Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Nanoparticles
2.2.1. Green Synthesis of Cu-NPs
2.2.2. Preparation of S-NPs
2.3. Transmission Electron Microscopy (TEM) and X-ray Diffraction (XRD) Analyses
2.4. Evaluation of the Antifungal Activity
2.4.1. Isolation and Purification
2.4.2. Pathogenicity Test
2.4.3. In Vitro Antifungal Activity
2.4.4. In Vivo Antifungal Activity
2.5. Determination of TPC and TSS
2.6. Cytotoxicity Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. NP Characterization
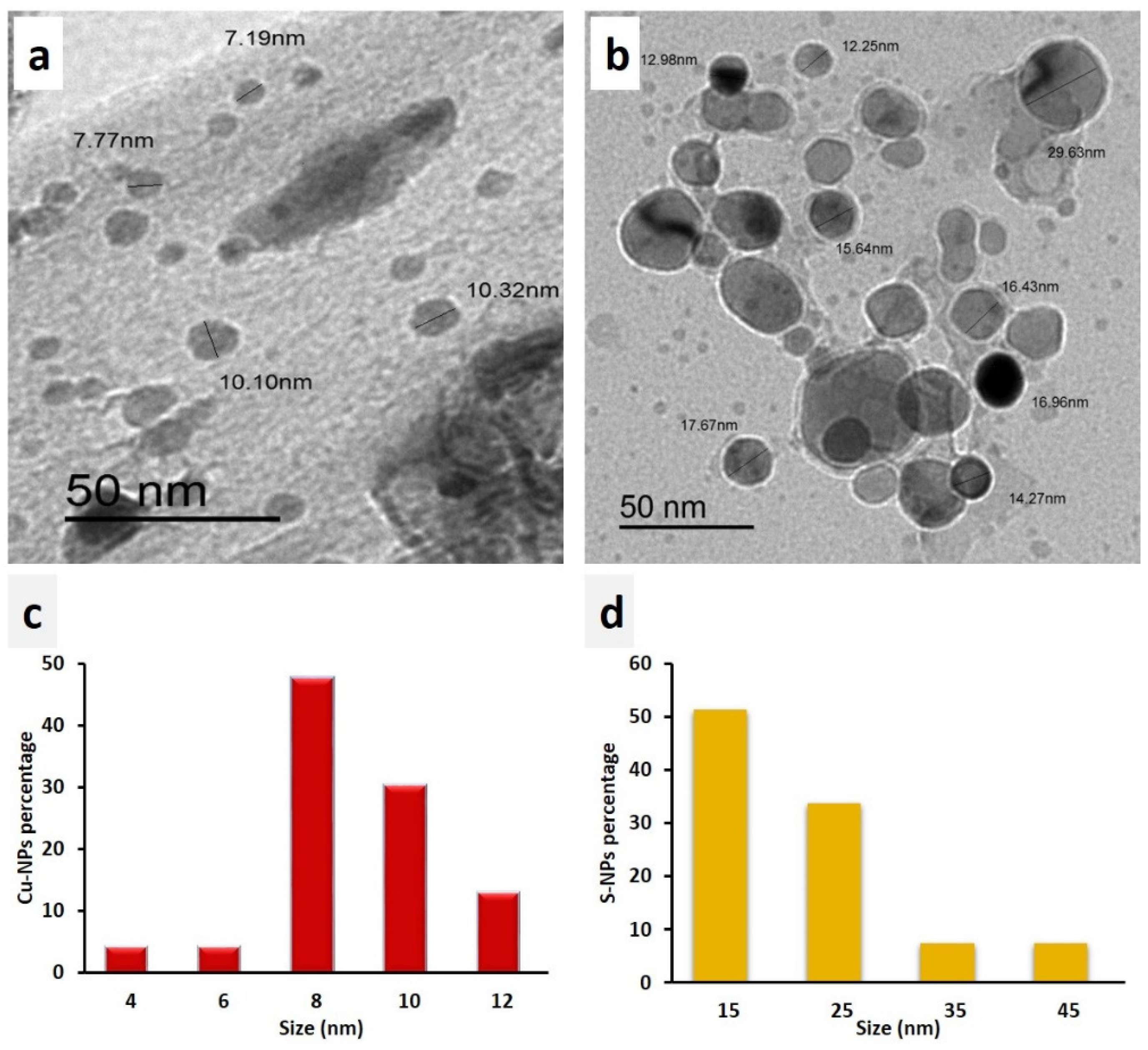
3.1.1. TEM Analysis
3.1.2. XRD Analysis
3.2. Evaluation of the Antifungal Activity
3.2.1. In Vitro Antifungal Activity
3.2.2. In Vivo Antifungal Activity
3.3. Total Phenolic (TPC) and Total Soluble Solids (TSS) Content
3.4. Cytotoxicity of the Synthesized Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, N.; Miyazaki, K. Nanotechnology innovation system: Understanding hidden dynamics of nanoscience fusion trajectories. Technol. Forecast. Soc. Chang. 2009, 76, 128–140. [Google Scholar] [CrossRef]
- Prasad, R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanoparticles 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, B.K.; Yadav, S.M.; Gupta, A.K. Applications of nanotechnology in agricultural and their role in disease management. Res. J. Nanosci. Nanotechnol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Duraisamy, P.; Govindarajan, M.; Buhroo, A.A.; Prasad, R. Nano-biofungicides: Emerging trend in insect pest control. In Advances and Applications through Fungal Nanobiotechnology; Springer: New York, NY, USA, 2016; pp. 307–319. [Google Scholar]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Atiq, M.; Naeem, I.; Sahi, S.T.; Rajput, N.A.; Haider, E.; Usman, M.; Fatimaa, K.; Arif, E.; Qayyum, A. Nanoparticles: A safe way towards fungal diseases. Arch. Phytopathol. Plant Prot. 2020, 53, 781–792. [Google Scholar] [CrossRef]
- Dik, A.J.; Elad, Y. Comparison of antagonists of Botrytis cinerea in greenhouse-grown cucumber and tomato under different climatic conditions. Eur. J. Plant Pathol. 1999, 105, 123–137. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Singh, S.P. Breeding common bean for resistance to white mold: A review. Crop Sci. 2013, 53, 1832–1844. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Rollins, J.A. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, Y.; Meng, Q.; Shi, F.; Ma, L.; Li, Y. Physiological and biochemical responses in sunflower leaves infected by Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 2017, 100, 41–48. [Google Scholar] [CrossRef]
- Hillocks, R.J. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Krasnow, C.; Ziv, C. Non-Chemical Approaches to Control Postharvest Gray Mold Disease in Bell Peppers. Agronomy 2022, 12, 216. [Google Scholar] [CrossRef]
- Schmid, G. Clusters and Colloids: From Theory to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–385. [Google Scholar]
- Chaudhuri, R.G.; Paria, S. Synthesis of sulfur nanoparticles in aqueous surfactant solutions. J. Colloid Interface Sci. 2010, 343, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Bhadauria, S.; Gaur, M.S.; Pasricha, R. Characterization of copper nanoparticles synthesized by a novel microbiological method. JOM 2010, 62, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Harne, S.; Sharma, A.; Dhaygude, M.; Joglekar, S.; Kodam, K.; Hudlikar, M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf. B Biointerfaces 2012, 95, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. The role of fungicides in the physiology of higher plants: Implications for defense responses. Bot. Rev. 2003, 69, 162–172. [Google Scholar] [CrossRef]
- Starner, K. Department of Pesticide Regulation Environmental Monitoring Branch 1001 I Street Sacramento, California 95812 Revised June 29, 2009. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.363.7331&rep=rep1&type=pdf (accessed on 1 February 2022).
- De Oliveira-Filho, E.C.; Lopes, R.M.; Paumgartten, F.J.R. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 2004, 56, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Mastin, B.J.; Rodgers, J.H. Toxicity and bioavailability of copper herbicides (Clearigate, Cutrine-Plus, and copper sulfate) to freshwater animals. Arch. Environ. Contam. Toxicol. 2000, 39, 445–451. [Google Scholar] [CrossRef]
- Liu, Q.M.; Yasunami, T.; Kuruda, K.; Okido, M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans. Nonferrous Met. Soc. China 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; El-Nagare, K.; Raouf, S.; Abdelfattah, K.; El-Kadi, S.; Abdelaziz, E. One-step green approach for functional printing and finishing of textiles using silver and gold NPs. RSC Adv. 2018, 8, 25546–25557. [Google Scholar] [CrossRef] [Green Version]
- Delcan, J.; Moyano, C.; Raposo, R.; Melgarejo, P. Storage of Botrytis cinerea using different methods. J. Plant Pathol. 2002, 84, 3–9. [Google Scholar]
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 1999; p. 76. [Google Scholar]
- Hanlin, R.T. Illustrated Genera of Ascomycetes, vol. I. Am. Phytopatho-Log. Soc. 1999, 64, 65. [Google Scholar]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer: New York, NY, USA, 2008; pp. 1–417. [Google Scholar]
- Hao, Y.; Cao, X.; Ma, C.; Zhang, Z.; Zhao, N.; Ali, A.; Hou, T.; Xiang, Z.; Zhuang, J.; Wu, S.; et al. Potential applications and antifungal activities of engineered nanomaterials against gray mold disease agent Botrytis cinerea on rose petals. Front. Plant Sci. 2017, 8, 1332. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Halim, K.Y.; El-Ghanam, A.A. Antifungal potent of some metallic nanoparticles against Sclerotinia sclerotiorum on common bean plants: An emphasis for biochemical alterations and metal accumulation. Acad. J. Life Sci. 2019, 5, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, Y.S.; Hashem, M.; Alshehri, A.M.; Alamri, S.; Eid, E.M.; Ziedan, E.S.H.; Alrumman, S.A. Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture 2021, 11, 1177. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Valverde-Miranda, D.; Díaz-Pérez, M.; Gómez-Galán, M.; Callejón-Ferre, Á.J. Total soluble solids and dry matter of cucumber as indicators of shelf life. Postharvest Biol. Technol. 2021, 180, 111603. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Costat. Version 6.311, Cohort Software 798 Lighthouse Ave., PMB 320 Monterery, CA, USA, 2005. Available online: https://www.researchgate.net/publication/335839765_Efficiency_of_some_Natural_Plant_Extracts_and_Ferrous_Sulphate_in_Controlling_the_Land_Snail_Monacha_cartusiana_under_Laboratory_and_Field_Conditions_at_Sharkia_Governorate_AR_Egypt (accessed on 1 February 2022).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F-tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Suleiman, M.; Al-Masri, M.; Al Ali, A.; Aref, D.; Hussein, A.; Saadeddin, I.; Warad, I. Synthesis of nano-sized sulfur nanoparticles and their antibacterial activities. J. Mater. Environ. Sci. 2015, 6, 513–518. [Google Scholar]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Kamrani, H. Synthesis and Characterization of Copper Nanoparticles by Bis-(Acetylacetonato)-Copper (II) Using Nonionic Surfactants and the Effect of Their Structures on Nanoparticles Size and Yield. Open J. Inorg. Non-Met. Mater. 2018, 8, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Sarma, J.V.N.; Pande, S.; Ababou-Girard, S.; Turban, P.; Lepine, B.; Gangopadhyay, S. Oxidation mechanism of thin Cu films: A gateway towards the formation of single oxide phase. AIP Adv. 2018, 8, 055114. [Google Scholar] [CrossRef]
- Colmenero, F.; Timón, V. Study of the structural, vibrational and thermodynamic properties of natroxalate mineral using density functional theory. J. Solid State Chem. 2018, 263, 131–140. [Google Scholar] [CrossRef]
- Schoonbeek, H.J.; Jacquat-Bovet, A.C.; Mascher, F.; Métraux, J.P. Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea. Mol. Plant-Microbe Interact. 2007, 20, 1535–1544. [Google Scholar] [CrossRef] [Green Version]
- Cessna, S.G.; Sears, V.E.; Dickman, M.B.; Low, P.S. Oxalic Acid, a Pathogenicity Factor for Sclerotinia sclerotiorum, Suppresses the Oxidative Burst of the Host Plant. Plant Cell 2000, 12, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Massalimov, I.A.; Medvedev, U.A.; Zaynitdinova, R.M.; Mufazalova, N.A.; Mustafin, A.G. Assessment of antifungal activity of micronized and nanosized elemental sulfur. Nanotechnol. Nanosci. 2012, 3, 55–58. [Google Scholar]
- Ouda, S.M. Antifungal Activity of Silver and Copper Nanoparticles on Two Plant Pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.R.; Ghosh, M.; Mandal, A.; Chakravorty, D.; Pal, M.; Pradhan, S.; Goswami, A. Surface-modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011, 90, 733–743. [Google Scholar] [CrossRef]
- Mlalila, N.G.; Swai, H.S.; Hilonga, A.; Kadam, D.M. Antimicrobial dependence of silver nanoparticles on surface plasmon resonance bands against Escherichia coli. Nanotechnol. Sci. Appl. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terefe, H.; Fininsa, C.; Sahile, S.; Fantaye, K.T. Effect of temperature on growth and sporulation of Botrytis fabae, and resistance reactions of faba bean against the pathogen. Plant Pathol. Microbiol. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Ahlem, H.; Mohammed, E.; Badoc, A.; Ahmed, L. Effect of pH, temperature and water activity on the inhibition of Botrytis cinerea by Bacillus amyloliquefaciens isolates. Afr. J. Biotechnol. 2012, 11, 2210–2217. [Google Scholar]
- Boscaiu, M.; Sánchez, M.; Bautista, I.; Donat, P.; Lidón, A.; Llinares, J.; Llul, C.; Mayoral, O.; Vicente, O. Phenolic compounds as stress markers in plants from gypsum habitats. Bull. UASVM Hortic. 2010, 67, 44–49. [Google Scholar]
- Hernández-Fuentes, A.D.; López-Vargas, E.R.; Pinedo-Espinoza, J.M.; Campos-Montiel, R.G.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest behavior of bioactive compounds in tomato fruits treated with Cu nanoparticles and NaCl stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef] [Green Version]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. Phenolic compounds in apple leaves after infection with apple scab. Biol. Plant. 2011, 55, 725–730. [Google Scholar] [CrossRef]
- Rusjan, D.; Veberič, R.; Mikulič-Petkovšek, M. The response of phenolic compounds in grapes of the variety ‘Chardonnay’ (Vitis vinifera L.) to the infection by phytoplasma Bois noir. Eur. J. Plant Pathol. 2012, 133, 965–974. [Google Scholar] [CrossRef]
- Mohammadi, S.; Aminifard, M.H. In vitro and in vivo antifungal activities of three essential oils against grey mould disease in cucumber (Cucumis sativus). Asian J. Plant Sci. 2011, 10, 287–293. [Google Scholar] [CrossRef] [Green Version]
- El Guilli, M.; Hamza, A.; Clément, C.; Ibriz, M.; Ait Barka, E. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Hassani, A.; Fathi, Z.; Ghosta, Y.; Abdollahi, A.L.I.; Meshkatalsadat, M.H.; Marandi, R.J. Evaluation of plant essential oils for control of postharvest brown and gray mold rots on apricot. J. Food Saf. 2012, 32, 94–101. [Google Scholar] [CrossRef]
- Sabela, M.I.; Makhanya, T.; Kanchi, S.; Shahbaaz, M.; Idress, D.; Bisetty, K. One-pot biosynthesis of silver nanoparticles using Iboza Riparia and Ilex Mitis for cytotoxicity on human embryonic kidney cells. J. Photochem. Photobiol. B Biol. 2018, 178, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firdhouse, J.; Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113–121. [Google Scholar]
- Schneider, T.; Baldauf, A.; Ba, L.A.; Jamier, V.; Khairan, K.; Sarakbi, M.B.; Reum, N.; Schneider, M.; Röseler, A.; Becker, K.; et al. Selective antimicrobial activity associated with sulfur nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 395–405. [Google Scholar] [CrossRef]




| Treatment | Concentration (µg/mL) | % Growth Inhibition | |
|---|---|---|---|
| Botrytis cinerea | Sclerotinia sclerotiorum | ||
| S-NPs | 5 | 76.47 f | 54.89 k |
| 10 | 78.82 e | 72.18 i | |
| 25 | 80.00 d | 83.08 e | |
| 50 | 88.24 c | 88.72 d | |
| 75 | 90.98 b | 90.60 c | |
| 100 | 94.12 a | 94.36 a | |
| Cu-NPs | 5 | 3.92 n | 8.27 o |
| 10 | 13.73 k | 13.53 n | |
| 25 | 23.53 j | 34.21 l | |
| 50 | 47.06 i | 63.91 j | |
| 75 | 80.39 d | 71.80 i | |
| 100 | 94.12 a | 92.48 b | |
| CuSO4 | 4000 | 58.82 g | 77.44 g |
| Micro sulfur (MS) | 1000 | 58.04 h | 82.33 f |
| Topsin-M 70 WP | 1000 | 9.80 m | 75.56 h |
| PVP (3 g/100 mL) | 11.76 l | 15.41 m | |
| Treatment | Conc. (µg/mL) | B. cinerea | S. sclerotiorum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 20 °C | 10 °C | 20 °C | ||||||
| DI a* (%) | DS b* (cm) | DI (%) | DS (cm) | DI (%) | DS (cm) | DI (%) | DS (cm) | ||
| Cu-NPs | 50 | 75 ab c* | 0.50 bc | 75 ab | 1 bcd | 0 b | 0 b | 50 a | 0.5 b |
| 100 | 25 ab | 0.25 bc | 50 ab | 0.5 cd | 0 b | 0 b | 50 a | 0.5 b | |
| S-NPs | 25 | 75 ab | 0.75 bc | 75 ab | 1.25 bc | 0 b | 0 b | 50 a | 0.5 b |
| 50 | 50 ab | 0.50 bc | 75 ab | 0.75 cd | 0 b | 0 b | 25 a | 0.25 b | |
| CuSO4 | 4000 | 100 a | 1 b | 100 a | 2 b | 0 b | 0 b | 50 a | 1 b |
| Micro sulfur (MS) | 1000 | 100 a | 1 b | 100 a | 1.25 bc | 25 ab | 0.25 ab | 50 a | 0.5 b |
| Positive control c* | 100 a | 1.75 a | 100 a | 4 a | 100 a | 1 a | 75 a | 3 a | |
| Treatment | Concentration (µg/mL) | B. cinerea | S. sclerotiorum | ||
|---|---|---|---|---|---|
| TPC (mg GAE/g FW) | TSS (%) | TPC (mg GAE/g FW) | TSS (%) | ||
| Cu-NPs | 50 | 0.137 c | 2.0 e | 0.174 a | 3.0 d |
| 100 | 0.152 a | 3.8 b | 0.169 a | 3.4 c | |
| S-NPs | 25 | 0.064 f | 3.9 a | 0.109 d | 3.3 c |
| 50 | 0.066 f | 3.0 d | 0.108 d | 3.56 b | |
| CuSO4 | 4000 | 0.141 b | 3.2 c | 0.093 d | 3.0 d |
| Microsulfur | 1000 | 0.104 e | 3.0 d | 0.108 d | 3.4 c |
| Positive control a* | 0.143 b | 0.7 f | 0.146 b | 2.8 e | |
| Negative control b* | 0.129 d | 4.0 a | 0.129 c | 4.0 a | |
| Tested NPs | Concentration (µg/mL) | WI 38 Cells | Vero Cells | ||
|---|---|---|---|---|---|
| Viability (%) | Cytotoxicity (%) | Viability (%) | Cytotoxicity (%) | ||
| S-NPs | 25 | 79.45 c | 20.55 c | 95.4 b | 4.60 e |
| 12.5 | 98.48 a | 1.52 e | 99 a | 1.00 f | |
| 6.25 | 98.98 a | 1.02 e | 99.14 a | 0.86 f | |
| 2.5 | 99.77 a | 0.23 e | 99.62 a | 0.38 f | |
| 1.25 | 100 a | 0.00 e | 100 a | 0.00 f | |
| 0.625 | 100 a | 0.00 e | 100 a | 0.00 f | |
| Cu-NPs | 25 | 6.81 e | 93.19 a | 11.24 f | 88.76 a |
| 12.5 | 32.52 d | 67.48 b | 15.02 e | 84.98 b | |
| 6.25 | 91.55 b | 8.45 d | 16.02 d | 83.98 c | |
| 2.5 | 98.82 a | 1.18 e | 60.99 c | 39.01 d | |
| 1.25 | 99.09 a | 0.91 e | 99.2 a | 0.80 f | |
| 0.625 | 99.18 a | 0.82 e | 100 a | 0.00 f | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadek, M.E.; Shabana, Y.M.; Sayed-Ahmed, K.; Abou Tabl, A.H. Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum. J. Fungi 2022, 8, 412. https://doi.org/10.3390/jof8040412
Sadek ME, Shabana YM, Sayed-Ahmed K, Abou Tabl AH. Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum. Journal of Fungi. 2022; 8(4):412. https://doi.org/10.3390/jof8040412
Chicago/Turabian StyleSadek, Mohamed E., Yasser M. Shabana, Khaled Sayed-Ahmed, and Ayman H. Abou Tabl. 2022. "Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum" Journal of Fungi 8, no. 4: 412. https://doi.org/10.3390/jof8040412
APA StyleSadek, M. E., Shabana, Y. M., Sayed-Ahmed, K., & Abou Tabl, A. H. (2022). Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum. Journal of Fungi, 8(4), 412. https://doi.org/10.3390/jof8040412





