Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing
Abstract
:1. Introduction
2. Characteristics and Biosynthesis of ZEA and Masked ZEA
2.1. ZEA
2.2. Masked ZEA
3. Incidence of ZEA and Masked ZEA in Cereal and Cereal-Based Food
4. Methods of Detection
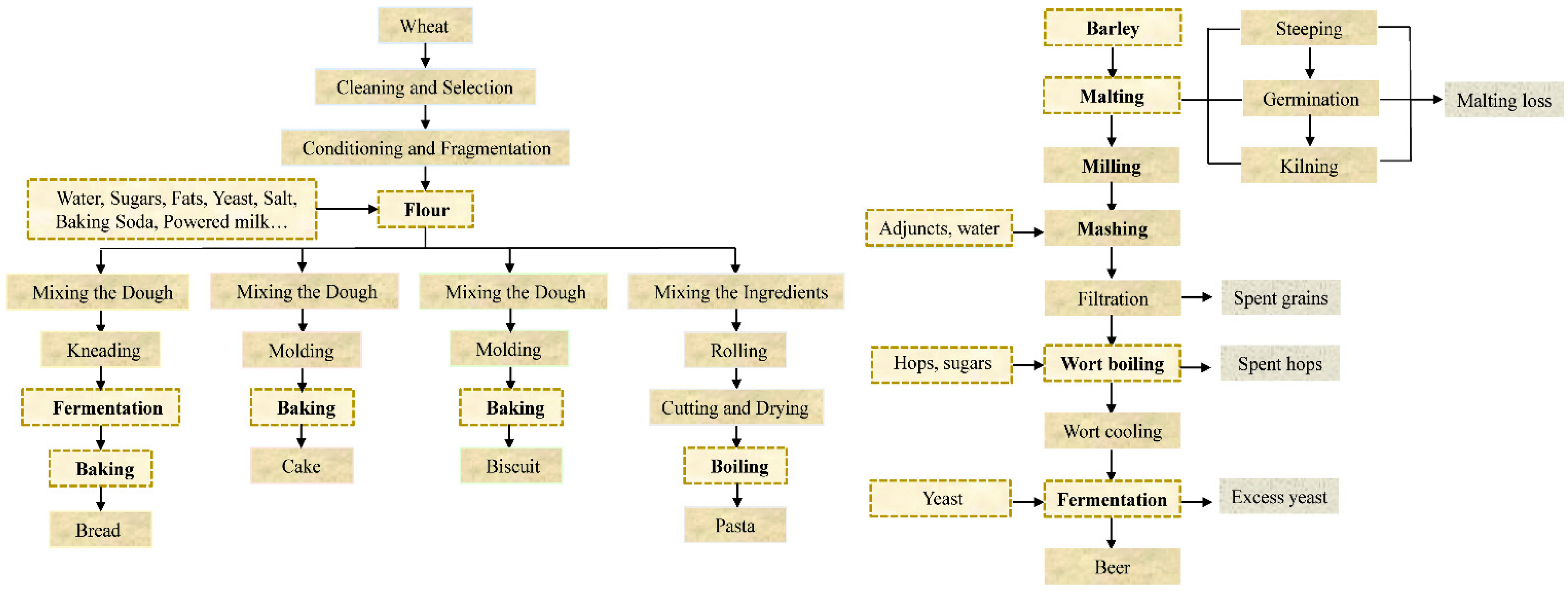
5. Fate of ZEA in Cereal-Based Food Processing
5.1. Bread
5.2. Biscuit and Cake
5.3. Pasta
5.4. Beer
6. Allowable Limit of ZEA in Cereal and Cereal-Based Food
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fast, R.B.; Caldwell, E.F. Breakfast Cereals, and How They Are Made; American Association of Cereal Chemists: Eagan, MN, USA, 2000. [Google Scholar]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and determination of acrylamide in the main group of cereal products using advanced microextraction method coupled with gas chromatography-mass spectrometry. J. Cereal Sci. 2019, 87, 157–164. [Google Scholar] [CrossRef]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Cereal Trade and Utilization in 2021/22 Revised Down. 2022. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 9 April 2022).
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Imran, M.; Cao, S.; Wan, S.; Chen, Z.; Saleemi, M.K.; Wang, N.; Naseem, M.; Munawar, J. Mycotoxins–a global one health concern: A review. Agrobiol. Rec. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Alborch, L.; Bragulat, M.R.; Castella, G.; Abarca, M.L.; Cabanes, F.J. Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 2012, 32, 97–103. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Gromadzka, K.; Wisniewska, H.; Golinski, P. Accumulation of zearalenone in genotypes of spring wheat after inoculation with Fusarium culmorum. Cereal Res. Commun. 2008, 36, 401–403. [Google Scholar]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, J.D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Paris, M.P.K.; Schweiger, W.; Hametner, C.; Stuckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A New Masked Mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Gbashi, S.; Njobeh, P.B. Occurrences of deoxynivalenol, zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. NFS J. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar]
- Poor, M.; Kunsagi-Mate, S.; Balint, M.; Hetenyi, C.; Gerner, Z.; Lemli, B. Interaction of mycotoxin zearalenone with human serum albumin. J. Photochem. Photobiol. B-Biol. 2017, 170, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Enzyme kinetics of zearalenone biotransformation: pH and cofactor effects. Arch. Toxicol. 2005, 79, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Said, S.; Sanchis, V. Influence of temperature, water activity and incubation time on fungal growth and production of ochratoxin A and zearalenone by toxigenic Aspergillus tubingensis and Fusarium incarnatum isolates in sorghum seeds. Int. J. Food Microbiol. 2017, 242, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, Y.R.; Jin, J.M.; Han, K.H.; Kim, H.; Kim, J.C.; Lee, T.; Yun, S.H.; Lee, Y.W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113. [Google Scholar] [CrossRef]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Environ. Microbiol. 2006, 72, 1793–1799. [Google Scholar] [CrossRef]
- Lysoe, E.; Bone, K.R.; Klemsdal, S.S. Real-Time Quantitative Expression Studies of the Zearalenone Biosynthetic Gene Cluster in Fusarium graminearum. Phytopathology 2009, 99, 176–184. [Google Scholar] [CrossRef]
- Lysoe, E.; Bone, K.R.; Klemsdal, S.S. Identification of up-regulated genes during zearalenone biosynthesis in Fusarium. Eur. J. Plant Pathol. 2008, 122, 505–516. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Atoui, A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant Pathol. 2021, 159, 247–258. [Google Scholar] [CrossRef]
- DallAsta, C.; Berthiller, F. Masked mycotoxins in food: Formation, occurrence and toxicological relevance. R. Soc. Chem. 2016, 24, 1–13. [Google Scholar]
- Kovac, M.; Subaric, D.; Bulaic, M.; Kovac, T.; Sarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arh. Hig. Rada. Toksikol. 2018, 69, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerkovich, N.; Palazzini, J.M.; Sulyok, M.; Chulze, S.N. Trichothecene genotypes, chemotypes and zearalenone production by Fusarium graminearum species complex strains causing Fusarium head blight in Argentina during an epidemic and non-epidemic season. Trop. Plant Pathol. 2017, 42, 190–196. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Brodehl, A.; Moller, A.; Kunte, H.J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A-Chem. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additives and Contaminants; WHO Technical Report Series; WHO: Geneva, Switzerland, 2001; 896p. [Google Scholar]
- Kirincic, S.; Skrjanc, B.; Kos, N.; Kozolc, B.; Pirnat, N.; Tavcar-Kalcher, G. Mycotoxins in cereals and cereal products in Slovenia—Official control of foods in the years 2008–2012. Food Control 2015, 50, 157–165. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Colovic, R.; Puvaca, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Duragic, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology; Academic Press: Cambrdige, MA, USA, 2018; pp. 1055–1063. [Google Scholar]
- Manstretta, V.; Rossi, V. Effects of Temperature and Moisture on Development of Fusarium graminearum Perithecia in Maize Stalk Residues. Appl. Environ. Microbiol. 2016, 82, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Aggarwal, A.; Paul, S.; Singh, V.; Singh, P.K.; Sharma, D.; Shaharan, M.S. Effect of temperature and pH on the growth of Fusarium spp. causing Fusarium head blight (FHB) in wheat. South Asian J. Exp. Biol 2016, 6, 186–193. [Google Scholar] [CrossRef]
- Edwards, S.G. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.X.; Xu, T.X.; Xu, W.T.; Asakiya, C.; Huang, K.L.; He, X.Y. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Berrada, H.; Miere, D.; Loghin, F.; Manes, J. Study on Trichothecene and Zearalenone Presence in Romanian Wheat Relative to Weather Conditions. Toxins 2019, 11, 163. [Google Scholar] [CrossRef]
- Marroquin-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Erbs, M.; Forrer, H.R.; Vogelgsang, S.; Wettstein, F.E.; Schwarzenbach, R.P.; Bucheli, T.D. Occurrence of zearalenone on Fusarium graminearum infected wheat and maize fields in crop organs, soil, and drainage water. Environ. Sci. Technol. 2008, 42, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xu, J.H.; Zhang, X.; Wang, S.F.; Xing, Y.J.; Mokoena, M.P.; Olaniran, A.O.; Shi, J.R. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvahuoko, J.; Malachova, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sievilainen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Bryla, M.; Waskiewicz, A.; Podolska, G.; Szymczyk, K.; Jedrzejczak, R.; Damaziak, K.; Sulek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Majerus, P.; Graf, N.; Kramer, M. Rapid determination of zearalenone in edible oils by HPLC with fluorescence detection. Mycotoxin Res. 2009, 25, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Pallaroni, L.; Bjorklund, E.; von Holst, C. Optimization of atmospheric pressure chemical ionization interface parameters for the simultaneous determination of deoxynivalenol and zearalenone using HPLC/MS. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 913–926. [Google Scholar] [CrossRef]
- Biselli, S.; Wegner, H.; Hummert, C. A multicomponent method for Fusarium toxins in cereal based food and feed samples using HPLC-MS/MS. Mycotoxin Res. 2005, 21, 18–22. [Google Scholar] [CrossRef]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Goryacheva, I.Y.; Basova, E.Y.; Franki, A.S.; Elewaut, D.; Van Beneden, K.; Deforce, D.; Van Peteghem, C.; De Saeger, S. Application of a new anti-zearalenone monoclonal antibody in different immunoassay formats. Anal. Bioanal. Chem. 2009, 395, 1301–1307. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Sahin, S.; Ustundag, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Beloglazova, N.V.; De Boevre, M.; Goryacheva, I.Y.; Werbrouck, S.; Guo, Y.; De Saeger, S. Immunochemical approach for zearalenone-4-glucoside determination. Talanta 2013, 106, 422–430. [Google Scholar] [CrossRef]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, H.G.; Sun, J.H.; Yan, Y.X. One-step rapid detection of fumonisin B-1, dexyonivalenol and zearalenone in grains. Food Control 2020, 117, 7. [Google Scholar] [CrossRef]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol’hakov, O.I.; De Saeger, S.; Beloglazova, N. Recent advances in electrochemical monitoring of zearalenone in diverse matrices. Food Chem. 2021, 353, 8. [Google Scholar] [CrossRef]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arevalo, F.J.; Fernandez, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuator B-Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Ji, X.D.; Yu, C.; Wen, Y.L.; Chen, J.; Yu, Y.J.; Zhang, C.L.; Gao, R.F.; Mu, X.Y.; He, J.L. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef]
- Sadrabadi, N.R.; Ensafi, A.A.; Heydari-Bafrooei, E.; Fazilati, M. Screening of Food Samples for Zearalenone Toxin Using an Electrochemical Bioassay Based on DNA-Zearalenone Interaction. Food Anal. Meth. 2016, 9, 2463–2470. [Google Scholar] [CrossRef]
- Afzali, D.; Padash, M.; Mostafavi, A. Determination of trace amounts of zearalenone in beverage samples with an electrochemical sensor. Mycotoxin Res. 2015, 31, 203–208. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Sant’Ana, A.S. Impact of unit operations during processing of cereal-based products on the levels of deoxynivalenol, total aflatoxin, ochratoxin A, and zearalenone: A systematic review and meta-analysis. Food Chem. 2018, 268, 611–624. [Google Scholar] [CrossRef]
- Heidari, S.; Milani, J.; Nazari, S. Effect of the bread-making process on zearalenone levels. Food Addit. Contam. Part A-Chem. 2014, 31, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of Zearalenone, Aflatoxin B-1, and Ochratoxin A by Yeast-Based Products: A Method for Quantification of Adsorption Performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef]
- El-Desouky, T.A.; Amer, M.M.; Naguib, K. Effects of pan bread making on zearalenone levels in artificial contaminated wheat flour. J. Agroaliment. Process. Technol. 2014, 20, 269–274. [Google Scholar]
- Cano-Sancho, G.; Sanchis, V.; Ramos, A.J.; Marin, S. Effect of food processing on exposure assessment studies with mycotoxins. Food Addit. Contam. Part A-Chem. 2013, 30, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bol, E.K.; Araujo, L.; Veras, F.F.; Welke, J.E. Estimated exposure to zearalenone, ochratoxin A and aflatoxin B1 through the consume of bakery products and pasta considering effects of food processing. Food Chem. Toxicol. 2016, 89, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J. Current views on the occurrence and significance of Fusarium toxins. J. Appl. Bacteriol. Symp. Suppl. 1989, 67, 89S–98S. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Raeisi, S.; Armoon, B.; Sant’Ana, A.S. Prevalence and concentration of ochratoxin A, zearalenone, deoxynivalenol and total aflatoxin in cereal-based products: A systematic review and meta-analysis. Food Chem. Toxicol. 2018, 118, 830–848. [Google Scholar] [CrossRef]
- Numanoglu, E.; Yener, S.; Gokmen, V.; Uygun, U.; Koksel, H. Modelling thermal degradation of zearalenone in maize bread during baking. Food Addit. Contam. Part A-Chem. 2013, 30, 528–533. [Google Scholar] [CrossRef]
- Numanoglu, E.; Uygun, U.; Koksel, H.; Solfrizzo, M. Stability of Fusarium toxins during traditional Turkish maize bread production. Qual. Assur. Saf. Crop. Foods 2010, 2, 84–92. [Google Scholar] [CrossRef]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Yoshinari, T.; Szymczyk, K.; Podolska, G.; Gwiazdowski, R.; Kubiak, K. Transformations of Selected Fusarium Toxins and Their Modified Forms During Malt Loaf Production. Toxins 2020, 12, 385. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Hazel, C.M.; Patel, S.; Scriven, F. Deoxynivalenol and other Fusarium mycotoxins in bread, cake, and biscuits produced from UK-grown wheat under commercial and pilot scale conditions. Food Addit. Contam. Part A-Chem. 2009, 26, 1191–1198. [Google Scholar] [CrossRef]
- Matsuura, Y.; Yoshizawa, T.; Morooka, N. Effects of food additives and heating on the decomposition of zearalenone in wheat flour. J. Food Hyg. Soc. Jpn. Shokuhin Eiseigaku Zasshi 1981, 22, 293–298. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Belakova, S.; Benesova, K.; Pernica, M.; Savi, G.D.; Rocha, L.O.; Hartman, I.; Caslavsky, J.; Correa, B. Fusarium Mycotoxins Stability during the Malting and Brewing Processes. Toxins 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Gil-Samarra, S.; Marin, S.; Ramos, A.J.; Sanchis, V. Fate of zearalenone, deoxynivalenol and deoxynivalenol-3-glucoside during malting process. LWT-Food Sci. Technol. 2019, 99, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, P.B.; Casper, H.H.; Beattie, S. Fate and Development of Naturally Occurring Fusarium Mycotoxins During Malting and Brewing. J. Am. Soc. Brew. Chem. 1995, 53, 121–127. [Google Scholar]
- El-Banna, A.A. Stability of citrinin and deoxynivalenol during germination process of barley. Mycotoxin Res. 1987, 3, 37–41. [Google Scholar] [CrossRef]
- Wall-Martinez, H.A.; Pascari, X.; Bigorda, A.; Ramos, A.J.; Marin, S.; Sanchis, V. The fate of Fusarium mycotoxins (deoxynivalenol and zearalenone) through wort fermenting by Saccharomyces yeasts (S. cerevisiae and S. pastorianus). Food Res. Int. 2019, 126, 8. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and Mycotoxin Content of Cereals in Southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Pascari, X.; Rodriguez-Carrasco, Y.; Juan, C.; Manes, J.; Marin, S.; Ramos, A.J.; Sanchis, V. Transfer of Fusarium mycotoxins from malt to boiled wort. Food Chem. 2019, 278, 700–710. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Stability of fumonisin B-1, deoxynivalenol, zearalenone, and T-2 toxin during processing of traditional Nigerian beer and spices. Mycotoxin Res. 2018, 34, 229–239. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marin, S.; Sanchis, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Wall-Martinez, H.A.; Pascari, X.; Ramos, A.J.; Marin, S.; Sanchis, V. Fate of the mycotoxins in the wort and yeast during ale and lager fermentation and their evaluation under different technological parameters. LWT-Food Sci. Technol. 2020, 132, 7. [Google Scholar] [CrossRef]
- Nkwe, D.O.; Taylor, J.E.; Siame, B.A. Fungi, aflatoxins, fumonisin B-1 and zearalenone contaminating sorghum-based traditional malt, wort and beer in Botswana. Mycopathologia 2005, 160, 177–186. [Google Scholar] [CrossRef]
- Scott, P.M.; Kanhere, S.R.; Daley, E.F.; Farber, J.M. Fermentation of wort containing deoxynivalenol and zearalenone. Mycotoxin Res. 1992, 8, 58–66. [Google Scholar] [CrossRef]
- Mizutani, K.; Nagatomi, Y.; Mochizuki, N. Metabolism of Zearalenone in the Course of Beer Fermentation. Toxins 2011, 3, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Fate of Fusarium mycotoxins during processing of Nigerian traditional infant foods (ogi and soybean powder). Food Res. Int. 2019, 116, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Vanegmond, H.P. Rationale for regulatory programmes for mycotoxins in human foods and animal feeds. Food Addit. Contam. 1993, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Correa, A.N.R.; Ferreira, C.D. Mycotoxins in Grains and Cereals Intended for Human Consumption: Brazilian Legislation, Occurrence Above Maximum Levels and Co-Occurrence. Food Rev. Int. 2022, 1–14. [Google Scholar] [CrossRef]
- Ji, F.; Xu, J.H.; Liu, X.; Yin, X.C.; Shi, J.R. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province. China Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. 2003. Available online: https://www.fao.org/3/y5499e/y5499e0d.htm (accessed on 9 April 2022).

| Masked ZEA | Cereal and Cereal Based Food | Incidence (%) | Mean (µg/kg) | Ref. |
|---|---|---|---|---|
| β-ZOL | Wheat | 10.0 | 3.5 | [48] |
| Wheat | 16.7 | 49 | [31] | |
| Wheat | 44.4 | 12 | [49] | |
| Wheat | 100 | 7 | [49] | |
| Wheat | 44.4 | 6 | [49] | |
| Maize | 100 | 11 | [14] | |
| Maize | 66.7 | 42.5 | [31] | |
| Oats | 32.3 | 3.0 | [48] | |
| Oats | 16.7 | 46 | [31] | |
| Millet | 100 | 16 | [14] | |
| Sorghum | 95 | 27 | [14] | |
| Barley | 2.9 | 2.0 | [48] | |
| Bread | 50 | 79 | [31] | |
| Cornflakes | 66.7 | 53.3 | [31] | |
| α-ZOL | Maize | 100 | 27 | [14] |
| Maize | 100 | 96.8 | [31] | |
| Wheat | 13.3 | 0.6 | [31] | |
| Wheat | 11.1 | 8 | [49] | |
| Oats | 9.7 | 1.9 | [48] | |
| Oats | 33.3 | 59.5 | [31] | |
| Sorghum | 100 | 8 | [14] | |
| Millet | 100 | 18 | [14] | |
| Barley | 2.9 | 0.6 | [48] | |
| Bread | 33.3 | 64 | [31] | |
| Cornflakes | 33.3 | 30 | [31] | |
| ZEA-4-sulfate | Maize | 16.7 | 51 | [31] |
| Wheat | 33.3 | 11 | [31] | |
| Oats | 16.7 | 12 | [31] | |
| Bread | 16.7 | 24 | [31] | |
| ZEA-14-sulfate | Barley | 8.8 | 10.6 | [48] |
| Oats | 29.0 | 31.6 | [48] | |
| Wheat | 40.0 | 4.9 | [48] | |
| ZEA-16-glucoside | Barley | 23.5 | <LOQ | [48] |
| Oats | 58.1 | 4.2 | [48] | |
| Wheat | 6.7 | 2.1 | [48] | |
| ZEA-14-glucoside | Barley | 17.6 | 2.7 | [48] |
| Oats | 3.2 | <LOQ | [48] | |
| Wheat | 6.7 | 0.6 | [48] | |
| ZEA-4-glucoside | Maize | 16.7 | 274 | [31] |
| Bread | 33.3 | 20 | [31] | |
| β-ZOL-4-glucoside | Maize | 50 | 152 | [31] |
| Oats | 16.7 | 20 | [31] | |
| α-ZOL-14-glucoside | Barley | 23.5 | 2.9 | [48] |
| Wheat | 16.7 | 3.1 | [48] | |
| α-ZOL-4-glucoside | Maize | 16.7 | 283 | [31] |
| β-ZOL-14-glucoside | Barley | 2.9 | 0.7 | [48] |
| Regulatory Bodies | Cereal and Cereal-Based Food | Maximum Levels (µg/kg) | Ref. |
|---|---|---|---|
| European Union | Wheat | 100 | [92] |
| Corn | 350 | ||
| Cereal, cereal flour, and bran for direct consumption | 75 | ||
| Corn flour | 200 | ||
| Bread and other bakeries | 50 | ||
| Cereal snacks | 50 | ||
| Corn-based snacks | 100 | ||
| Brazil | Wheat flour, pasta, crackers and bakery products, cereals and cereal products (except wheat), and malted barley | 100 | [93] |
| Processed rice and derivates | 100 | ||
| Brown rice | 400 | ||
| Rice bran | 600 | ||
| Corn-based products | 150 | ||
| Whole wheat, whole wheat flour, wheat bran | 200 | ||
| Corn and wheat in grains | 400 | ||
| China | Wheat, wheat flour | 60 | [94] |
| Corn, corn flour | 60 | ||
| Australia | Cereals | 50 | [95] |
| Chile | All foods | 200 | [95] |
| Armenia | All foods | 1000 | |
| Belarus | Barley, wheat, maize | 1000 | |
| Colombia | Sorghum | 1000 | |
| Indonesia | Maize | Not detectable | |
| Iran | Barley | 400 | |
| Maize, wheat, rice | 200 | ||
| Moldova | Wheat and wheat flour, barley and barley flour, maize and maize flour | 1000 | |
| Morocco | Cereals | 200 | |
| Russia | Wheat, barley, maize, corn | 1000 | |
| Serbia and Montenegro | Corn | 1000 | |
| Ukraine | Grains, beans, sunflower press, flour, bread, all nuts, all seeds to be used for immediate human consumption and for processing into the products for human consumption, wheat middlings | 1000 | |
| Uruguay | Corn, barley | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Zhang, J.; Chen, Y.; Zhu, J. Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing. J. Fungi 2022, 8, 976. https://doi.org/10.3390/jof8090976
Yu H, Zhang J, Chen Y, Zhu J. Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing. Journal of Fungi. 2022; 8(9):976. https://doi.org/10.3390/jof8090976
Chicago/Turabian StyleYu, Huilin, Junhui Zhang, Yixuan Chen, and Jiajin Zhu. 2022. "Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing" Journal of Fungi 8, no. 9: 976. https://doi.org/10.3390/jof8090976
APA StyleYu, H., Zhang, J., Chen, Y., & Zhu, J. (2022). Zearalenone and Its Masked Forms in Cereals and Cereal-Derived Products: A Review of the Characteristics, Incidence, and Fate in Food Processing. Journal of Fungi, 8(9), 976. https://doi.org/10.3390/jof8090976






