New Insights into Methyl Jasmonate Regulation of Triterpenoid Biosynthesis in Medicinal Fungal Species Sanghuangporusbaumii (Pilát) L.W. Zhou & Y.C. Dai
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Plasmid Vector
2.2. MeJA Treatment of Cultures at Different Concentrations
2.3. Quantification of Terpenoids
2.4. Measurement of Gene Transcript Levels by qRT-PCR
2.5. Amplification the Gene Promoter Regions
2.6. Statistical Analysis
3. Results
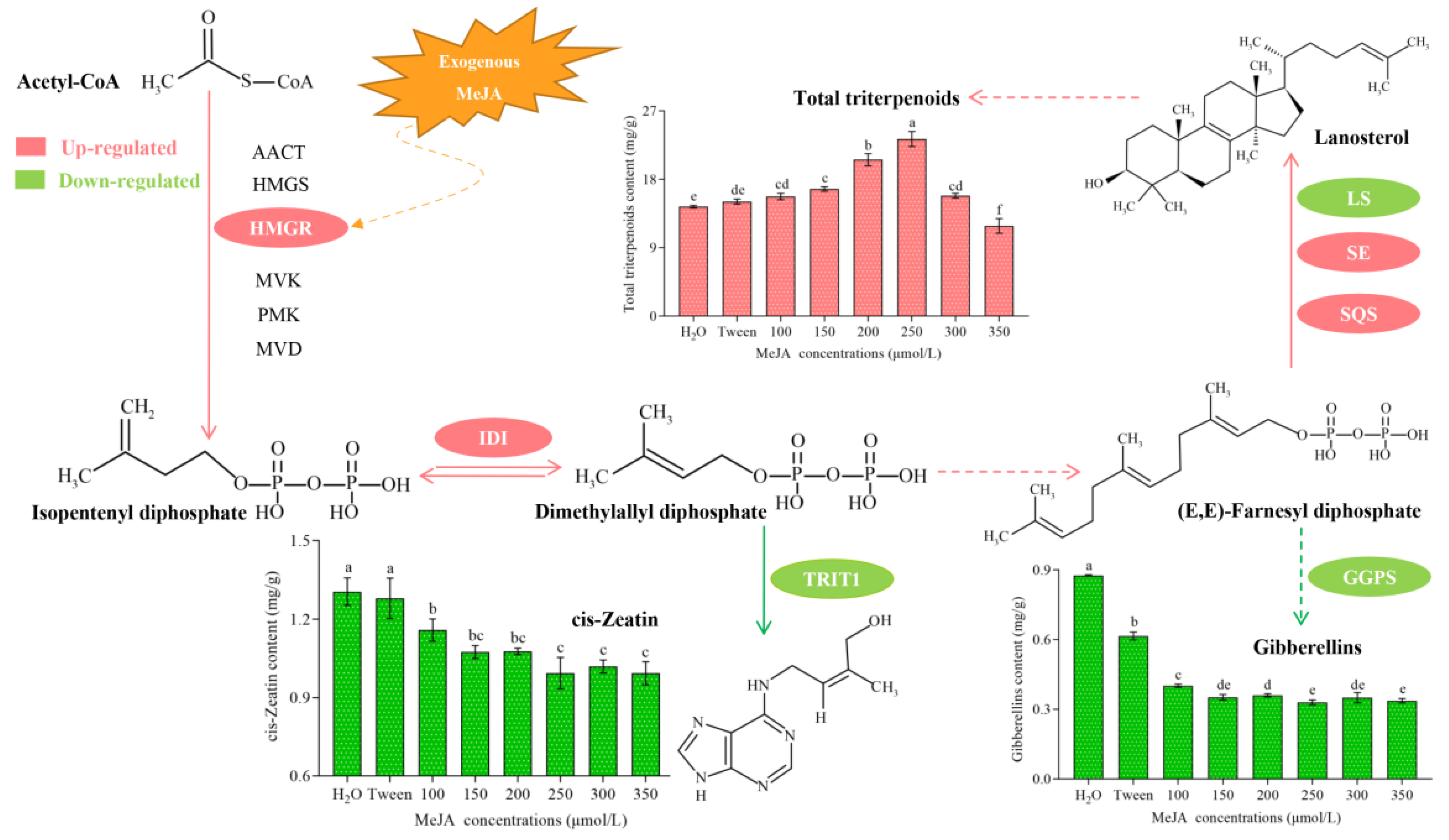
3.1. Total Triterpenoid Profiles in Response to MeJA Treatment in S. baumii
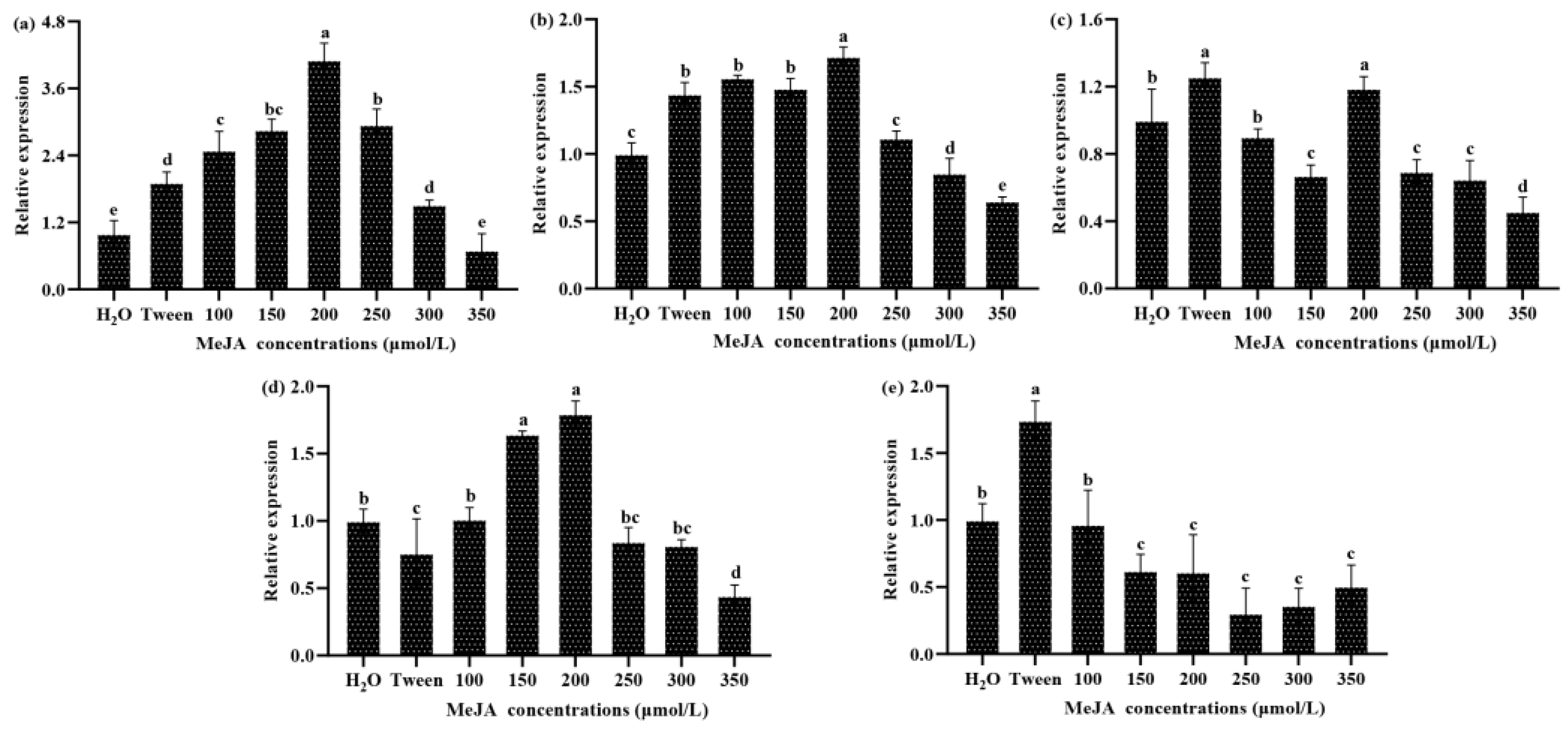
3.2. Relative Changes in the Expression of Five Genes in Response to MeJA Treatment in S. baumii
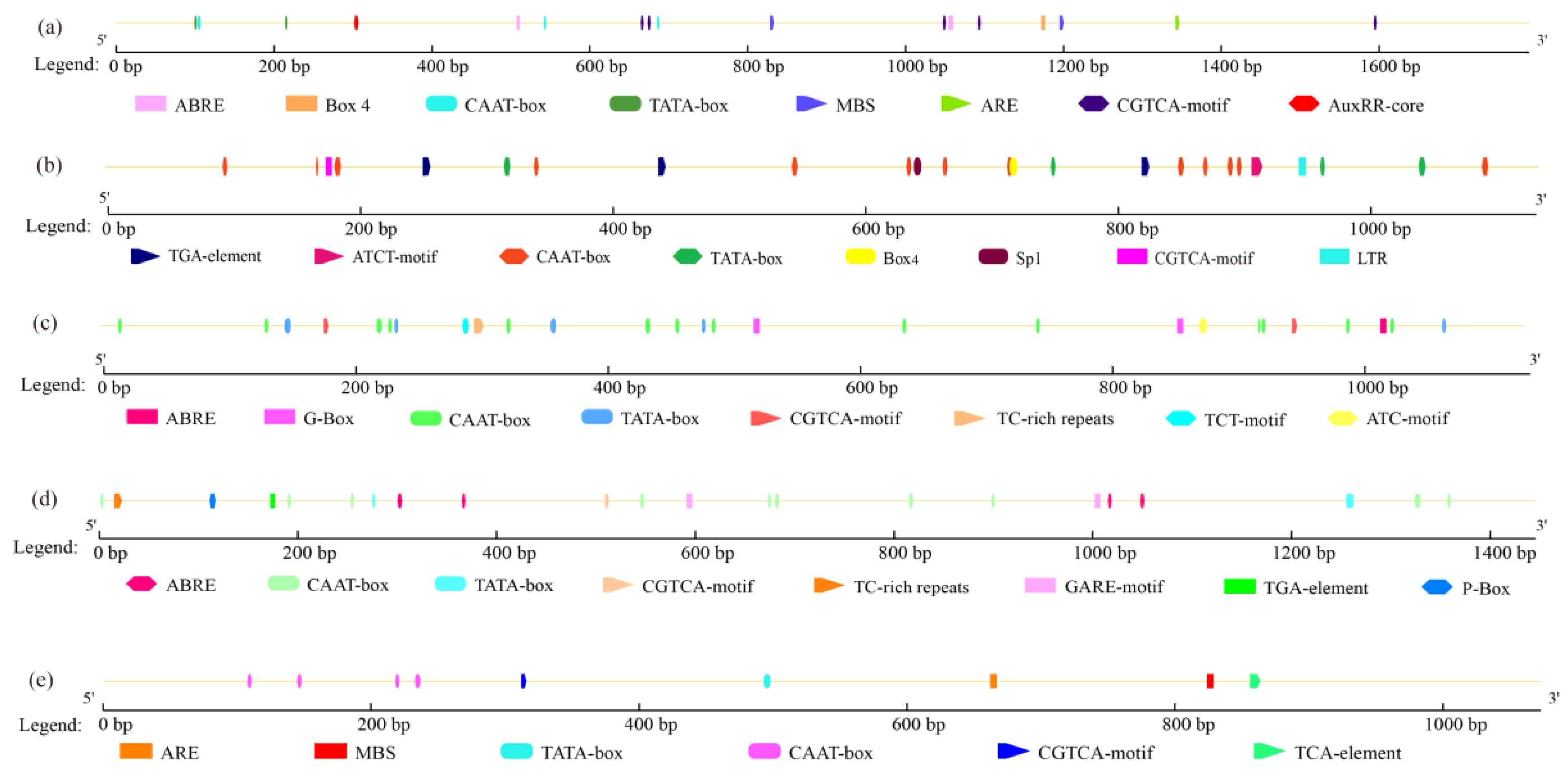
3.3. Analysis of the Promoter Regions of Five Genes Involved in Triterpenoid Biosynthesis
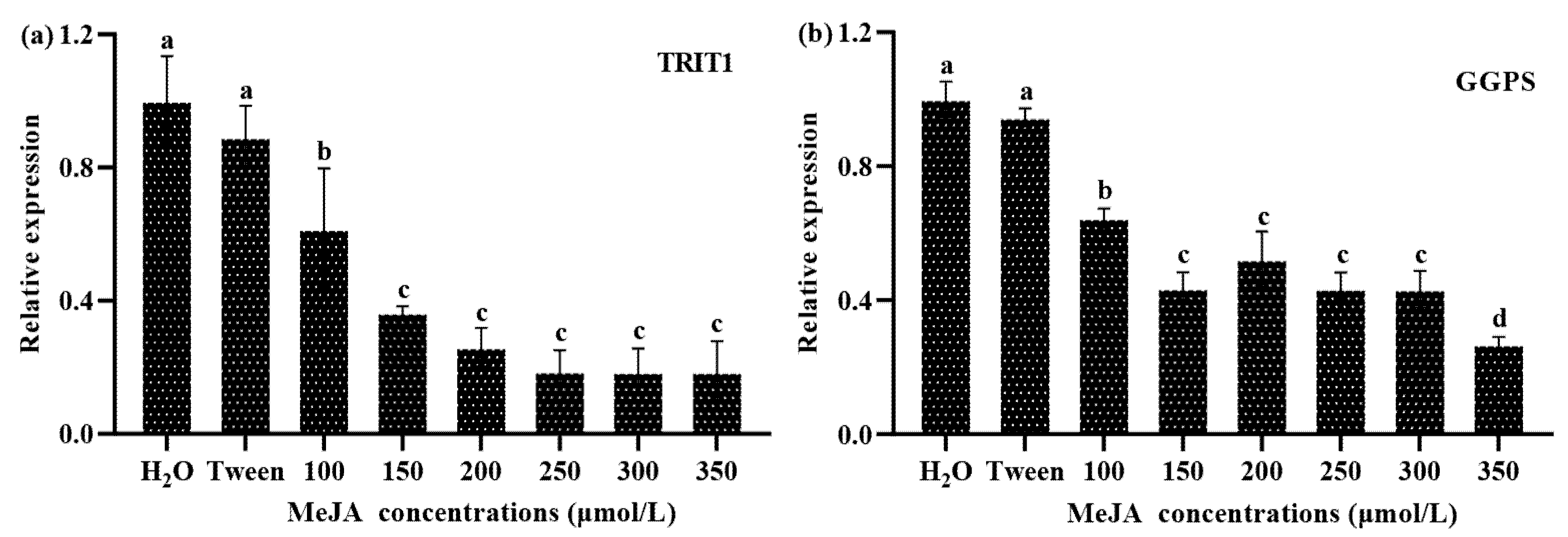
3.4. cZ Profile and TRIT1 Gene Expression Changes in Response to MeJA Treatment in S. baumii
3.5. GAs Profile and GGPS Gene Transcription Changes in Response to MeJA Treatment in S. baumii
3.6. Analysis of the Promoter Regions of the TRIT1 and GGPS Genes
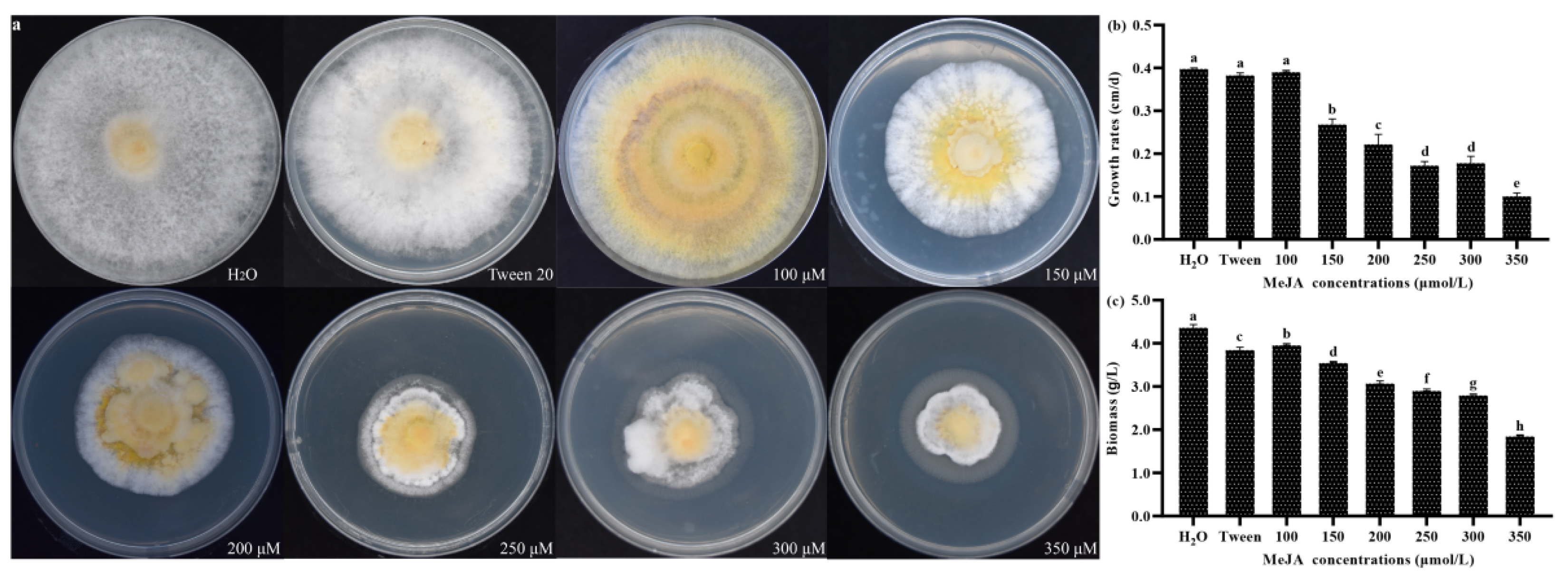
3.7. Effects of MeJA on Mycelial Growth in S. baumii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.H.; Dai, Y.C. Species clarification of the medicinal fungus Sanghuang. Mycosystema 2020, 39, 781–794. [Google Scholar] [CrossRef]
- Oue-artorn, R.; Aphidech, S.; Kwanjai, K.; Sarawut, T.; Vittaya, A.; Somdej, K. Cyclofarnesane sesquiterpenoids from the fungus Sanghuangporus sp. Phytochem. Lett. 2020, 37, 17–20. [Google Scholar] [CrossRef]
- Hou, R.R.; Zhou, L.J.; Fu, Y.; Wang, T.; Li, Z.; Zhou, L.W.; Zhang, G.L.; Tian, X.M. Chemical characterization of two fractions from Sanghuangporus sanghuang and evaluation of antidiabetic activity. J. Funct. Foods 2021, 87, 104825. [Google Scholar] [CrossRef]
- Cheng, J.W.; Song, J.L.; Wang, Y.B.; Wei, H.L.; He, L.; Liu, Y.; Ding, H.M.; Huang, Q.R.; Hu, C.J.; Huang, X.B.; et al. Conformation and anticancer activity of a novel mannogalactan from the fruiting bodies of Sanghuangporus sanghuang on HepG2 cells. Food Res. Int. 2022, 156, 111336. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guo, H.W.; Zhang, J.P.; Liu, H.; Wang, Y. The genome of the medicinal macro fungus Sanghuang provides insights into the synthesis of diverse secondary metabolites. Front. Microbiol. 2020, 10, 3035. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, K.; Zuo, S.; Chen, L.; Ding, Z.Y.; El-shazly, M.; Zhang, B.B. Unsaturated fatty acid promotes the production of triterpenoids in submerged fermentation of Sanghuangporus baumii. Food Biosci. 2020, 37, 100712. [Google Scholar] [CrossRef]
- Zheng, N.; Ming, Y.F.; Chu, J.Z.; Yang, S.D.; Wu, G.C.; Li, W.H.; Zhang, R.; Cheng, X.H. Optimization of extraction process and the antioxidant activity of phenolics from Sanghuangporus baumii. Molecules 2021, 26, 3850. [Google Scholar] [CrossRef]
- Liu, Z.C.; Sun, T.T.; Wang, S.X.; Zou, L. Cloning, molecular properties and differential expression analysis of the isopentenyl diphosphate isomerase gene in Sanghuangporus baumii. Biotechnol. Biotechnol. Equip. 2020, 34, 623–630. [Google Scholar] [CrossRef]
- Cai, C.S.; Ma, J.X.; Han, C.R.; Jin, Y.; Zhao, G.G.; He, X.W. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Ren, P.; Yongze, F.; Jiao, Z.; Hai, Q.; Gan, L.T.; Yi, H.L.; Yao, R.Y.; Luo, X.Y. Improvement of polysaccharide and triterpenoid production of Ganoderma lucidum through mutagenesis of protoplasts. Biotechnol. Biotechnol. Equip. 2016, 30, 381–387. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Pongkitwitoon, B.; Pathomwichaiwat, T.; Viboonjun, U.; Prathanturarug, S. Effects of methyl jasmonate on the growth and triterpenoid production of diploid and tetraploid Centella asiatica (L.) Urb. hairy root cultures. Sci. Rep. 2019, 9, 18665. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Sun, L.; Li, Y.; Xiao, J.L.; Wang, S.Y.; Yang, J.; Qu, Z.Y.; Zhan, Y. Functional identification of BpMYB21 and BpMYB61 transcription factors responding to MeJA and SA in birch triterpenoid synthesis. BMC Plant Biol. 2020, 20, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.L.; Liu, Y.N.; Liu, R.; Ren, A.; Ma, H.Y.; Shu, L.B.; Shi, L.; Zhu, J.; Zhao, M.W. Integrated proteomics and metabolomics analysis provides insights into ganoderic acid biosynthesis in response to methyl jasmonate in Ganoderma Lucidum. Int. J. Mol. Sci. 2019, 20, 6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Yue, S.N.; Gao, T.; Zhu, J.; Ren, A.; Yu, H.S.; Wang, H.; Zhao, M.W. Nitrate reductase-dependent nitric oxide plays a key role on MeJA-induced ganoderic acid biosynthesis in Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2020, 104, 10737–10753. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Zhang, X.; Chen, C. Stimulated production of triterpenoids of Inonotus obliquus using methyl jasmonate and fatty acids. Ind. Crops Prod. 2016, 85, 49–57. [Google Scholar] [CrossRef]
- Wang, J.R.; Lin, J.F.; Guo, L.Q.; You, L.F.; Zeng, X.L.; Wen, J.M. Cloning and characterization of squalene synthase gene from Poria cocos and its up-regulation by methyl jasmonate. World J. Microbiol. Biotechnol. 2014, 30, 613–620. [Google Scholar] [CrossRef]
- Jennifer, F.J.; Jacob, P.; Tessa, M.; Philipp, A.; Noelia, B.M.; Eduardo, M.; Venancio, A.; Maria de Lourdes, T.; Alain, G.; Antonio, L.R. Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves. Plant Sci. 2016, 250, 188–197. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.X.; Zhao, M.W. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hussain, R.M.; Rehman, N.U.; She, G.B.; Li, P.H.; Wan, X.C.; Guo, L.; Zhao, J. De novo transcriptome sequencing and metabolite profiling analyses reveal the complex metabolic genes involved in the terpenoid biosynthesis in Blue Anise Sage (Salvia guaranitica L.). DNA Res. 2018, 25, 597–617. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xu, M.M.; Zhao, L.T.; Wang, F.; Li, Y.R.; Shi, G.Y.; Ding, Z.Y. Transcriptome dynamics and metabolite analysis revealed the candidate genes and regulatory mechanism of ganoderic acid biosynthesis during liquid superficial-static culture of Ganoderma lucidum. Microb. Biotechnol. 2020, 14, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Z.; Yang, J.H.; Xing, H.H.; Jiang, S.Y. A spectrophotometric method for the determination of gibberellin. J. Yantai Univ. 1997, 10, 33–36. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ali, Q.; Salisu, I.B.; Raza, A.; Shahid, A.A.; Rao, A.Q.; Husnain, T. A modified protocol for rapid DNA isolation from cotton (Gossypium spp.). Methods 2019, 6, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, T.; Miura, T.; Shimizu, K.; Terada, T. Identification and characterization of functional antioxidant response elements in the promoter of the aldo-keto reductase AKR1B10 gene. Chem. Biol. Interact. 2017, 276, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Wang, X.T.; Liu, Z.C.; Sun, T.T.; Zou, L. Expression analysis of squalene synthase gene in mevalonate pathway of Sanghuangporus baumii. Biotechnol. Biotechnol. Equip. 2022, 36, 176–183. [Google Scholar] [CrossRef]
- Wang, X.T.; Sun, T.T.; Sun, J.; Wang, S.X.; Zou, L. Expression analysis of lanosterol synthase gene in dynamic accumulation of triterpenoids in Sanghuangporus baumii. Protein Pept. Lett. 2022, 29, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 2003, 15, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Bahrawi, S.A. Effect of gibberellic acid on some microbial growth and spore germination of some fungi. Zent. Für Mikrobiol. 1982, 137, 238–240. [Google Scholar] [CrossRef]
- Kaori, M.; Petr, T.; Miho, M.K.; Tomohiko, K.; Shusei, S.; Danuse, T.; Satoshi, T.; Göran, S.; Tatsuo, K. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wang, Z.; Zheng, J.Y.; Xu, Z.J.; Tang, X.; Huang, Z.X.; Zhang, N.N.; Dong, Y.; Li, T. The effects of methyl jasmonate on growth, gene expression and metabolite accumulation in Isatis indigotica Fort. Ind. Crops Prod. 2022, 177, 114482. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.N.; Wang, J.; Zuo, X.X.; Li, Y.F.; Li, M.L.; Wang, K.T.; Jin, P.; Zheng, Y.H. PpWRKY45 is involved in methyl jasmonate primed disease resistance by enhancing the expression of jasmonate acid biosynthetic and pathogenesis-related genes of peach fruit. Postharvest Biol. Technol. 2021, 172, 111390. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Lin, S.Z.; Zhang, Q.; Lei, Y.; Hou, L.; Zhang, Z.Y. Functional identification and regulation of the PtDrl02 gene promoter from triploid white poplar. Plant Cell. Rep. 2010, 29, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, H.; Gao, X.H.; Fu, X.D. New insights into gibberellin signaling in regulating plant growth-metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Ji, S.L.; Ren, M.F.; He, Y.L.; Jing, X.R.; Xu, J.W. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem. Eng. J. 2014, 90, 178–183. [Google Scholar] [CrossRef]
- Zhang, D.H.; Li, N.; Yu, X.; Zhao, P.; Li, T.; Xu, J.W. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynthesis in Ganoderma lingzhi. Phytochemistry 2017, 134, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Jiang, L.X.; Li, N.; Yu, X.; Zhao, P.; Li, T.; Xu, J.W. Overexpression of the squalene epoxidase gene alone and in combination with the 3-hydroxy-3-methylglutaryl coenzyme a gene increases ganoderic acid production in Ganoderma lingzhi. J. Agric. Food Chem. 2017, 65, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, R.; Tong, X.; Zou, L. New Insights into Methyl Jasmonate Regulation of Triterpenoid Biosynthesis in Medicinal Fungal Species Sanghuangporusbaumii (Pilát) L.W. Zhou & Y.C. Dai. J. Fungi 2022, 8, 889. https://doi.org/10.3390/jof8090889
Liu Z, Liu R, Tong X, Zou L. New Insights into Methyl Jasmonate Regulation of Triterpenoid Biosynthesis in Medicinal Fungal Species Sanghuangporusbaumii (Pilát) L.W. Zhou & Y.C. Dai. Journal of Fungi. 2022; 8(9):889. https://doi.org/10.3390/jof8090889
Chicago/Turabian StyleLiu, Zengcai, Ruipeng Liu, Xinyu Tong, and Li Zou. 2022. "New Insights into Methyl Jasmonate Regulation of Triterpenoid Biosynthesis in Medicinal Fungal Species Sanghuangporusbaumii (Pilát) L.W. Zhou & Y.C. Dai" Journal of Fungi 8, no. 9: 889. https://doi.org/10.3390/jof8090889




