Fungal Diversity and Its Relationship with Environmental Factors in Coastal Sediments from Guangdong, China
Abstract
:1. Introduction
2. Materials and Methods
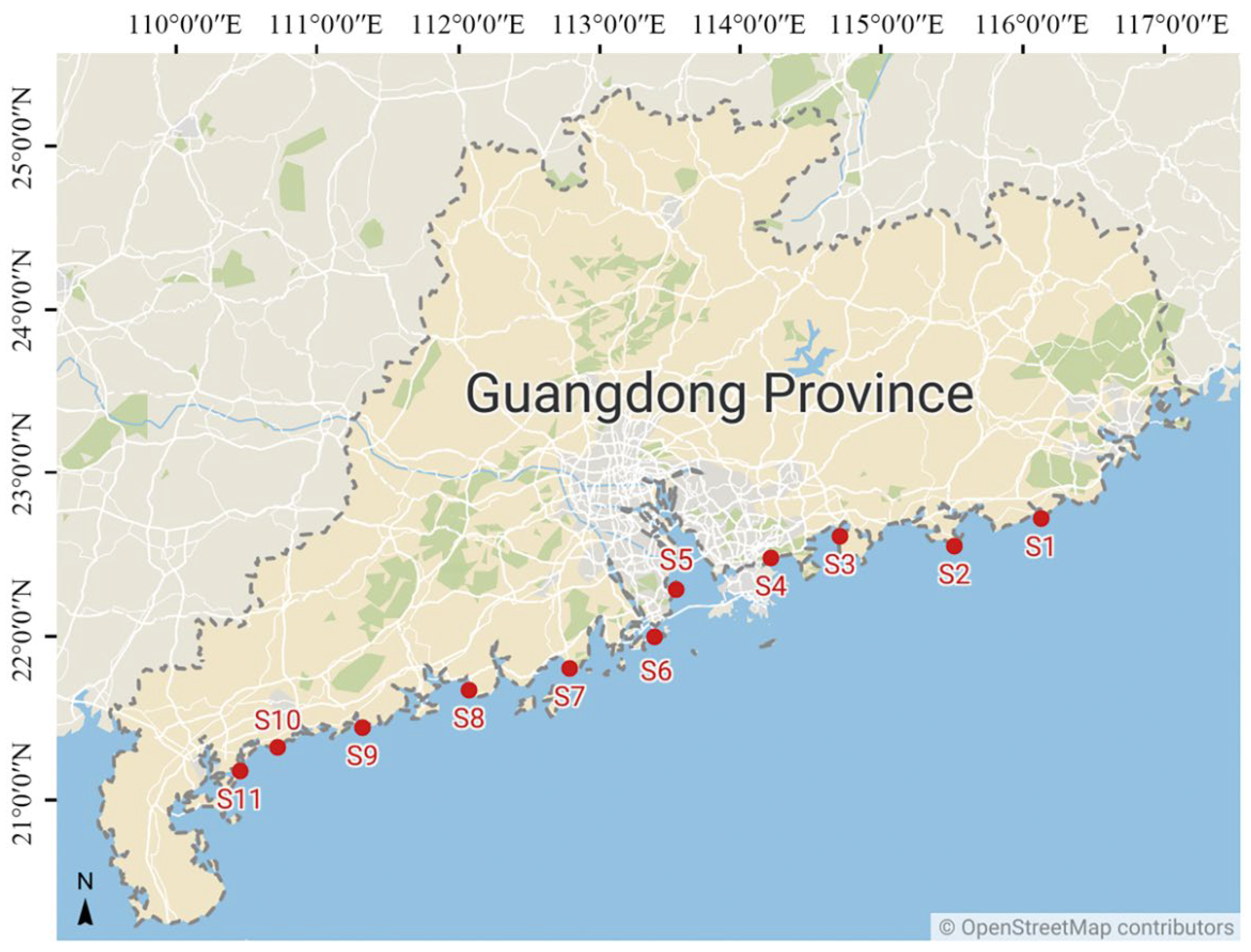
2.1. Study Sites and Sample Collection
2.2. Determination of Environmental Factors
2.3. DNA Extraction and Sequencing Analysis of Fungi
2.4. Statistical Analyses
2.5. Accession Numbers for Nucleotide Sequences
3. Results
3.1. Physicochemical Properties of Coastal Sediments
3.2. Composition and Distribution of Fungi in Coastal Sediments
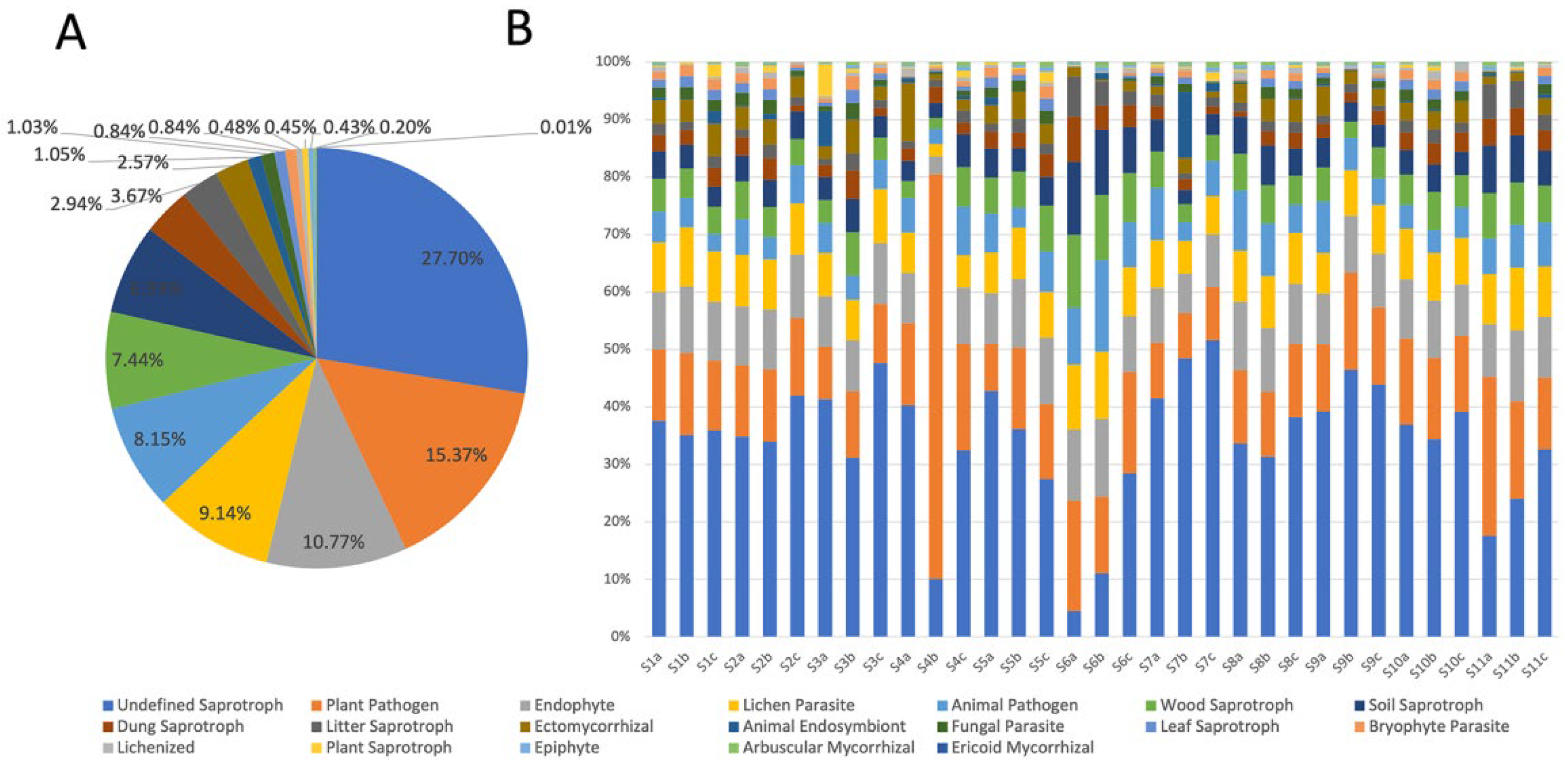
3.3. Functional Prediction of Fungi by FUNGuild
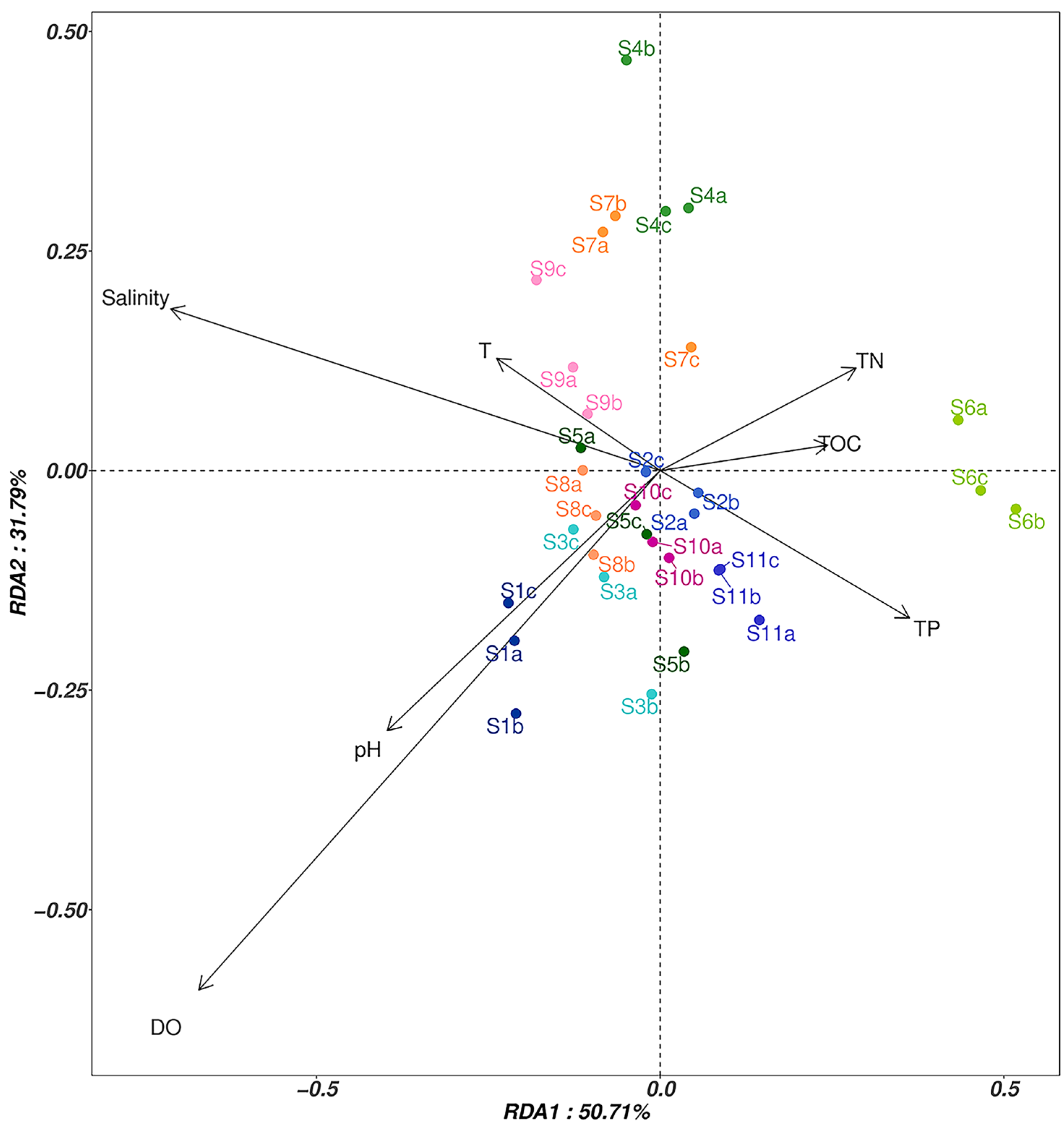
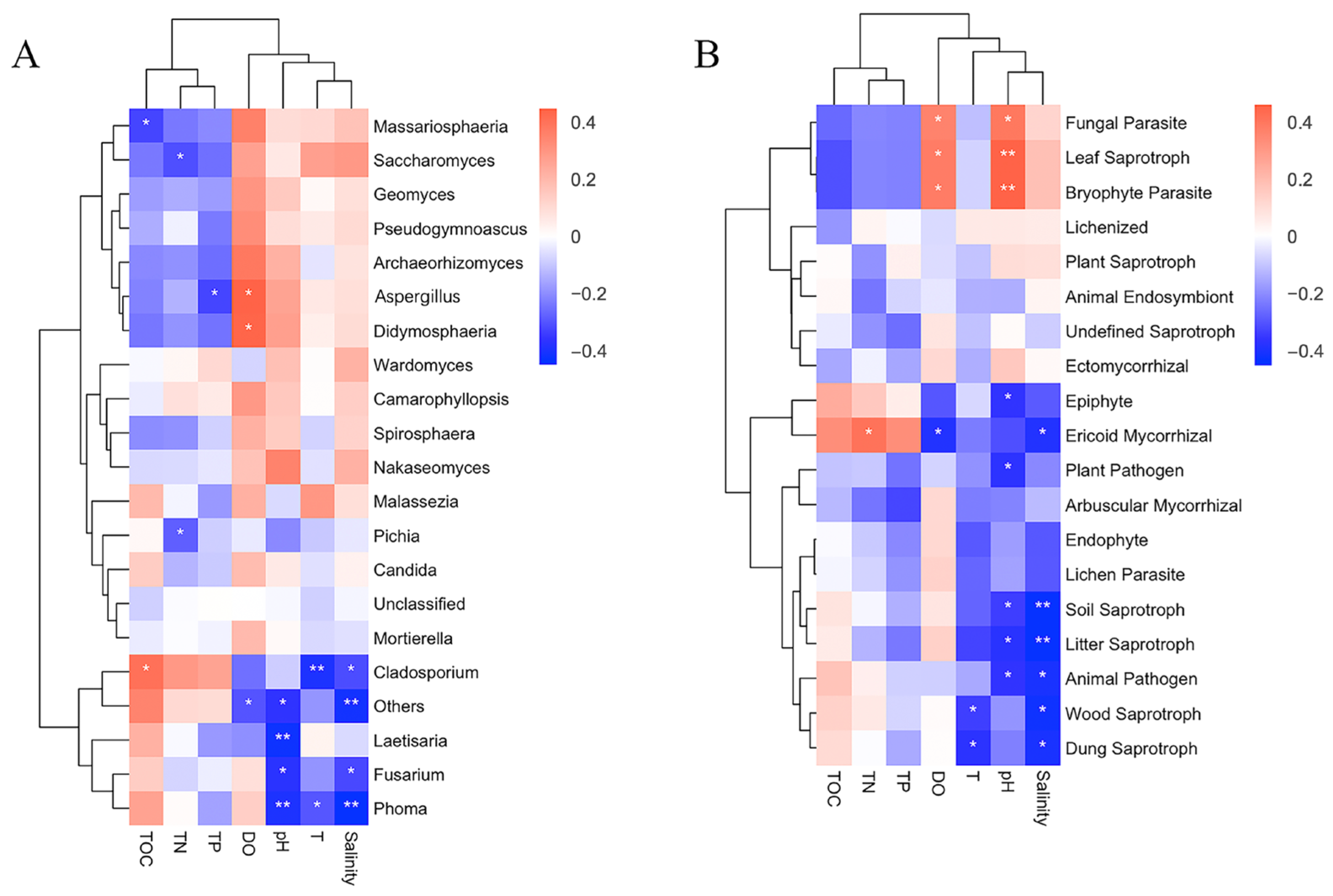
3.4. Correlation Analysis between Fungi and Environmental Factors
3.5. Correlation Analysis between Trophic Modes and Environmental Factors
4. Discussion
4.1. Environmental Factors in Coastal Sediments
4.2. Fungal Diversity and Distribution
4.3. Correlation Analysis between the Fungal Communities and Environmental Factors
4.4. Correlation Analysis between Fungal Function Prediction and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, M.S.; Oh, S.; Fong, J.J.; Houbraken, J.; Lim, Y.W. The Diversity and ecological roles of penicillium in intertidal zones. Sci. Rep. 2019, 9, 13540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, J.P.; Buser, T.J.; Burns, M.D.; Simons, A.M.; Hundt, P.J. Patterns of body shape diversity and evolution in intertidal and subtidal lineages of Combtooth Blennies (Blenniidae). Integr. Org. Biol. 2021, 3, obab004. [Google Scholar] [CrossRef]
- Fozia; Zheng, Y.; Hou, L.; Zhang, Z.; Chen, F.; Gao, D.; Yin, G.; Han, P.; Dong, H.; Liang, X.; et al. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in sediments of the Indus Estuary. Estuar. Coast. Shelf S. 2020, 243, 106925. [Google Scholar] [CrossRef]
- Miettinen, H.; Bomberg, M.; Nyyssönen, M.; Reunamo, A.; Jørgensen, K.S.; Vikman, M. Oil degradation potential of microbial communities in water and sediment of Baltic Sea coastal area. PLoS ONE 2019, 14, e0218834. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Fang, X.; Wu, H.; Cai, X.; Xiao, H. Effects of plant cultivars on the structure of bacterial and fungal communities associated with ginseng. Plant Soil. 2021, 465, 143–156. [Google Scholar] [CrossRef]
- Hartmann, M.; Niklaus, P.A.; Zimmermann, S.; Schmutz, S.; Kremer, J.; Abarenkov, K.; Lüscher, P.; Widmer, F.; Frey, B. Resistance and resilience of the forest soil microbiome to logging-associated compaction. ISME J. 2014, 8, 226–244. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Wang, J.; Wang, L.; Li, X.; Zhang, D. Unique functional responses of fungal communities to various environments in the mangroves of the Maowei Sea in Guangxi, China. Mar. Pollut. Bull. 2021, 173, 113091. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Bio. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.; He, Y.; Wu, J.; Jiang, Y.; Tam, N.F.; Zhou, H. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; McIlroy, S.E.; Archana, A.; Baker, D.M.; Panagiotou, G. A Pollution gradient contributes to the taxonomic, functional, and resistome diversity of microbial communities in marine sediments. Microbiome 2019, 7, 104. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Li, H.; Wang, H.; Wang, Y.; Xu, J.; Ding, K.; Zhu, Y. Bacterial communities are more sensitive to ocean acidification than fungal communities in estuarine sediments. FEMS Microbio. Ecol. 2021, 97, fiab058. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, Y.; Xiong, S.; Yu, H.; Zhao, X.; Tan, W.; Cui, D.; Xi, B. Untangling the response of fungal community structure, composition and function in soil aggregate fractions to food waste compost addition. Sci. Total Environ. 2021, 769, 145248. [Google Scholar] [CrossRef]
- Hui, E.C.M.; Li, X.; Chen, T.; Lang, W. Deciphering the spatial structure of China’s megacity region: A new bay area—The Guangdong-Hong Kong-Macao Greater Bay Area in the making. Cities 2020, 105, 102168. [Google Scholar] [CrossRef]
- Yu, S.; Pang, Y.; Wang, Y.; Li, J.; Qin, S. Spatial variation of microbial communities in sediments along the environmental gradients from Xiaoqing River to Laizhou Bay. Mar. Pollut. Bull. 2017, 120, 90–98. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Z.G.; Liu, S.M.; Xu, H.; Wen, Q.B.; Shao, B.; Chen, J.F. Dominance of terrigenous particulate organic carbon in the high-turbidity Shuangtaizihe estuary. Chem. Geol. 1997, 138, 211–219. [Google Scholar] [CrossRef]
- Xu, C.; Yang, B.; Dan, S.F.; Zhang, D.; Liao, R.; Lu, D.; Li, R.; Ning, Z.; Peng, S. Spatiotemporal variations of biogenic elements and sources of sedimentary organic matter in the largest oyster mariculture bay (Maowei Sea), Southwest China. Sci. Total Environ. 2020, 730, 139056. [Google Scholar] [CrossRef]
- Yang, B.; Song, G.; Liu, S.; Jin, J. Phosphorus recycling and burial in core sediments of the East China Sea. Mar. Chem. 2017, 192, 59–72. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Liu, M.; Gong, J.; Zhang, X.; Yin, G.; You, L. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary: Anammox in the Yangtze Estuary. J. Geophys. Res. Biogeosci. 2013, 118, 1237–1246. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the dermination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 678–681. [Google Scholar]
- Thiyahuddin, N.M.; Lamping, E.; Rich, A.M.; Cannon, R.D. Yeast species in the oral cavities of older people: A comparison between people living in their own homes and those in rest homes. J. Fungi 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Tao, J. Distribution of the potential pathogenic alternaria on plant leaves determines foliar fungal communities around the disease spot. Environ. Res. 2021, 11, 111715. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sen, K.; He, Y.; Xie, Y.; Wang, G. Impact of environmental gradients on the abundance and diversity of planktonic fungi across coastal habitats of contrasting trophic status. Sci. Total Environ. 2019, 683, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Mbareche, H.; Veillette, M.; Bilodeau, G.J. In Silico Study Suggesting the Bias of Primers Choice in the Molecular Identification of Fungal Aerosols. J. Fungi 2021, 7, 99. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef] [Green Version]
- Diederich, P.; Lawrey, J.D.; Sikaroodi, M.; Gillevet, P.M. A new lichenicolous teleomorph is related to plant pathogens in Laetisaria and Limonomyces (Basidiomycota, Corticiales). Mycologia 2011, 103, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Fournier, J.; Crous, P.W.; Pointing, S.B.; Hyde, K.D. Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales). Persoonia 2009, 23, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-González, J.A.; Vargas-García, M.d.C.; López, M.J.; Suárez-Estrella, F.; Jurado, M.d.M.; Moreno, J. Biodiversity and succession of mycobiota associated to agricultural lignocellulosic waste-based composting. Bioresour. Technol. 2015, 187, 305–313. [Google Scholar] [CrossRef]
- Meerupati, T.; Andersson, K.M.; Friman, E.; Kumar, D.; Tunlid, A.; Ahrén, D. Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet. 2013, 9, e1003909. [Google Scholar] [CrossRef] [Green Version]
- Samarakoon, B.C.; Phookamsak, R.; Wanasinghe, D.N.; Chomnunti, P.; Hyde, K.D.; Mckenzie, E.H.C.; Promputtha, I.; Xu, J.C.; Li, Y.J. Taxonomy and phylogenetic appraisal of Spegazzinia Musae sp. Nov. and S. Deightonii (Didymosphaeriaceae, Pleosporales) on Musaceae from Thailand. MycoKeys 2020, 70, 19–37. [Google Scholar] [CrossRef]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araújo, W.L.; dos Santos, D.R.; Azevedo, J.L. Diversity of endophytic fungal community of cacao (Theobromacacao L.) and biological control of CrinipellisPerniciosa, causal agent of Witches’ Broom Disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Duan, G.; Li, G.; Zhu, D. Insights into the role of the fungal community in variations of the antibiotic resistome in the soil collembolan gut microbiome. Environ. Sci. Technol. 2021, 55, 11784–11794. [Google Scholar] [CrossRef]
- Rajashekhar, M.; Kaveriappa, K.M. Studies on the aquatic hyphomycetes of a sulfur spring in the Western Ghats, India. Microb. Ecol. 1996, 32, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Li, J.; Zhang, Y.J. Mitochondrial genome, comparative analysis and evolutionary insights into the entomopathogenic fungus Hirsutella Thompsonii: Mitogenome and intron evolution in H. Thompsonii. Environ. Microbiol. 2018, 20, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.; Coetzee, M.P.A. First report of pink disease on native trees in South Africa and phylogenetic placement of Erythricium Salmonicolor in the homobasidiomycetes. Plant Dis. 2005, 89, 1158–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yang, X.; Zhang, L.; Cao, H.; Cao, P.; Wang, X.; Xiang, W.; Zhao, J. First report of leaf blight caused by Limonomyces Roseipellis on maize (Zea Mays) in China. Plant Dis. 2021, 105, 2730. [Google Scholar] [CrossRef] [PubMed]
- Riess, K.; Bauer, R.; Kellner, R.; Kemler, M.; Piątek, M.; Vánky, K.; Begerow, D. Identification of a new order of root-colonising fungi in the Entorrhizomycota: Talbotiomycetalesord. nov. on eudicotyledons. IMA Fungus 2015, 6, 129–133. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Mohammed, C. Pathogenicity of fungi associated with stem cankers of eucalypts in Tasmania, Australia. Plant Dis. 1999, 83, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, X.; Cui, Y.; Wang, A.; Lai, D.; Liu, Y.; Li, Q.X.; Wang, B.; Zhou, L. A monoclonal antibody-based enzyme-linked immunosorbent assay for detection of ustiloxin A in rice false smut balls and rice samples. Food Chem. 2015, 181, 140–145. [Google Scholar] [CrossRef]
- Tian, K.; Wu, Q.; Liu, P.; Hu, W.; Huang, B.; Shi, B.; Zhou, Y.; Kwon, B.O.; Choi, K.; Ryu, J.; et al. Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ. Int. 2020, 136, 105512. [Google Scholar] [CrossRef]
- Gao, C.; Yu, F.; Chen, J.; Huang, Z.; Jiang, Y.; Zhuang, Z.; Xia, T.; Kuehl, S.A.; Zong, Y. Anthropogenic impact on the organic carbon sources, transport and distribution in a subtropical semi-enclosed bay. Sci. Total Environ. 2021, 767, 145047. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.; Cartwright, I.; Poh, S.C.; Cook, P. Sources and cycling of nitrogen revealed by stable isotopes in a highly populated large temperate coastal embayment. Sci. Total Environ. 2022, 806, 150408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, B.; Xu, G.; Liu, F. Effects of organic phosphorus on methylotrophic methanogenesis in coastal lagoon sediments with seagrass (Zostera Marina) colonization. Front. Microbiol. 2020, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.; Pan, H.; Burgaud, G.; Liang, S.; Guo, J.; Luo, T.; Li, Z.; Zhang, S.; Cai, L. Highlighting patterns of fungal diversity and composition shaped by ocean currents using the East China Sea as a model. Mol. Ecol. 2018, 27, 564–576. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Bian, X.; Guo, J.; Cai, L. A high-level fungal diversity in the intertidal sediment of chinese seas presents the spatial variation of community composition. Front. Microbiol. 2016, 7, 2098. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Huang, J.; Luo, K.; Lin, X.; Su, M.; Lu, J. Bacterial, archaeal, and fungal community structure and interrelationships of deep-sea shrimp intestine and the surrounding sediment. Environ. Res. 2022, 205, 112461. [Google Scholar] [CrossRef]
- Alburae, N.A.; Mohammed, A.E.; Alorfi, H.S.; Turki, A.J.; Asfour, H.Z.; Alarif, W.M.; Abdel-Lateff, A. Nidulantes of Aspergillus (formerly Emericella): A treasure trove of chemical diversity and biological activities. Metabolites 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosling, A.; Cox, F.; Cruz-Martinez, K.; Ihrmark, K.; Grelet, G.A.; Lindahl, B.D.; Menkis, A.; James, T.Y. Archaeorhizomycetes: Unearthing an ancient class of ubiquitous soil fungi. Science 2011, 333, 876–879. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D.; Bruno, J.F.; Li, B.; Chen, G.; Coverdale, T.C.; Altieri, A.H.; Bai, J.; Sun, T.; Pennings, S.C.; et al. Economic development and coastal ecosystem change in China. Sci. Rep. 2015, 4, 5995. [Google Scholar] [CrossRef] [Green Version]
- Cathrine, S.J.; Raghukumar, C. Anaerobic denitrification in fungi from the coastal marine sediments off Goa, India. Mycol. Res. 2009, 113, 100–109. [Google Scholar] [CrossRef]
- Barik, S.K.; Bramha, S.; Behera, D.; Bastia, T.K.; Cooper, G.; Rath, P. Ecological health assessment of a coastal ecosystem: Case study of the largest brackish water lagoon of Asia. Mar. Pollut. Bull. 2019, 138, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhao, H.; Edwards, N.; Peyer, L.; Tao, Y.; Arneborg, N. The effects of cell-cell contact between Pichia Kluyveri and Saccharomyces Cerevisiae on amino acids and volatiles in mixed culture alcoholic fermentations. Food Microbiol. 2022, 103, 103960. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, W.T.; van Mastrigt, O.; Block, D.E.; Notebaart, R.A.; Smid, E.J.; Cuomo, C.A. Nitrogenous compound utilization and production of volatile organic compounds among commercial wine yeasts highlight strain-specific metabolic diversity. Microbiol. Spectr. 2021, 9, e00485-21. [Google Scholar] [CrossRef] [PubMed]
- Arab, G.; Razaviarani, V.; Sheng, Z.; Liu, Y.; McCartney, D. Benefits to decomposition rates when using digestate as compost co-feedstock: Part II—Focus on microbial community dynamics. Waste Manage. 2017, 68, 85–95. [Google Scholar] [CrossRef]
- Tian, H.; Wang, H.; Hui, X.; Wang, Z.; Drijber, R.A.; Liu, J. Changes in soil microbial communities after 10 years of winter wheat cultivation versus fallow in an organic-poor soil in the loess plateau of China. PLoS ONE 2017, 12, e0184223. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiyarifar, M.; Enayatizamir, N.; Mehdi Khanlou, K. Biochemical and molecular investigation of non-rhizobial endophytic bacteria as potential biofertilisers. Arch. Microbiol. 2021, 203, 513–521. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.; Zhang, Y.; Liu, H.; Yu, L. Diversity and distribution of aquatic fungal communities in the Ny-Ålesund region, Svalbard (high arctic): Aquatic fungi in the arctic. Microb. Ecol. 2016, 71, 543–554. [Google Scholar] [CrossRef]
- Chung, D.; Kim, H.; Choi, H.S. Fungi in salterns. J. Microbiol. 2019, 57, 717–724. [Google Scholar] [CrossRef]
- Retter, A.; Nilsson, R.H.; Bourlat, S.J. Exploring the taxonomic composition of two fungal communities on the Swedish West Coast through metabarcoding. Biodivers. Data J. 2019, 7, e35332. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gao, L.; Zhang, S. Effects of bird aggregation on the soil properties and microbial community diversity of urban forest fragments. Sci. Total Environ. 2020, 737, 140250. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiang, W.; Wu, H.; Ouyang, S.; Lei, P.; Hu, Y.; Ge, T.; Ye, J.; Kuzyakov, Y. Contrasting patterns and drivers of soil fungal communities in subtropical deciduous and evergreen broadleaved forests. Appl. Microbiol. Biotechnol. 2019, 103, 5421–5433. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, H.; Liu, Z.; Sui, G.; Li, D.; Kan, H.; Zhao, Z.; Hu, W.; Chen, J. The decay of airborne bacteria and fungi in a constant temperature and humidity test chamber. Environ. Int. 2021, 157, 106816. [Google Scholar] [CrossRef] [PubMed]
- Schröter, K.; Wemheuer, B.; Pena, R.; Schöning, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly processes of trophic guilds in the root mycobiome of temperate forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (high arctic). Sci. Rep. 2015, 5, 14524. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Pang, K.L.; Luo, Z.H. High fungal diversity and abundance recovered in the deep-sea sediments of the Pacific Ocean. Microb. Ecol. 2014, 68, 688–698. [Google Scholar] [CrossRef]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.; Barac, A.; Bostanaru, A.C.; Brito, S.; Bull, M.; Çerikçioğlu, N.; Chapman, B.; Efstratiou, M.A.; et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef]
- Wentzel, L.C.P.; Inforsato, F.J.; Montoya, Q.V.; Rossin, B.G.; Nascimento, N.R.; Rodrigues, A.; Sette, L.D. Fungi from Admiralty bay (King George Island, Antarctica) soils and marine sediments. Microb. Ecol. 2019, 77, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Micalizzi, E.W.; Smith, M.L. Volatile organic compounds kill the white-nose syndrome fungus, Pseudogymnoascus Destructans, in hibernaculum sediment. Can. J. Microbiol. 2020, 66, 593–599. [Google Scholar] [CrossRef]
- VanWormer, E.; Carpenter, T.E.; Singh, P.; Shapiro, K.; Wallender, W.W.; Conrad, P.A.; Largier, J.L.; Maneta, M.P.; Mazet, J.A.K. Coastal development and precipitation drive pathogen flow from land to sea: Evidence from a toxoplasma gondii and felid host system. Sci. Rep. 2016, 6, 29252. [Google Scholar] [CrossRef]
- Krause, E.; Wichels, A.; Giménez, L.; Gerdts, G. Marine fungi may benefit from ocean acidification. Aquat. Microb. Ecol. 2013, 69, 59–67. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, Y.; Zhang, X.; Hu, H.; Cheng, Y.; Borjigin, S. Comparison of the responses of soil fungal community to straw, inorganic fertilizer, and compost in a farmland in the Loess Plateau. Microbiol. Spectr. 2022, 10, e02230-21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 7. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhang, X.L.; Wang, Y.; Zheng, J.Y.; Wang, C.Y.; Shao, C.L. Aspergivones A and B, two new flavones isolated from a gorgonian-derived Aspergillus candidus fungus. Nat. Prod. Res. 2017, 31, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Pei, Z.; Meng, L.; Zheng, Y.; Wang, L.; Feng, R.; Li, Q.; Liu, Y.; Zhao, X.; Wei, Q.; et al. Determination of community structure and diversity of seed-vectored endophytic fungi in Alpinia zerumbet. Front. Microbiol. 2022, 13, 814864. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]

| Sites | Sequence Read | OTUs | Chao1 | ACE | Shannon | Simpson |
|---|---|---|---|---|---|---|
| S1 | 83,567 ± 4507 ab | 151 ± 4 c | 486 ± 50 ab | 466 ± 39 ab | 5.8 ± 0.55 ab | 0.91 ± 0.05 ab |
| S2 | 78,526 ± 9626 ab | 150 ± 3 c | 444 ± 72 ab | 434 ± 70 ab | 4.6 ± 1.40 b | 0.80 ± 0.18 ab |
| S3 | 70,227 ± 8075 b | 179 ± 1 a | 514 ± 35 a | 495 ± 27 a | 6.1 ± 0.27 a | 0.95 ± 0.01 a |
| S4 | 66,106 ± 5547 b | 156 ± 5 bc | 423 ± 42 b | 418 ± 44 b | 6.0 ± 0.41 a | 0.94 ± 0.02 ab |
| S5 | 73,033 ± 9366 ab | 154 ± 4 bc | 319 ± 14 c | 296 ± 14 c | 4.0 ± 0.36 b | 0.69 ± 0.06 b |
| S6 | 86,543 ± 16,540 a | 181 ± 21 a | 369 ± 65 bc | 366 ± 66 bc | 5.2 ± 1.07 ab | 0.90 ± 0.08 ab |
| S7 | 86,324 ± 12,343 ab | 158 ± 6 bc | 421 ± 50 b | 411 ± 50 b | 5.0 ± 0.34 ab | 0.86 ± 0.05 ab |
| S8 | 83,899 ± 7918 ab | 158 ± 11 bc | 428 ± 55 b | 424 ± 55 ab | 5.2 ± 1.23 ab | 0.81 ± 0.15 ab |
| S9 | 70,458 ± 3473 ab | 139 ± 3 c | 396 ± 33 bc | 386 ± 36 b | 4.6 ± 0.74 b | 0.79 ± 0.11 b |
| S10 | 64,059 ± 13,164 b | 149 ± 2 c | 387 ± 30 bc | 380 ± 32 b | 5.6 ± 0.92 ab | 0.89 ± 0.10 ab |
| S11 | 72,766 ± 7313 ab | 165 ± 5 b | 370 ± 20 bc | 365 ± 20 bc | 4.2 ± 0.37 b | 0.74 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Liu, Y.; Liao, X.; Yang, X.; Chen, Z.; Mo, L.; Zhong, S.; Zhang, X. Fungal Diversity and Its Relationship with Environmental Factors in Coastal Sediments from Guangdong, China. J. Fungi 2023, 9, 101. https://doi.org/10.3390/jof9010101
Wu K, Liu Y, Liao X, Yang X, Chen Z, Mo L, Zhong S, Zhang X. Fungal Diversity and Its Relationship with Environmental Factors in Coastal Sediments from Guangdong, China. Journal of Fungi. 2023; 9(1):101. https://doi.org/10.3390/jof9010101
Chicago/Turabian StyleWu, Keyue, Yongchun Liu, Xinyu Liao, Xinyue Yang, Zihui Chen, Li Mo, Saiyi Zhong, and Xiaoyong Zhang. 2023. "Fungal Diversity and Its Relationship with Environmental Factors in Coastal Sediments from Guangdong, China" Journal of Fungi 9, no. 1: 101. https://doi.org/10.3390/jof9010101





