Author Contributions
Conceptualization, S.A., S.K.J., F.R.; methodology, S.A., S.K.J., S.A.A., U.B.; software, O.A.W., R.A.R., M.M.; validation, S.K.J., F.R., S.A.A., U.B., O.A.W.; formal analysis, S.A., S.K.J., O.A.W., R.A.R.; investigation, S.A., S.K.J., F.R., S.A.A., U.B., M.M; resources, S.A.A., M.M., M.T.Y., A.A.-F.M., A.M.E., E.E.-H., A.M.E.-S., R.C., H.O.E., U.B.; writing—original draft preparation, S.A., S.A.A., U.B., R.A.R.; writing—review and editing, M.M., M.T.Y., A.A.-F.M., A.M.E., E.E.-H., A.M.E.-S., R.C., H.O.E.; All authors have read and agreed to the published version of the manuscript.
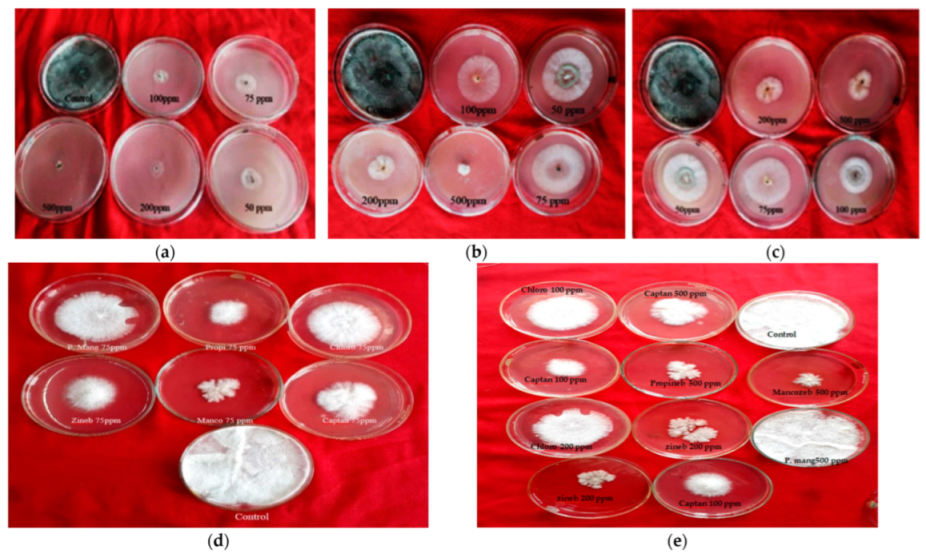
Figure 1.
Effect of non-systemic fungitoxicants on radial mycelial growth of T. harzianum and A. bisporus: (a) Chlorothalonil fungitoxicant against T. harzianum; (b) Prochloraz manganese fungitoxicant against T. harzianum; (c) Captan fungitoxicant against T. harzianum; (d,e) P. mang (=Prochloraz manganese), Propi (=propineb), Chloro (=chlorothalonil), zineb, Manco (=mancozeb) and captan fungitoxicant against A. bisporus.
Figure 1.
Effect of non-systemic fungitoxicants on radial mycelial growth of T. harzianum and A. bisporus: (a) Chlorothalonil fungitoxicant against T. harzianum; (b) Prochloraz manganese fungitoxicant against T. harzianum; (c) Captan fungitoxicant against T. harzianum; (d,e) P. mang (=Prochloraz manganese), Propi (=propineb), Chloro (=chlorothalonil), zineb, Manco (=mancozeb) and captan fungitoxicant against A. bisporus.
Figure 2.
Comparative bar chart (left) and Boxplots (right) describe the effect of non-systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
Figure 2.
Comparative bar chart (left) and Boxplots (right) describe the effect of non-systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
Figure 3.
Comparative bar chart (left) and boxplots (right) designate the effect of non-systemic fungi toxicants under in vitro conditions on host A. bisporus mycelium.
Figure 3.
Comparative bar chart (left) and boxplots (right) designate the effect of non-systemic fungi toxicants under in vitro conditions on host A. bisporus mycelium.
Figure 4.
Impact of non-systemic fungitoxicants in casing on percent intensity of green mold disease of button mushroom, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II (uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 4.
Impact of non-systemic fungitoxicants in casing on percent intensity of green mold disease of button mushroom, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II (uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 5.
Boxplot designates the effect of non-systemic fungitoxicants on T. harzianum infested casing pooled during spring 2017 and 2018 on the number and weight of fruit bodies and button yield, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II(uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 5.
Boxplot designates the effect of non-systemic fungitoxicants on T. harzianum infested casing pooled during spring 2017 and 2018 on the number and weight of fruit bodies and button yield, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II(uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 6.
Boxplots for impact of non-systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II(uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 6.
Boxplots for impact of non-systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus, where T1 = Prochloraz manganese 0.05% conc., T10 = Check I(infested-untreated), T11 = Check II(uninfested-untreated), T2 = Prochloraz manganese 0.1% conc., T3 = Prochloraz manganese 0.2% conc., T4 = Chlorothalonil 0.05% conc., T5 = Chlorothalonil 0.1% conc., T6 = Chlorothalonil 0.2% conc., T7 = Captan 0.05% conc., T8 = Captan 0.1% conc., T9 = Captan 0.2% conc.
Figure 7.
Effect of systemic fungitoxicants on radial mycelial growth of T. harzianum and A. bisporus: (a) thiophanate methyl against T. harzianum; (b) myclobutanil against T. harzianum; (c) carbendazim against T. harzianum; (d,e) Thio (= thiophanate methyl), Myclo (= myclobutanil), Hexa (= hexaconazole), Carben (= carbendazim) and Difeno (= difenoconazole) fungitoxicant against A. bisporus.
Figure 7.
Effect of systemic fungitoxicants on radial mycelial growth of T. harzianum and A. bisporus: (a) thiophanate methyl against T. harzianum; (b) myclobutanil against T. harzianum; (c) carbendazim against T. harzianum; (d,e) Thio (= thiophanate methyl), Myclo (= myclobutanil), Hexa (= hexaconazole), Carben (= carbendazim) and Difeno (= difenoconazole) fungitoxicant against A. bisporus.
Figure 8.
Comparative bar chart (left) designates the effect of systemic fungitoxicants under in vitro conditions against T. harzianum mycelium, where Carben = Carbendazim 50 WP, Myclo = Myclobutanil 10 WP, Thiopha = Thiophanate methyl 70 WP, Hexacona = Hexaconazole 5 EC, Difenoc = Difenoconazole 25 EC, Sys INHP = mycelial inhibition of Pathogen.
Figure 8.
Comparative bar chart (left) designates the effect of systemic fungitoxicants under in vitro conditions against T. harzianum mycelium, where Carben = Carbendazim 50 WP, Myclo = Myclobutanil 10 WP, Thiopha = Thiophanate methyl 70 WP, Hexacona = Hexaconazole 5 EC, Difenoc = Difenoconazole 25 EC, Sys INHP = mycelial inhibition of Pathogen.
Figure 9.
Comparative bar chart designates the effect of systemic fungitoxicants under in vitro conditions on host A. bisporus mycelium, where Carben = Carbendazim 50 WP, Myclo = Myclobutanil 10 WP, Thiopha = Thiophanate methyl 70 WP, Hexacona = Hexaconazole 5 EC, Difenoc = Difenoconazole 25 EC, Sys INHH = mycelial inhibition of host.
Figure 9.
Comparative bar chart designates the effect of systemic fungitoxicants under in vitro conditions on host A. bisporus mycelium, where Carben = Carbendazim 50 WP, Myclo = Myclobutanil 10 WP, Thiopha = Thiophanate methyl 70 WP, Hexacona = Hexaconazole 5 EC, Difenoc = Difenoconazole 25 EC, Sys INHH = mycelial inhibition of host.
Figure 10.
Effect of fungitoxicants under in vivo conditions: (a) experiment plan of layout; (b) prochloraz manganese treatment; (c) carbendazim treatment; (d) casing soil (uninfested).
Figure 10.
Effect of fungitoxicants under in vivo conditions: (a) experiment plan of layout; (b) prochloraz manganese treatment; (c) carbendazim treatment; (d) casing soil (uninfested).
Figure 11.
Boxplots depict the impact of systemic fungitoxicants in casing on percent intensity of green mold disease, where T1 = Carbendazim 0.025% conc., T10 = Check I (infested-untreated), T11 = Check II (uninfested-untreated), T2 = Carbendazim 0.05% conc., T3 = Carbendazim 0.1% conc., T4 = Thiophanate methyl 0.025% conc., T5 = Thiophanate methyl 0.05% conc., T6 = Thiophanate methyl 0.1% conc., T7 = Myclobutanil 0.025% conc., T8 = Myclobutanil 0.05% conc., T9 = Myclobutanil 0.1% conc.
Figure 11.
Boxplots depict the impact of systemic fungitoxicants in casing on percent intensity of green mold disease, where T1 = Carbendazim 0.025% conc., T10 = Check I (infested-untreated), T11 = Check II (uninfested-untreated), T2 = Carbendazim 0.05% conc., T3 = Carbendazim 0.1% conc., T4 = Thiophanate methyl 0.025% conc., T5 = Thiophanate methyl 0.05% conc., T6 = Thiophanate methyl 0.1% conc., T7 = Myclobutanil 0.025% conc., T8 = Myclobutanil 0.05% conc., T9 = Myclobutanil 0.1% conc.
Figure 12.
Comparative bar chart designates the effect of systemic fungitoxicants on T. harzianum infested casing pooled during spring 2017 and 2018 on the number and weight of fruit bodies and button yield, where T1 = Carbendazim 0.025% conc., T10 = Check I (infested-untreated), T11 = Check II (uninfested-untreated), T2 = Carbendazim 0.05% conc., T3 = Carbendazim 0.1% conc., T4 = Thiophanate methyl 0.025% conc., T5 = Thiophanate methyl 0.05% conc., T6 = Thiophanate methyl 0.1% conc., T7 = Myclobutanil 0.025% conc., T8 = Myclobutanil 0.05% conc., T9 = Myclobutanil 0.1% conc.
Figure 12.
Comparative bar chart designates the effect of systemic fungitoxicants on T. harzianum infested casing pooled during spring 2017 and 2018 on the number and weight of fruit bodies and button yield, where T1 = Carbendazim 0.025% conc., T10 = Check I (infested-untreated), T11 = Check II (uninfested-untreated), T2 = Carbendazim 0.05% conc., T3 = Carbendazim 0.1% conc., T4 = Thiophanate methyl 0.025% conc., T5 = Thiophanate methyl 0.05% conc., T6 = Thiophanate methyl 0.1% conc., T7 = Myclobutanil 0.025% conc., T8 = Myclobutanil 0.05% conc., T9 = Myclobutanil 0.1% conc.
Figure 13.
Boxplots for impact of systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
Figure 13.
Boxplots for impact of systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
Table 1.
Fungitoxicants evaluated against the green mold pathogen of white button mushroom (A. bisporus).
Table 1.
Fungitoxicants evaluated against the green mold pathogen of white button mushroom (A. bisporus).
| Common Name | Manufacture Name | Trade Name |
| (a) Non-systemic |
| Prochloraz Manganese | FMC company | Sportek 50 WP |
| Chlorothalonil | M/S Syngenta India Ltd. | Kavach 75 WP |
| Mancozeb | M/S Dow Agro Science Mumbai | Dithane M 45 |
| Captan | M/S Rallis India Ltd. | Captaf 50 WP |
| Propineb | M/S Bayer Crop Science Ltd. | Antracol 70 WP |
| Zineb | M/S Indofil Industries Ltd. | Indofil Z-78 (75 WP) |
| (b) Systemic |
| Carbendazim | Crystal Crop Protection Pvt Limited | Bavistin 50 WP |
| Thiophanate methyl | Mahashantam Pesticides Private limited | Topsin M 70 WP |
| Difenoconazole | M/S Syngenta India Ltd. | Score 25 EC |
| Myclobutanil | M/S Dow Agro Science Mumbai Ltd. | Systhane 10 WP |
| Hexaconazole | M/S Rallis India Ltd. | Contaf 5 EC |
Table 2.
Effect of non-systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
Table 2.
Effect of non-systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
| | Conc. (µg mL−1) | Inhibition of Growth over Control (%) * | Mean |
|---|
| Fungicide | | 50 | 75 | 100 | 200 | 500 |
|---|
| Prochloraz manganese 50 WP | 79.86 (63.30) | 86.80 (68.96) | 100.00 (89.42) | 100.00 (89.42) | 100.00 (89.42) | 93.40 (80.10) a |
| Chlorothalonil 75 WP | 46.52 (42.98) | 68.66 (55.93) | 83.88 (66.25) | 90.56 (72.07) | 94.73 (76.69) | 76.87 (62.78) b |
| Mancozeb 75 WP | 11.57 (19.87) | 13.63 (21.65) | 17.90 (25.01) | 22.06 (28.00) | 31.88 (34.36) | 19.40 (25.78) f |
| Captan 50 WP | 34.68 (36.06) | 42.85 (40.86) | 48.70 (44.23) | 52.35 (46.32) | 68.65 (55.92) | 49.44 (44.68) c |
| Propineb 75 WP | 19.94 (26.51) | 25.52 (30.33) | 38.48 (38.32) | 51.12 (45.62) | 61.43 (51.58) | 39.29 (38.47) e |
| Zineb 75 WP | 23.98 (29.30) | 31.01 (33.82) | 41.88 (40.31) | 55.60 (48.19) | 67.19 (55.03) | 43.93 (41.33) d |
| Mean | 36.09 (36.33) e | 44.74 (41.92) d | 55.14 (50.59) c | 61.94 (54.93) b | 70.64 (60.50) a | |
| | S.E± | CD (p ≤ 0.05) |
| Fungicides | (0.074) | (0.020) |
| Concentration | (0.067) | (0.019) |
| Fungicide × Concentration | (0.165) | (0.047) |
Table 3.
Effect of non-systemic fungitoxicants under in vitro conditions on host A. bisporus mycelium.
Table 3.
Effect of non-systemic fungitoxicants under in vitro conditions on host A. bisporus mycelium.
| Conc. (µg mL−1) | Inhibition of Growth over Control (%) * | Mean |
|---|
| Fungitoxicant | | 50 | 75 | 100 | 200 | 500 |
|---|
| Prochloraz manganese 50 WP | 0.0 (0.57) | 3.32 (10.49) | 5.80 (13.93) | 11.61 (19.91) | 18.56 (25.50) | 7.85 (14.08) b |
| Chlorothalonil 75 WP | 0.0 (0.57) | 2.88 (9.76) | 4.94 (12.83) | 10.98 (19.34) | 17.03 (24.36) | 7.16 (13.37) a |
| Mancozeb 75 WP | 4.69 (12.50) | 8.01 (16.43) | 14.34 (22.24) | 23.31 (28.85) | 33.52 (35.36) | 16.77 (23.08) f |
| Captan 50 WP | 0.0 (0.57) | 3.27 (10.41) | 6.66 (14.95) | 13.42 (21.48) | 22.19 (28.09) | 9.10 (15.1) c |
| Propineb 75 WP | 3.86 (11.32) | 7.41 (15.79) | 13.74 (21.74) | 22.54 (28.33) | 31.34 (34.03) | 15.77 (22.24) e |
| Zineb 75 WP | 2.84 (9.69) | 5.28 (13.27) | 12.51 (20.70) | 20.89 (27.18) | 28.13 (32.01) | 14.24 (20.57) d |
| Mean | 1.89 (5.87) a | 5.20 (12.69) b | 9.66 (17.73) c | 17.12 (24.18) d | 25.12 (29.89) e | |
| | S.E± | CD (p ≤ 0.05) |
| Fungicides | (0.005) | (0.015) |
| Concentration | (0.004) | (0.013) |
| Fungicide × Concentration | (0.012) | (0.034) |
Table 4.
Impact of non-systemic fungitoxicants in casing on percent intensity of green mold disease of button mushroom.
Table 4.
Impact of non-systemic fungitoxicants in casing on percent intensity of green mold disease of button mushroom.
| Treatments | Concentration | Spring 2017 * | Spring 2018 * | Pool * | Disease Control |
|---|
| Prochloraz manganese 50 WP | 0.05% | 6.28 (2.69) | 7.42 (2.90) | 6.85 (2.79) b | 63.81 |
| 0.1% | 1.72 (1.64) | 1.84 (1.68) | 1.78 (1.66) a | 90.59 |
| 0.2% | 0.54 (1.24) | 0.86 (1.36) | 0.70 (1.30) a | 96.30 |
| Sub mean | 2.84 (1.85) | 3.37 (1.98) | 3.11 (1.91) | |
| Chlorothalonil 75 WP | 0.05% | 5.34 (2.51) | 7.63 (2.93) | 6.48 (2.72) b | 65.76 |
| 0.1% | 2.87 (1.96) | 3.73 (2.17) | 3.30 (2.07) b | 82.56 |
| 0.2% | 2.12 (1.76) | 2.02 (1.73) | 2.07 (1.75) a | 89.06 |
| Sub mean | 3.44 (2.07) | 4.46 (2.27) | 3.95 (2.18) | |
| Captan 50 WP | 0.05% | 5.67 (2.58) | 7.93 (2.98) | 6.80 (2.78) b | 64.07 |
| 0.1% | 3.13 (2.03) | 4.11 (2.26) | 3.62 (2.14) b | 80.87 |
| 0.2% | 2.42 (1.84) | 2.12 (1.76) | 2.27 (1.80) a | 88.00 |
| Sub mean | 3.74 (2.15) | 4.72 (2.33) | 4.23 (2.24) | |
| Check I (infested-untreated) | 18.23 (4.38) | 19.63 (4.54) | 18.93 (4.46) c | - |
| Check II (uninfested-untreated) | 0.0 (1.00) | 0.0 (1.00) | 0.0 (1.00)a | - |
| CD (p ≤ 0.05) |
| Treatment combination | 0.0091 | 0.0086 | 0.0060 | |
| Control v/s rest | 0.0082 | 0.0077 | 0.0054 | |
| Fungicides | 0.0198 | 0.0196 | 0.0196 | |
| Concentration | 0.0198 | 0.0196 | 0.0197 | |
| Fungicide × Concentration | 0.0342 | 0.0339 | 0.0338 | |
Table 5.
Effect of non-systemic fungicides on T. harzianum infested casing pooled during spring, 2017 and 2018 on the number of fruit bodies per kg mushroom, fruit bodies weight (g) and yield of button (kg/quintal compost).
Table 5.
Effect of non-systemic fungicides on T. harzianum infested casing pooled during spring, 2017 and 2018 on the number of fruit bodies per kg mushroom, fruit bodies weight (g) and yield of button (kg/quintal compost).
| Fungitoxicant | Concentration | Number of Fruit Bodies per kg Mushroom * | Fruit Bodies Weight (g) * | Yield of Button (kg/quintal Compost) * |
|---|
| Prochloraz manganese 50 WP | 0.05% | 94.63 g | 9.82 e | 9.13 e |
| 0.1% | 93.22 ef | 10.14 d | 11.67 cd |
| 0.2% | 90.46 ab | 10.52 c | 14.88 a |
| Sub mean | 92.77 | 10.16 | 11.89 |
| Chlorothalonil 75 WP | 0.05% | 95.11 f | 9.26 e | 8.37 f |
| 0.1% | 94.68 e | 10.02 d | 11.83 cd |
| 0.2% | 91.36 c | 10.24 cd | 13.52 ab |
| Sub mean | 93.71 | 9.84 | 11.24 |
| Captan 50 WP | 0.05% | 92.66 e | 10.16 cd | 12.33 c |
| 0.1% | 91.68 cd | 11.54 b | 14.18 ab |
| 0.2% | 90.08 a | 12.16 a | 14.96 a |
| Sub mean | 91.47 | 11.28 | 13.82 |
| Check I (infested-untreated) | 94.48 g | 11.32 c | 7.46 g |
| Check II (uninfested-untreated) | 91.73 cd | 11.86 a | 13.78 ab |
| CD (p ≤ 0.05) |
| Control v/s rest | 0.0638 | 0.0333 | 0.0328 |
| Fungicides | 0.0336 | 0.0196 | 0.0206 |
| Concentration | 0.0336 | 0.0196 | 0.0206 |
| Fungicide × Concentration | 0.0538 | 0.0340 | 0.0357 |
Table 6.
Impact of non-systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
Table 6.
Impact of non-systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
| Fungicide | Concentration | Pileus Weight (g) * | Pileus Diameter (cm) * | Weight of Stipe (g) * | Diameter of Stipe (cm) * |
|---|
| Prochloraz manganese 50 WP | 0.05% | 6.37 d | 3.47 f | 4.43 d | 1.28 |
| 0.1% | 7.14 c | 3.67 b | 4.56 c | 1.31 |
| 0.2% | 7.37 ab | 3.81 a | 4.67 b | 1.35 |
| Sub mean | 6.96 | 3.65 | 4.55 | 1.31 |
| Chlorothalonil 75 WP | 0.05% | 5.87 e | 3.46 f | 4.45 d | 1.24 |
| 0.1% | 6.13 de | 3.53 e | 4.59 c | 1.26 |
| 0.2% | 6.22 de | 3.59 c | 4.75 a | 1.27 |
| Sub mean | 6.07 | 3.52 | 4.59 | 1.25 |
| Captan 50 WP | 0.05% | 6.39 d | 3.55 d | 4.59 c | 1.21 |
| 0.1% | 7.07 c | 3.57 d | 4.64 b | 1.22 |
| 0.2% | 7.35 ab | 3.62 c | 4.79 a | 1.24 |
| Sub mean | 6.93 | 3.58 | 4.67 | 1.22 |
| Check I (infested-untreated) | 5.98 de | 3.49 f | 4.29 e | 1.24 |
| Check II (uninfested-untreated) | 7.67 a | 3.67 b | 4.17 f | 1.27 |
| CD (p ≤ 0.05) |
| Control v/s rest | 0.0338 | 0.0338 | 0.0340 | 0.0338 |
| Fungicides | 0.0197 | 0.0198 | 0.0199 | 0.0197 |
| Concentration | 0.0197 | 0.0198 | 0.0199 | 0.0197 |
| Fungicide × Concentration | 0.0342 | 0.0343 | 0.0344 | NS |
Table 7.
Effect of systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
Table 7.
Effect of systemic fungitoxicants under in vitro conditions against T. harzianum mycelium.
| Conc. (µg mL−1) | Inhibition of Growth over Control (%) * | Mean |
|---|
| Fungitoxicant | | 10 | 25 | 50 | 75 | 100 |
|---|
| Carbendazim 50 WP | 74.27 (61.46) | 81.04 (64.29) | 90.81 (72.34) | 100.00 (89.42) | 100.00 (89.42) | 89.22 (75.38) a |
| Myclobutanil 10 WP | 48.27 (44.11) | 59.86 (50.51) | 68.77 (55.96) | 77.69 (61.37) | 85.76 (67.80) | 68.07 (55.95) c |
| Thiophanate methyl 70 WP | 71.14 (57.91) | 79.96 (63.32) | 85.05 (67.70) | 93.32 (74.99) | 100.00 (89.42) | 85.89 (70.66) b |
| Hexaconazole 5 EC | 33.52 (35.36) | 35.65 (36.64) | 37.62 (37.81) | 40.19 (39.32) | 42.52 (40.68) | 37.90 (37.96) e |
| Difenoconazole 25 EC | 44.73 (41.91) | 56.44 (49.12) | 64.35 (53.91) | 73.30 (58.95) | 81.42 (64.46) | 64.04 (53.67) d |
| Mean | 54.38 (48.15) e | 62.59 (61.68) d | 69.32 (57.54) c | 76.9 (64.81) b | 81.94 (70.35) a | |
| | S.E± | CD (p ≤ 0.05) |
| Fungitoxicant | (1.41) | (0.0049) |
| Concentration | (1.41) | (0.0049) |
| Fungitoxicant × concentration | (3.17) | (0.0109) |
Table 8.
Effect of systemic fungitoxicants on host A. bisporus mycelium under in vitro conditions.
Table 8.
Effect of systemic fungitoxicants on host A. bisporus mycelium under in vitro conditions.
| Conc. (µg mL−1) | Inhibition of Growth over Control (%)* | Mean |
|---|
| Fungicide | | 10 | 25 | 50 | 75 | 100 |
|---|
| Carbendazim 50 WP | 0.0 (0.57) | 0.0 (0.57) | 1.72 (7.53) | 2.54 (9.16) | 3.57 (10.88) | 1.56 (5.74) a |
| Myclobutanil 10 WP | 12.34 (20.55) | 14.41 (22.30) | 18.51 (25.47) | 21.70 (27.75) | 24.13 (29.40) | 18.21 (25.09) c |
| Thiophanate methyl 70 WP | 0.0 (0.57) | 1.85 (7.81) | 2.68 (9.41) | 4.14 (11.73) | 5.83 (13.96) | 2.90 (8.69 )b |
| Hexaconazole 5 EC | 23.81 (29.19) | 28.34 (32.15) | 38.55 (38.36) | 47.37 (43.47) | 61.90 (51.86) | 39.99 (39.01) e |
| Difenoconazole 25 EC | 14.10 (22.04) | 17.26 (24.53) | 20.65 (27.01) | 24.20 (29.45) | 31.19 (33.93) | 21.48 (27.39) d |
| Mean | 10.05 (14.58) e | 12.37 (17.36) d | 16.42 (21.56) c | 19.99(24.31) b | 25.32 (28.01)a | |
| | S.E± | CD (p ≤ 0.05) |
| Fungitoxicant | (0.005) | (0.015) |
| Concentration | (0.005) | (0.015) |
| Fungitoxicant× concentration | (0.012) | (0.035) |
Table 9.
Impact of systemic fungitoxicants in casing on percent intensity of green mold disease.
Table 9.
Impact of systemic fungitoxicants in casing on percent intensity of green mold disease.
| Treatments | Concentration | Spring 2017 * | Spring 2018 * | Pool * | Disease Control |
|---|
| Carbendazim 50 WP | 0.025% | 7.21 (2.86) | 6.01 (2.64) | 6.61(2.75) f | 64.38 |
| 0.05% | 5.86 (2.61) | 4.17 (2.27) | 5.01 (2.44) d | 73.00 |
| 0.1% | 2.96 (1.98) | 1.63 (1.62) | 2.29 (1.80) b | 87.66 |
| Sub mean | 5.34 (2.48) | 3.93 (2.17) | 4.63 (2.33) | |
| Thiophanate methyl 70 WP | 0.025% | 8.65 (3.10) | 7.10 (2.84) | 7.87 (2.97) g | 57.59 |
| 0.05% | 7.08 (2.84) | 5.29 (2.50) | 6.18(2.67) de | 66.70 |
| 0.1% | 4.70 (2.38) | 2.69 (1.92) | 3.69 (2.15) c | 80.11 |
| Sub mean | 6.81 (2.77) | 5.02 (2.42) | 5.91 (2.59) | |
| Myclobutanil 10 WP | 0.025% | 10.90 (3.44) | 8.50 (3.08) | 9.70 (3.26) h | 47.73 |
| 0.05% | 7.92 (2.98) | 6.62 (2.76) | 7.27 (2.87) f | 60.82 |
| 0.1% | 4.62 (2.37) | 2.84 (1.95) | 3.73 (2.16) c | 79.90 |
| Sub mean | 7.81 (2.93) | 5.98 (2.59) | 6.90 (2.76) | |
| Check I (infested-untreated) | 18.16 (4.37) | 18.96(4.46) | 18.56(4.42) i | - |
| Check II (uninfested-untreated) | 0.0 (1.00) | 0.0 (1.00) | 0.0 (1.00) a | - |
| CD (p ≤ 0.05) |
| Treatment combination | 0.0061 | 0.0074 | 0.0046 | |
| Control v/s rest | 0.0056 | 0.0066 | 0.0042 | |
| Fungicides | 0.0198 | 0.0192 | 0.0195 | |
| Concentration | 0.0198 | 0.0192 | 0.0196 | |
| Fungicide × Concentration | 0.0344 | 0.0333 | 0.0338 | |
Table 10.
Effect of systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on the number of fruit bodies per kg mushroom, fruit bodies weight (g) and yield of button (kg/quintal compost).
Table 10.
Effect of systemic fungicides on T. harzianum infested casing pooled during spring 2017 and 2018 on the number of fruit bodies per kg mushroom, fruit bodies weight (g) and yield of button (kg/quintal compost).
| Treatments | Concentration | Number of Fruit Bodies per kg Mushroom | Fruit Bodies Weight (g) | Yield of Button kg/qt Compost |
|---|
| Carbendazim 50 WP | 0.025% | 97.83 h | 10.44 bc | 10.05 c |
| 0.05% | 95.58 f | 10.66 b | 11.10 b |
| 0.1% | 90.07 b | 11.21 a | 12.63 a |
| Sub mean | 94.49 | 10.77 | 11.26 |
| Thiophanate methyl 70 WP | 0.025% | 96.33 g | 10.67 b | 7.63 e |
| 0.05% | 92.43 d | 10.82 b | 9.12 d |
| 0.1% | 88.50 a | 11.26 a | 11.44 b |
| Sub mean | 92.42 | 10.91 | 9.39 |
| Myclobutanil 10 WP | 0.025% | 95.32 f | 10.33 d | 9.33 d |
| 0.05% | 93.47 e | 10.56 b | 11.08 b |
| 0.1% | 90.67 bc | 10.96 a | 12.87 a |
| Sub mean | 93.15 | 10.61 | 11.09 |
| Check I (infested-untreated) | 95.32 f | 10.33 d | 9.33 d |
| Check II (uninfested-untreated) | 93.47 e | 10.56 b | 11.08 b |
| CD(p ≤ 0.05) |
| Control v/s rest | 0.0638 | 0.0343 | 0.0338 |
| Fungicides | 0.0505 | 0.0201 | 0.0197 |
| Concentration | 0.0505 | 0.0201 | 0.0197 |
| Fungicide × Concentration | 0.0875 | 0.0349 | 0.0342 |
Table 11.
Impact of systemic fungitoxicants on T. harzianum-infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
Table 11.
Impact of systemic fungitoxicants on T. harzianum-infested casing pooled during spring 2017 and 2018 on quality parameters of A. bisporus.
| Fungicide | Concentration | Pileus Weight (g) * | Pileus Diameter (cm) * | Weight of Stipe (g) * | Diameter of Stipe (cm)* |
|---|
| Carbendazim 50 WP | 0.025% | 6.13 | 3.47 d | 4.77 ab | 1.32 |
| 0.05% | 6.25 | 3.39 d | 4.83 a | 1.37 |
| 0.1% | 6.47 | 3.73 b | 5.01 a | 1.41 |
| Sub mean | 6.28 | 3.53 | 4.87 | 1.36 |
| Thiophanate methyl 70WP | 0.25% | 5.47 | 3.45 d | 4.71 ab | 1.27 |
| 0.05% | 5.77 | 3.67 bc | 4.73 ab | 1.31 |
| 0.1% | 6.03 | 3.77 b | 4.87 a | 1.37 |
| Sub mean | 5.75 | 3.63 | 4.77 | 1.31 |
| Myclobutanil 10 WP | 0.025% | 5.70 | 3.66 bc | 4.73 ab | 1.23 |
| 0.05% | 5.91 | 3.67 bc | 4.83 a | 1.26 |
| 0.1% | 6.10 | 3.82 b | 4.91 a | 1.29 |
| Sub mean | 5.90 | 3.71 | 4.82 | 1.26 |
| Check I (infested-untreated) | 5.93 | 3.43 d | 4.37 d | 1.29 |
| Check II (uninfested-untreated) | 6.67 | 4.12 a | 4.69 c | 1.31 |
| CD (p ≤ 0.05) | | | | |
| Control v/s rest | 0.0338 | 0.0339 | 0.0338 | 0.0338 |
| Fungicides | 0.0198 | 0.0198 | 0.0199 | 0.0198 |
| Concentration | 0.0198 | 0.0198 | 0.0199 | 0.0198 |
| Fungicide × Concentration | 0.0343 | 0.0343 | 0.0344 | NS |