An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Nucleic Acid Extraction
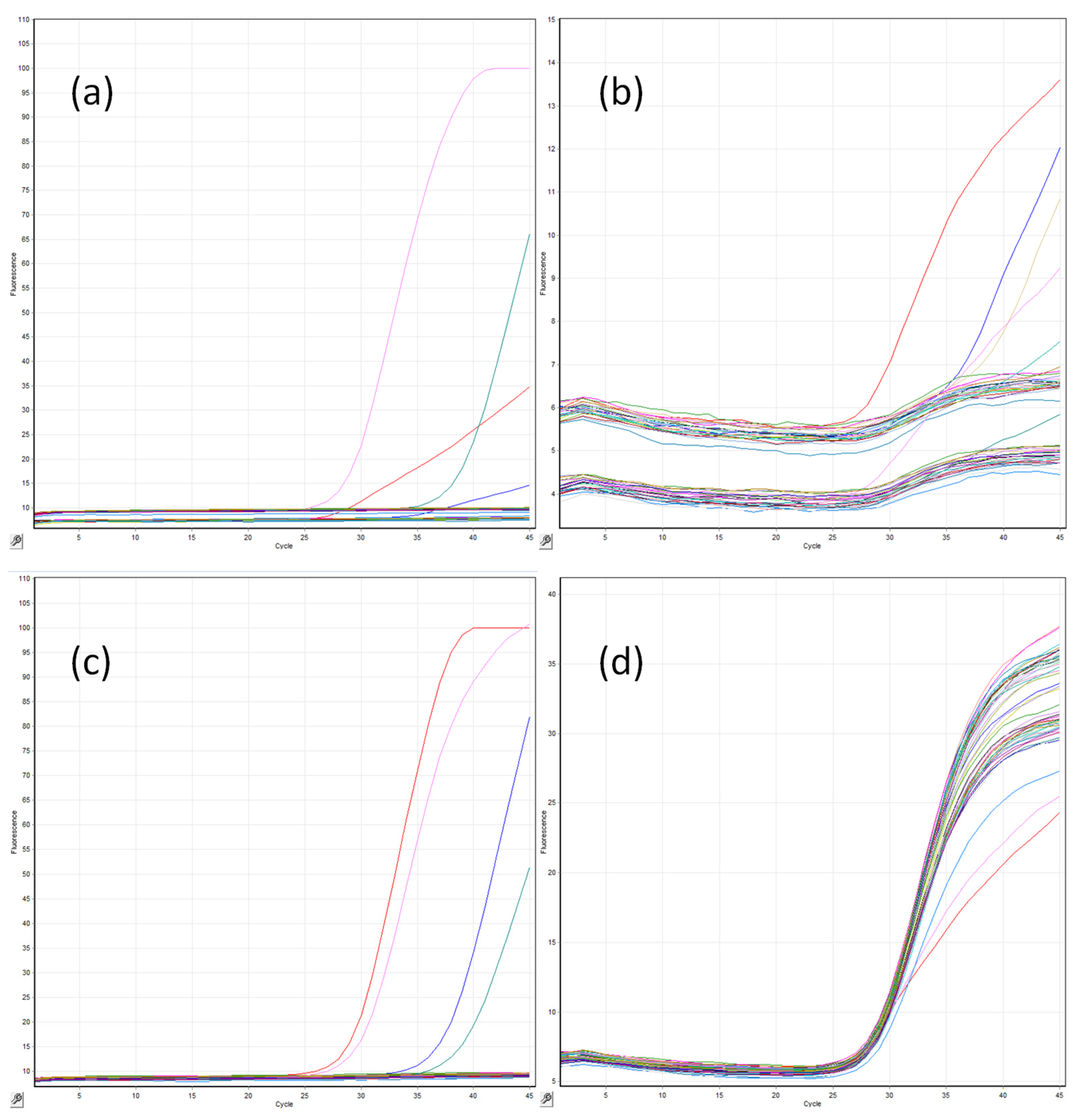
2.3. CandID Real-Time PCR Amplification
2.4. (1-3)-β-D-Glucan Testing
2.5. Statistical Analysis
3. Results
3.1. Retrospective Performance Evaluation
3.2. Prospective Performance Evaluation Using Nucleic acid Extracted Using the BioMerieux eMag Extractor
3.3. Prospective Performance Evaluation Using Nucleic acid Extracted Using the Roche MagNA Pure 96 Extractor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D. Time to initiation of fluconazole therapy impacts mortality in patients with Candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the “Missing 50%” of invasive Candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Hauser, P.; Lagrou, K.; Melchers, W.J.G.; Helweg-Larsen, J.; Matos, O.; Cesaro, S.; Maschmeyer, G.; Einsele, H.; Donnelly, J.P.; et al. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 2016, 71, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Bretagne, S.; Caliendo, A.M.; Loeffler, J.; Patterson, T.F.; Slavin, M.; Wingard, J.R. Aspergillus polymerase chain reaction—An update on technical recommendations, clinical applications, and justification for inclusion in the second revision of the EORTC/MSGERC definitions of invasive fungal disease. Clin. Infect. Dis. 2021, 72 (Suppl. 2), S95–S101. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Price, J.S.; Cordey, A.; Backx, M. Molecular diagnosis of yeast infections. Curr. Fungal Infect. Rep. 2021, 15, 67–80. [Google Scholar] [CrossRef]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive Candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; Silveira, F.P.; et al. Performance of Candida real-time polymerase chain reaction, -D-Glucan Assay, and blood cultures in the diagnosis of invasive Candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef]
- Tang, D.-L.; Chen, X.; Zhu, C.-G.; Li, Z.-W.; Xia, Y.; Guo, X.-G. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect. Dis. 2019, 19, 798. [Google Scholar] [CrossRef]
- Muñoz, P.; Vena, A.; Machado, M.; Gioia, F.; Martínez-Jiménez, M.C.; Gómez, E.; Origüen, J.; Orellana, M.; López-Medrano, F.; Fernández-Ruiz, M.; et al. T2Candida MR as a predictor of outcome in patients with suspected invasive candidiasis starting empirical antifungal treatment: A prospective pilot study. J. Antimicrob. Chemother. 2018, 73, iv6–iv12. [Google Scholar] [CrossRef]
- White, P.L.; Perry, M.D.; Loeffler, J.; Melchers, W.; Klingspor, L.; Bretagne, S.; McCulloch, E.; Cuenca-Estrella, M.; Finnstrom, N.; Donnelly, J.P.; et al. Critical stages of extracting DNA from Aspergillus fumigatus in whole-blood specimens. J. Clin. Microbiol. 2010, 48, 3753–3755. [Google Scholar] [CrossRef] [Green Version]
- Clancy, C.J.; Nguyen, M.H. Diagnosing invasive Candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17. [Google Scholar] [CrossRef]
- Loeffler, J.; Hebart, H.; Bialek, R.; Hagmeyer, L.; Schmidt, D.; Serey, F.-P.; Hartmann, M.; Eucker, J.; Einsele, H. Contaminations occurring in fungal PCR assays. J. Clin. Microbiol. 1999, 37, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Rimek, D.; Garg, A.P.; Haas, W.H.; Kappe, R. Identification of Contaminating Fungal DNA Sequences in Zymolyase. J. Clin. Microbiol. 1999, 37, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, E.E.M.; Carroll, N.M.; Choudhury, S.; Dunlop, A.A.S.; Towler, H.M.A.; Matheson, M.M.; Adamson, P.; Okhravi, N.; Lightman, S. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J. Clin. Microbiol. 2000, 38, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.D.; White, P.L.; Barnes, R.A. Comparison of four automated nucleic acid extraction platforms for the recovery of DNA from Aspergillus fumigatus. J. Med. Microbiol. 2014, 63, 1160–1166. [Google Scholar] [CrossRef]
- White, P.L.; Alanio, A.; Brown, L.; Cruciani, M.; Hagen, F.; Gorton, R.; Lackner, M.; Millon, L.; Morton, C.O.; Rautemaa-Richardson, R.; et al. An overview of using fungal DNA for the diagnosis of invasive mycoses. Expert Rev. Mol. Diagn. 2022, 22, 169–184. [Google Scholar] [CrossRef]
- Clancy, C.J.; Pappas, P.G.; Vazquez, J.; Judson, M.A.; Kontoyiannis, D.P.; Thompson, G.R., III; Garey, K.W.; Reboli, A.; Greenberg, R.N.; Apewokin, S.; et al. Detecting infections rapidly and easily for Candidemia trial, Part 2 (DIRECT2): A prospective, multicenter study of the T2Candida panel. Clin. Infect. Dis. 2018, 66, 1678–1686. [Google Scholar] [CrossRef]
- Nieto, M.; Robles, J.C.; Causse, M.; Gutiérrez, L.; Perez, M.C.; Ferrer, R.; Xercavins, M.; Herrero, E.; Sirvent, E.; Fernández, C.; et al. Polymerase chain reaction versus blood culture to detect Candida species in high-risk patients with suspected invasive Candidiasis: The MICAFEM Study. Infect. Dis. Ther. 2019, 8, 429–444. [Google Scholar] [CrossRef]
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 2011, 49, 2879–2883. [Google Scholar] [CrossRef]
- Telenti, A.; Steckelberg, J.M.; Stockman, L.; Edson, R.S.; Roberts, G.D. Quantitative blood cultures in Candidemia. Mayo Clin. Proc. 1991, 66, 1120–1123. [Google Scholar] [CrossRef]
- Bloos, F.; Held, J.; Kluge, S.; Simon, P.; Kogelmann, K.; de Heer, G.; Kuhn, S.-O.; Jarczak, D.; Motsch, J.; Hempel, G.; et al. (1 → 3)-β-d-Glucan-guided antifungal therapy in adults with sepsis: The CandiSep randomized clinical trial. Intensive Care Med. 2022, 48, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Karageorgopoulos, D.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. -D-Glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Sugiyama, D.; Kogata, Y.; Saegusa, J.; Sugimoto, T.; Kawano, S.; Morinobu, A.; Nishimura, K.; Kumagai, S. Diagnostic accuracy of serum 1,3-β- d -Glucan for Pneumocystis Jiroveci Pneumonia, invasive Candidiasis, and invasive Aspergillosis: Systematic review and meta-analysis. J. Clin. Microbiol. 2012, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, M.A. Specificity influences in (1→3)-β-d-Glucan-supported diagnosis of invasive fungal disease. J. Fungi 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, Z.; Mattiuzzi, G.N.; Estey, E.; Kantarjian, H.M.; Saeki, F.; Ridge, R.J.; Ketchum, P.A.; Finkelman, M.A.; Rex, J.; Ostrosky-Zeichner, L. -D-Glucan as a diagnostic adjunct for invasive fungal infections: Validation, cutoff development, and performance in patients with acute Myelogenous Leukemia and Myelodysplastic Syndrome. Clin. Infect. Dis. 2004, 39, 199–205. [Google Scholar] [CrossRef]
- Fuchs, S.; Lass-Flörl, C.; Posch, W. Diagnostic performance of a novel multiplex PCR assay for candidemia among ICU patients. J. Fungi 2019, 5, 86. [Google Scholar] [CrossRef] [Green Version]

| Population (n = 83) | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Se (%, 95% CI) | Sp (%, 95% CI) | LR +tive | LR -tive | DOR | ||||||
| CID | BFC | CID | BFC | CID | BFC | CID | BFC | CID | BFC | |
| Candidemia (10) vs. no IC (45) | 80 (49–94) | 70 (40–89) | 93 (82–98) | 62 (48–75) | 11.4 | 1.8 | 0.2 | 0.5 | 53.1 | 3.8 |
| Probable IC (12) vs. no IC (45) | 92 (65–99) | 75 (47–91) | 93 (82–98) | 62 (48–75) | 13.1 | 2.0 | 0.1 | 0.4 | 152.8 | 4.9 |
| Candida peritonitis (2) vs. no IC (45) | 0 (0–66) | 50 (10–91) | 93 (82–98) | 62 (48–75) | 0 | 1.3 | 1.1 | 0.8 | 0 | 1.6 |
| Combined proven/prob IC (24) vs. no IC (45) | 79 (60–91) | 71 (51–95) | 93 (82–98) | 62 (48–75) | 11.3 | 1.9 | 0.2 | 0.5 | 50.0 | 4.0 |
| Possible IC (14) vs. no IC (45) | 43 (21–67) | 64 (39–84) | 93 (82–98) | 62 (48–75) | 6.1 | 1.7 | 0.6 | 0.6 | 10.0 | 2.9 |
| All IC (38) vs. no IC (45) | 66 (50–79) | 68 (53–81) | 93 (82–98) | 62 (48–75) | 9.4 | 1.8 | 0.4 | 0.5 | 25.8 | 3.5 |
| Population (n = 103) | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| Se (%, 95% CI) | Sp (%, 95% CI) | PPV (%, 95% CI) | NPV (%, 95% CI) | LR +tive | LR -tive | DOR | |
| Candidemia (4) vs. no IC (79) | 100 (51–100) | 82 (72–89) | 22 (9–45) | 100 (94–100) | 5.6 | <0.001 | >4571 |
| Probable/chronic IC (2) vs. no IC (79) | 100 (34–100) | 82 (72–89) | 13 (4–36) | 100 (94–100) | 5.6 | <0.001 | >4571 |
| Combined candidemia/prob/chronic IC (6) vs. no IC (79) | 100 (61–100) | 82 (72–89) | 30 (15–52) | 100 (94–100) | 5.6 | <0.001 | >4571 |
| Possible IC (18) vs. no IC (79) | 83 (61–94) | 82 (72–89) | 52 (34–69) | 96 (88–98) | 4.7 | 0.20 | 22.9 |
| All IC (24) vs. no IC (79) | 88 (69–96) | 82 (72–89) | 60 (44–74) | 96 (88–98) | 4.9 | 0.15 | 32.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, J.S.; Fallon, M.; Posso, R.; Backx, M.; White, P.L. An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples. J. Fungi 2022, 8, 935. https://doi.org/10.3390/jof8090935
Price JS, Fallon M, Posso R, Backx M, White PL. An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples. Journal of Fungi. 2022; 8(9):935. https://doi.org/10.3390/jof8090935
Chicago/Turabian StylePrice, Jessica S., Melissa Fallon, Raquel Posso, Matthijs Backx, and P. Lewis White. 2022. "An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples" Journal of Fungi 8, no. 9: 935. https://doi.org/10.3390/jof8090935
APA StylePrice, J. S., Fallon, M., Posso, R., Backx, M., & White, P. L. (2022). An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples. Journal of Fungi, 8(9), 935. https://doi.org/10.3390/jof8090935







