Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. albicans Strains and Growth Conditions
2.2. Biofilm Formation
2.3. Photoinactivation of C. albicans
2.4. Cell Labeling and Confocal Microscopy
2.5. Scanning Electron Microscopy
2.6. Cytotoxicity on Mammalian Cells
2.7. Statistical Analysis
3. Results
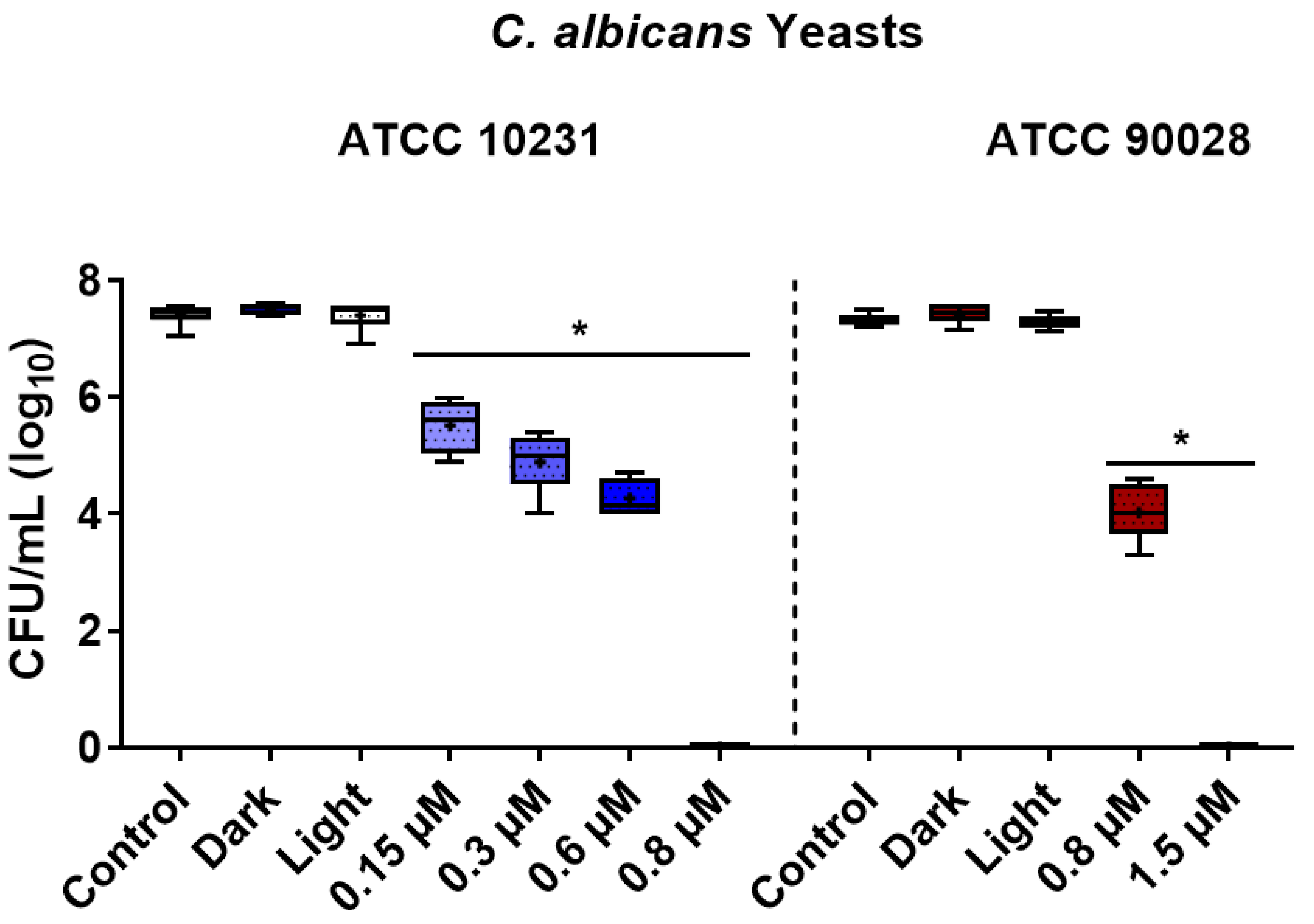
3.1. Photoinactivation Effect on Yeast Cells
3.2. Photoinactivation Effect on Biofilms
3.3. Cytotoxicity on Mammalian Cells
4. Discussion
4.1. Photoinactivation Effect on Yeast Cells
4.2. Photoinactivation Effect on Biofilms
4.3. Cytotoxicity on Mammalian Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51 (Suppl. 4), 2–15. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [Green Version]
- Hurley, R.; De Louvois, J. Candida Vaginitis. Postgrad. Med. J. 1979, 55, 645–647. [Google Scholar] [CrossRef] [Green Version]
- Sobel, J.D. Vulvovaginal Candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Souza, T.H.S.; Sarmento-Neto, J.F.; Souza, S.O.; Raposo, B.L.; Silva, B.P.; Borges, C.P.F.; Santos, B.S.; Cabral Filho, P.E.; Rebouças, J.S.; Fontes, A. Advances on Antimicrobial Photodynamic Inactivation Mediated by Zn(II) Porphyrins. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100454. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy–What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Al-Mutairi, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Sublethal Photodynamic Treatment Does Not Lead to Development of Resistance. Front. Microbiol. 2018, 9, 1699. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Rojz, J.C.C.; Cotomacio, C.C.; Caran, E.M.M.; Chen, M.J.; Figueiredo, M.L.S. Photodynamic Therapy to Control Oral Candidiasis in a Pediatric Patient Undergoing Head and Neck Radiotherapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102627. [Google Scholar] [CrossRef]
- Suzuki, L.C.; Kato, I.T.; Prates, R.A.; Sabino, C.P.; Yoshimura, T.M.; Silva, T.O.; Ribeiro, M.S. Glucose Modulates Antimicrobial Photodynamic Inactivation of Candida albicans in Biofilms. Photodiagnosis Photodyn. Ther. 2017, 17, 173–179. [Google Scholar] [CrossRef]
- Ma, J.; Shi, H.; Sun, H.; Li, J.; Bai, Y. Antifungal Effect of Photodynamic Therapy Mediated by Curcumin on Candida albicans Biofilms in Vitro. Photodiagnosis Photodyn. Ther. 2019, 27, 280–287. [Google Scholar] [CrossRef]
- Davies, A.; Gebremedhin, S.; Yee, M.; Padilla, R.J.; Duzgunes, N.; Konopka, K.; Dorocka-Bobkowska, B. Cationic Porphyrin-Mediated Photodynamic Inactivation of Candida Biofilms and the Effect of Miconazole. J. Physiol. Pharmacol. 2016, 67, 777–783. [Google Scholar]
- Chabrier-Roselló, Y.; Foster, T.H.; Pérez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida albicans Germ Tubes and Biofilms to Photofrin-Mediated Phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [Green Version]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic Inactivation of Candida albicans Sensitized by Tri- and Tetra-Cationic Porphyrin Derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic Therapy in the Treatment of Microbial Infections: Basic Principles and Perspective Applications. Lasers. Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Souza, T.H.S.; Andrade, C.G.; Cabral, F.V.; Sarmento-Neto, J.F.; Rebouças, J.S.; Santos, B.S.; Ribeiro, M.S.; Figueiredo, R.C.B.Q.; Fontes, A. Efficient Photodynamic Inactivation of Leishmania Parasites Mediated by Lipophilic Water-Soluble Zn(II) Porphyrin ZnTnHex-2-PyP4+. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129897. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, M.; Zhang, J.; Zhu, S.; Shi, M.; Wang, K. Metalloporphyrin–Indomethacin Conjugates as New Photosensitizers for Photodynamic Therapy. JBIC Biol. Inorg. Chem. 2019, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Marydasan, B.; Nair, A.K.; Ramaiah, D. Optimization of Triplet Excited State and Singlet Oxygen Quantum Yields of Picolylamine–Porphyrin Conjugates through Zinc Insertion. J. Phys. Chem. B 2013, 117, 13515–13522. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Pucelik, B.; Pereira, M.M.; Arnaut, L.G.; Stochel, G. Towards Tuning PDT Relevant Photosensitizer Properties: Comparative Study for the Free and Zn2+ Coordinated meso-tetrakis[2,6-difluoro-5-(N-methylsulfamylo)phenyl]Porphyrin. J. Coord. Chem. 2015, 68, 3116–3134. [Google Scholar] [CrossRef]
- Moghnie, S.; Tovmasyan, A.; Craik, J.; Batinic-Haberle, I.; Benov, L. Cationic Amphiphilic Zn-Porphyrin with High Antifungal Photodynamic Potency. Photochem. Photobiol. Sci. 2017, 16, 1709–1716. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Alenezi, K.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Optimizing Zn Porphyrin-Based Photosensitizers for Efficient Antibacterial Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2017, 17, 154–159. [Google Scholar] [CrossRef]
- Andrade, C.G.; Figueiredo, R.C.B.Q.; Ribeiro, K.R.C.; Souza, L.I.O.; Sarmento-Neto, J.F.; Rebouças, J.S.; Santos, B.S.; Ribeiro, M.S.; Carvalho, L.B.; Fontes, A. Photodynamic Effect of Zinc Porphyrin on the Promastigote and Amastigote Forms of Leishmania braziliensis. Photochem. Photobiol. Sci. 2018, 17, 482–490. [Google Scholar] [CrossRef]
- Viana, O.S.; Ribeiro, M.S.; Rodas, A.C.D.; Rebouças, J.S.; Fontes, A.; Santos, B.S. Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules 2015, 20, 8893–8912. [Google Scholar] [CrossRef] [Green Version]
- Ezzeddine, R.; Al-Banaw, A.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Effect of Molecular Characteristics on Cellular Uptake, Subcellular Localization, and Phototoxicity of Zn(II) N-Alkylpyridylporphyrins*. J. Biol. Chem. 2013, 288, 36579–36588. [Google Scholar] [CrossRef] [Green Version]
- Odeh, A.M.; Craik, J.D.; Ezzeddine, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Targeting Mitochondria by Zn(II)N-Alkylpyridylporphyrins: The Impact of Compound Sub-Mitochondrial Partition on Cell Respiration and Overall Photodynamic Efficacy. PLoS ONE 2014, 9, e108238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jett, B.D.; Hatter, K.L.; Huycke, M.M.; Gilmore, M.S. Simplified Agar Plate Method for Quantifying Viable Bacteria. Biotechniques 1997, 23, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Aliança, A.S.; Anjos, K.F.; De Vasconcelos Reis, T.N.; Higino, T.M.; Brelaz-de-Castro, M.C.; Bianco, É.M.; De Figueiredo, R.C. The in Vitro Biological Activity of the Brazilian Brown Seaweed Dictyota Mertensii against Leishmania amazonensis. Molecules 2014, 19, 14052–14065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, M.M.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Important Cellular Targets for Antimicrobial Photodynamic Therapy. Appl. Microbiol. Biotechnol. 2016, 100, 7679–7688. [Google Scholar] [CrossRef]
- Costa, A.R.; Rodrigues, M.E.; Silva, F.; Henriques, M.; Azeredo, J.; Faustino, A.; Oliveira, R. MIC Evaluation of Candida Reference Strains and Clinical Isolates by E-Test. J. Chemother. 2009, 21, 351–355. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of Clinical Isolates of Candida to Photodynamic Effects of Curcumin. Lasers. Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; Carvalho, M.T.; Mima, E.G.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic Inactivation of Clinical Isolates of Candida Using Photodithazine®. Biofouling 2013, 29, 1057–1067. [Google Scholar] [CrossRef]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design Features for Optimization of Tetrapyrrole Macrocycles as Antimicrobial and Anticancer Photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Guo, J.; Zhuang, J.J.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. Highly Positive-Charged Zinc(II) Phthalocyanine as Non-Aggregated and Efficient Antifungal Photosensitizer. Bioorg. Med. Chem. Lett. 2015, 25, 2386–2389. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.-Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive Cationic Functionalization of Chlorin Derivatives for Antimicrobial Photodynamic Inactivation and Related Vancomycin Conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Ozturk, I.; Tunçel, A.; Yurt, F.; Biyiklioglu, Z.; Ince, M.; Ocakoglu, K. Antifungal Photodynamic Activities of Phthalocyanine Derivatives on Candida albicans. Photodiagnosis Photodyn. Ther. 2020, 30, 101715. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell Wall Architecture in Yeast: New Structure and New Challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K. Photochemistry of Water-Soluble Porphyrins: Comparative Study of Isomeric Tetrapyridyl- and Tetrakis(N-methylpyridiniumyl)Porphyrins. Inorg. Chem. 1984, 23, 2453–2459. [Google Scholar] [CrossRef]
- Espitia-Almeida, F.; Díaz-Uribe, C.; Vallejo, W.; Gómez-Camargo, D.; Romero Bohórquez, A.R. In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania braziliensis and Leishmania panamensis Promastigotes Properties. Molecules 2020, 25, 1887. [Google Scholar] [CrossRef] [Green Version]
- Mang, T.S.; Mikulski, L.; Hall, R.E. Photodynamic Inactivation of Normal and Antifungal Resistant Candida Species. Photodiagnosis Photodyn. Ther. 2010, 7, 98–105. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; de Oliveira Mima, E.G.; Giampaolo, E.T.; Vergani, C.E.; Bagnato, V.S. Fungicidal Effect of Photodynamic Therapy against Fluconazole-Resistant Candida albicans and Candida glabrata. Mycoses 2011, 54, 123–130. [Google Scholar] [CrossRef]
- Azizi, A.; Amirzadeh, Z.; Rezai, M.; Lawaf, S.; Rahimi, A. Effect of Photodynamic Therapy with Two Photosensitizers on Candida albicans. J. Photochem. Photobiol. B Biol. 2016, 158, 267–273. [Google Scholar] [CrossRef]
- Sakita, K.M.; Conrado, P.C.V.; Faria, D.R.; Arita, G.S.; Capoci, I.R.G.; Rodrigues-Vendramini, F.A.V.; Pieralisi, N.; Cesar, G.B.; Gonçalves, R.S.; Caetano, W.; et al. Copolymeric Micelles as Efficient Inert Nanocarrier for Hypericin in the Photodynamic Inactivation of Candida Species. Future Microbiol. 2019, 14, 519–531. [Google Scholar] [CrossRef]
- Valkov, A.; Zinigrad, M.; Nisnevitch, M. Photodynamic Eradication of Trichophyton rubrum and Candida albicans. Pathogens 2021, 10, 263. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wu, S.; Gu, Y. In Vitro Sensitivity of Candida spp. to Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Inactivation. In Proceedings of the SPIE 9268, Optics in Health Care and Biomedical Optics VI, Beijing, China, 18 November 2014; Volume 9268. [Google Scholar] [CrossRef]
- Romano, R.A.; Pratavieira, S.; da Silva, A.P.; Kurachi, C.; Guimarães, F.E.G. Light-Driven Photosensitizer Uptake Increases Candida albicans Photodynamic Inactivation. J. Biophotonics 2017, 10, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.C.A.; Corrêa, T.Q.; Pratavieira, S.; Uliana, M.P.; de Oliveira, K.T.; Bagnato, V.S.; de Souza, C.W.O. Photodynamic Inactivation of Candida albicans Using a Synthesized Bacteriochlorin as a Photosensitizer. In Proceedings of the SPIE 11070, 17th International Photodynamic Association World Congress, Cambridge, MA, USA, 7 August 2019; Volume 11070. [Google Scholar] [CrossRef]
- Garcia, B.A.; Panariello, B.H.D.; de Freitas-Pontes, K.M.; Duarte, S. Candida Biofilm Matrix as a Resistance Mechanism against Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102525. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.P.; da Silva, F.C.; Viana, M.S.; Meira, G.A. In Vitro Effectiveness of 455-nm Blue LED to Reduce the Load of Staphylococcus aureus and Candida albicans Biofilms in Compact Bone Tissue. Lasers Med. Sci. 2016, 31, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm Visible Light for the Elimination of Candida albicans in Vitro and in Vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, P.; Singh, G.; Nobile, C.J. Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms. Microorganisms 2021, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Imamura, T.; Tatehara, S.; Ide, S.; Wakabayashi, N.; Satomura, K. Microbicidal Effect of 405-nm Blue LED Light on Candida albicans and Streptococcus mutans Dual-Species Biofilms on Denture Base Resin. Lasers Med. Sci. 2022, 37, 857–866. [Google Scholar] [CrossRef]
- Jackson, Z.; Meghji, S.; MacRobert, A.; Henderson, B.; Wilson, M. Killing of the Yeast and Hyphal Forms of Candida albicans Using a Light-Activated Antimicrobial Agent. Lasers Med. Sci. 1999, 14, 150–157. [Google Scholar] [CrossRef]
- Manavathu, M.; Manavathu, E.; Gunasekaran, S.; Porte, Q.; Gunasekaran, M. Changes in Glutathione Metabolic Enzymes during Yeast-to-Mycelium Conversion of Candida albicans. Can. J. Microbiol. 1996, 42, 76–79. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Zhang, H.; Zhang, J.; Sun, H. Effect of 5-Aminolevulinic Acid Photodynamic Therapy on Candida albicans Biofilms: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2016, 15, 40–45. [Google Scholar] [CrossRef]
- de Carvalho Leonel, L.; Carvalho, M.L.; da Silva, B.M.; Zamuner, S.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT) Using Methylene Blue Inhibits the Viability of the Biofilm Produced by Candida albicans. Photodiagnosis Photodyn. Ther. 2019, 26, 316–323. [Google Scholar] [CrossRef]
- Huang, M.-C.; Shen, M.; Huang, Y.-J.; Lin, H.-C.; Chen, C.-T. Photodynamic Inactivation Potentiates the Susceptibility of Antifungal Agents against the Planktonic and Biofilm Cells of Candida albicans. Int. J. Mol. Sci. 2018, 19, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.C.B.P.; de Campos Rasteiro, V.M.; Pereira, C.A.; da Silva Hashimoto, E.S.H.; Beltrame, M.; Junqueira, J.C.; Jorge, A.O.C. Susceptibility of Candida albicans and Candida dubliniensis to Erythrosine- and LED-Mediated Photodynamic Therapy. Arch. Oral Biol. 2011, 56, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.P.; Yip, H.K.; Samaranayake, L.P. The Use of New Probes and Stains for Improved Assessment of Cell Viability and Extracellular Polymeric Substances in Candida albicans Biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Dižová, S.; Bujdáková, H. Employment of Methylene Blue Irradiated with Laser Light Source in Photodynamic Inactivation of Biofilm Formed by Candida albicans Strain Resistant to Fluconazole. Med. Mycol. 2017, 55, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Yan, G.; Gong, R.; Zhang, L.; Liu, T.; Feng, C.; Du, W.; Wang, Y.; Yang, F.; Li, Y.; et al. Effects of Blue Light Emitting Diode Irradiation on the Proliferation, Apoptosis and Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2017, 43, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.-X.; Zhou, W.-C.; Zhu, X.-G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxid. Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; et al. Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. J. Fungi 2022, 8, 556. https://doi.org/10.3390/jof8060556
Souza SO, Raposo BL, Sarmento-Neto JF, Rebouças JS, Macêdo DPC, Figueiredo RCBQ, Santos BS, Freitas AZ, Cabral Filho PE, Ribeiro MS, et al. Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. Journal of Fungi. 2022; 8(6):556. https://doi.org/10.3390/jof8060556
Chicago/Turabian StyleSouza, Sueden O., Bruno L. Raposo, José F. Sarmento-Neto, Júlio S. Rebouças, Danielle P. C. Macêdo, Regina C. B. Q. Figueiredo, Beate S. Santos, Anderson Z. Freitas, Paulo E. Cabral Filho, Martha S. Ribeiro, and et al. 2022. "Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin" Journal of Fungi 8, no. 6: 556. https://doi.org/10.3390/jof8060556
APA StyleSouza, S. O., Raposo, B. L., Sarmento-Neto, J. F., Rebouças, J. S., Macêdo, D. P. C., Figueiredo, R. C. B. Q., Santos, B. S., Freitas, A. Z., Cabral Filho, P. E., Ribeiro, M. S., & Fontes, A. (2022). Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. Journal of Fungi, 8(6), 556. https://doi.org/10.3390/jof8060556





