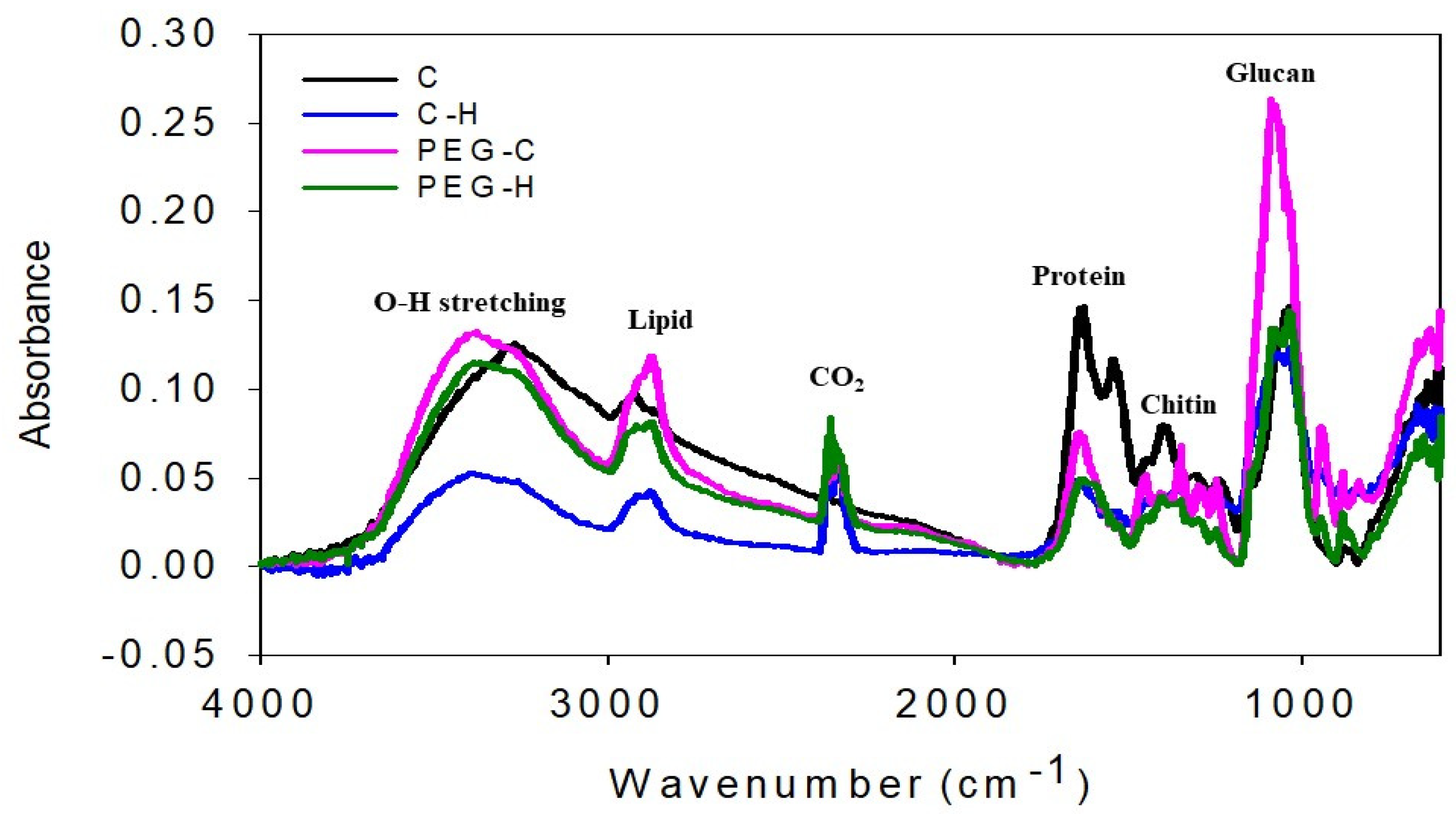
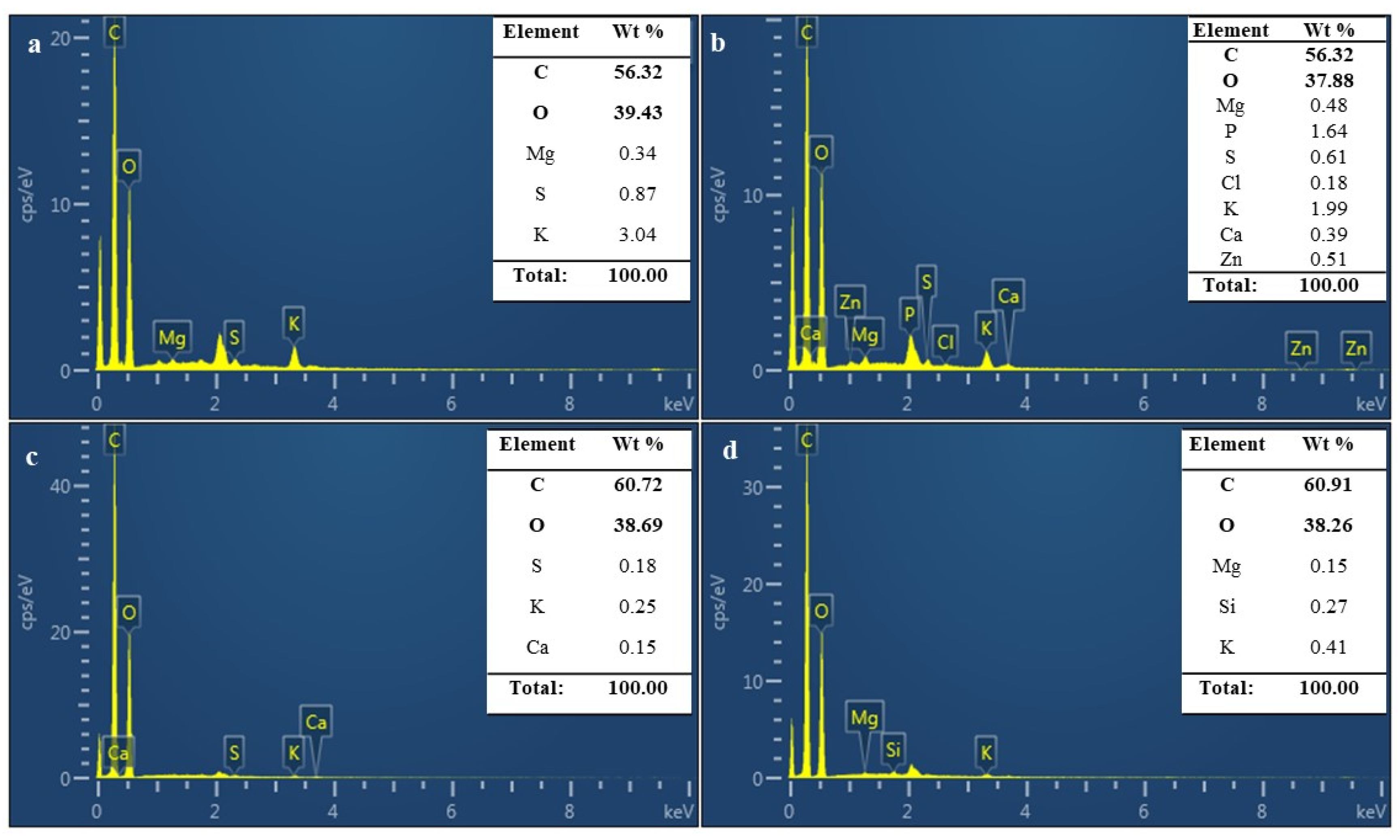
3.8. Chemical Properties of MBLs from F. Fraxinea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kahlig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-derived low-cost mycelium nanopaper with tunable mechanical and surface properties. Biomacromolecules 2019, 20, 3513–3523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attias, N.; Danai, O.; Ezov, N.; Tarazi, E.; Grobman, Y.J. Developing novel applications of mycelium based biocomposite materials for design and architecture. In Final COST FP1303 Conference “Building with Bio-Based Materials: Best Practice and Performance Specification”; The University of Zagreb: Zagreb, Croatia, 2017. [Google Scholar]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R.; Li, B. Bioresin infused then cured mycelium-based sandwich-structure biocomposites: Resin transfer molding (RTM) process, flexural properties, and simulation. J. Clean. Prod. 2019, 207, 123–135. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Bustillos, J.; Loganathan, A.; Agrawal, R.; Gonzalez, B.A.; Perez, M.G.; Ramaswamy, S.; Boesl, B.; Agarwal, A. Uncovering the mechanical, thermal, and chemical characteristics of biodegradable mushroom leather with intrinsic antifungal and antibacter ial properties. ACS Appl. Bio Mater. 2020, 3, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Still, B.; White, M.; Amstislavski, P. Physical and mechanical properties of fungal mycelium-based biofoam. J. Mater. Civ. Eng. 2017, 29, 04017030. [Google Scholar] [CrossRef]
- Agustina, W.; Aditiawati, P.; Kusumah, S.S.; Dungani, R. Physical and mechanical properties of composite boards from the mixture of palm sugar fiber and cassava bagasse using mycelium of Ganoderma lucidum as a biological adhesive. IOP Conf. Ser. Earth Environ. Sci. 2019, 374, 012012. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [Green Version]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Latge, J.P. Tasting the fungal cell wall. Cell Microbiol. 2010, 12, 863–872. [Google Scholar] [CrossRef]
- Jegadeesh, R.; Da-Song, K.; Sung-Won, K.; Sun-Jin, S.; Hyun-Jae, S. Mechanical and physical properties of mycelium-based leather from Fomitella sp. In Proceedings of the Fall Meeting and International Symposium, The Korean Institute of Chemical Engineers, Gwangju, Korea, 27–29 October 2021. [Google Scholar]
- Chang, H.Y.; Cha, D.Y.; Kang, A.S.; Hong, I.P.; Kim, K.P.; Seok, S.J.; Ryu, Y.J.; Sung, J.M. Cultural characteristics of Fomitella fraxinea (Fr.) Imaz. Korean J. Mycol. 1995, 23, 238–245. [Google Scholar]
- Mswaka, A.; Magan, N. Temperature and water potential relations of tropical Trametes and other wood-decay fungi from the indigenous forests of Zimbabwe. Mycol. Res. 1999, 103, 1309–1317. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Jones, M.; Gandia, A.; John, S.; Bismarck, A. Leather-like material biofabrication using fungi. Nat. Sustain. 2021, 4, 9–16. [Google Scholar] [CrossRef]
- Gdula, A.K.; Skubala, P.; Zawieja, P.; Gwiazdowicz, D.J. Mite communities 9Acari: Mesostigmata, Oribatida) in the red belt conk, Fomitopsis pinicola (Polyporales), in Polish forest. Exp. Appl. Acarol. 2021, 84, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Kim, M.; Kim, S.; Ro, H.S. Growth Characteristics of polyporales mushrooms for the mycelial mat formation. Mycobiology 2021, 26, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Li, C.; Cheng, S.; Zhou, J.; Wu, Z. A novel biodegradable film from edible mushroom (F. velutipes) by product: Microstructure, mechanical and barrier properties associated with the fiber morphology. Innov. Food Sci. Emerg. Technol. 2018, 47, 153–160. [Google Scholar] [CrossRef]
- Song, H.Y.; Choi, H.J.; Jeong, H.; Choi, D.; Kim, D.H.; Kim, J.M. Viral Effects of a dsRNA Mycovirus (PoV-ASI2792) on the Vegetative Growth of the Edible Mushroom Pleurotus ostreatus. Mycobiology 2016, 44, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, G.H.; Shrestha, B.; Han, S.K.; Sung, J.M. Growth and Cultural Characteristics of Ophiocordyceps longissima Collected in Korea. Mycobiology 2011, 39, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Hu, D.D.; Ma, X.T.; Li, S.G.; Gu, J.G.; Hu, Q.X. Adapting stick spawn reduced the spawn running time and improved mushroom yield and biological efficiency of Pleurotus eryngii. Sci. Hortic. 2014, 175, 156–159. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Ismail, R.; Johari, N.M.; Annuar, M.S. Production of liquid spawn of an edible grey oyster mushroom, Pleurotus pulmonarius (Fr.) Quel by submerged fermentation and sporophore yield on rubber wood sawdust. Sci. Hortic. 2013, 161, 65–69. [Google Scholar] [CrossRef]
- Zhang, W.R.; Liu, S.R.; Kuang, Y.B.; Zheng, S.Z. Development of a Novel Spawn (Block Spawn) of an Edible Mushroom, Pleurotus ostreatus, in Liquid Culture and its Cultivation Evaluation. Mycobiology 2019, 47, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Stamets, P. Growing Gourment and Medicinal Mushrooms, 4th ed.; Ten Speed Press: Toronto, ON, Canada, 2000. [Google Scholar]
- Brezani, A.; Svobodova, K.; Jablonsky, I.; Tlustos, P. Cultivation of medicinal mushrooms on spruce sawdust fermented with a liquid digestate from biogas stations. Int. J. Med. Mushrooms 2019, 21, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cortina-Escribano, M.; Pihlava, J.M.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules 2020, 25, 4732. [Google Scholar] [CrossRef] [PubMed]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Expr. 2016, 6, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obodai, M.; Sawyerr, L.C.B.; Johnson, P.N.T. Yield of seven strains of oyster mushrooms (Pleurotus spp.) grown on composted sawdust of Triplochiton scleroxylon. Trop. Sci. 2002, 40, 95–99. [Google Scholar]
- Utamia, C.P.; Susilawati, P.R. Rice straw addition as sawdust substitution in oyster mushroom (Pleurotus ostreatus) planted media. AIP Conf. Proc. 2017, 1868, 090002. [Google Scholar]
- Naidu, Y.; Siddiqui, Y.; Idris, A.S. Comprehensive studies on optimization of lingo-hemicellulolytic enzymes by indigenous white rot hymenomycetes under solid-state cultivation using agro-industrial wastes. J. Environ. Manage. 2020, 259, 110056. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, A.; Behera, H.T.; Ray, L. Mushroom mycelia-based material: An environmental friendly alternative to synthetic packaging. In Microbial Polymers; Vaishnav, A., Choudhary, D.K., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Karana, E.; Blauwhoff, D.; Hultink, E.; Camere, S. When the material grows: A case study on designing (with) mycelium-based materials. Int. J. Des. 2018, 12, 119–136. [Google Scholar]
- Callister, J.W.D. Materials Science and Engineering: An Introduction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Sejidov, F.T.; Mansoori, Y.; Goodarzi, N. Esterification reaction using solid heterogeneous acid catalysts under solvent-less condition. J. Mol. Catal. A Chem. 2005, 240, 186–190. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Krochta, J.M. Edible packaging materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef] [PubMed]
- Cartabia, M.; Girometta, C.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and characterization of wood decay fungal strains for developing pure mycelium mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef] [PubMed]
- Albers, P.T.M.; van der Ven, L.G.J.; van Benthem, R.A.T.M.; Esteves, A.C.C.; de With, G. Water Swelling Behavior of Poly(ethylene glycol)-Based Polyurethane Networks. Macromolecules 2020, 53, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; Howell, C. All-natural smart mycelium surface with tunable wettability. ACS Appl. Bio Mater. 2021, 4, 1015–1022. [Google Scholar] [CrossRef]
- Antinori, M.E.; Contardi, M.; Suarato, G.; Armirotti, A.; Bertorelli, R.; Mancini, G.; Debellis, D.; Athanassiou, A. Advanced mycelium materials as potential self-growing biomedical scaffolds. Sci. Rep. 2021, 11, 12630. [Google Scholar] [CrossRef]
- Appels, F.V.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A. Fungal mycelium classified in different material families based on glycerol treatment. Commun. Biol. 2020, 3, 334. [Google Scholar] [CrossRef]
- Ziegler, A.R.; Bajwa, S.G.; Holt, G.A.; Mcntyre, G.; Bajwa, D.S. Evaluation of physico-mechanical properties of mycelium reinforced green biocomposites made from cellulosic fibers. Appl. Eng. Agric 2016, 32, 931–938. [Google Scholar]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.H. Thermal degradation and fire properties of fungal mycelium and mycelium—Biomass composite materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A. Protein-Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucan of fungi. A review. Cell. Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Li, J.; Du, G.; Chen, J. Enhanced glucosamine production by Aspergillus sp. BCRC 31742 based on the time-variant kinetics analysis of dissolved oxygen level. Bioresour. Technol. 2012, 111, 507–511. [Google Scholar] [CrossRef]
- Zhou, C.; Qiao, Y.; Tang, Q.; Jia, W.; Liu, Y.; Wu, A. Purification and characterization of a novel small-molecule polysaccharide from the Maitake medicinal mushroom Grifola frondosa (higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Appels, F.V.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Mycotech Lab. Mylea Technical Data Sheet. Available online: https://mycl.bio/storage/app/media/mylea/Mylea%20Technical%20Data%20Sheet.pdf (accessed on 29 November 2021).
- Von Hoven, T.M. Characterization of Alligator, Ostrich Andemu Skins and Comparisons to Traditional Leathers. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2002. [Google Scholar]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]



| Strain | Culture Number | Radial Growth (mm in 4 d) * | Average Linear Growth (mm in 16 d) # | Fresh Weight $ | Dry Weight $ | Initial Moisture Content (%) $ | Mycelial Characteristics * |
|---|---|---|---|---|---|---|---|
| G. lucidum | JF 17-01 | 20.33 ± 0.68 f | 46.46 ± 2.85 e | 7.17 ± 0.34 de | 0.98 ± 0.04 abc | 94.74 ± 0.51 b | Whitest brown, compact, and highly dense with brown patches in the middle |
| G. applanatum | KMCC 02967 | 41.42 ± 0.13 i | 80.88 ± 7.52 i | 6.45 ± 0.61 bcd | 0.98 ± 0.03 abc | 94.06 ± 1.12 b | Yellowish-white, compact, and rapid growth Mycelium and highly dense |
| Elfvingia applanate | JF 26-01 | 11.75 ± 0.77 a | 59.90 ± 2.00 g | 5.54 ± 1.27 b | 1.26 ± 0.37 bc | 86.28 ± 8.14 a | Creamy white, compact, highly dense, exudate on surface, and wrinkled mycelium |
| Fomitella fraxinea | ASTI 17001 | 18.17 ± 0.79 de | 42.77 ± 0.78 de | 4.07 ± 0.34 a | 0.81 ± 0.02 a | 95.15 ± 0.39 b | White, highly dense, and slow growth |
| Fomitopsis pinicola | JF 79-01 | 17.42 ± 0.47 d | 33.85 ± 0.50 bc | 5.54 ± 0.41 b | 1.12 ± 0.07 abc | 90.10 ± 1.21 ab | Creamy white, thin, fluffy in corner, and dense |
| F. pinicola | KCTC 6208 | 18.75 ± 0.67d ef | 45.63 ± 2.43 e | 7.60 ± 0.66 ef | 1.02 ± 0.09 abc | 94.57 ± 1.09 b | Yellowish-white, thin, and dense |
| F. rosea | KCTC 26226 | 13.58 ± 0.52 b | 28.10 ± 1.13 a | 5.74 ± 0.09 bc | 1.12 ± 0.01 abc | 90.87 ± 0.56 ab | White to pinkish-white, cottony, fluffy in corner, and thin |
| Tramets versicolor | JF 52-01 | 25.33 ± 0.56 g | 53.67 ± 4.74 f | 10.92 ± 0.28 h | 1.33 ± 0.51 c | 93.38 ± 4.75 b | Yellowish-white and dense |
| T. suaveolens | KCTC 26205 | 19.42 ± 0.47 ef | 31.90 ± 0.67 ab | 8.38 ± 0.27 fg | 1.15 ± 0.02 abc | 93.51 ± 0.35 b | Creamy white and dense |
| T. hirsute | KCTC 26200 | 26.17 ± 0.68 g | 42.81 ± 1.75 de | 11.33 ± 0.17 h | 1.36 ± 0.02 c | 93.25 ± 0.11 b | Creamy white and dense, and colonies are powdery |
| Wolfiporia extensa | JF 46-01 | 32.17 ± 0.68 h | 69.16 ± 0.83 h | 4.33 ± 0.76 a | 0.91 ± 0.08ab | 92.05 ± 3.96 ab | Brownish-white, fluffy, upright mycelial, thin, and moderate growth |
| Microporus affinis | JF 47-01 | 15.17 ± 0.47 c | 44.19 ± 1.80 e | 6.80 ± 0.81 cde | 1.21 ± 0.01 abc | 90.49 ± 2.70 ab | White-pink and dense |
| Bjerkandera adusta | JF 78-01 | 41.00 ± 0.39 i | 68.17 ± 4.26 h | 5.68 ± 0.10 bc | 1.19 ± 0.24 abc | 89.07 ± 5.07 ab | Creamy white, thin, floccose texture, and rapid growth |
| Postia balsamea | JF 80-01 | 24.83 ± 1.81 g | 38.10 ± 0.69 cd | 9.32 ± 0.38 g | 1.27 ± 0.05 bc | 92.73 ± 0.69 ab | White, dense, in vitro teleomorph formation was observed, and exudate drops |
| No. | MBL Samples (MBLs) | Elongation Rate (%) | Tensile Strength (MPa) | Strain | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| Without hotpress | |||||
| 1 | Control | 4.59 ± 1.29 a | 1.40 ± 0.22 ab | 1.03 ± 0.01 ab | 1.37 ± 0.20 ab |
| 2 | 15% G | 69.74 ± 5.33 j | 1.60 ± 0.07 ab | 1.35 ± 0.03 j | 1.18 ± 0.05 ab |
| 3 | 15% EG | 37.88 ± 5.28 gh | 7.00 ± 1.74 h | 1.19 ± 0.03 gh | 5.91 ± 1.61 gh |
| 4 | 20% PEG | 29.29 ± 2.15 ef | 1.74 ± 0.05 ab | 1.49 ± 0.03 k | 1.16 ± 0.06 ab |
| Hotpress (60 °C) | |||||
| 5 | Control | 25.41 ± 3.29 de | 2.65 ± 0.26 ab | 1.13 ± 0.02 def | 2.48 ± 0.20 bc |
| 6 | 15% G | 58.86 ± 5.19 i | 4.92 ± 1.05 efg | 1.29 ± 0.03 i | 3.79 ± 0.74 de |
| 7 | 15% EG | 33.56 ± 5.96 fg | 5.09 ± 1.11 efg | 1.17 ± 0.03 fg | 4.35 ± 0.90 ef |
| 8 | 15% G + 5% tannic acid | 22.64 ± 1.15 bcde | 3.13 ± 0.62 bcd | 1.11 ± 0.01 de | 2.81 ± 0.54 cd |
| 9 | 15% EG + 5% tannic acid | 23.55 ± 3.17 cde | 2.58 ± 0.06 ab | 1.07 ± 0.06 bcd | 2.41 ± 0.18 abc |
| 10 | 15% EG + 20% corn zein | 43.73 ± 7.82 h | 1.35 ± 0.26 a | 1.22 ± 0.02 h | 1.10 ± 0.20 a |
| 11 | 20% PEG | 18.88 ± 6.33 bcd | 5.06 ± 0.78 efg | 1.09 ± 0.03 d | 4.62 ± 0.67 efg |
| Hotpress (120 °C) | |||||
| 12 | Control | 3.62 ± 1.00 a | 3.99 ± 0.45 cde | 1.02 ± 0.01 a | 3.94 ± 0.46 de |
| 13 | 15% G | 20.32 ± 1.90 bcde | 4.54 ± 0.23 def | 1.11 ± 0.02 de | 4.08 ± 0.18 de |
| 14 | 15% GCO | 16.73 ± 5.65 bc | 6.16 ± 0.80 gh | 1.08 ± 0.03 cd | 5.68 ± 0.66 fgh |
| 15 | 15% EG | 29.92 ± 1.67 ef | 5.74 ± 0.09 fgh | 1.15 ± 0.01 efg | 4.99 ± 0.11 efg |
| 16 | 15% EGCO | 20.9 ± 1.82b cd | 6.28 ± 0.89 gh | 1.10 ± 0.01 de | 5.60 ± 0.97 fgh |
| 17 | 20% PEG | 15.89 ± 2.02 b | 7.21 ± 0.93 hi | 1.08 ± 0.01 bcd | 6.69 ± 0.67 h |
| 18 | 20% PEGCO | 8.6 ± 0.44 a | 8.49 ± 0.90 i | 1.04 ± 0 abc | 8.14 ± 0.88 i |
| Type | Elongation (%) | Strain | Tensile Strength (MPa) | Young’s Modulus (MPa) | Ref. |
|---|---|---|---|---|---|
| Fungal mycelium (S. c) # | - | 2.2 | 12.3 | 1048 | [49] |
| Fungal mycelium (G. l) # | 14–33 | - | 0.8–1.1 | - | [36] |
| Mycelium composite # | - | - | 0.1–0.2 | 66.14–71.77 | [45] |
| Mycelium-based composite (T. m) # | 0.9 | 4.7 | 0.15 | 100 | [51] |
| Mycelium leather * | 22–35 | - | 8–11 | - | [52] |
| Mycelium based leather * | 4.59–58.86 | 1.03–1.49 | 1.40–8.49 | 1.37–8.14 | Present study |
| Animal leather | <40 | 6–16 | 39.5 | 1–13 | [53,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, J.; Kim, D.-S.; Kim, H.-S.; Oh, D.-S.; Shin, H.-J. Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi. J. Fungi 2022, 8, 317. https://doi.org/10.3390/jof8030317
Raman J, Kim D-S, Kim H-S, Oh D-S, Shin H-J. Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi. Journal of Fungi. 2022; 8(3):317. https://doi.org/10.3390/jof8030317
Chicago/Turabian StyleRaman, Jegadeesh, Da-Song Kim, Hyun-Seok Kim, Deuk-Sil Oh, and Hyun-Jae Shin. 2022. "Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi" Journal of Fungi 8, no. 3: 317. https://doi.org/10.3390/jof8030317
APA StyleRaman, J., Kim, D.-S., Kim, H.-S., Oh, D.-S., & Shin, H.-J. (2022). Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi. Journal of Fungi, 8(3), 317. https://doi.org/10.3390/jof8030317






