Taxonomic and Phylogenetic Characterizations Reveal Four New Species, Two New Asexual Morph Reports, and Six New Country Records of Bambusicolous Roussoella from China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Sampling and Morphology
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Phylogeny
2.3. Phylogenetic Analyses
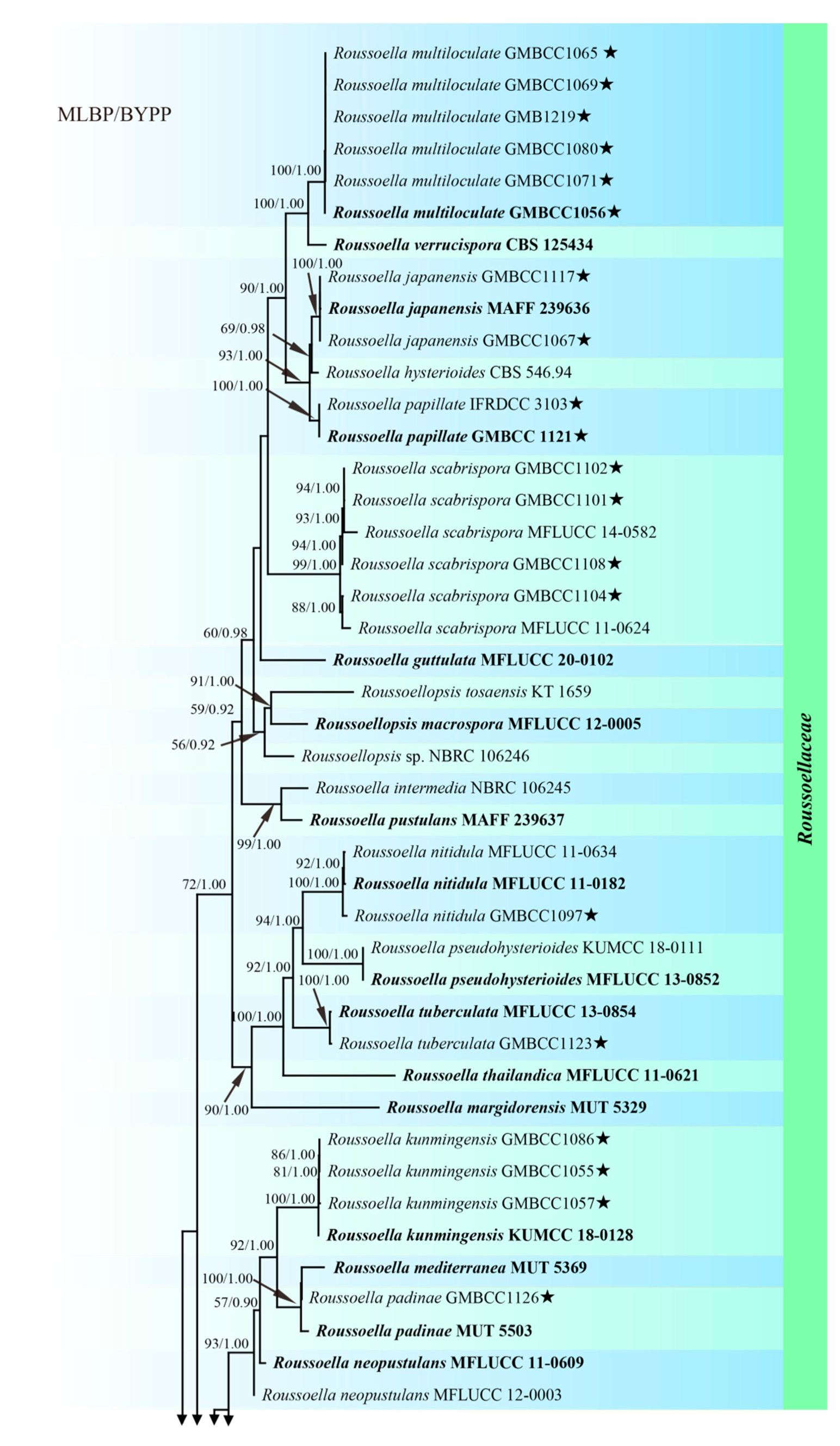
3. Results
3.1. Phylogenetic Analyses
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.K.; Phookamsak, R.; Dai, D.Q.; Tanaka, K.; Jones, E.G.; Xu, J.C.; Chukeatirote, E.; Hyde, K.D. Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa 2014, 181, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Jaklitsch, W.M.; Voglmayr, H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud. Mycol. 2016, 85, 35–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.; Liu, J.K.; Bhat, D.J.; Jones, E.B.; McKenzie, E.H.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chaiwan, N.; Norphanphoun, C.; Boonmee, S.; Camporesi, E.; Chethana, K.W.T.; Dayarathne, M.C.; De Silva, N.I.; Dissanayake, A.J.; Ekanayaka, A.H.; et al. Mycosphere notes 169–224. Mycosphere 2018, 9, 271–430. [Google Scholar] [CrossRef]
- Jiang, H.B.; Hyde, K.D.; Jayawardena, R.S.; Doilom, M.; Xu, J.; Phookamsak, R. Taxonomic and phylogenetic characterizations reveal two new species and two new records of Roussoella (Roussoellaceae, Pleosporales) from Yunnan, China. Mycol. Prog. 2019, 18, 577–591. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.; Maharachchikumbura, S.S.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef] [Green Version]
- Mapook, A.; Hyde, K.D.; McKenzie, E.H.; Jones, E.B.; Bhat, D.J.; Jeewon, R.; Stadler, M.; Samarakoon, M.C.; Malaithong, M.; Tanunchai, B.; et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers. 2020, 101, 1–175. [Google Scholar] [CrossRef]
- Poli, A.; Bovio, E.; Ranieri, L.; Varese, G.C.; Prigione, V. News from the sea: A new genus and seven new species in the Pleosporalean families Roussoellaceae and Thyridariaceae. Diversity 2020, 12, 144. [Google Scholar] [CrossRef] [Green Version]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa-2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Saccardo, P.A.; Paoletti, G. Mycetes Malacenses. Funghi della penisola di Malacca raccolti nel 1885 dell’ Ab. Benedetto Scortechini. Atti Accad. Sci. Veneto-Trentino-Istriana 1885, 6, 387–428. [Google Scholar]
- Höhnel, F. Fragmente zur Mykologie XXIII. Sitzungsber Akad Wiss Wien Math-Naturwiss KI 1919, 128, 535–625. [Google Scholar]
- Aptroot, A. Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwig. 1995, 60, 325–379. [Google Scholar]
- Müller, E.; von Arx, J.A. Die Gattungen der Didymosporen Pyrenomyceten; Kommissionsverlag Buchdruckerei Büchler: Wabern, Switzerland, 1962. [Google Scholar]
- Aptroot, A. A monograph of Didymosphaeria. Stud. Mycol. 1995, 37, 1–160. [Google Scholar]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Hatakeyama, S.; Harada, Y.; Sano, T.; Shirouzu, T.; Hosoya, T. Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Stud. Mycol. 2009, 64, 175–209. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2017, 82, 1–105. [Google Scholar] [CrossRef]
- Karunarathna, A.; Phookamsak, R.; Jayawardena, R.S.; Cheewangkoon, R.; Hyde, K.D.; Kuo, C.H. The holomorph of Neoroussoella alishanense sp. nov. (Roussoellaceae, Pleosporales) on Pennisetum purpureum (Poaceae). Phytotaxa 2019, 406, 218–236. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; McKenzie, E.H.; Phillips, A.J.; Gareth Jones, E.B.; Jayarama Bhat, D.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: http://www.indexfungorum.org/names/Names.asp (accessed on 20 April 2022).
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Rehner, S. Primers for Elongation Factor 1-α (EF1-α). Available online: http://ocid.NACSE.ORG/research/deephyphae/EF1primer.pdf/ (accessed on 30 March 2022).
- Liu, Y.; Whelen, S.; Hall, B. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Hall, T. BioEdit. Ibis Therapeutics. 2004. Available online: http://www.mbio.ncsu.edu/BioEdit/bioedit.html/ (accessed on 30 March 2022).
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Schumacher, R.K.; Akulov, A.; Thangavel, R.; Hernández-Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfield, M.J.; Summerell, B.A.; Quaedvlieg, W.; et al. New and interesting fungi. 2. Fungal Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2011, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. MrModeltest 2.0; Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Zhaxybayeva, O.; Gogarten, J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Maharachchikumbura, S.S.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Huang, S.K.; Abdel-Wahab, M.A.; Daranagama, D.A.; Dayarathne, M.; D’souza, M.J.; Goonasekara, I.D.; et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015, 72, 199–301. [Google Scholar] [CrossRef]
- Page, R.D.M. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [PubMed] [Green Version]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- Hyde, K.D.; Eriksson, O.E.; Yue, J.Z. Roussoëlla, an ascomycete genus of uncertain relationships with a Cytoplea anamorph. Mycol. Res. 1996, 100, 1522–1528. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Wanasinghe, D.N.; Phillips, A.J.L.; Camporesi, E.; Bulgakov, T.S.; Phukhamsakda, C.; Ariyawansa, H.A.; Goonasekara, I.D.; Phookamsak, R.; Dissanayake, A.; et al. Mycosphere notes 1–50: Grass (Poaceae) inhabiting Dothideomycetes. Mycosphere 2017, 8, 697–796. [Google Scholar] [CrossRef]
- Hyde, K.D. The genus Roussoella, including two new species from palms in Cuyabeno, Ecuador. Mycol. Res. 1997, 101, 609–616. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Cai, L.; Hyde, K.D. Astrosphaeriella and Roussoella species on bamboo from Hong Kong and Yunnan, China including a new species of Roussoella. Cryptogam. Mycol. 2003, 24, 191–197. [Google Scholar]
- Monod, M. Monographie taxonomique des Gnomoniaceae (Ascomycètes de l’ordre des Diaporthales). I. Beihefte Sydowia 1983, 9, 1–314. [Google Scholar] [CrossRef]
- Hyde, K.D.; Aptroot, A.; Fröhlich, J.; Taylor, J.E. Fungi from palms. XLII. Didymosphaeria and similar ascomycetes from palms. Nova Hedwig. 1999, 69, 449–471. [Google Scholar] [CrossRef]
- Candoussau, F.; Katumoto, K.; Sherwood-Pike, M.A. Bambusicolous fungi from the southwest of France I. Two new species of Pyrenomycetes and a new genus of the Phacidiaceae. Sydowia 1985, 38, 28–34. [Google Scholar]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, J.D.; Liu, N.; et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1553–2107. [Google Scholar] [CrossRef]
- Species Fungorum. Available online: http://www.speciesfungorum.org/Names/Names.asp (accessed on 20 April 2022).
- Theissen, F.; Sydow, H. Die Dothideales. Kritisch-systematische Originaluntersuchungen. Ann. Mycol. 1915, 13, 147–746. [Google Scholar]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Ju, Y.M.; Rogers, J.D.; Huhndorf, S.M. Valsaria and notes on Endoxylina, Pseudothyridaria, Pseudovalsaria, and Roussoella. Mycotaxon 1996, 58, 419–481. [Google Scholar] [CrossRef]












| Genes | Primers and Base Pairs | References |
|---|---|---|
| Internal transcribed spacers (ITS) | Forward: ITS5 TCCTCCGCTTATTGATATGC Reverse: ITS4 GGAAGTAAAAGTCGTAACAAGG | [21] |
| Large subunit rDNA (LSU) | Forward: LROR GTACCCGCTGAACTTAAGC Reverse: LR5 ATCCTGAGGGAAACTTC | [22] |
| Small subunit rDNA (SSU) | Forward: NS1 GTAGTCATATGCTTGTCTC Reverse: NS4 CTTCCGTCAATTCCTTTAAG | [21] |
| Translation elongation factor 1-α gene region (tef1) | Forward: EF1-983F GCYCCYGGHCAYCGTGAYTTYAT Reverse: EF1-2218R ATGACACCRACRGCRACRGTYTG | [23] |
| RNA polymerase II second largest subunit (rpb2) | Forward: fRPB2-5f GAYGAYMGWGATCAYTTYGG Reverse: fRPB2-7cr CCCATRGCTTGTYYRCCCAT | [24] |
| Genes | Initial Period | Cycles, Denaturation, Annealing and Elongation | Final Extension | References |
|---|---|---|---|---|
| ITS, LSU, SSU, tef1 | 94 °C for 3 min | 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 50 s, elongation at 72 °C for 1 min | 72 °C for 10 min | [17] |
| rpb2 | 95 °C for 5 min | 40 cycles of denaturation at 95 °C for 1 min, annealing at 52 °C for 2 min, elongation at 72 °C for 90 s | 72 °C for 10 min | [17] |
| Taxa | Strain/Voucher No. | GenBank Accession Numbers | |||
|---|---|---|---|---|---|
| ITS | LSU | tef1 | rpb2 | ||
| Arthopyrenia sp. | UTHSC DI16-362 | LT796905 | LN907505 | LT797145 | LT797065 |
| Arthopyrenia sp. | UTHSC DI16-334 | LT796887 | LN907477 | LT797127 | - |
| Neoroussoella alishanense | FU31016 | MK503816 | MK503822 | MK336181 | MN037756 |
| Neoroussoella bambusae | MFLUCC 11-0124 | KJ474827 | KJ474839 | KJ474848 | KJ474856 |
| Neoroussoella bambusae ★ | GMBCC1116 | OM891810 | OM884022 | ON098358 | ON098377 |
| Neoroussoella bambusae ★ | GMBCC1118 | OM891812 | OM801294 | ON098359 | ON098376 |
| Neoroussoella entadae | MFLUCC 15-0098 | MH275075 | MH260309 | - | - |
| Neoroussoella heveae | MFLUCC 17-0338 | MH590693 | MH590689 | - | - |
| Neoroussoella heveae | MFLUCC 17-2069 | MT310634 | MT214589 | MT394647 | MT394703 |
| Neoroussoella lenispora | GZCC 16-0020 | - | KX791431 | - | - |
| Neoroussoella leucaenae | MFLUCC 18-1544 | MK347767 | MK347984 | MK360067 | MK434876 |
| Neoroussoella solani | CPC 26331 | KX228261 | KX228312 | - | - |
| Pararoussoella mangrovei | MFLUCC 16-0424 | MH025951 | MH023318 | MH028246 | MH028250 |
| Pararoussoella mukdahanensis | KUMCC 18-0121 | MH453489 | MH453485 | MH453478 | MH453482 |
| Pararoussoella mukdahanensis | MFLUCC 11-0201 | KU940129 | KU863118 | - | - |
| Pararoussoella rosarum | MFLUCC 17-0796 | NR_157529 | NG059872 | MG829224 | - |
| Parathyridaria percutanea | CBS 128203 | KF322117 | KF366448 | KF407988 | KF366453 |
| Parathyridaria percutanea | CBS 868.95 | KF322118 | KF366449 | KF407987 | KF366452 |
| Parathyridaria ramulicola | CBS 141479 | KX650565 | KX650565 | KX650536 | KX650584 |
| Parathyridaria ramulicola | MF4 | KX650564 | KX650564 | KX650535 | - |
| Parathyridaria robiniae | MFLUCC 14-1119 | KY511142 | KY511141 | KY549682 | - |
| Pseudoneoconiothyrium euonymi | CBS 143426 | MH107915 | MH107961 | - | MH108007 |
| Pseudoneoconiothyrium rosae | MFLUCC 15-0052 | NR_157523 | NG059868 | - | - |
| Pseudoroussoella chromolaenae | MFLUCC 17-1492 | MT214345 | MT214439 | MT235769 | - |
| Pseudoroussoella elaeicola | MFLUCC 17-1483 | MT214348 | MT214442 | MT235772 | MT235808 |
| Pseudoroussoella elaeicola | MFLUCC 15-0276b | MH742330 | MH742327 | - | - |
| Pseudoroussoella elaeicola | MFLUCC 15-0276a | MH742329 | MH742326 | - | - |
| Roussoella angusta | MFLUCC 15-0186 | - | KT281979 | - | - |
| Roussoella aquatica | MFLUCC 18-1040 | NR171975 | NG073797 | - | - |
| Roussoella arundinacea | CBS 146088 | MT223838 | MT223928 | MT223723 | - |
| Roussoella chiangraina | MFLUCC 10-0556 | KJ474828 | KJ474840 | KJ474849 | KJ474857 |
| Roussoella doimaesalongensis | MFLUCC 14-0584 | KY026584 | KY000659 | KY651249 | KY678394 |
| Roussoella elaeicola | MFLUCC 15-0276a | MH742329 | MH742326 | - | - |
| Roussoella elaeicola | MFLUCC 15-0276b | MH742330 | MH742327 | - | - |
| Roussoella guttulata | MFLUCC 20-0102 | NR_172428 | NG_075383 | MW022188 | MW022187 |
| Roussoella hysterioides | CBS 546.94 | KF443405 | KF443381 | KF443399 | KF443392 |
| Roussoella intermedia | NBRC 106245 | KJ474831 | AB524624 | - | - |
| Roussoella intermedia | CBS 170.96 | KF443407 | KF443382 | KF443398 | KF443394 |
| Roussoella japanensis | MAFF 239636 | KJ474829 | AB524621 | AB539114 | AB539101 |
| Roussoella japanensis ★ | GMBCC1067 | OM891802 | OM884018 | ON098344 | ON098381 |
| Roussoella japanensis ★ | GMBCC1117 | OM891811 | OM884023 | ON098345 | ON098382 |
| Roussoella kunmingensis | KUMCC 18-0128 | MH453491 | MH453487 | MH453480 | MH453484 |
| Roussoella kunmingensis ★ | GMBCC1055 | OM891797 | OM884013 | ON098353 | ON098385 |
| Roussoella kunmingensis ★ | GMBCC1057 | OM891798 | OM884014 | ON098354 | ON098362 |
| Roussoella kunmingensis ★ | GMBCC1086 | OM891804 | OM801287 | ON098355 | ON098363 |
| Roussoella magnatum | MFLUCC 15-0185 | - | KT281980 | - | - |
| Roussoella margidorensis | MUT 5329 | KU314944 | MN556322 | MN605897 | MN605917 |
| Roussoella mediterranea | MUT 5369 | KU314947 | MN556324 | MN605899 | MN605919 |
| Roussoella mexicana | CPC 25355 | KT950848 | KT950862 | - | - |
| Roussoella multiloculate ★ | GMB1219 | OM891801 | OM884017 | ON098341 | ON098366 |
| Roussoella multiloculate★ | GMBCC1056 | OM891799 | OM884015 | ON098343 | ON098369 |
| Roussoella multiloculate ★ | GMBCC1065 | OM891800 | OM884016 | ON098338 | ON098364 |
| Roussoella multiloculate ★ | GMBCC1069 | OM891803 | OM884019 | ON098340 | ON098365 |
| Roussoella multiloculate ★ | GMBCC1071 | ON159383 | OM755586 | ON098342 | ON098368 |
| Roussoella multiloculate ★ | GMBCC1080 | ON159384 | OM755589 | ON098339 | ON098367 |
| Roussoella neopustulans | MFLUCC 11-0609 | KJ474833 | KJ474841 | KJ474850 | - |
| Roussoella neopustulans | MFLUCC 12-0003 | KU940130 | KU863119 | - | - |
| Roussoella nitidula | MFLUCC 11-0182 | KJ474835 | KJ474843 | KJ474852 | KJ474859 |
| Roussoella nitidula | MFLUCC 11-0634 | KJ474834 | KJ474842 | KJ474851 | KJ474858 |
| Roussoella nitidula ★ | GMBCC1097 | OM891805 | OM884020 | ON098351 | ON098384 |
| Roussoella padinae | MUT 5503 | KU158170 | MN556327 | MN605902 | MN605922 |
| Roussoella padinae ★ | GMBCC1126 | OM891816 | OM884025 | ON098356 | ON098383 |
| Roussoella papillate★ | GMBCC1121 | OM891814 | OM755608 | ON098346 | ON098378 |
| Roussoella papillate ★ | IFRDCC 3103 | ON228188 | ON228184 | ON244452 | ON244450 |
| Roussoella pseudohysterioides | MFLUCC 13-0852 | KU940131 | KU863120 | KU940198 | - |
| Roussoella pseudohysterioides | KUMCC 18-0111 | MH453490 | MH453486 | MH453479 | MH453483 |
| Roussoella pustulans | MAFF 239637 | KJ474830 | AB524623 | AB539116 | AB539103 |
| Roussoella scabrispora | MFLUCC 11-0624 | KJ474836 | KJ474844 | KJ474853 | KJ474860 |
| Roussoella scabrispora | MFLUCC 14-0582 | KY026583 | KY000660 | - | - |
| Roussoella scabrispora ★ | GMBCC1101 | ON159385 | OM755615 | ON098347 | ON098371 |
| Roussoella scabrispora ★ | GMBCC1102 | OM891806 | OM884021 | ON098348 | ON098370 |
| Roussoella scabrispora ★ | GMBCC1104 | OM891807 | OM755616 | ON098349 | ON098373 |
| Roussoella scabrispora ★ | GMBCC1108 | OM891808 | OM755614 | ON098350 | ON098372 |
| Roussoella siamensis | MFLUCC 11-0149 | KJ474837 | KJ474845 | KJ474854 | KJ474861 |
| Roussoella sinensis★ | GMBCC1119 | OM891813 | OM884024 | ON098357 | ON098379 |
| Roussoella sinensis ★ | IFRDCC 3101 | ON228187 | ON228183 | ON244453 | ON244451 |
| Roussoella thailandica | MFLUCC 11-0621 | KJ474838 | KJ474846 | - | - |
| Roussoella tuberculata | MFLUCC 13-0854 | KU940132 | KU863121 | KU940199 | - |
| Roussoella tuberculata ★ | GMBCC1123 | OM891815 | OM755613 | ON098352 | ON098380 |
| Roussoella uniloculata★ | GMBCC1110 | OM891809 | OM801286 | ON098360 | ON098374 |
| Roussoella uniloculata ★ | DDQ01005-2 | OM891817 | OM884026 | ON098361 | ON098375 |
| Roussoella verrucispora | CBS 125434 | KJ474832 | AB524622 | AB539115 | AB539102 |
| Roussoella yunnanensis | KUMCC 18-0115 | MH453492 | MH453488 | MH453481 | - |
| Roussoellopsis macrospora | MFLUCC 12-0005 | - | KJ474847 | KJ474855 | KJ474862 |
| Roussoellopsis sp. | NBRC 106246 | - | AB524626 | - | - |
| Roussoellopsis tosaensis | KT 1659 | - | AB524625 | MG829199 | AB539104 |
| Setoarthopyrenia chromolaenae | MFLUCC 17-1444 | MT214344 | MT214438 | MT235768 | MT235805 |
| Thyridaria acaciae | CBS 138873 | KP004469 | KP004497 | - | - |
| Thyridaria broussonetiae | CBS 141481 | NR_147658 | KX650568 | KX650539 | KX650586 |
| Torula herbarum | CBS 111855 | KF443409 | KF443386 | KF443403 | KF443396 |
| Torula hollandica | CBS 220.69 | KF443406 | KF443384 | KF443401 | KF443393 |
| Xenoroussoella triseptata | MFLUCC 17-1438 | MT214343 | MT214437 | MT235767 | MT235804 |
| Analyses | Parameters | Value |
|---|---|---|
| ML | Final ML Optimization Likelihood | −30,685.326261 |
| No of characters | 3372 | |
| Alignment patterns | 1513 | |
| Proportion of Undetermined characters or gaps | 29.97% | |
| Substitution model | GTR | |
| Tree length | 3.629102 | |
| Estimated base frequencies | A = 0.240311 | |
| C = 0.267630 | ||
| G = 0.270582 | ||
| T = 0.221477 | ||
| Substitution rates | AC = 1.886299 | |
| AG = 5.163488 | ||
| AT = 1.984078 | ||
| CG = 1.308792 | ||
| CT = 9.477974 | ||
| GT = 1.000000 | ||
| Gamma distribution shape parameter | α = 0.180205 |
| Characters | R. japanensis [1] | R. papillate (in This Study) | R. hysterioides [39] |
|---|---|---|---|
| Ascostromata | 500–2000 μm diam., immersed under a clypeus, raised, visible, black, dome-shape areas on host surface, uni-biloculate | 250–350 μm high, 500–900 μm long, 500–700 μm wide deeply immersed under a brown area, becoming raised at maturity, ellipsoidal to irregular coriaceous, solitary to gregarious, brown, with black papilla, uniloculate | 230–280 µm high, 2–2.5 mm wide, immersed, flattened at the base, multilocular |
| Locules | 190–210 μm high, 500–560 μm diam., depressed globose with a flattened base, single or 2–3 grouped, ostiolate | 200–300 μm high, 450–500 μm diam., solitary, subglobose, brown to dark brown, with a central ostiole | 75–150 × 35–50 µm, with the ostiole erumpent through the host epidermis |
| Peridium (Wall of locules) | 10–15 μm thick at sides, composed of 3–5 layers of polygonal flattened cells (3.5–12.5 × 1.5–2.5 μm), surrounded by wedge-shaped stromatic region (450–800 μm wide at sides) composed of rectangular to polygonal cells (3.5–15 × 4–10 μm) | 9–20 μm wide, composed of 1–2 layers of textura angularis, thin-walled flattened at the base, light brown to brown | |
| Asci | 107–132 × 8–9.5 μm, cylindrical, short pedicellate | 108–125 × 7–10 μm, cylindrical, short pedicellate | 105–120 × 4–6 µm, cylindrical, short- pedicellate |
| Ascospores | 16–22 × 5.5–7 μm, uniseriate, fusiform to ellipsoidal, with a median septum, 2-celled, brown, rough-walled more or less, covered with longitudinal striations and surrounded by an entire sheath of 0.5–4 μm wide | 15–17 × 5.5–7 μm, uniseriate, ellipsoidal to broad fusiform, 2-celled, constricted at the septum, brown to dark brown, with longitudinal striations, surrounded by a mucilaginous sheath | 13–20 × 4–6 µm, uniseriate, overlapping, fusiform, uniseptate, constricted at the septum, brown, slightly pointed at the ends, upper cell larger, with striate ornamentation on surface |
| Species | ITS | rpb2 |
|---|---|---|
| R. chiangraina | 7.22% (33/457) | 3.68% (34/925) |
| R. kunmingensis | 5.29% (25/472) | 4.61% (39/845) |
| R. mediterranea | 4.26% (20/469) | 3.84% (25/651) |
| R. neopustulans | 5.56% (27/469) | 3.80% (35/922) |
| R. padinae | 4.71% (22/467) | 3.78% (35/925) |
| Taxa | Ascospores | Host | Known Distribution | References |
|---|---|---|---|---|
| Roussoella aequatoriensis Hyde | 26–33 × 9–11 µm, fusiform-ellipsoidal, 1-septate, constricted at the septum, brown, with oblique wall, striations running the entire length of the ascospore and with yellow coloured mucilaginous, pad-like appendages at each end | Palm | Ecuador, Puerto Rico | [41] |
| R. alveolata Ju, Rogers, and Huhndorf | 34–42 × 11–13 µm, with ridges between the longitudinal striations. | Bamboo | Indonesia (Java) | [41] |
| R. angustispora Zhou, Cai, and Hyde | 24–28 × 6–8 µm, ellipsoid-fusiform, 1-septate, constricted at the septum, brown, with reticulate wall ornamentations | Bamboo (Bambusa changii) | China (Hong Kong) | [42] |
| R. bambusae (Pat.) Monod | 23 × 5 µm, elliptical elongated, often acute at both ends, not constricted at the septum, colorless and surrounded by a fleeting hyaline sheath | - | - | [43] |
| R. calamicola Fröhl., Hyde, and Aptroot | 20–27(–29.5) × 7–8.5 μm, ellipsoidal, 1-septatae, brown, verrucose, surrounded by a mucilaginous sheath | Calamus | Australian (Queensland) | [44] |
| R. chilensis (Speg.) Ju, Rogers, and Huhndorf | asci contain only four 20–25(–28) × 6–8 µm, ascospores with longitudinal wall striations This fungus is unique amongst Roussoella in having four ascospores per ascus | Bamboo (Chusquea) | Chile | [41] |
| R. donacicola (Speg.) Ju, Rogers, and Huhndorf | ascospores are (6–)6.5–8(–8.5) × 3–3.5 µm, with longitudinal striations | Bamboo (Arundo, Phyllostachys) | Argentina, France | [41] |
| R. palmicola Fröhl., Hyde, and Aptroot | 12.5–24 × 2.5–4 μm, fusiform, 1-septate, brown, striate, with small pads of mucilage at both ends | Rattan (Calamus flabellatus) | Brunei | [44] |
| R. saltuensis Hyde | 25–30 × 8–11 µm, overlapping ellipsoidal, 1-septate, constricted at the central septum, dark-brown, covered with irregular longitudinal striations and surrounded by a mucilaginous sheath which spreads in water | Palm (indet.) | Ecuador | [41] |
| R. serrulata (Ellis and Martin) Hyde and Aptroot | 18–20 × 5–6 µm, characterized by the often deeply (up to 1 mm) immersed ascomata | Pam (Serenoa serrulata) | USA, Florida | [12] |
| R. verruculosa Cand. and Katum | 7–8 × 5 µm, fusoid, septate, slightly constricted at the septum, rounded at the ends, verruculose | Bamboo (Phyllostachys mitis) | France | [45] |
| R. papillate Dai and Wijayaw | 15–17 × 5.5–7 μm, brown to dark brown, rough-walled, with longitudinal striations | Bamboo | China (Yunnan) | In this study |
| R. sinensis Dai and Wijayaw | 16.5–20.5 × 6–7.5 μm, ellipsoid to broad fusiform, upper cells bigger, constricted at the septum, narrowly at both ends, with longitudinal striations | Bamboo | China (Yunnan) | In this study |
| R. uniloculata Dai and Wijayaw | 8.5–12× 3.5–4.5, ellipsoid to broad fusiform, 2-celled, upper cells bigger, occasionally curve, brown, constricted at the septum, with longitudinal striations | Bamboo | China (Yunnan) | In this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.-Q.; Wijayawardene, N.N.; Dayarathne, M.C.; Kumla, J.; Han, L.-S.; Zhang, G.-Q.; Zhang, X.; Zhang, T.-T.; Chen, H.-H. Taxonomic and Phylogenetic Characterizations Reveal Four New Species, Two New Asexual Morph Reports, and Six New Country Records of Bambusicolous Roussoella from China. J. Fungi 2022, 8, 532. https://doi.org/10.3390/jof8050532
Dai D-Q, Wijayawardene NN, Dayarathne MC, Kumla J, Han L-S, Zhang G-Q, Zhang X, Zhang T-T, Chen H-H. Taxonomic and Phylogenetic Characterizations Reveal Four New Species, Two New Asexual Morph Reports, and Six New Country Records of Bambusicolous Roussoella from China. Journal of Fungi. 2022; 8(5):532. https://doi.org/10.3390/jof8050532
Chicago/Turabian StyleDai, Dong-Qin, Nalin N. Wijayawardene, Monika C. Dayarathne, Jaturong Kumla, Li-Su Han, Gui-Qing Zhang, Xian Zhang, Ting-Ting Zhang, and Huan-Huan Chen. 2022. "Taxonomic and Phylogenetic Characterizations Reveal Four New Species, Two New Asexual Morph Reports, and Six New Country Records of Bambusicolous Roussoella from China" Journal of Fungi 8, no. 5: 532. https://doi.org/10.3390/jof8050532
APA StyleDai, D.-Q., Wijayawardene, N. N., Dayarathne, M. C., Kumla, J., Han, L.-S., Zhang, G.-Q., Zhang, X., Zhang, T.-T., & Chen, H.-H. (2022). Taxonomic and Phylogenetic Characterizations Reveal Four New Species, Two New Asexual Morph Reports, and Six New Country Records of Bambusicolous Roussoella from China. Journal of Fungi, 8(5), 532. https://doi.org/10.3390/jof8050532







