Elicitor-Induced Metabolomics Analysis of Halodule pinifolia Suspension Culture for an Alternative Antifungal Screening Approach against Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Surface Sterilization of Explant
2.3. Callus Induction and Suspension-Cultured Cells (SCC) of H. pinifolia
2.4. Preparation of MeJA
2.5. Extraction of H. pinifolia SCC and Derivatization
2.6. GC-MS Based Metabolomics Analysis of H. pinifolia SCC
2.7. HPLC Chromatographic Separation and Structural Elucidation of SCC Metabolites
2.8. Alternative Screening Approach for Growth Inhibition of C. albicans in YNB Broth
2.9. Isocitrate Lyase (ICL) Enzyme Assay and Inhibition Studies
2.10. Minimum Inhibitory Concentration of Identified Lead Compounds
2.11. Drug Likeliness and ADMET Properties of RA
2.12. Statistical Analysis
3. Results
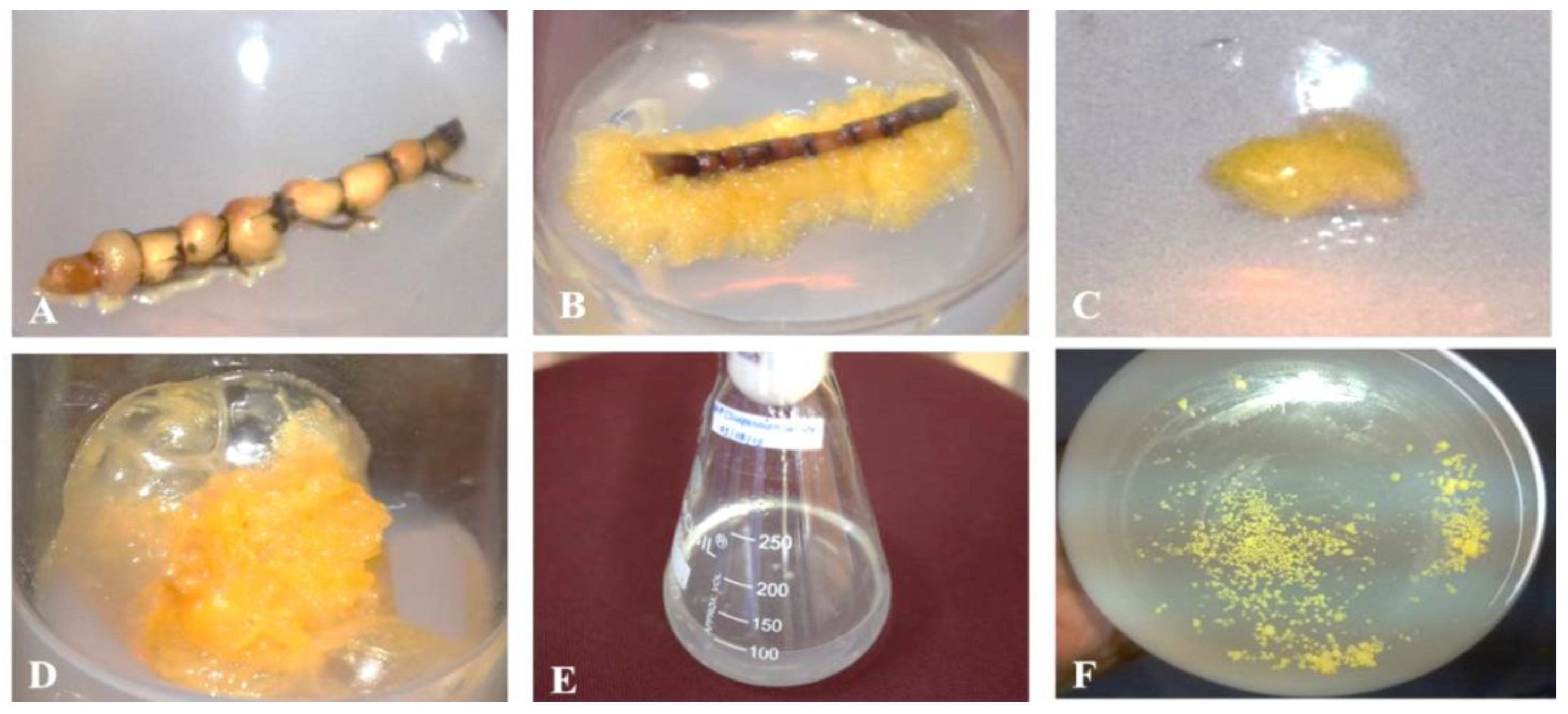
3.1. Callus Induction and Suspension-Cultured Cells of H. pinifolia
3.2. Influence of MeJA Concentration on Rosmarinic Acid Biosynthesis
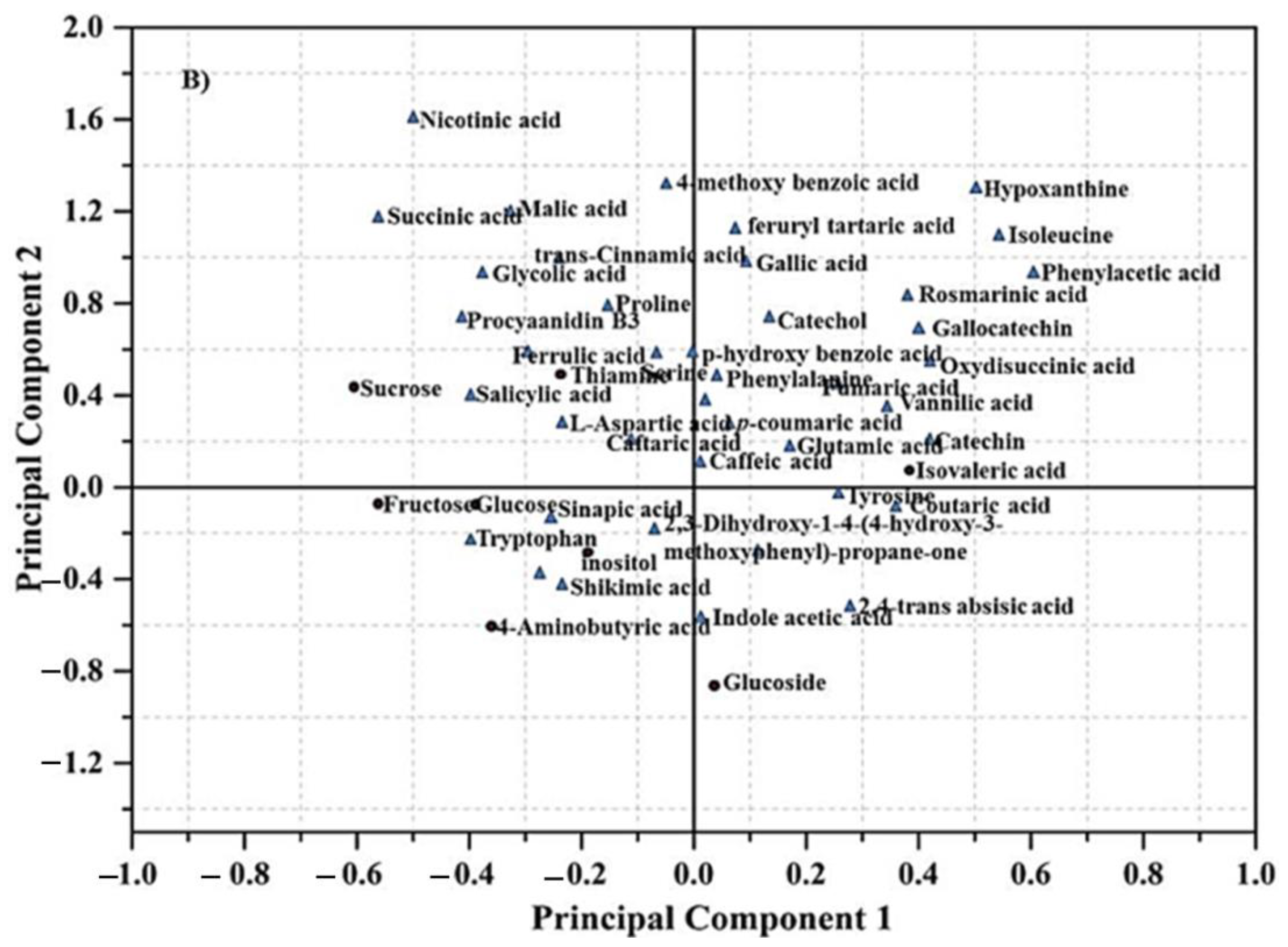
3.3. GC-MS Based Metabolomics Analysis Following MeJA Elicitation
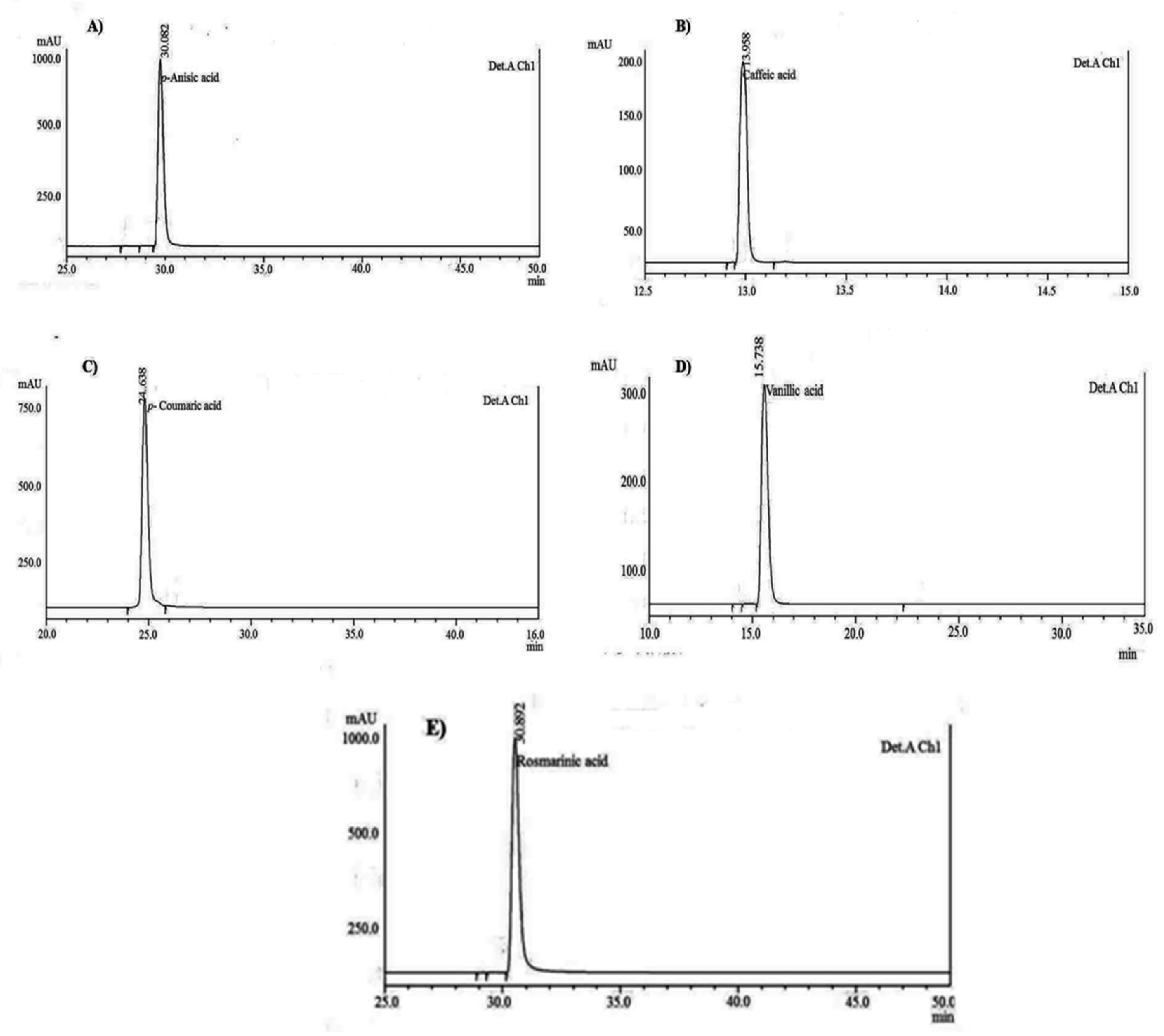
3.4. HPLC Chromatographic Separation and Structural Elucidation of SCC Metabolites
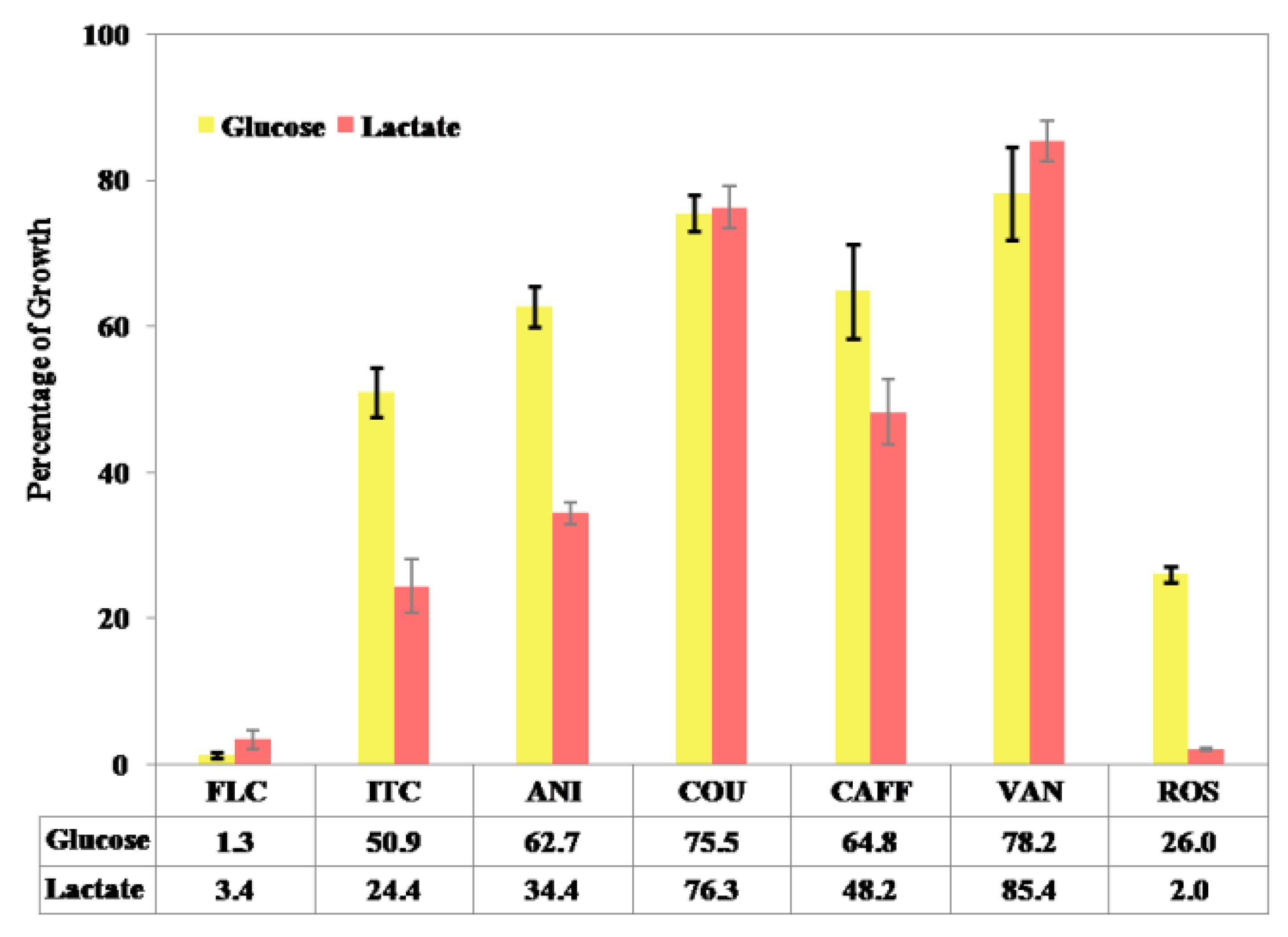
3.5. Alternative Screening Approach for C. albicans Growth in YNB Broth
3.6. ICL 1 Enzyme Assay Confirmed Potential Inhibitors
3.7. Determination of Minimum Inhibitory Concentration
3.8. Drug Likeliness and ADMET Properties of RA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubois, M.G.; Rezzonico, B. First Phytochemical Evidence of Chemophytes for the Seagrass Zostera noltii. Plants 2012, 1, 27–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papenbrock, J. Highlights in Seagrsses Phylogeny, Physiology, and Metabolism: What makes them special? ISRN Bot. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.R.R.; Arumugam, R.; Meenakshi, S.; Anantharaman, P. Thin layer chromatography analysis of antioxidant constituents from seagrasses of Gulf of Mannar Biosphere Reserve, South India. Int. J. Chem. Technol. Res. 2010, 2, 1526–1530. [Google Scholar]
- Weckwerth, W. Metabolomics in systems biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T. Metabolomics Analysis and Biosynthesis of Rosmarinic Acid in Agastache rugosa Kuntze Treated with Methyl Jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.; Simmonds, M.S.J. Molecules of interest: Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Oriente, G. Isolamente e connstitizibrudellacidorosmarinico (dal Rosmarinus off.). Ric Sci. 1958, 28, 2329–2333. [Google Scholar]
- Ravn, H.; Petersen, M.F.; Andary, J.B.C.; Anthoni, U.; Christophersen, C.; Nielsen, P.H. Seasonal variation and distribution of two phenolic compounds, rosmarinic acid and caffeic acid, in leaves and roots-rhizomes of eelgrass (Zostera marina L.). Ophelia 1994, 40, 51–61. [Google Scholar] [CrossRef]
- Aquino, R.; Morelli, S.; Lauro, M.R.; Abdo, S.; Saija, A.; Tomaino, A. Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J. Nat. Prod. 2001, 64, 1019–1023. [Google Scholar] [CrossRef]
- Yun, I.A.; Wakabayashi, K.T.; Fields, H.L.; Nicola, S.M. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J. Neurosci. 2004, 24, 2923–2933. [Google Scholar] [CrossRef]
- Mika, J.; Rojewska, E.; Makuch, W.; Korostynski, M.; Luvisetto, S.; Marinelli, S.; Pavone, F.; Przewlocka, B. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuroscience 2011, 175, 301–310. [Google Scholar]
- Abdullah, G.H.; Khan, I.A.; Khan, S.A.; Ali, H. Impact of planting methods and herbicides on weed biomass and some agronomic traits of maize. Pak. J. Weed Sci. Res. 2008, 14, 121–130. [Google Scholar]
- Petersen, M. Characterization of rosmarinic acid synthase from cell cultures of Coleus blumei. Phytochem 1991, 30, 2877–2881. [Google Scholar] [CrossRef]
- Jeyapragash, D.; Saravanakumar, A.; Thangaradjou, T. GC-MS based metabolomics analysis and characterization of rosmarinic acid from tropical seagrass Halodulepinifolia. Int. J. Pharm. Res. 2018, 10, 277–285. [Google Scholar]
- Petersen, M.; Alfermann, A.W. Two new enzymes of rosmarinic acid biosynthesis from cell cultures of Coleus blumei: Hydroxyphenylpyruvate reductase and rosmarinic acid synthase. Z. Nat. C 1988, 43, 501–504. [Google Scholar] [CrossRef]
- Ellis, B.E.; Towers, G.H.N. Biogenesis of rosmarinic acid in Mentha. Biochem. J. 1970, 118, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.; Hausler, E.; Meinhard, J.; Karwatzki, B.; Gertlowski, C. The biosynthesis of rosmarinic acid in suspension-cultures of Coleus blumei. Plant Cell Tissue Organ Cult. 1994, 38, 171–179. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Razzaque, A.; Ellis, B.E. Rosmarinic acid production in Coleus cell cultures. Planta 1997, 137, 287–291. [Google Scholar]
- Zenk, M.H.; El-shagi, H.; Ulbrich, B. Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharan thus roseus. Naturwissenshaften 1977, 64, 585–586. [Google Scholar] [CrossRef]
- Hippolyte, I.; Marin, B.; Baccou, J.C.; Jonard, R. Growth and rosmarinic acid production in cell suspension cultures of Salvia officinalis L. Plant Cell Rep. 1992, 11, 109–112. [Google Scholar] [CrossRef]
- Vogelsang, K.; Schneider, B.; Petersen, M. Production of rosmarinic acid and a new rosmarinic acid 3-O-beta-D-glucoside in suspension cultures of the hornwort Anthoceros agrestis Paton. Planta 2006, 223, 369–373. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Kuzeva, S.L.; Pavlov, A.I.; Kovacheva, E.G.; Ilieva, M.P. Elicitation of rosmarinic acid by Lavandula vera MM cell suspension culture with abiotic elicitors. World J. Microbiol. Biotechnol. 2007, 23, 301–304. [Google Scholar] [CrossRef]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production of rosmarinic acid and structiurally related compounds. Appl. Microbiol. Biotechnol. 2019, 99, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Kalyani, S.; Essa, M.; Girija, S.; Song, H. Production of rosmarinic in Ocimum sanctum cell culures by the influence of sucrose, phenylalanine, yeast extract, and methyl jasmonate. Int. J. Biol. Med. Res. 2011, 2, 1070–1074. [Google Scholar]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Engin. Biotechnol. 2008, 111, 187–228. [Google Scholar]
- Mulabagal, V.; Tsay, H. Plant cell cultures as a source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Liu, X.N.; Zhang, X.Q.; Zhang, S.X.; Sun, J.S. Regulation of metabolite production by precursors and elicitors in liquid cultures of Hypericum perforatum. Plant Cell Tissue Organ Cult. 2007, 91, 1–7. [Google Scholar] [CrossRef]
- Coste, A.; Vlase, L.; Halmagyi, A.; Deliu, C.; Coldea, G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult. 2011, 106, 279–288. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Korsangruang, S.; Soonthornchareonnon, N.; Chintapakorn, Y.; Saralamp, P.; Prathanturarug, S. Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tissue Organ Cult. 2010, 103, 333–342. [Google Scholar] [CrossRef]
- Subhashini, P.; Raja, S.; Thangaradjou, T. Establishment of cell suspension culture protocol for a seagrass (Halodulepinifolia): Growth kinetics and histomorphological characterization. Aquaic Bot. 2014, 117, 33–40. [Google Scholar] [CrossRef]
- Bird, K.T.; Brown, M.S.; Henderson, T.T.; Hara, C.E.O.; Robbie, J.M. Studies of Ruppia maritima L. bicarbonate and sucrose-based media. J. Exp. Mar. Biol. Ecol. 1996, 199, 153–164. [Google Scholar] [CrossRef]
- Jeyapragash, D.; Yosuva, M.; Saravanakumar, A. Comparative metabolomics analysis of wild and suspension cultured cells of seagrass Halodule pinifolia of Cymodoceaceae family. Aquat. Bot. 2020, 147, 104392. [Google Scholar]
- Moussaoui, A.E.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Benbrahim, K.F.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- Ouadi, Y.E.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.A.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from north-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Modeling 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Ellender, R.D. Initiation of Callus Cultures and Plantlet Regeneration from Seagrasses and Marine Coastal Plants: Final Report; University of Southern Mississippi: Hattiesburg, MS, USA, 1991; p. 108. [Google Scholar]
- Mante, S. Plant Biotechnology; Mantell, S.H., Smith, H., Eds.; Cambridge University Press: NewYork, NY, USA, 1983; p. 334. [Google Scholar]
- Wang, Y.; Jeknic, Z.; Ernst, R.C.; Chen, T.H.H. Improved plant regeneration from suspension-cultured cells of Iris germanica L. Hort. Sci. 1999, 34, 1271–1276. [Google Scholar]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Szabo, M.R.; Iditoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Mizukami, H.; Tabira, Y.; Ellis, B.E. Methyl Jasmonate induced rosmarinic acid Biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 1993, 2, 706–709. [Google Scholar] [CrossRef]
- Sumaryono, W.; Proksch, P.; Wray, V.; Writte, L.; Hartman, T. Qualitative and quantitative analysis of the constituents from Orthosiphon aristatus. Planta Med. 1991, 57, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Bae, M.J.; Lim, S.; Lee, W.; Kim, S. A Water-Soluble Extract from Actinidia Arguta Ameliorates Psoriasis-like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration. Nutrients 2018, 10, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.S.; Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Verpoorte, R. Metabolomic analysis of methyl jasmonate treated Brassica rapa leaves by 2- dimensional NMR spectroscopy. Phytochemistry 2006, 67, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Huhman, D.V.; Farag, M.A.; Smith, J.T.; May, G.D.; Mendes, P.; Dixon, R.A.; Sumner, L.W. Metabolic Profiling of Medicago Truncatula Cell Cultures Reveals the Effects of Biotic and Abiotic Elicitors on Metabolism. J. Exp. Bot. 2005, 56, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.Y.; Li, D.; Han, Z.C.; Gao, H.Y.; Wu, L.J. Chemical constituents of Rhodiola rosea and inhibitory effect on UV-induced A375–S2 cell death. Shenyang Yaoke Daxue Xuebao 2007, 24, 280–287. [Google Scholar]
- Li, P.; Qi, L.W.; Liu, E.H. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Anal. Chem. 2008, 27, 66–77. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.C.; Fink, G.R. Life and death in a macrophage: Role of the glyoxylate cycle in virulence. Eukaryot. Cell 2002, 1, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.F.; Ramı’rez-Trujillo, J.A.; Herna’ndez-Lucas, I. Major roles of isocitratelyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 2009, 155, 3166–3175. [Google Scholar] [CrossRef] [Green Version]
- Cheah, H.L.; Lim, V.; Sandai, D. Inhibitors of the Glyoxylate Cycle Enzyme ICL1 in Candida albicans for Potential Use as Antifungal Agents. PLoS ONE 2014, 9, 95951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krátký, M.; Vinšová, D.J. Advances in mycobacterial isocitrate lyase targeting and inhibitors. Curr. Med. Chem. 2012, 19, 6126–6137. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Rao, S.P.S.; Pethe, K.; Dick, T. Nutrient-starved, nonreplicating Mycobacterium tuberculosis requires respiration, ATP synthase andisocitratelyase for maintenance of ATP homeostasis and viability. Microbiology 2010, 156, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, A.; Someya, K.; Hata, M.; Nakajima, R.; Takemura, M. Discovery of a Small-Molecule Inhibitor of β-1,6-Glucan Synthesis. Antimicrob. Agents Chemother. 2009, 53, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha x piperita cell suspension cultures. Plant Cell Tissue Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Cruz, A.H.; Brock, M.; Zambuzzi-Carvalho, P.F.; Santos-Silva, L.K.; Troian, R.F.; Góes, A.M.; Soares, C.M.; Pereira, M. Phosphorylation is the major mechanism regulating isocitrate lyase activity in Paracoccidioides brasiliensis yeast cells. FEBS J. 2011, 278, 2318–2332. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Levin, G.M. P-glycoprotein: Why this drug transporter may be clinically important. Curr. Psychiatry 2012, 11, 38–40. [Google Scholar]
- Gülçin, I. Antioxidant and antiradical activities of L-Carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Hirose, M.; Tanaka, Y.; Tamano, S.; Kato, T.; Shirai, T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogeniesis 1998, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition ofp22phox NADPH oxidase in fructose-fed hypertensive rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521. [Google Scholar] [CrossRef]
- Verma, A. Lead finding from Phyllanthus debelis with hepatoprotective potentials. Asian Pac. J. Trop. Biomed. 2012, 2, 1735–1737. [Google Scholar] [CrossRef]
- Jeyapragash, D.; Yosuva, M.; Saravanakumar, A.; Vijayakumar, K. Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb. Pathog. 2020, 147, 104392. [Google Scholar]
- Jeyapragash, D.; Yosuva, M.; Rajiv, P.; Jayachandran, K.; Madhan, R.; Saravanakumar, A.; Ramachandran, S.; Aran, I. Phenylpropanoid biosynthetic gene expression and nitrate uptake kinetics for enhanced rosmarinic acid production in suspension cultured cells of Halodule pinifolia. Algal Res. 2022, 64, 102675. [Google Scholar]

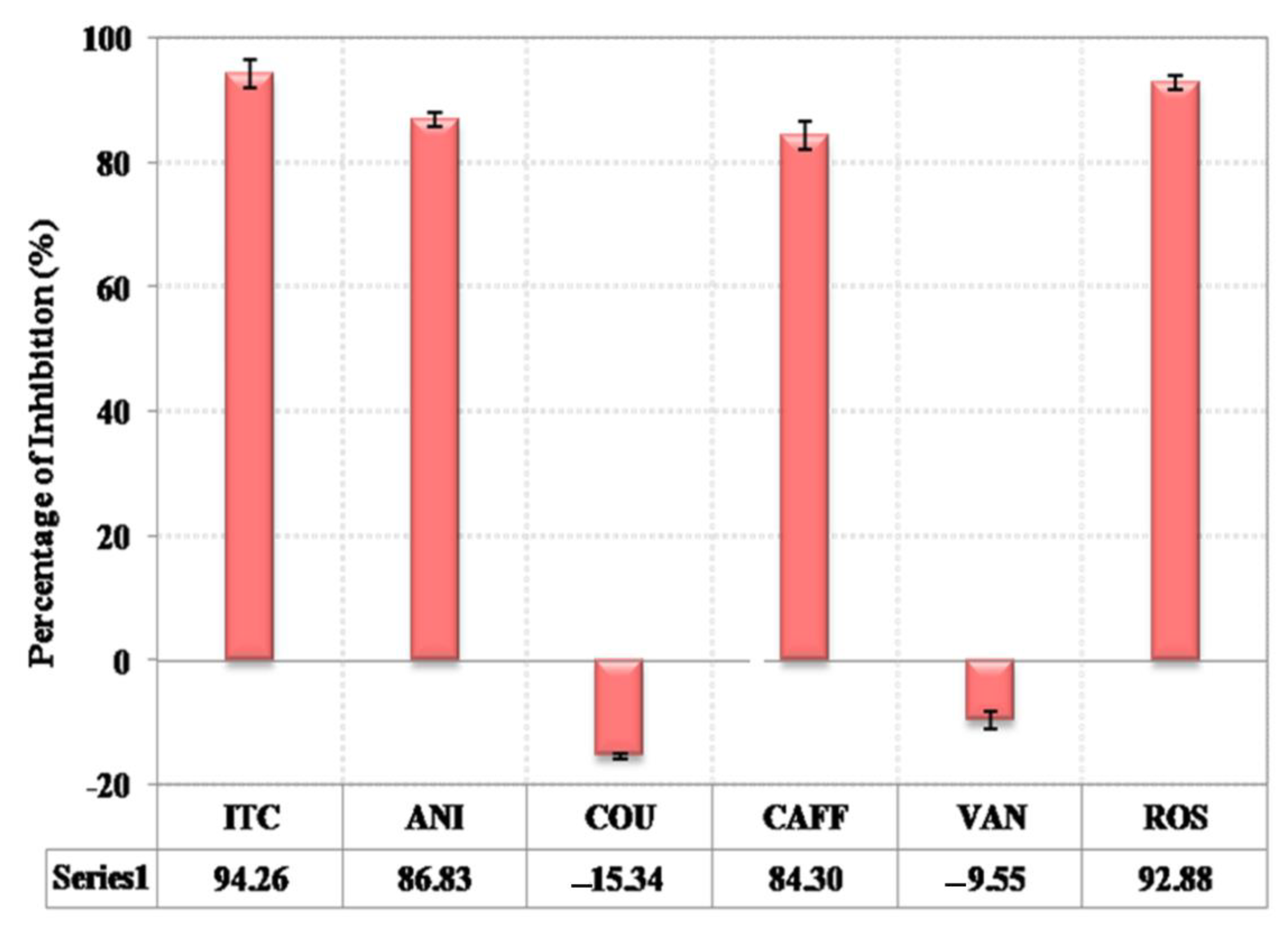
| Potential ICL Inhibitors/Test Compounds | Minimum Inhibitory Concentration (mg/L) |
|---|---|
| Fluconazole | 16 |
| Itaconic acid | 250 |
| p-Anisic acid | 500 |
| Caffeic acid | 1000 |
| Rosmarinic acid | 31.25 |
| Compounds | Fluconazole | Itaconic Acid | p-Anisic Acid | Caffeic Acid | Rosmarinic Acid |
|---|---|---|---|---|---|
| mi LogP | −0.12 | −0.343 | 1.91 | 0.941 | 1.63 |
| TPSA | 81.664 | 74.598 | 46.53 | 77.755 | 144.52 |
| n atoms | 22 | 9 | 11 | 13 | 26 |
| MW | 306.276 | 130.099 | 152.15 | 180.16 | 360.32 |
| n ON | 7 | 4 | 3 | 4 | 8 |
| n OHNH | 1 | 2 | 1 | 3 | 5 |
| n violations | 0 | 0 | 0 | 0 | 0 |
| n rotb | 5 | 3 | 2 | 2 | 7 |
| MV | 248.97 | 111.171 | 136.59 | 154.50 | 303.54 |
| S. No | Admet | Fluconazole | Itaconic Acid | p-Anisic Acid | Caffeic Acid | Rosmarinic Acid |
|---|---|---|---|---|---|---|
| 1. | Absorption | |||||
| BBB | + | + | + | - | + | |
| HIA | + | + | + | + | + | |
| Caco-2 permeability | + | - | + | + | - | |
| Aqueous solubility | −1.8626 | −0.7363 | −1.8579 | −1.6939 | −3.2050 | |
| P-glycoprotein | ||||||
| Substrate | - | - | - | - | + | |
| Inhibitor | - | - | - | - | - | |
| ROCT | - | - | - | - | - | |
| 2. | Distribution | |||||
| Subcellular localization | Mitochondria | Mitochondria | Mitochondria | Mitochondria | Mitochondria | |
| 3. | Metabolism | |||||
| CYP450 Substrate | ||||||
| CYP450 2C9 | - | - | - | - | - | |
| CYP450 2D6 | - | - | - | - | - | |
| CYP450 3A4 | - | - | - | - | - | |
| CYP450 Inhibitor | ||||||
| CYP450 1A2 | - | - | - | - | - | |
| CYP450 2C9 | + | |||||
| YP450 2D6 | - | - | - | - | - | |
| CYP450 2C19 | - | - | - | - | - | |
| CYP450 3A4 | - | - | - | - | - | |
| CYP Inhibitory Promiscuity | Low | Low | Low | Low | Low | |
| 4. | Excretion and Toxicity | |||||
| HERG Inhibition | ||||||
| HERG 1 | Weak | Weak | Weak | Weak | Weak | |
| HERG 2 | - | - | - | - | - | |
| AMES Toxicity | - | - | - | - | - | |
| Carcinogens | - | - | - | - | - | |
| Rat Acute Toxicity (LD50, mol/kg) | 2.4136 | 2.4525 | 1.8917 | 1.4041 | 2.6983 | |
| Fish Toxicity (pLC50, mg/L) | 1.4529; high | 0.8180; high | 1.9309; high | 0.7921; high | −0.1231; high |
| Drug Target | Fluconazole | Itaconic Acid | p-Anisic Acid | Caffeic Acid | Rosmarinic Acid |
|---|---|---|---|---|---|
| GPCR ligand | 0.04 | −1.78 | −1.02 | −0.48 | 0.17 |
| Ion channel Modulator | 0.01 | 0.97 | −0.55 | −0.23 | −0.08 |
| Kinase inhibitor | −0.09 | −2.82 | −1.22 | −0.81 | −0.18 |
| Nuclear receptor ligand | −0.23 | −1.54 | −0.79 | −0.10 | 0.57 |
| Protease inhibitor | −0.09 | −2.08 | −1.16 | −0.79 | 0.15 |
| Enzyme inhibitor | 0.03 | −1.23 | −0.52 | −0.09 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuthaymi, M.A.; Danaraj, J.; Albarakaty, F.M.; Periakaruppan, R.; Vajravelu, M.; Ayyappan, S.; Selvaraj, K.; Abd-Elsalam, K.A. Elicitor-Induced Metabolomics Analysis of Halodule pinifolia Suspension Culture for an Alternative Antifungal Screening Approach against Candida albicans. J. Fungi 2022, 8, 609. https://doi.org/10.3390/jof8060609
Alghuthaymi MA, Danaraj J, Albarakaty FM, Periakaruppan R, Vajravelu M, Ayyappan S, Selvaraj K, Abd-Elsalam KA. Elicitor-Induced Metabolomics Analysis of Halodule pinifolia Suspension Culture for an Alternative Antifungal Screening Approach against Candida albicans. Journal of Fungi. 2022; 8(6):609. https://doi.org/10.3390/jof8060609
Chicago/Turabian StyleAlghuthaymi, Mousa A., Jeyapragash Danaraj, Fawziah M. Albarakaty, Rajiv Periakaruppan, Manigandan Vajravelu, Saravanakumar Ayyappan, Kumaralingam Selvaraj, and Kamel A. Abd-Elsalam. 2022. "Elicitor-Induced Metabolomics Analysis of Halodule pinifolia Suspension Culture for an Alternative Antifungal Screening Approach against Candida albicans" Journal of Fungi 8, no. 6: 609. https://doi.org/10.3390/jof8060609







