Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition
Abstract
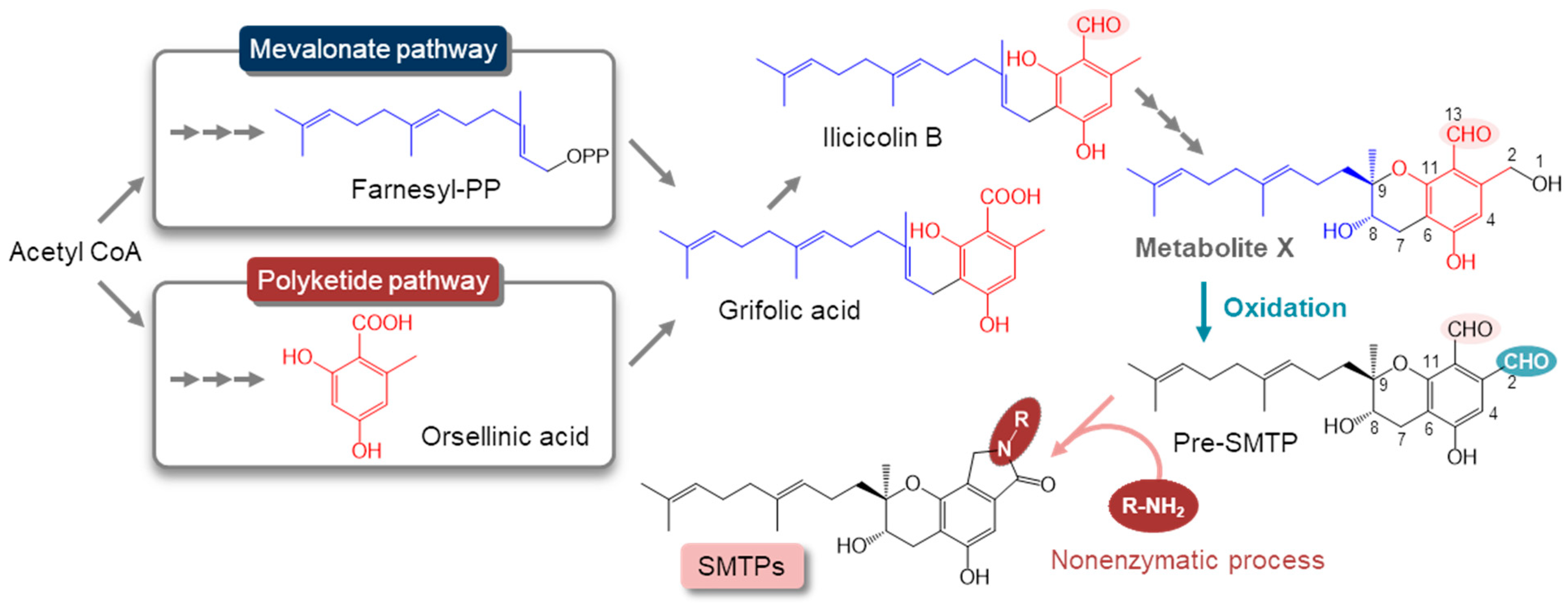
:1. Introduction
2. Materials and Methods
2.1. Materials
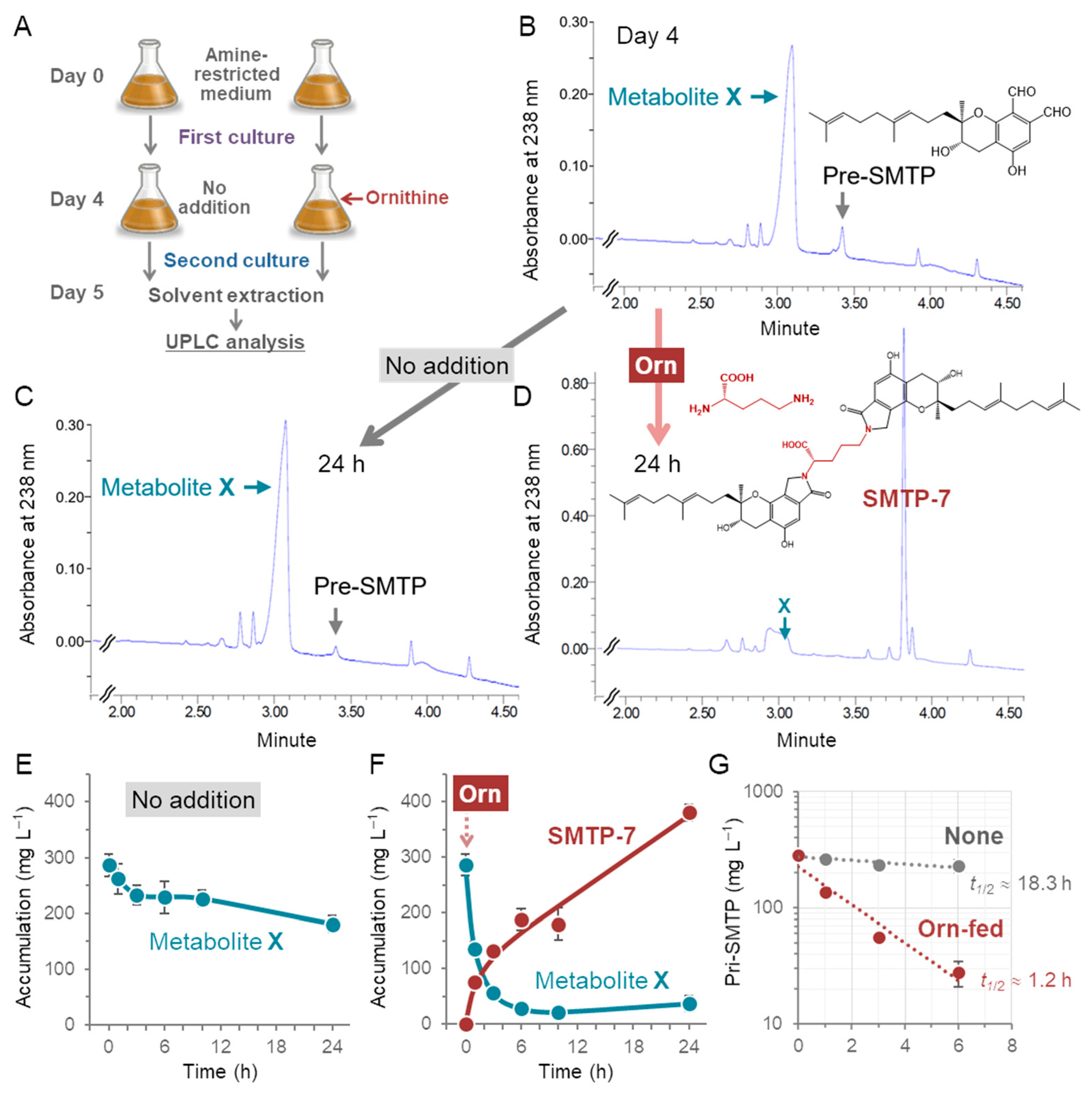
2.2. Production and Determination of the Precursor of SMTP Biosynthesis (Metabolite X)
2.3. Production and Determination of SMTP-7, an SMTP Family Member That Is Produced through the Conjugation of pre-SMTP with l-ornithine
2.4. UPLC Analysis of the Metabolites
2.5. Isolation of Metabolite X (pri-SMTP)
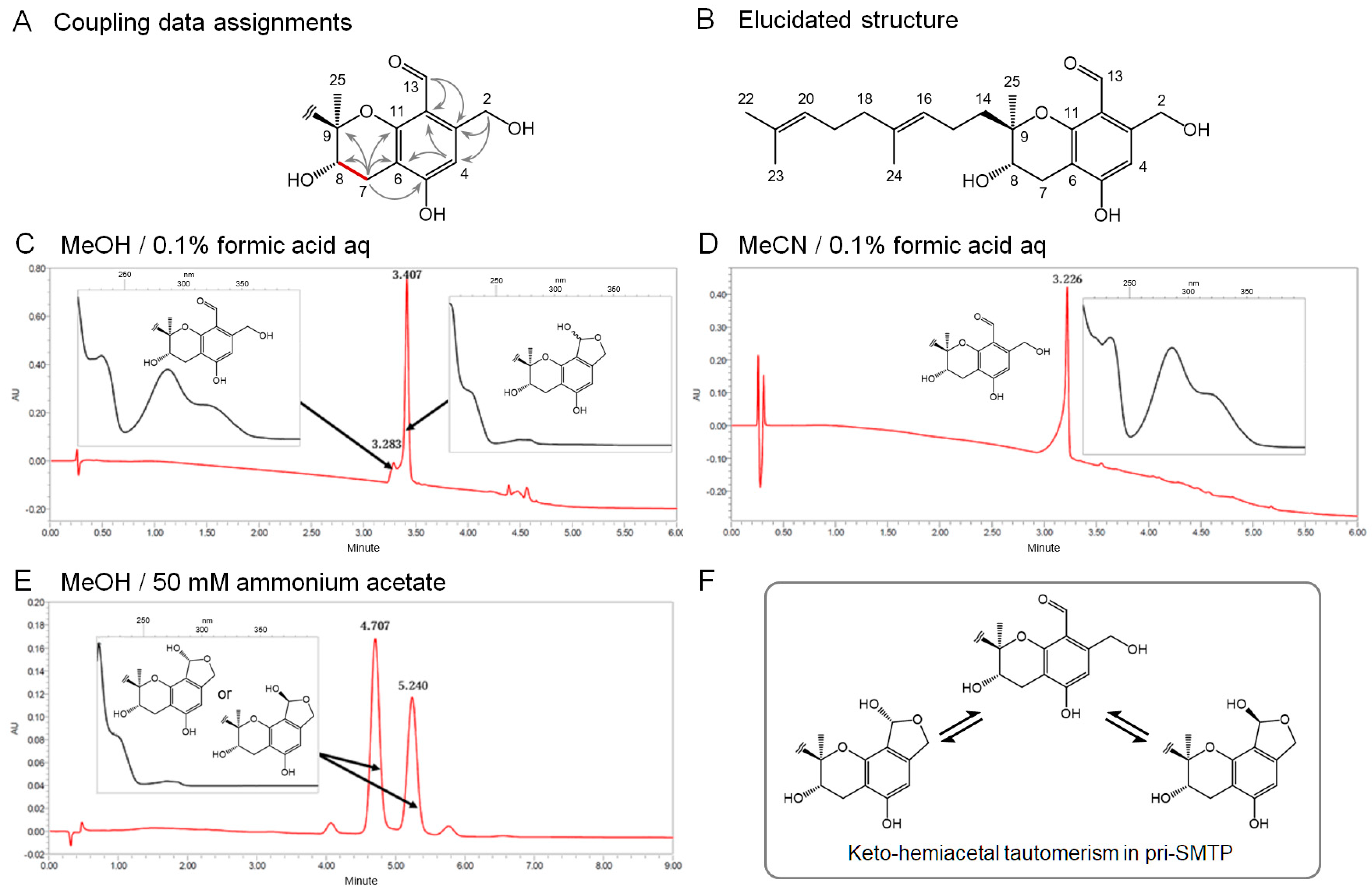
2.6. Spectroscopic Analyses of pri-SMTP
2.7. Analysis of the Tautomerism in pri-SMTP
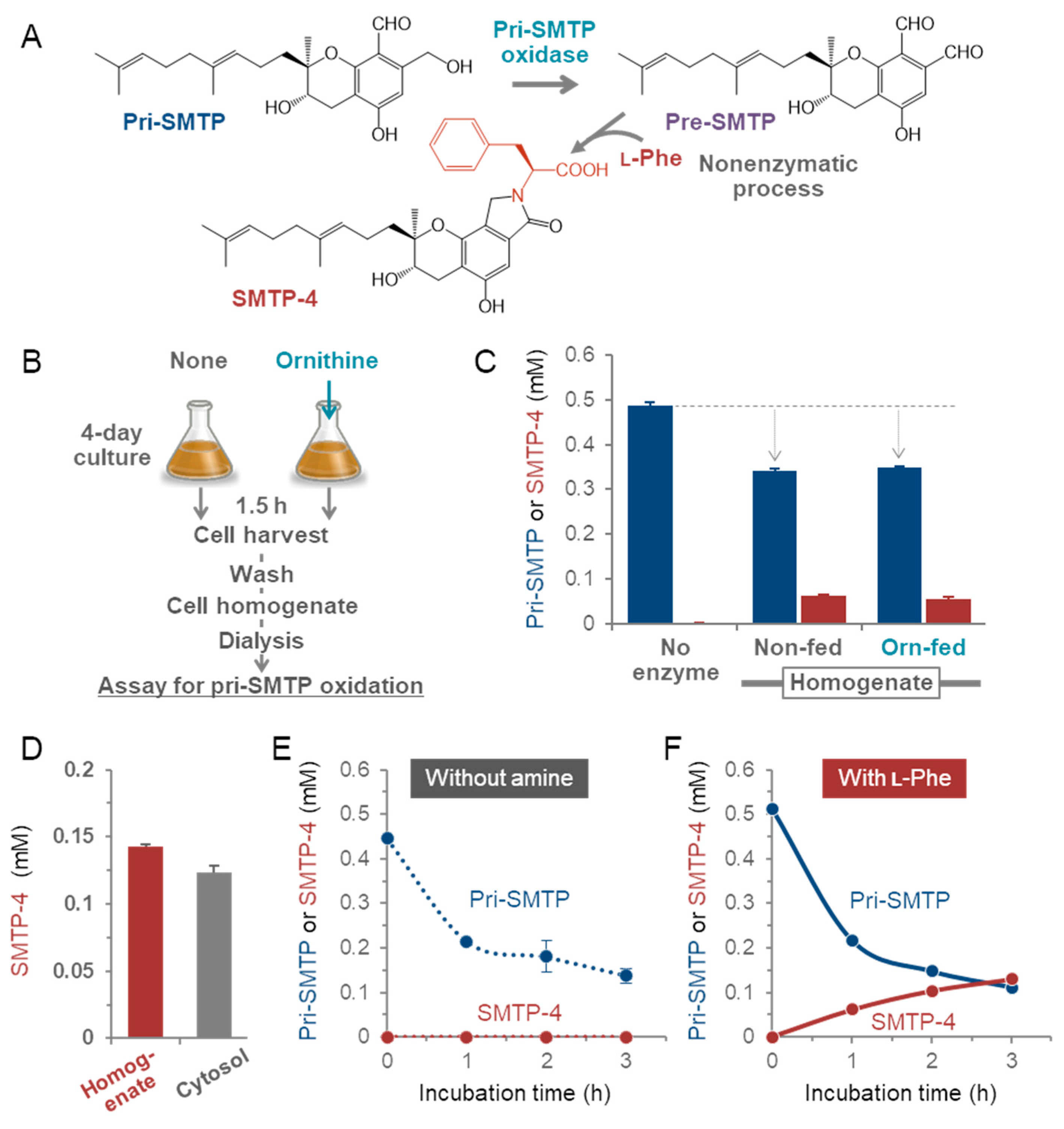
2.8. Preparation of Cell Extracts
2.9. Assay for pri-SMTP Oxidation (Cell-Free Enzyme Assay)
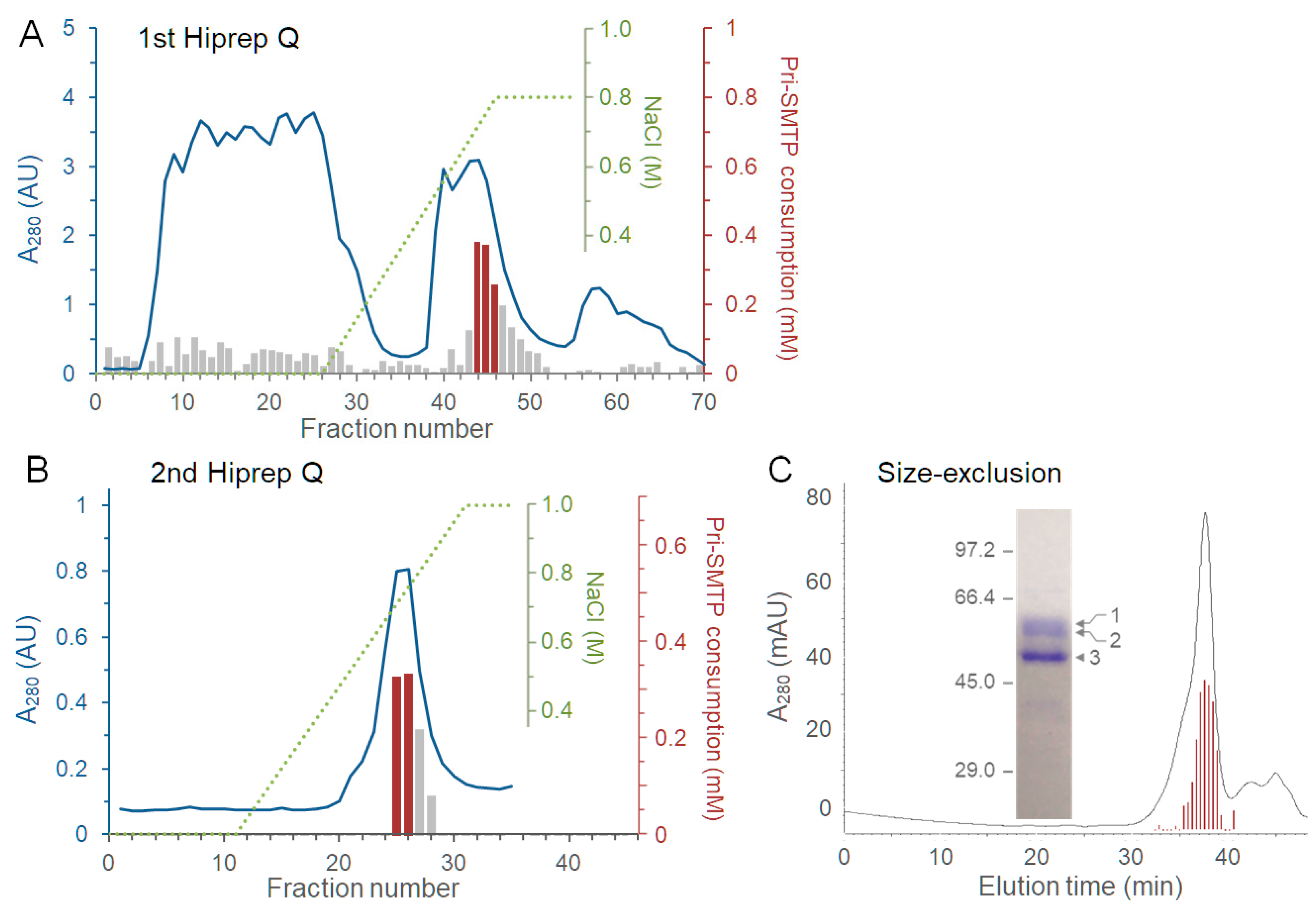
2.10. Purification of pri-SMTP Oxidase
2.11. Protein and Gene Analysis
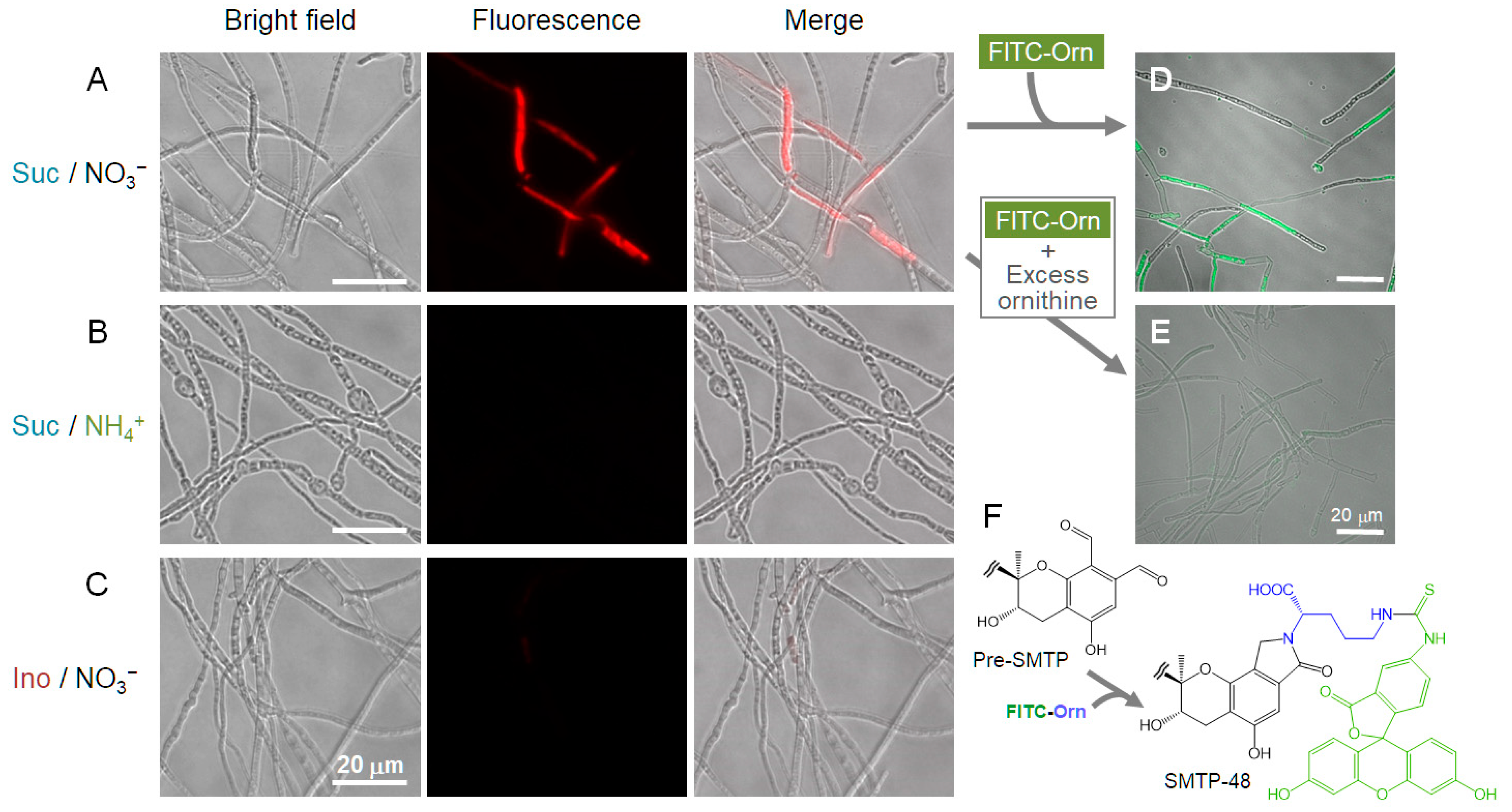
2.12. Microscopic Localization of pri-SMTP Accumulation
3. Results and Discussion
3.1. Metabolite X Accumulates in the Amine-Restricted Culture of S. microspora
3.2. Isolation and the Structure of Metabolite X (pri-SMTP)
3.3. Tautomerism in pri-SMTP
3.4. Enzymatic Oxidation of pri-SMTP
3.5. Purification of the Enzyme Responsible for the Oxidation of pri-SMTP
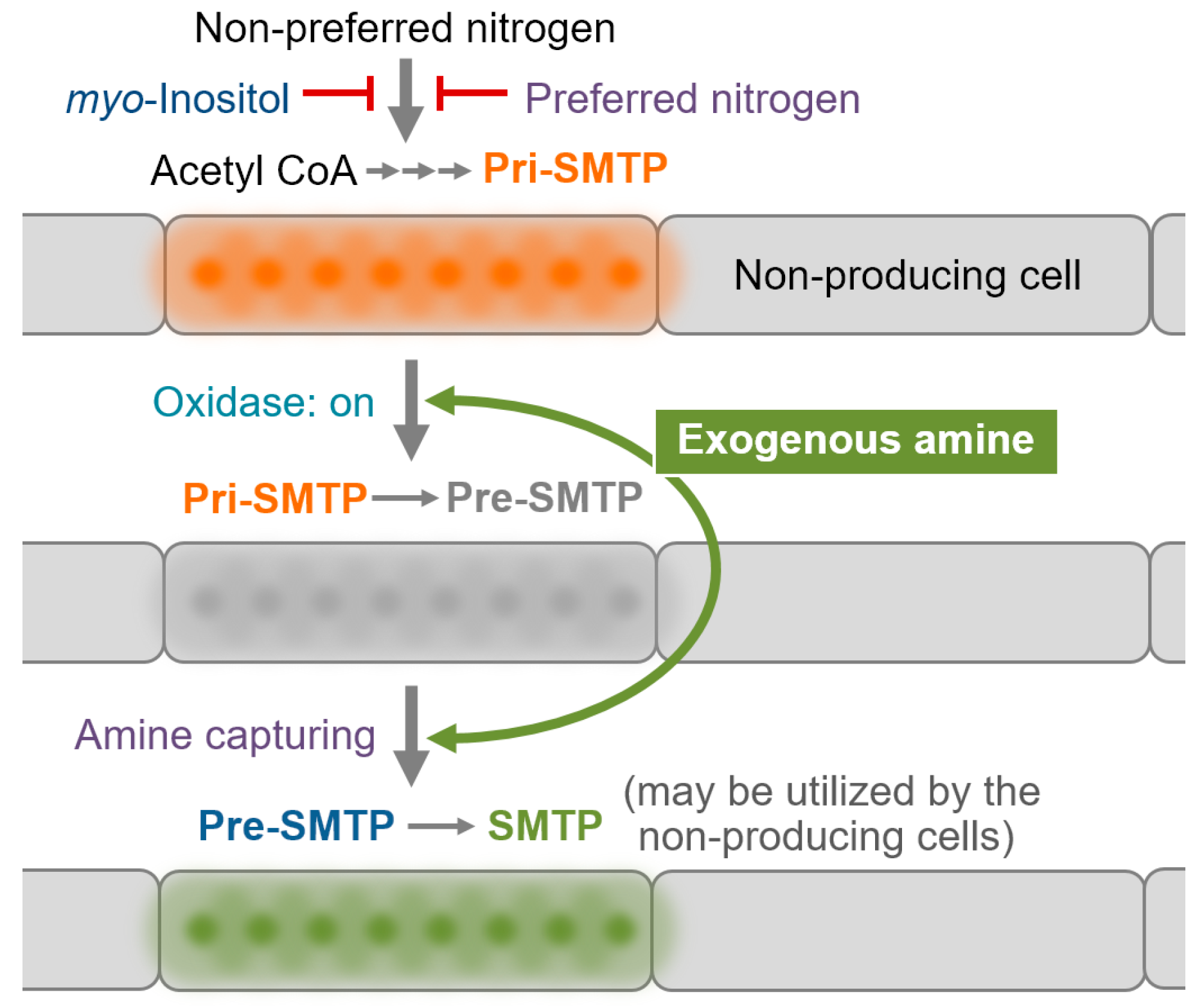
3.6. Regulation of the Biosynthesis of SMTP
3.7. Nitrogen-Related Nutritional Aspects of the Biosynthesis of SMTP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasumi, K.; Suzuki, E. Impact of SMTP targeting plasminogen and soluble epoxide hydrolase on thrombolysis, inflammation, and ischemic stroke. Int. J. Mol. Sci. 2021, 22, 954. [Google Scholar] [CrossRef] [PubMed]
- Hasumi, K.; Yamamichi, S.; Harada, T. Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems. FEBS J. 2010, 277, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, Y.; Gao, Y.; Huang, Q.; Luo, X.; An, H.; Dong, J. Chemical and bioactive diversities of the genera Stachybotrys and Memnoniella secondary metabolites. Phytochem. Rev. 2015, 14, 623–655. [Google Scholar] [CrossRef]
- Hu, W.; Narasaki, R.; Nishimura, N.; Hasumi, K. SMTP (Stachybotrys microspora triprenyl phenol) enhances clot clearance in a pulmonary embolism model in rats. Thromb. J. 2012, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Suzuki, E.; Ishikawa, M.; Shirafuji, T.; Hasumi, K. Soluble epoxide hydrolase as an anti-inflammatory target of the thrombolytic stroke drug SMTP-7. J. Biol. Chem. 2014, 289, 35826–35838. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Hashimoto, T.; Nobe, K.; Hasumi, K.; Honda, K. A novel finding of a low-molecular-weight compound, SMTP-7, having thrombolytic and anti-inflammatory effects in cerebral infarction of mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2010, 382, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kimura, Y.; Ohata, H.; Hashimoto, T.; Shibata, K.; Hasumi, K.; Honda, K. Distinct effects of tissue-type plasminogen activator and SMTP-7 on cerebrovascular inflammation following thrombolytic reperfusion. Stroke 2011, 42, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Saito, A.; Fujimura, M.; Shimizu, H.; Mekawy, M.; Hasumi, K.; Tominaga, T. Stachybotrys microspora triprenyl phenol-7, a novel fibrinolytic agent, suppresses superoxide production, matrix metalloproteinase-9 expression, and thereby attenuates ischemia/reperfusion injury in rat brain. Neurosci. Lett. 2011, 503, 110–114. [Google Scholar] [CrossRef]
- Huang, Y.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Shi, X.; Feng, T.; Yamashita, T.; et al. Antineuroinflammatory Effect of SMTP-7 in Ischemic Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Feng, T.; Huang, Y.; Sato, K.; et al. Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling. J. Neurosci. Res. 2018, 96, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Hashimoto, T.; Hasumi, K.; Honda, K.; Nobe, K. Evaluation of the effects of a new series of SMTPs in the acetic acid-induced embolic cerebral infarct mouse model. Eur. J. Pharmacol. 2018, 818, 221–227. [Google Scholar] [CrossRef]
- Li, C.; Matsuda, Y.; Gao, H.; Hu, D.; Yao, X.S.; Abe, I. Biosynthesis of LL-Z1272β: Discovery of a New Member of NRPS-like Enzymes for Aryl-Aldehyde Formation. ChemBioChem 2016, 17, 904–907. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Cleto, S.; Machado, I.; Pereira, M.O.; Vieira, M.J. Antimicrobial mechanisms of ortho-phthalaldehyde action. J. Basic Microbiol. 2007, 47, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.; Walsh, S.E.; Maillard, J.Y.; Russell, A.D. A NOTE: Ortho-phthalaldehyde: Proposed mechanism of action of a new antimicrobial agent. Lett. Appl. Microbiol. 2000, 31, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Hasumi, K.; Ohyama, S.; Kohyama, T.; Ohsaki, Y.; Takayasu, R.; Endo, A. Isolation of SMTP-3, 4, 5 and -6, novel analogs of staplabin, and their effects on plasminogen activation and fibrinolysis. J. Antibiot. (Tokyo) 1998, 51, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ohyama, S.; Hasumi, K. Activation of fibrinolysis by SMTP-7 and -8, novel staplabin analogs with a pseudosymmetric structure. J. Antibiot. (Tokyo) 2000, 53, 241–2417. [Google Scholar] [CrossRef]
- Hasumi, K.; Hasegawa, K.; Kitano, Y. Isolation and absolute configuration of SMTP-0, a simplest congener of the SMTP family nonlysine-analog plasminogen modulators. J. Antibiot. (Tokyo) 2007, 60, 463–468. [Google Scholar] [CrossRef]
- Nishimura, Y.; Suzuki, E.; Hasegawa, K.; Nishimura, N.; Kitano, Y.; Hasumi, K. Pre-SMTP, a key precursor for the biosynthesis of the SMTP plasminogen modulators. J. Antibiot. (Tokyo) 2012, 65, 483–485. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genom. 2014, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- Kistler, H.C.; Broz, K. Cellular compartmentalization of secondary metabolism. Front. Microbiol. 2015, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [Google Scholar] [CrossRef]
- Nair, A.; Sarma, S.J. The impact of carbon and nitrogen catabolite repression in microorganisms. Microbiol. Res. 2021, 251, 126831. [Google Scholar] [CrossRef]
- Fernandez, J.; Wright, J.D.; Hartline, D.; Quispe, C.F.; Madayiputhiya, N.; Wilson, R.A. Principles of Carbon Catabolite repression in the rice blast fungus: Tps1, Nmr1-3, and a MATE-family pump regulate glucose metabolism during infection. PLoS Genet. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon catabolite repression in filamentous Fungi. Int. J. Mol. Sci. 2018, 19, 48. [Google Scholar] [CrossRef]
- Reynolds, T.B. Strategies for acquiring the phospholipid metabolite inositol in pathogenic bacteria, fungi and protozoa: Making it and taking it. Microbiology 2009, 155, 1386–1396. [Google Scholar] [CrossRef]
- Nelson, B.D. Stachybotrys chartarum: The Toxic Indoor Mold. APSnet Featur. Artic. 2001. Online. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwama, R.; Sasano, Y.; Hiramatsu, T.; Otake, S.; Suzuki, E.; Hasumi, K. Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition. J. Fungi 2022, 8, 975. https://doi.org/10.3390/jof8090975
Iwama R, Sasano Y, Hiramatsu T, Otake S, Suzuki E, Hasumi K. Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition. Journal of Fungi. 2022; 8(9):975. https://doi.org/10.3390/jof8090975
Chicago/Turabian StyleIwama, Ryota, Yu Sasano, Taichi Hiramatsu, Shinya Otake, Eriko Suzuki, and Keiji Hasumi. 2022. "Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition" Journal of Fungi 8, no. 9: 975. https://doi.org/10.3390/jof8090975
APA StyleIwama, R., Sasano, Y., Hiramatsu, T., Otake, S., Suzuki, E., & Hasumi, K. (2022). Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition. Journal of Fungi, 8(9), 975. https://doi.org/10.3390/jof8090975





