Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Growing Substrate
2.3. Inoculation, Incubation, Growth, and Processing of Fruiting Bodies
2.4. Polymorphism Analysis of Pleurotus ostreatus by CDDP Markers
2.5. Determination of Glucans
2.6. Statistical Analysis
3. Results and Discussion
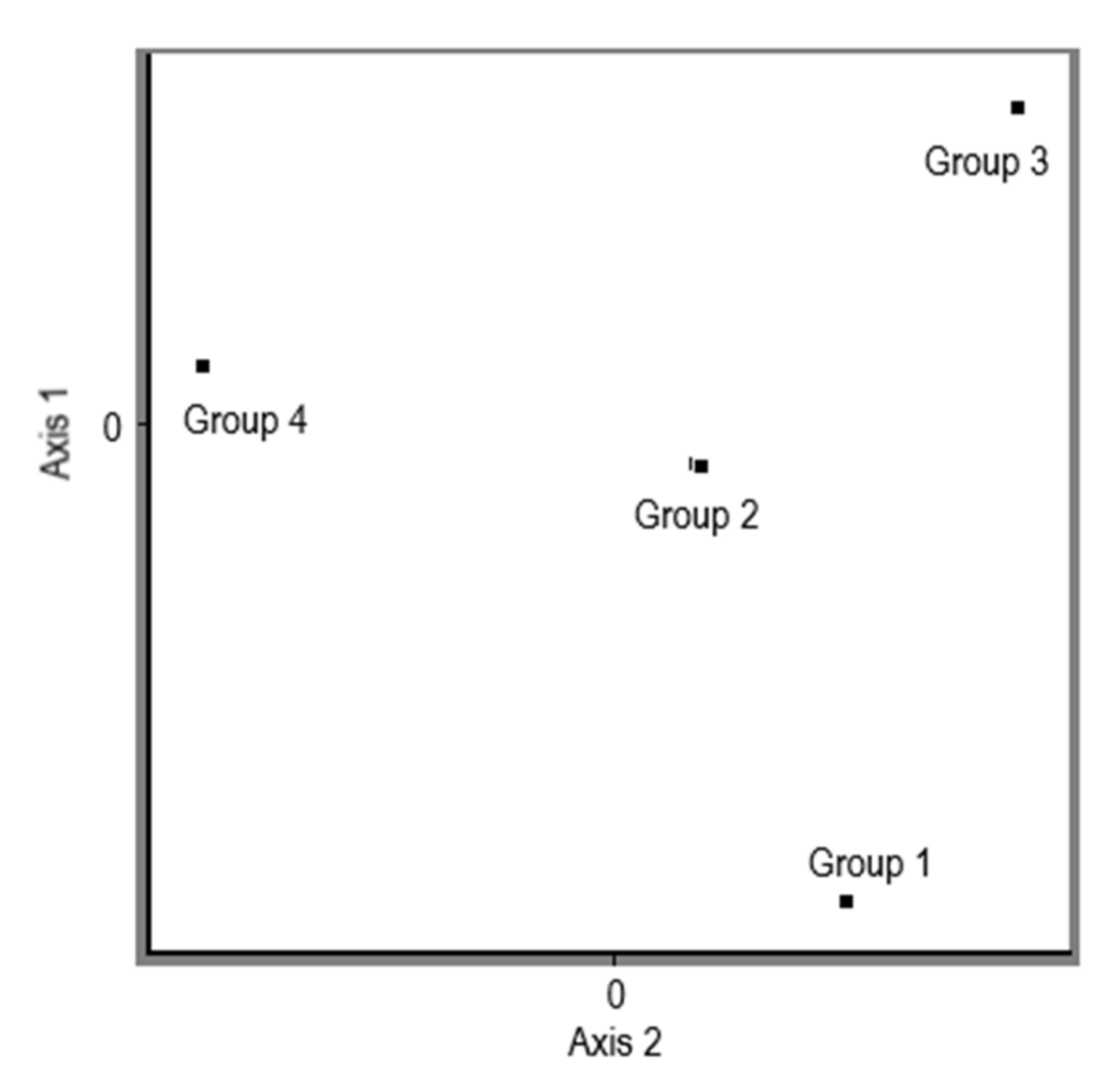
3.1. CDDP Fingerprinting of Pleurotus ostreatus
3.2. Concentration of Glucans in Production Strains of Oyster Mushroom (Pleurotus ostreatus)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bobade, H.; Gupta, A.; Sharma, S. Beta-Glucan. Nutraceuticals Health Care 2022, 343–358. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Pogue, R.; Simonassi-Paiva, B.; Abidin, I.I.Z.; Fehrenbach, G.W.; O’Neil, E.; Major, I.; Laffey, J.G.; Rowan, N. Immunomodulatory Activity of β-Glucan Polysaccharides Isolated from Different Species of Mushroom-A Potential Treatment for Inflammatory Lung Conditions. Sci. Total Environ. 2022, 809, 152177. [Google Scholar] [CrossRef]
- Sivieri, K.; de Oliveira, S.M.; de Souza Marquez, A.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-Glucan as a Prebiotic Coadjuvant in the Treatment of Diabetes Mellitus: A Review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Iqbal, H.M.N.; Mu, B.Z. Prospects of Microbial Polysaccharides-Based Hybrid Constructs for Biomimicking Applications. J. Basic Microbiol. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, N.; He, F.; Liu, Q.; Xu, X. Specific β-Glucans in Chain Conformations and Their Biological Functions. Polym. J. 2022, 54, 427–453. [Google Scholar] [CrossRef]
- Lee, D.; Park, S.D.; Jun, S.J.; Park, J.T.; Chang, P.S.; Yoo, S.H. Differentiated Structure of Synthetic Glycogen-like Particle by the Combined Action of Glycogen Branching Enzymes and Amylosucrase. Int. J. Biol. Macromol. 2022, 195, 152–162. [Google Scholar] [CrossRef]
- Da Silva Milhorini, S.; Simas, F.F.; Smiderle, F.R.; de Jesus, L.I.; Rosado, F.R.; Longoria, E.L.; Iacomini, M. β-Glucans from the Giant Mushroom Macrocybe Titans: Chemical Characterization and Rheological Properties. Food Hydrocoll. 2022, 125, 107392. [Google Scholar] [CrossRef]
- Hong, M.G.; Yoo, S.H.; Lee, B.H. Effect of Highly Branched α-Glucans Synthesized by Dual Glycosyltransferases on the Glucose Release Rate. Carbohydr. Polym. 2022, 278, 119016. [Google Scholar] [CrossRef]
- Giacomin, C.E.; Kim, K.; Wagner, N.J. Rheological Behavior for α-1,3-Glucan Derived from Enzymatic Polymerization of Sucrose. ACS Food Sci. Technol. 2022, 2, 240–248. [Google Scholar] [CrossRef]
- Bai, L.; Kim, J.; Son, K.-H.; Shin, D.-H.; Ku, B.-H.; Kim, D.Y.; Park, H.-Y. Novel Anti-Fungal D-Laminaripentaose-Releasing Endo-β-1,3-Glucanase with a RICIN-like Domain from Cellulosimicrobium Funkei HY-13. Biomolecules 2021, 11, 1080. [Google Scholar] [CrossRef]
- Adachi, Y.; Kanno, T.; Ishibashi, K.I.; Yamanaka, D.; Motoi, A.; Motoi, M.; Ohno, N. Binding Specificity of a New Artificial β-Glucan Recognition Protein and Its Application to β-Glucan Detection in Mushroom Extracts. Int. J. Med. Mushrooms 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.S.D.; Gómez-Delgado, L.; Curto, M.Á.; Moreno, M.B.; Pérez, P.; Ribas, J.C.; Cortés, J.C.G. Analysis and Application of a Suite of Recombinant Endo-β(1,3)-D-Glucanases for Studying Fungal Cell Walls. Microb. Cell Fact. 2021, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, C.; Nunes, J.; Dias, R.; Mendes, C.; de Andrade, G.; Bule, P. From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1,3-Glucanases. Int. J. Mol. Sci. 2022, 23, 3156. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwijk, H.P.; Bast, A.; de Boer, A. Immunomodulating Effects of Fungal Beta-Glucans: From Traditional Use to Medicine. Nutrients 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Itto-Nakama, K.; Ohnuki, S.; Yashiroda, Y.; Li, S.C.; Kimura, H.; Kawamura, Y.; Shimamoto, Y.; Tominaga, K.-I.; Yamanaka, D.; et al. Jerveratrum-Type Steroidal Alkaloids Inhibit β-1,6-Glucan Biosynthesis in Fungal Cell Walls. Microbiol. Spectr. 2022, 10, e00873-21. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.C.G.; Curto, M.Á.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The Fungal Cell Wall as a Target for the Development of New Antifungal Therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Teplyakova, T.V.; Shintyapina, A.B.; Korolenko, T.A. Effects of Medicinal Fungi-Derived β-Glucan on Tumor Progression. J. Fungi 2021, 7, 250. [Google Scholar] [CrossRef]
- Sadoughi, F.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B. Beta-Glucans Is a Potential Inhibitor of Ovarian Cancer: Based on Molecular and Biological Aspects. Curr. Pharm. Biotechnol. 2021, 22, 1142–1152. [Google Scholar] [CrossRef]
- Kremmyda, A.; MacNaughtan, W.; Arapoglou, D.; Eliopoulos, C.; Metafa, M.; Harding, S.E.; Israilides, C. Mushroom and Cereal β-D-Glucan Solid State NMR and FTIR Datasets. Data Brief 2022, 40, 107765. [Google Scholar] [CrossRef]
- Zhao, Y.; Jalili, S. Dextran, as a Biological Macromolecule for the Development of Bioactive Wound Dressing Materials: A Review of Recent Progress and Future Perspectives. Int. J. Biol. Macromol. 2022, 207, 666–682. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhao, J.; Wang, J.; Song, Q.; Zhao, C. The Phagocytic Receptors of β-Glucan. Int. J. Biol. Macromol. 2022, 205, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical Functions of Beta-Glucans in Human Nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dejean, G.; van Petegem, F.; Brumer, H. Distinct Protein Architectures Mediate Species-Specific Beta-Glucan Binding and Metabolism in the Human Gut Microbiota. J. Biol. Chem. 2021, 296, 100415. [Google Scholar] [CrossRef] [PubMed]
- Colaço, M.; Panão Costa, J.; Borges, O. Glucan Particles: Choosing the Appropriate Size to Use as a Vaccine Adjuvant. Methods Mol. Biol. 2022, 2412, 269–280. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Spacek, J.; Vocka, M.; Zavadova, E.; Konopasek, B.; Petruzelka, L. Immunomodulation with β-Glucan from Pleurotus ostreatus in Patients with Endocrine-Dependent Breast Cancer. Immunotherapy 2021, 14, 31–40. [Google Scholar] [CrossRef]
- Larraya, L.M.; Gúgúmer, G.; Rez, P.É.; Ritter, E.; Pisabarro, A.G.; Lucí, L.; Ramírez, L.; Ramírez, R. Genetic Linkage Map of the Edible Basidiomycete Pleurotus ostreatus. Appl. Environ. Microbiol. 2000, 66, 5290–5300. [Google Scholar] [CrossRef]
- Vieira, F.R.; Pereira, D.M.; Cristina, M.; de Andrade, N.; Paulo, S. Molecular Characterization of Pleurotus ostreatus Commercial Strains by Random Amplified Polymorphic DNA (RAPD). Afr. J. Agric. Res. 2013, 8, 3146–3150. [Google Scholar] [CrossRef]
- Familoni, T.V.; Ogidi, C.O.; Akinyele, B.J.; Onifade, A.K. Genetic Diversity, Microbiological Study and Composition of Soil Associated with Wild Pleurotus ostreatus from Different Locations in Ondo and Ekiti States, Nigeria. Chem. Biol. Technol. Agric. 2018, 5, 7. [Google Scholar] [CrossRef]
- Lin, P.; Yan, Z.-F.; Kook, M.; Li, C.-T.; Yi, T.-H. Genetic and Chemical Diversity of Edible Mushroom Pleurotus Species. BioMed Res. Int. 2022, 2022, 6068185. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Yin, Y.; Xu, F. Evaluation of Genetic Diversity of Chinese Pleurotus ostreatus Cultivars Using DNA Sequencing Technology. Ann. Microbiol. 2013, 63, 571–576. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Conserved DNA-Derived Polymorphism (CDDP): A Simple and Novel Method for Generating DNA Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 558. [Google Scholar] [CrossRef]
- Aouadi, M.; Guenni, K.; Abdallah, D.; Louati, M.; Chatti, K.; Baraket, G.; Salhi Hannachi, A. Conserved DNA-Derived Polymorphism, New Markers for Genetic Diversity Analysis of Tunisian Pistacia vera L. Physiol. Mol. Biol. Plants 2019, 25, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Saidi, A.; Hajkazemian, M.; Emami, S.N. Evaluation of Genetic Diversity in Gerbera Genotypes Revealed Using SCoT and CDDP Markers. Pol. J. Nat. Sci. 2020, 35, 21–34. [Google Scholar]
- Bilčíková, J.; Farkasová, S.; Žiarovská, J. Genetic Variability of Commercially Important Apple Varieties (Malus × Domestica Borkh.) Assessed by CDDP Markers. Acta Fytotech. Zootech. 2021, 2021, 21–26. [Google Scholar]
- Ahmed, A.A.; Qadir, S.A.; Tahir, A.-R.N. CDDP and ISSR Markers-Assisted Diversity and Structure Analysis in Iraqi Mazu (Quercus Infectoria Oliv.) Accessions. All Life 2022, 15, 247–261. [Google Scholar] [CrossRef]
- Golian, M.; Hegedűsová, A.; Mezeyová, I.; Chlebová, Z.; Hegedűs, O.; Urminská, D.; Vollmannová, A.; Chlebo, P. Accumulation of Selected Metal Elements in Fruiting Bodies of Oyster Mushroom. Foods 2022, 11, 76. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from Milligram Amounts of Fresh, Herbarium and Mummified Plant Tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Smith, J.S.C.; Chin, E.C.L.; Shu, H.; Smith, O.S.; Wall, S.J.; Senior, M.L.; Mitchell, S.E.; Kresovich, S.; Ziegle, J. An Evaluation of the Utility of SSR Loci as Molecular Markers in Maize (Zea Mays L.): Comparisons with Data from RFLPS and Pedigree. Theor. Appl. Genet. 1997, 95, 163–173. [Google Scholar] [CrossRef]
- Petrovicová, L.; Balážová, Ž.; Vivodík, M.; Gálová, Z. Detection Genetic Variability of Secale Cereale L. by Scot Markers. Potravin. Slovak J. Food Sci. 2017, 11, 197–202. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Zang, D. Analysis of Genetic Relationships in Rosa Rugosa Using Conserved DNA-Derived Polymorphism Markers. Biotechnol. Biotechnol. Equip. 2018, 32, 88–94. [Google Scholar] [CrossRef]
- Vivodík, M.; Balážová, Ž.; Gálová, Z. Genetic Divergence in Tunisian Castor Bean Genotypes Based on Trap Markers. Potravin. Slovak J. Food Sci. 2020, 14, 510–518. [Google Scholar] [CrossRef]
- Drisya Ravi, R.S.; Siril, E.A.; Nair, B.R. The Efficiency of Cytochrome P450 Gene-Based Markers in Accessing Genetic Variability of Drumstick (Moringa oleifera Lam.) Accessions. Mol. Biol. Rep. 2020, 47, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Hlozáková, T.K.; Gálová, Z.; Gregová, E.; Vivodík, M.; Balážová, Ž.; Miháliková, D. RAPD Analysis of the Genetic Polymorphism in European Wheat Genotypes. Potravinarstvo 2016, 10, 1–6. [Google Scholar] [CrossRef][Green Version]
- Žiarovská, J.; Ražná, K.; Labajová, M. Using of Inter Microsatellite Polymorphism to Evaluate Gamma-Irradiated Amaranth Mutants. Emir. J. Food Agric. 2013, 25, 673–681. [Google Scholar] [CrossRef]
- Labajová, M.; Žiarovská, J.; Ražná, K.; Ovesná, J.; Hricová, A. Using of AFLP to Evaluate Gamma-Irradiated Amaranth Mutants. Genetika 2013, 45, 825–835. [Google Scholar] [CrossRef]
- Doungous, O.; Kalendar, R.; Filippova, N.; Ngane, B.K. Utility of IPBS Retrotransposons Markers for Molecular Characterization of African Gnetum Species. Plant Biosyst. 2020, 154, 587–592. [Google Scholar] [CrossRef]
- Amiteye, S. Basic Concepts and Methodologies of DNA Marker Systems in Plant Molecular Breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef]
- Fonseca, G.G.; Gandra, A.E.; Sclowitz, F.L.; Correa, A.A.P.; Costa, A.V.J.; Levy, A.J. Oyster Mushrooms Species Differentiation Through Molecular Markers RAPD. Int. J. Plant Breed. Genet. 2007, 2, 13–18. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, C.S.; Yang, M.F.; Chen, S.; Soni, M.; Mahadevan, B. Subchronic Toxicity and Genotoxicity Studies of Hericium erinaceus β-Glucan Extract Preparation. Curr. Res. Toxicol. 2022, 3, 100068. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-Glucans from Edible and Medicinal Mushrooms: Characteristics, Physicochemical and Biological Activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Chlebová, Z.; Golian, M.; Chlebo, P.; Štefániková, J.; Hovaňáková, L.; Valková, V.; Tirpáková, M.; Fialková, V.; Ďúranová, J.; Adamec, S. Hodnotenie obsahu celkového glukánu vo vybraných vzorkách hlivy ustricovitej (Pleurotus ostreatus). In NUTRITION-HUMAN-HEALTH 2021. Peer-Reviewed Proceedings of Scientifica Papers of th Institute of Nutrition and Genomics FAFR SUA in Nitra; GaŽarová, M., Ed.; Slovenská Poľnohospodárska Univerzita v Nitre: Nitra, Slovakia, 2021; p. 318. ISBN 978-80-552-2412-1. [Google Scholar]
- Assemie, A.; Abaya, G. The Effect of Edible Mushroom on Health and Their Biochemistry. Int. J. Microbiol. 2022, 2022, 8744788. [Google Scholar] [CrossRef]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A.; et al. Immunomodulating Activity of Pleurotus Eryngii Mushrooms Following Their In Vitro Fermentation by Human Fecal Microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Mironczuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-Glucans from Fungi: Biological and Health-Promoting. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Nakayama, Y.; Tanaka, A.; Naito, K.; Konishi, M. Antitumor Activity of Orally Administered Maitake α-Glucan by Stimulating Antitumor Immune Response in Murine Tumor. PLoS ONE 2017, 12, e0173621. [Google Scholar] [CrossRef]
- Širić, I.; Falandysz, J. Contamination, Bioconcentration and Distribution of Mercury in Tricholoma spp. Mushrooms from Southern and Northern Regions of Europe. Chemosphere 2020, 251, 126614. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Jablonský, I.; Sluková, M.; Čopíková, J. Mushrooms of Genus Pleurotus as a Source of Dietary Fibres and Glucans for Food Supplements. Czech J. Food Sci. 2008, 26, 441–446. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of Beta-Glucan Contents in Commercially Cultivated and Wild Growing Mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Saetang, N.; Rattanapot, T.; Manmai, N.; Amornlerdpison, D.; Ramaraj, R.; Unpaprom, Y. Effect of Hot Water Extraction Process on Schizophyllan from Split Gill Mushroom. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Bandura, I.I.; Kulik, A.S.; Bisko, N.A.; Khareba, O.V.; Tsyz, O.M.; Khareba, V.V. Analysis of the Biological Efficiency and Quality Factors of Mushrooms of the Genus Pleurotus (Fr.) P.Kumm as a Model of Effective Cultivation of Lignicolous Fungi with High Functional Value. Plant Var. Stud. Prot. 2020, 16, 334–342. [Google Scholar] [CrossRef]
- Szabová, E.; Rohaľová, Ľ.; Hedvigy, M. Semi-Solid Fermentation of Pleurotus ostreatus. J. Microbiol. Biotechnol. Food Sci. 2013, 7, 1950–1958. [Google Scholar]
- Luque, J.M.R.; Rugolo, M.; Rajchenberg, M.; Barroetaveña, C. Assessment of Lignocellulosic Residues from Northern Patagonian Andes (Argentina) for Cultivation of Pleurotus ostreatus. Univ. Sci. 2021, 26, 159–177. [Google Scholar] [CrossRef]
- Ahmad Zakil, F.; Xuan, L.H.; Zaman, N.; Alan, N.I.; Salahutheen, N.A.A.; Sueb, M.S.M.; Isha, R. Growth Performance and Mineral Analysis of Pleurotus ostreatus from Various Agricultural Wastes Mixed with Rubber Tree Sawdust in Malaysia. Bioresour. Technol. Rep. 2022, 17, 100873. [Google Scholar] [CrossRef]
- Utami, C.P.; Susilawati, P.R.; Pamardining Utami, C. Rice Straw Addition as Sawdust Substitution in Oyster Mushroom (Pleurotus ostreatus) Planted Media. AIP Conf. Proc. 2017, 1868, 20053. [Google Scholar] [CrossRef]
- Ali, N.; Khairudin, H.; Mohamed, M.; Hassan, O. Cultivation of Pleurotus Ostreatus on Oil Palm Fronds Mixed with Rubber Tree Sawdust. Chem. Eng. Trans. 2018, 63, 547–552. [Google Scholar] [CrossRef]
- Ali, N.; Mohd Tabi, A.N.; Zakil, A.F.; Mohd Fauzai, W.N.F.; Hassan, O. Yield Performance and Biological Efficiency of Empty Fruit Bunch (EFB) and Palm Pressed Fibre (PPF) as Substrates for… Jurnal Teknologi Yield Performance and Biological Efficiency of Empty Fruit Bunch (EFB). J. Teknol. 2013, 64, 93–99. [Google Scholar]
- Ahmad Zakil, F.; Muhammad Hassan, K.H.; Mohd Sueb, M.S.; Isha, R. Growth and Yield of Pleurotus ostreatus Using Sugarcane Bagasse as an Alternative Substrate in Malaysia. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022021. [Google Scholar] [CrossRef]
- Rambey, R.; Sitepu, I.D.B.; Siregar, E.B.M. Productivity of Oyster Mushrooms (Pleurotus ostreatus) on Media Corncobs Mixed with Sawdust. IOP Conf. Ser. Earth Environ. Sci. 2019, 260, 012076. [Google Scholar] [CrossRef]
- Nisha; Chhatbar, A.; Chhatbar, H.; Arya, A. Biochemical Aspects and Cultivation of Medicinal Mushroom on Cellulosic Waste of Cotton and Paper. In Biology, Cultivation and Applications of Mushrooms; Springer: Singapore, 2022; pp. 629–652. [Google Scholar] [CrossRef]


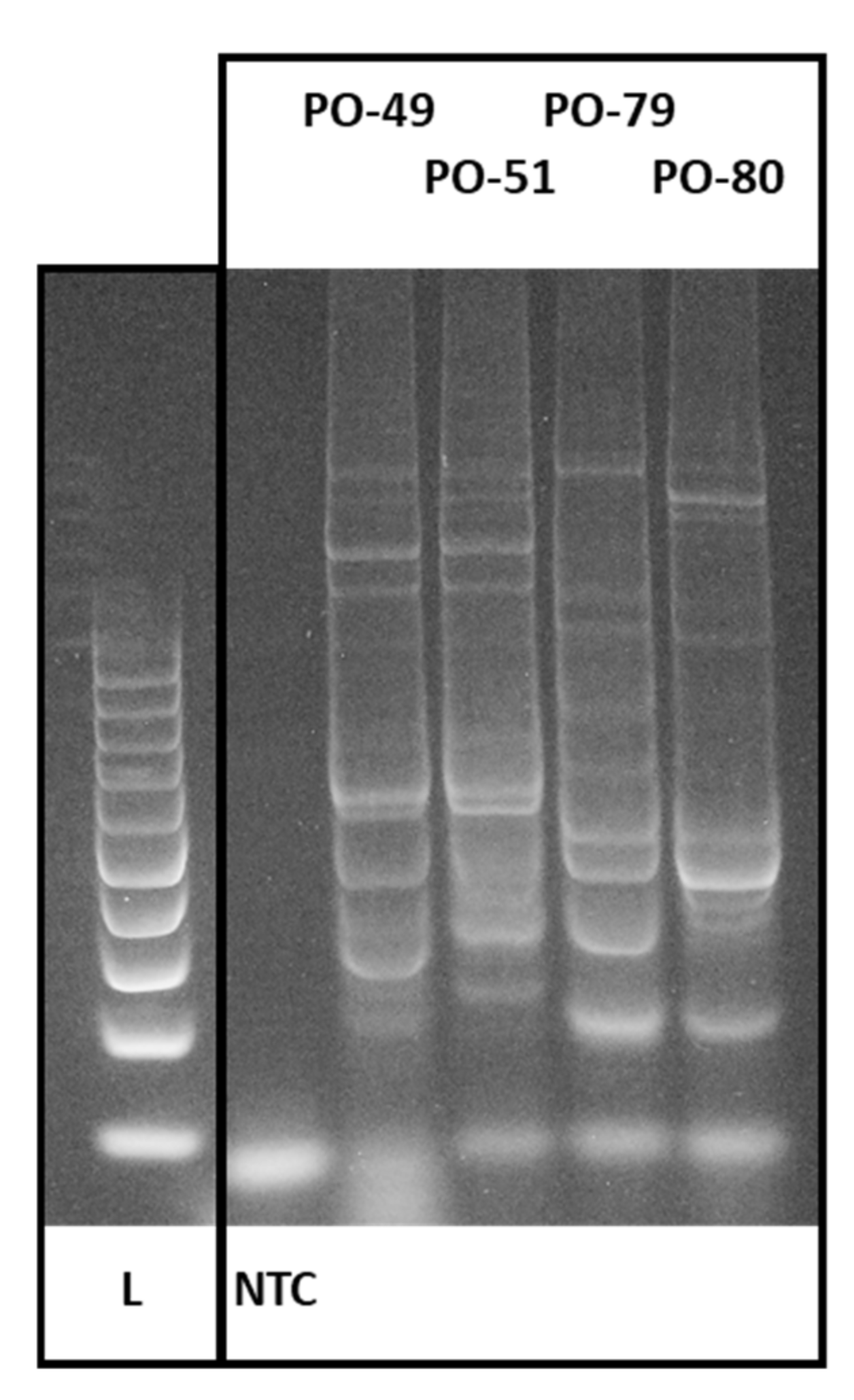


| Number | Designation in the Collection | Identification | Further Identification and Description |
|---|---|---|---|
| 1 | Pleurotus ostreatus HK35 | dr. Jablonský, Czech University of Life Sciences Prague, Czech Republic | |
| 2 | Pleurotus ostreatus Kryos B | dr. Jablonský, Czech University of Life Sciences Prague, Czech Republic | |
| 5 | Pleurotus ostreatus P-80 | Mr. Rajtár, Mycoforest Company, Slovakia, | |
| 8 | Pleurotus ostreatus | dr. Pavlík, Zvolen, spruce harvest, Slovakia | |
| 19 | Pleurotus ostreatus 2175 | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 20 | Pleurotus ostreatus CHINA BLACK | Mr. Rajtár, Mycoforest Company, Slovakia | |
| PL-28 | Pleurotus ostreatus PL-28 | commercial strain | |
| 28 | Pleurotus osteratus | isolate from the market, Slovakia | |
| 29 | Pleurotus osteratus | origin unknown | |
| 42 | Pleurotus osteratus MEY 2191 | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 43 | Pleurotus ostreatus GIZA | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 44 | Pleurotus ostreatus K12 | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 45 | Pleurotus ostreatus RH | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 46 | Pleurotus ostreatus K6 | Mr. Rajtár, Mycoforest Company, Slovakia | |
| 48 | Pleurotus osteratus | origin unknown | |
| 49 | Pleurotus osteratus | origin unknown | |
| 51 | CPPF-5001 | Pleurotus ostreatus | Crop Research Institute, Czech Republic |
| 53 | CPPF-5002 | Pleurotus ostreatus Pl2 | Crop Research Institute, Czech Republic |
| 54 | CPPF-5019 | Pleurotus ostreatus P-84 | Crop Research Institute, Czech Republic |
| 55 | CPPF-5022 | Pleurotus osteratus | Crop Research Institute, Czech Republic |
| 56 | CPPF-5075 | Pleurotus osteratus | Crop Research Institute, Czech Republic |
| 57 | CPPF-5117 | Pleurotus osteratus | Chna 4, Crop Research Institute, Czech Republic |
| 58 | CPPF-5141 | Pleurotus osteratus | PO-DD-1/1, Crop Research Institute, Czech Republic |
| 59 | CPPF-5142 | Pleurotus osteratus | PO-SV-1/1, Crop Research Institute, Czech Republic |
| 60 | CPPF-5143 | Pleurotus osteratus | PO-PH-1/1A, Crop Research Institute, Czech Republic |
| 61 * | CPPF-5144 | Pleurotus osteratus | PO-HOR-1/2, Crop Research Institute, Czech Republic |
| 62 | CPPF-5145 | Pleurotus osteratus | PO-HOR-2/4, Crop Research Institute, Czech Republic |
| 63 | CPPF-5146 | Pleurotus osteratus | PO-CB-1/2, Crop Research Institute, Czech Republic |
| 64 | CPPF-5147 | Pleurotus osteratus | PO-HD-1/1A, Crop Research Institute, Czech Republic |
| 65 | CPPF-5148 | Pleurotus osteratus | PO-HD-2/1, Crop Research Institute, Czech Republic |
| 66 | CPPF-5149 | Pleurotus osteratus | PO-LZ-1/1, Crop Research Institute, Czech Republic |
| 67 | CPPF-5150 | Pleurotus osteratus | PO-MV-1/1A, Crop Research Institute, Czech Republic |
| 68 | CPPF-5151 | Pleurotus osteratus | PO-SK-1, Crop Research Institute, Czech Republic |
| 69 * | CPPF-5153 | Pleurotus osteratus | PO-SK-3, Crop Research Institute, Czech Republic |
| 70 * | CPPF-5155 | Pleurotus osteratus | PO-SK-5, Crop Research Institute, Czech Republic |
| 71 * | CPPF-5156 | Pleurotus osteratus | PO-PSB, Crop Research Institute, Czech Republic |
| 72 * | CPPF-5173 | Pleurotus osteratus | Po-OH--JR1A, Crop Research Institute, Czech Republic |
| 73 | CPPF-5177 | Pleurotus osteratus | PO ŠMA, Crop Research Institute, Czech Republic |
| 74 | CPPF-5179 | Pleurotus osteratus | Crop Research Institute, Czech Republic |
| 75 | CPPF-5192 | Pleurotus osteratus | from Hlíva Huť, Crop Research Institute, Czech Republic |
| 76 | CEMM012 (VURV-M12) | Pleurotus osteratus | 210-ENV, dr. Havránek, 2009, Olomouc, Crop Research Institute, Czech Republic |
| 77 | CEMM013 (VURV-M13) | Pleurotus osteratus | 93-PLV, dr. Havranek 2008, Pohořany, Crop Research Institute, Czech Republic |
| 78 | CEMM118 (VURV-M118) | Pleurotus osteratus | PLM pl, dr. Petrželová 2016, PR Doubrava (from Mora-vičany-Bradlec), Crop Research Institute, Czech Republic |
| 79 | CEMM119 (VURV-M119) | Pleurotus osteratus | PLNZ sp1, dr. Petrželová, 2016, CHKO Litovelské Pomoraví (from Nové Zámky a Nový Dvůr), Crop Research Institute, Czech Republic |
| 80 | CEMM120 (VURV-M120) | Pleurotus osteratus | PLO sp, Dr. Egertová, Sochor 2015, Olomoučany, Crop Research Institute, Czech Republic |
| 81 | CEMM121 (VURV-M121) | Pleurotus osteratus | PLP pl, dr. Jurková, 2013, Pohořany, Crop Research Institute, Czech Republic |
| 82 | CCBAS 278 | Pleurotus osteratus | dr. Semerdžieva, 1993, Crop Research Institute, Czech Republic |
| 83 | CCBAS 459 | Pleurotus osteratus | G. Ritter 1956, Schierke, Harz mountains, Germany, Crop Research Institute, Czech Republic |
| 84 * | CCBAS 462 | Pleurotus osteratus | E. Jones 1966, England, Great Britain, Crop Research Institute, Czech Republic |
| 85 | CCBAS 472 | Pleurotus osteratus | W. Luthart 1959, České Budějovice, Crop Research Institute, Czech Republic |
| 86 | CCBAS 473 | Pleurotus osteratus | W. Luthart 1960, České Budějovice, Crop Research Institute, Czech Republic |
| 87 | CCBAS 474 | Pleurotus osteratus | dr. A. Torev, 1965, Plovdiv, Bulgaria, Crop Research Institute, Czech Republic |
| 88 | CCBAS 476 | Pleurotus osteratus | dr. A. Ginterová, 1973, Svatý Jur near Bratislava, Slovakia, Crop Research Institute, Czech Republic |
| 89 | CCBAS 684 | Pleurotus osteratus | dr. M. Semerdžieva, 1983, Gaštanica near Nitra, Slovakia, Crop Research Institute, Czech Republic |
| 90 | CCBAS 692 | Pleurotus osteratus | dr. I. Ohira, 1975, Shuzenzi-cho, Pref. Shizuoka, Japan, Crop Research Institute, Czech Republic |
| 91 | CCBAS 757 | Pleurotus osteratus | dr. M. Semerdžieva, 1987, near Trutnov, Crop Research Institute, Czech Republic |
| 92 | CCBAS 766 | Pleurotus osteratus | dr. M. Semerdžieva, 1985, Crop Research Institute, Czech Republic |
| 94 | Pleurotus osteratus | isolate from the market, Slovakia, 2019, SPOREA, origin Poland | |
| 95 | Pleurotus osteratus | isolate from the market, Slovakia, 2019, origin Slovakia | |
| 96 | Pleurotus osteratus | isolate from the market, Slovakia, 2019, České houby, Czech Republic | |
| 97 * | Pleurotus osteratus | origin Czech Republic | |
| 102 * | Pleurotus osteratus | isolate from the market, Slovakia, 2019, České houby, from ČR, Czech Republic |
| Locus Number | Lf | Ne | H | I |
|---|---|---|---|---|
| 1 | Allele 0: 0.4118 | 1.9396 | 0.4844 | 0.6775 |
| Allele 1: 0.5882 | ||||
| 2 | Allele 0: 0.5490 | 1.9810 | 0.4952 | 0.6883 |
| Allele 1: 0.4510 | ||||
| 3 | Allele 0: 0.5882 | 1.9396 | 0.4844 | 0.6775 |
| Allele 1: 0.4118 | ||||
| 4 | Allele 0: 0.4706 | 1.9931 | 0.4983 | 0.6914 |
| Allele 1: 0.5294 | ||||
| 5 | Allele 0: 0.4510 | 1.9810 | 0.4952 | 0.6883 |
| Allele 1: 0.5490 | ||||
| 6 | Allele 0: 0.4902 | 1.9992 | 0.4998 | 0.6930 |
| Allele 1: 0.5098 | ||||
| 7 | Allele 0: 0.0 | 1.0 | 0.0 | 0.0 |
| Allele 1: 1.0 | ||||
| 8 | Allele 0: 0.5490 | 1.9810 | 0.4952 | 0.6883 |
| Allele 1: 0.4510 | ||||
| 9 | Allele 0: 0.2353 | 1.5622 | 0.3599 | 0.5456 |
| Allele 1: 0.7647 | ||||
| 10 | Allele 0: 0.1176 | 1.2620 | 0.2076 | 0.3622 |
| Allele 1: 0.8824 | ||||
| 11 | Allele 0: 0.4314 | 1.9630 | 0.4906 | 0.6837 |
| Allele 1: 0.5686 | ||||
| 12 | Allele 0: 0.3922 | 1.9111 | 0.4767 | 0.6697 |
| Allele 1: 0.6078 |
| Production Strain of Pleurotus ostreatus | TG | AG | BG | Evaluation Considering β-Glucans Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | Cap | ±SD | Stipe | ±SD | Cap | ±SD | Stipe | ±SD | Cap | ±SD | Stipe | ±SD | average value | Concentration * |
| 43 | 40 | 3.3 | 58 | 4 | 1 | 0.49 | 2 | 0.38 | 39 | 3.8 | 56 | 3.9 | 48 | high |
| 44 | 42 * | 7.3 | 54 * | 7.5 | 0.9 | 0.088 | 0.6 | 0.061 | 41 | 7.2 | 54 | 7 | 48 | high |
| 74 | 37 | 3.1 | 58 | 2.5 | 1.9 | 0.11 | 2.9 | 0.49 | 35 | 1.1 | 55 | 2.6 | 45 | high |
| 81 | 39 | 3.6 | 58 | 6.2 | 3 | 0.53 | 2.8 | 0.063 | 36 | 7.3 | 55 | 9.5 | 46 | high |
| 61 | 42 | 3.7 | 53 | 5.8 | 1.7 | 0.2 | 1 | 0.083 | 40 | 5.1 | 52 | 5.7 | 46 | high |
| 67 | 42 | 2.9 | 57 | 4.8 | 2.4 | 0.51 | 5.1 | 0.75 | 39 | 5.3 | 52 | 4.2 | 46 | high |
| 79 | 42 | 6.4 | 52 | 8.1 | 1.6 | 0.35 | 1.6 | 0.36 | 41 | 8.2 | 51 | 9.4 | 46 | high |
| 97 | 46 | 6.2 | 50 | 6.3 | 0.7 | 0.11 | 1.6 | 0.18 | 45 | 6.3 | 48 | 7.8 | 47 | high |
| 8 | 37 | 2.9 | 54 | 5.6 | 1.2 | 0.27 | 1.8 | 0.31 | 36 | 2.6 | 52 | 7.7 | 44 | high |
| 96 (** 95) | 39 | 3.3 | 54 | 3.0 | 1.5 | 0.16 | 1.8 | 0.21 | 37 | 3.2 | 52 | 2 | 45 | high |
| 19 | 40 | 5.1 | 55 | 5.9 | 1.5 | 0.14 | 3.4 | 0.054 | 38 | 5 | 51 | 5.9 | 45 | high |
| 66 | n.d. | n.d. | 51 | 4.4 | n.d. | n.d. | 0.8 | 0.012 | n.d. | n.d. | 51 | 4.9 | n.d. | high |
| 88 | 36 | 7.4 | 52 | 7.4 | 0.5 | 0.012 | 0.6 | 0.067 | 36 | 7.2 | 51 | 12 | 44 | high |
| 92 | 38 | 2.5 | 52 | 3.9 | 0.5 | 0.028 | 0.5 | 0.059 | 38 | 2.5 | 51 | 3.9 | 45 | high |
| 28 | 40 | 6.5 | 52 | 5.9 | 1.5 | 0.24 | 3.1 | 0.47 | 38 | 6.1 | 49 | 6.2 | 44 | high |
| 69 | 40 | 3.3 | 56 | 4.3 | 0.7 | 0.065 | 6.8 | 0.65 | 39 | 3.2 | 49 | 4.3 | 44 | high |
| 83 | 42 | 4.4 | 51 | 6.9 | 1.2 | 0.24 | 1.4 | 0.11 | 41 | 5.3 | 49 | 5.9 | 45 | high |
| 29 | 42 | 4.9 | 50 | 3.2 | 1 | 0.22 | 1.4 | 0.17 | 41 | 4.2 | 48 | 4.2 | 45 | high |
| 90 | 40 | 2.3 | 49 | 3.1 | 0.8 | 0.11 | 1 | 0.14 | 39 | 2.8 | 48 | 3.2 | 44 | high |
| 46 | 40 | 7.7 | 48 | 7.3 | 1.4 | 0.17 | 1.2 | 0.13 | 39 | 4.6 | 47 | 7.6 | 43 | high |
| 51 | 46 * | 6.4 | 50 * | 5.4 | 2.8 | 0.37 | 3 | 0.33 | 43 | 4.3 | 47 | 5.2 | 45 | high |
| 59 | 41 | 3.2 | 48 | 4.7 | 1 | 0.17 | 1 | 0.14 | 40 | 2.9 | 47 | 4.1 | 44 | high |
| 60 | 42 | 5.4 | 50 | 5.5 | 1 | 0.19 | 3.1 | 0.12 | 41 | 3.6 | 47 | 4.9 | 44 | high |
| 75 | 46 | 4.9 | 50 | 5.1 | 3.1 | 0.47 | 4.4 | 0.34 | 43 | 5.5 | 46 | 5.7 | 45 | high |
| 76 | 44 | 4.7 | 47 | 4.2 | 2.7 | 0.46 | 2.2 | 0.42 | 42 | 1.7 | 44 | 7.5 | 43 | medium |
| 89 (** 45) | 44 | 6.2 | 45 | 6.6 | 1 | 0.28 | 1 | 0.26 | 43 | 4.1 | 44 | 7 | 44 | medium |
| 56 | 34 * | 2.7 | 56 * | 5.9 | 1.8 | 0.16 | 3.3 | 0.39 | 33 | 2.5 | 52 | 5.5 | 43 | high |
| 86 | 36 | 7.4 | 55 | 6.2 | 1.6 | 0.15 | 4 | 0.32 | 34 | 7.2 | 51 | 10 | 43 | high |
| 45 (** 89) | 36 | 3.3 | 53 | 3.5 | 1.5 | 0.22 | 4.2 | 0.33 | 35 | 4.2 | 49 | 3.9 | 42 | high |
| 58 | 37 | 4.6 | 50 | 5.1 | 1.5 | 0.23 | 1.7 | 0.21 | 35 | 5.1 | 49 | 5.7 | 42 | high |
| 62 | 38 | 2.7 | 51 | 3.4 | 2 | 0.29 | 2 | 0.34 | 36 | 3.6 | 49 | 2.2 | 43 | high |
| PL-28 | 37 | 7.5 | 50 | 5.3 | 1.1 | 0.32 | 0.7 | 0.077 | 36 | 3.4 | 49 | 6.4 | 43 | high |
| 102 | 35 | 6.2 | 51 | 5.6 | 1.9 | 0.42 | 3.3 | 0.36 | 33 | 4.1 | 48 | 5.7 | 41 | high |
| 57 | 37 | 4.2 | 52 | 5.4 | 1.7 | 0.34 | 3.8 | 0.55 | 35 | 5.3 | 48 | 7.7 | 42 | high |
| 80 | 35 | 2.3 | 51 | 6.5 | 1.6 | 0.31 | 2.7 | 0.19 | 33 | 1.8 | 48 | 6.5 | 41 | high |
| 94 | 34 | 4.6 | 50 | 4.9 | 1.7 | 0.13 | 2.2 | 0.19 | 32 | 3.3 | 48 | 5.5 | 40 | high |
| 73 | 38 | 3.6 | 51 | 4.9 | 2.4 | 0.56 | 4.2 | 0.41 | 36 | 3.6 | 47 | 4.2 | 42 | high |
| 82 | 38 | 5.8 | 49 | 6.6 | 1.3 | 0.41 | 2.3 | 0.25 | 37 | 5.6 | 47 | 6.8 | 42 | high |
| 65 | 38 | 6.3 | 48 | 5.7 | 1.4 | 0.37 | 2.2 | 0.31 | 37 | 3.5 | 46 | 5.2 | 42 | high |
| 85 | 38 | 5.9 | 49 | 5.5 | 2.1 | 0.68 | 3.6 | 0.28 | 36 | 5.2 | 46 | 5.6 | 41 | high |
| 91 | 39 | 3.0 | 46 | 3.3 | 0.8 | 0.11 | 0.9 | 0.012 | 38 | 4.2 | 46 | 5.1 | 42 | high |
| 95 (** 96) | 37 | 7 | 47 | 6.7 | 1.3 | 0.22 | 1.5 | 0.16 | 36 | 4.3 | 46 | 6.0 | 41 | high |
| 64 | 34 | 0.93 | 46 | 2.4 | 0.6 | 0.027 | 0.7 | 0.035 | 34 | 0.9 | 45 | 5.3 | 40 | high |
| 42 | 41 | 5.8 | 46 | 3.6 | 1.6 | 0.23 | 2 | 0.11 | 39 | 4.5 | 44 | 3.6 | 42 | medium |
| 78 | 35 | 4.6 | 45 | 5.2 | 0.6 | 0.074 | 1 | 0.19 | 35 | 5.4 | 44 | 4.1 | 40 | medium |
| 84 | 40 | 7.7 | 44 | 6.8 | 0.4 | 0.037 | 0.3 | 0.022 | 40 | 7.7 | 44 | 6.8 | 42 | medium |
| 70 | 37 | 5.4 | 42 | 6.5 | 0.7 | 0.078 | 0.5 | 0.083 | 37 | 5.3 | 41 | 5.4 | 39 | medium |
| 68 | 42 | 2.5 | 42 | 3.4 | 0.5 | 0.087 | 1.5 | 0.14 | 42 | 3.8 | 40 | 3.8 | 41 | medium |
| 1 | 27 * | 1.2 | 55 * | 7.1 | 1.3 | 0.067 | 3.4 | 0.47 | 25 | 1.2 | 52 | 7 | 39 | high |
| 5 | 30 * | 2.7 | 51 * | 6.4 | 0.7 | 0.062 | 2.2 | 0.34 | 29 | 1.9 | 48 | 6.7 | 39 | high |
| 55 | 32 * | 3.6 | 51 * | 4.6 | 1.3 | 0.11 | 3.8 | 0.29 | 31 | 3.4 | 47 | 6.1 | 39 | high |
| 87 | 34 | 4.4 | 48 | 5.2 | 1.5 | 0.14 | 3.4 | 0.52 | 33 | 1.7 | 45 | 5.2 | 39 | medium |
| 20 | 29 * | 2.9 | 51 * | 4.6 | 1 | 0.16 | 3.1 | 0.15 | 28 | 3.3 | 48 | 4.7 | 38 | high |
| 48 | 31 * | 2.7 | 50 * | 7.9 | 1 | 0.34 | 2.7 | 0.37 | 30 | 1.9 | 47 | 7.3 | 39 | high |
| 54 | 33 | 5.9 | 47 | 4.9 | 1.1 | 0.061 | 2.5 | 0.55 | 31 | 3.4 | 44 | 5.3 | 38 | medium |
| 71 | 35 | 4.6 | 42 | 5.8 | 0.6 | 0.13 | 0.7 | 0.036 | 35 | 4.5 | 41 | 5.5 | 38 | medium |
| 77 | 36 | 3.4 | 43 | 3.9 | 1.9 | 0.34 | 1.9 | 0.39 | 34 | 3.2 | 41 | 3.8 | 38 | medium |
| 49 | 29 | 4.8 | 46 | 4.1 | 0.6 | 0.062 | 2.1 | 0.34 | 28 | 4.8 | 44 | 3.9 | 36 | medium |
| 2 | 24 * | 1.9 | 47 * | 3.8 | 1.2 | 0.11 | 2.8 | 0.29 | 23 | 2.1 | 44 | 4.2 | 34 | medium |
| 72 | 38 | 6.2 | 24 | 5.6 | 1 | 0.19 | 1.1 | 0.11 | 37 | 2.1 | 22 | 2.8 | 30 | low |
| Total Glucans (%) | α-Glucans (%) | β-Glucans (%) | |||||
|---|---|---|---|---|---|---|---|
| Variant | n | Average | ±SD | Average | ±SD | Average | ±SD |
| Cap | 59 | 38 a | 4.6 | 1.4 a | 0.63 | 36 a | 4.4 |
| Stipe | 60 | 50 b | 5.2 | 2.2 b | 1.3 | 48 b | 4.8 |
| Level | Content of β-Glucans in % |
|---|---|
| Low | from 22 to 33 |
| Medium | from 34 to 45 |
| High | from 46 to 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golian, M.; Chlebová, Z.; Žiarovská, J.; Benzová, L.; Urbanová, L.; Hovaňáková, L.; Chlebo, P.; Urminská, D. Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers. J. Fungi 2022, 8, 563. https://doi.org/10.3390/jof8060563
Golian M, Chlebová Z, Žiarovská J, Benzová L, Urbanová L, Hovaňáková L, Chlebo P, Urminská D. Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers. Journal of Fungi. 2022; 8(6):563. https://doi.org/10.3390/jof8060563
Chicago/Turabian StyleGolian, Marcel, Zuzana Chlebová, Jana Žiarovská, Lenka Benzová, Lucia Urbanová, Lucia Hovaňáková, Peter Chlebo, and Dana Urminská. 2022. "Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers" Journal of Fungi 8, no. 6: 563. https://doi.org/10.3390/jof8060563
APA StyleGolian, M., Chlebová, Z., Žiarovská, J., Benzová, L., Urbanová, L., Hovaňáková, L., Chlebo, P., & Urminská, D. (2022). Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers. Journal of Fungi, 8(6), 563. https://doi.org/10.3390/jof8060563






