Volatile Metabolites in Lavage Fluid Are Correlated with Cytokine Production in a Valley Fever Murine Model
Abstract
1. Introduction
2. Methods and Materials
2.1. Mice
2.2. Pulmonary Coccidioidal Infections
2.3. Cytokine Analysis
2.4. Volatile Metabolomic Analysis by SPME-GC×GC-TOFMS
2.5. Processing and Analysis of Chromatographic Data
2.6. Data Postprocessing and Statistical Analyses
2.6.1. Cytokine Data
2.6.2. Volatile Data
3. Results
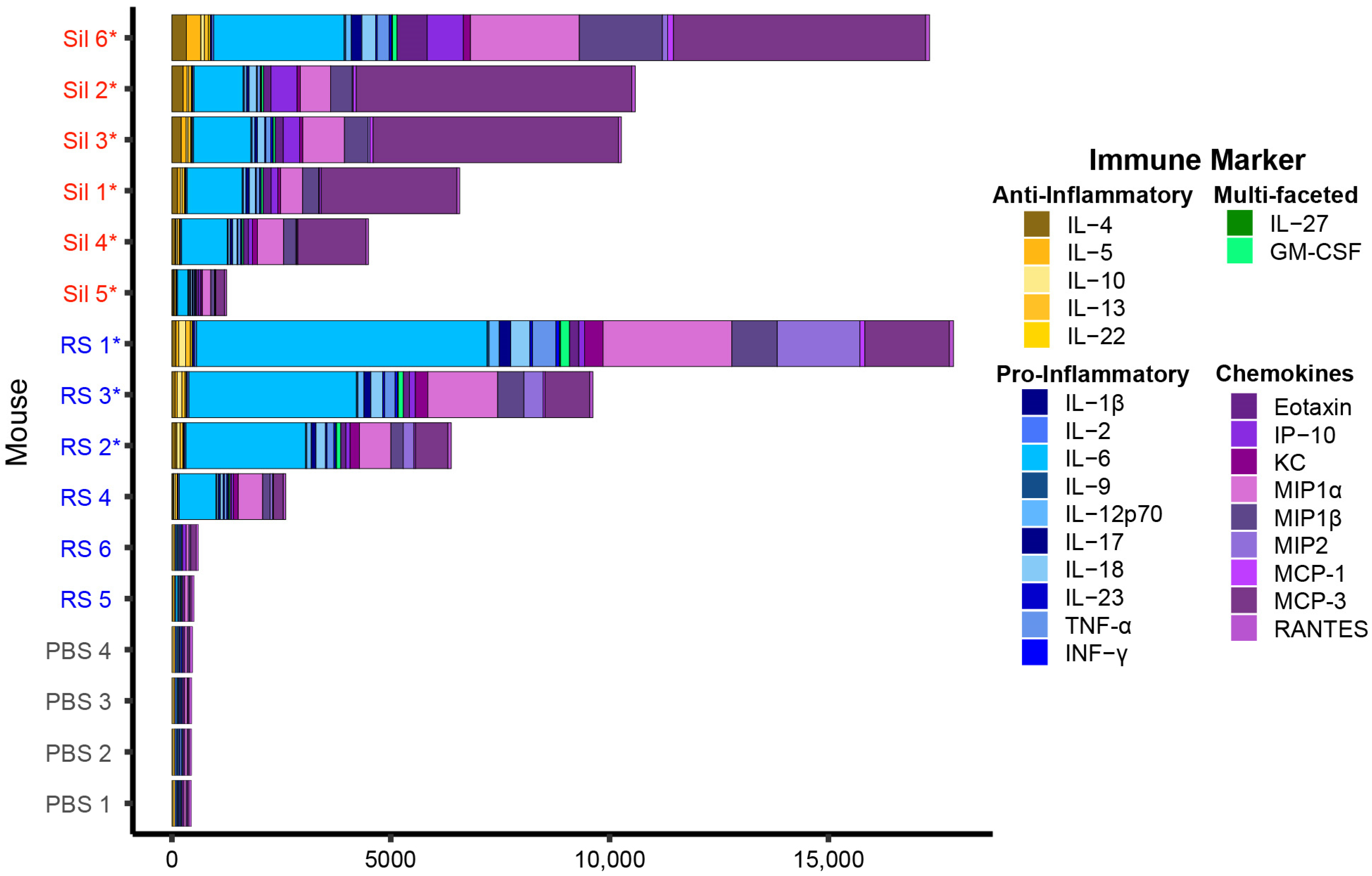
3.1. Coccidioides-Infected C57BL/6J Mice Exhibit a Gradient of Cytokine Production
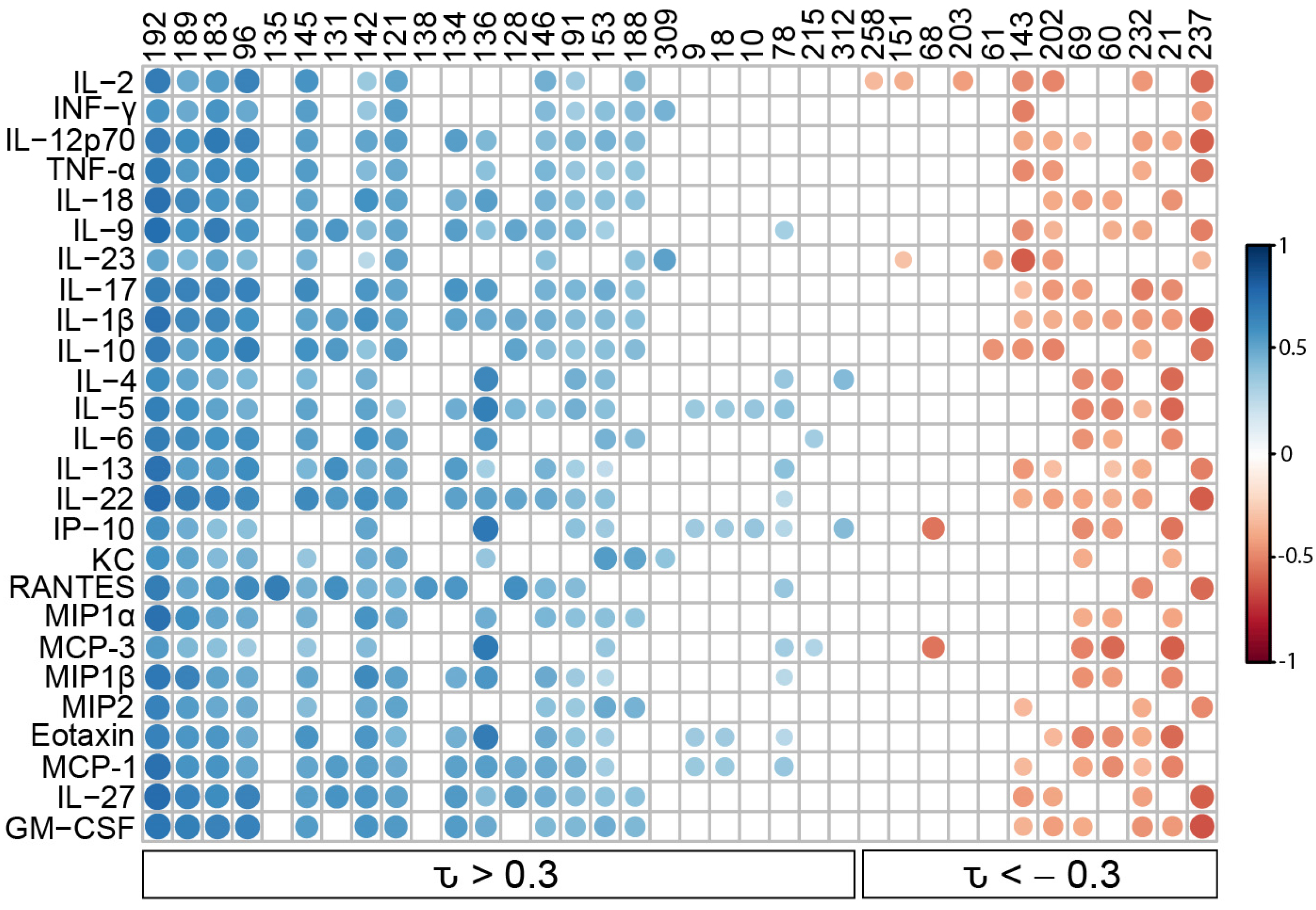
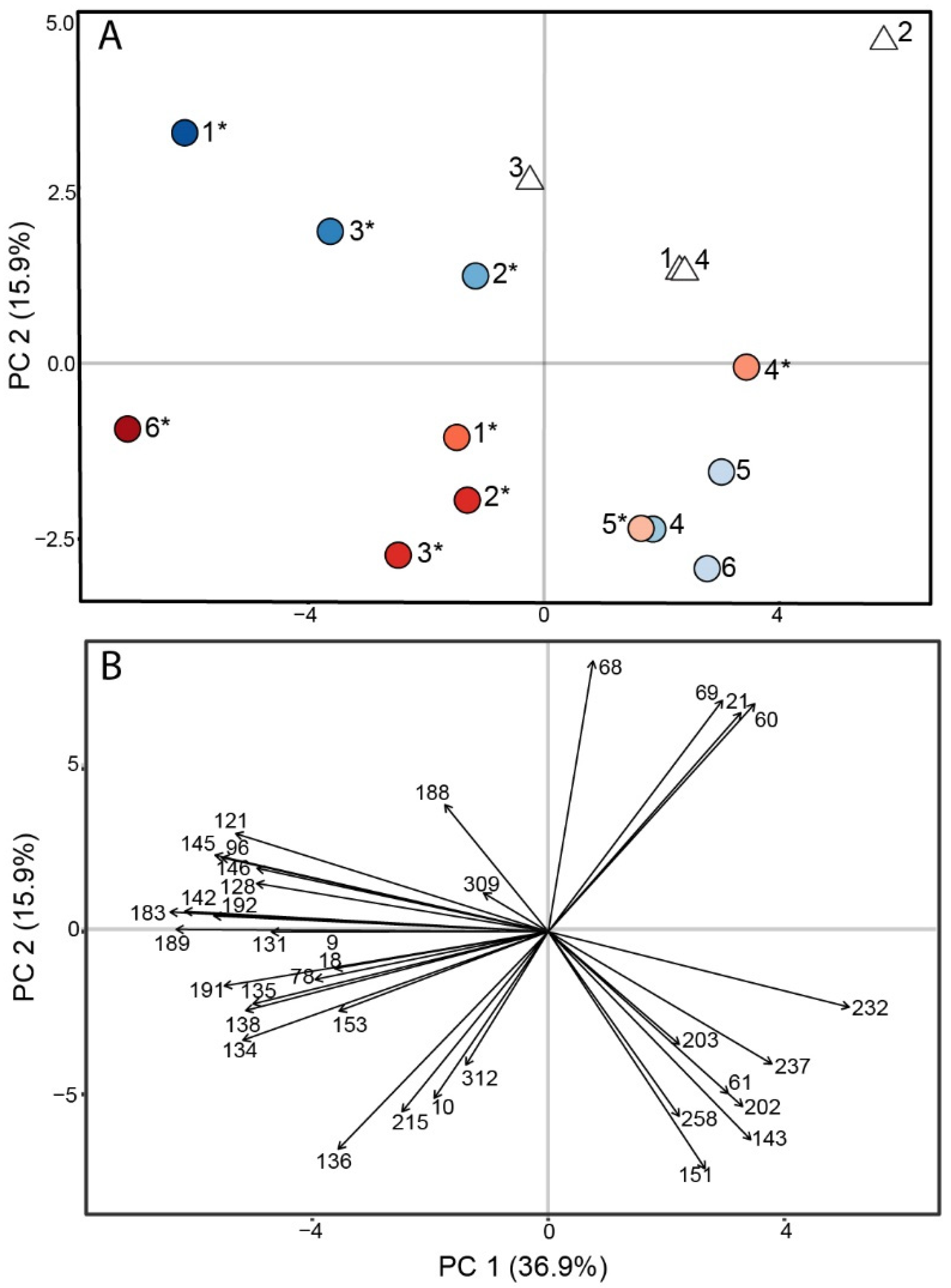
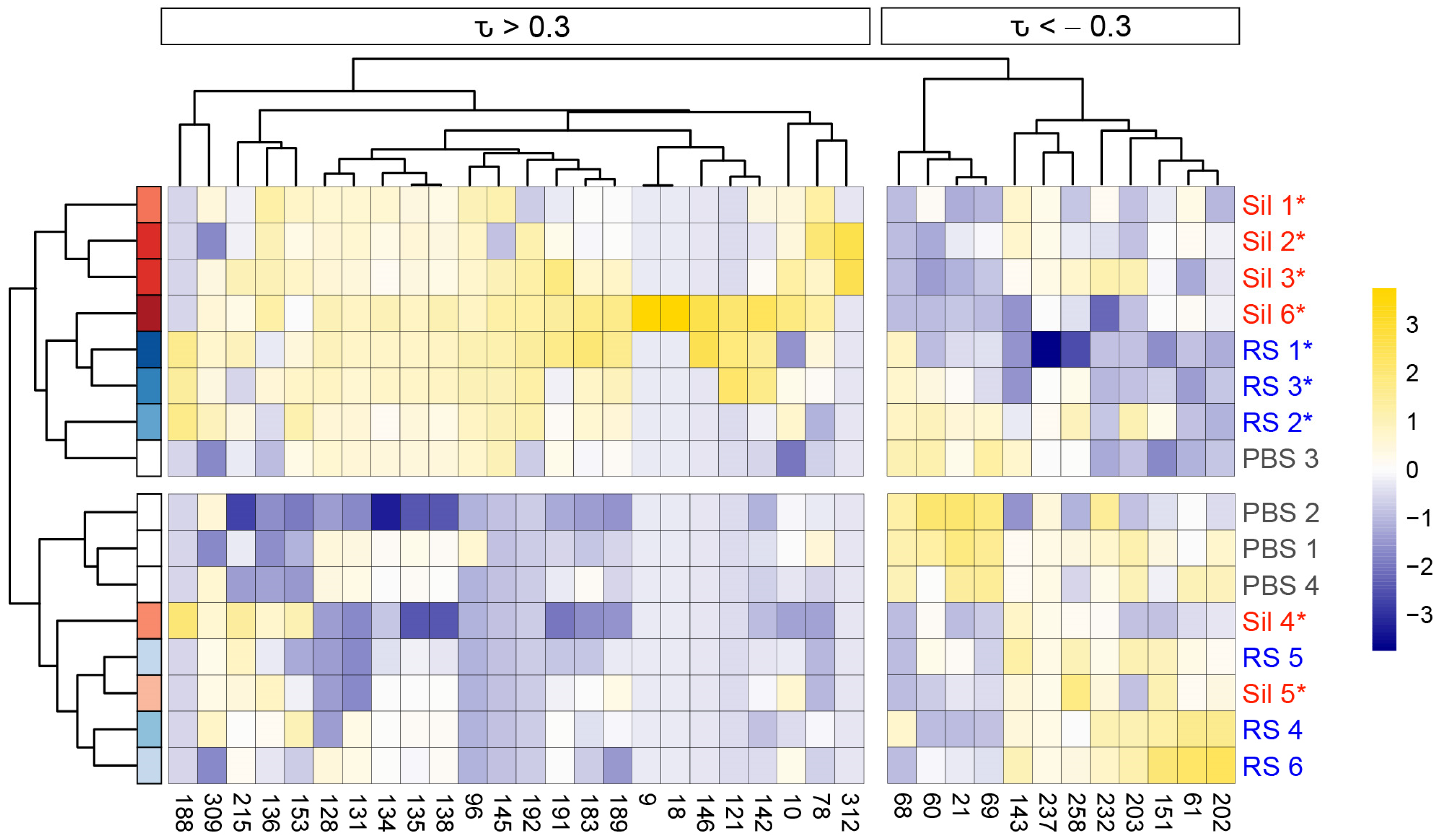
3.2. Murine Coccidioidomycosis Volatilome and Its Correlation with Cytokine Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galgiani, J.N.; Ampel, N.M.; Catanzaro, A.; Johnson, R.H.; Stevens, D.A.; Williams, P.L. Practice Guidelines for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2000, 30, 658–661. [Google Scholar] [CrossRef]
- Williams, S.L.; Chiller, T. Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. J. Fungi 2022, 8, 666. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Le, T.; Chindamporn, A.; Kauffman, C.A.; Alastruey-Izquierdo, A.; Ampel, N.M.; Andes, D.R.; Armstrong-James, D.; Ayanlowo, O.; Baddley, J.W.; et al. Global guideline for the diagnosis and management of the endemic mycoses: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect. Dis. 2021, 21, e364–e374. [Google Scholar] [CrossRef] [PubMed]
- Ginn, R.; Mohty, R.; Bollmann, K.; Goodsell, J.; Mendez, G.; Bradley, B.; Galgiani, J.N. Delays in Coccidioidomycosis Diagnosis and Relationship to Healthcare Utilization, Phoenix, Arizona, USA(1). Emerg. Infect. Dis. 2019, 25, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Ireland, M.; Weinberg, M.; Gruninger, R.; Weigand, J.; Chen, L.; Perez-Lockett, K.; Bledsoe, C.; Denny, L.; Cibulskas, K.; et al. Enhanced Surveillance for Coccidioidomycosis, 14 US states, 2016. Emerg. Infect. Dis. 2018, 24, 1444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bean, H.D.; Jiménez-Díaz, J.; Hill, J.E. Secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting of multiple bacterial lung pathogens, a mouse model study. J. Appl. Physiol. 2013, 114, 1544–1549. [Google Scholar] [CrossRef]
- Zhu, J.; Bean, H.D.; Wargo, M.J.; Leclair, L.W.; Hill, J.E. Detecting bacterial lung infections: In vivo evaluation of in vitro volatile fingerprints. J. Breath Res. 2013, 7, 016003. [Google Scholar] [CrossRef]
- Zhu, J.; Jiménez-Díaz, J.; Bean, H.D.; Daphtary, N.A.; Aliyeva, M.I.; Lundblad, L.K.A.; Hill, J.E. Robust detection of P. aeruginosa and S. aureus acute lung infections by secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting: From initial infection to clearance. J. Breath Res. 2013, 7, 037106. [Google Scholar] [CrossRef]
- Bean, H.D.; Zhu, J.; Sengle, J.C.; Hill, J.E. Identifying methicillin-resistant Staphylococcus aureus (MRSA) lung infections in mice via breath analysis using secondary electrospray ionization-mass spectrometry (SESI-MS). J. Breath Res. 2014, 8, 041001. [Google Scholar] [CrossRef] [PubMed]
- Bean, H.D.; Jiménez-Díaz, J.; Zhu, J.; Hill, J.E. Breathprints of model murine bacterial lung infections are linked with immune response. Eur. Respir. J. 2015, 45, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mellors, T.R.; Blanchet, L.; Flynn, J.L.; Tomko, J.; Malley, M.; Scanga, C.A.; Lin, P.L.; Hill, J.E. A new method to evaluate macaque health using exhaled breath: A case study of in a BSL-3 setting. J. Appl. Physiol. 2017, 122, 695–701. [Google Scholar] [CrossRef]
- Mellors, T.R.; Nasir, M.; Franchina, F.A.; Smolinska, A.; Blanchet, L.; Flynn, J.L.; Tomko, J.; O’Malley, M.; Scanga, C.A.; Lin, P.L.; et al. Identification of Mycobacterium tuberculosis using volatile biomarkers in culture and exhaled breath. J. Breath Res. 2018, 13, 016004. [Google Scholar] [CrossRef]
- Franchina, F.A.; Mellors, T.R.; Aliyeva, M.; Wagner, J.; Daphtary, N.; Lundblad, L.K.A.; Fortune, S.M.; Rubin, E.J.; Hill, J.E. Towards the use of breath for detecting mycobacterial infection: A case study in a murine model. J. Breath Res. 2018, 12, 026008. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Nasir, M.; Franchina, F.A.; Rees, C.A.; Aliyeva, M.; Daphtary, N.; Wargo, M.J.; Lundblad, L.K.A.; Hill, J.E. Breath metabolome of mice infected with Pseudomonas aeruginosa. Metabolomics 2019, 15, 10. [Google Scholar] [CrossRef]
- van Oort, P.M.; Brinkman, P.; Slingers, G.; Koppen, G.; Maas, A.; Roelofs, J.J.; Schnabel, R.; Bergmans, D.C.; Raes, M.; Goodacre, R.; et al. Exhaled breath metabolomics reveals a pathogen-specific response in a rat pneumonia model for two human pathogenic bacteria: A proof-of-concept study. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L751–L756. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Leon, A.; Koshy, S.; Aloum, O.; Al-Jabawi, Y.; Ismail, N.; Weiss, Z.F.; Koo, S. Breath-Based Diagnosis of Infectious Diseases: A Review of the Current Landscape. Clin. Lab. Med. 2021, 41, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Hérivaux, A.; Gonçalves, S.M.; Carvalho, A.; Cunha, C. Microbiota-derived metabolites as diagnostic markers for respiratory fungal infections. J. Pharm. Biomed. Anal. 2020, 189, 113473. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Mogaddam, M.R.A.; Rahimpour, E.; Soleymani, J.; Saei, A.A.; Jouyban, A. Breathomics: Review of Sample Collection and Analysis, Data Modeling and Clinical Applications. Crit. Rev. Anal. Chem. 2021, 52, 1461–1487. [Google Scholar] [CrossRef]
- Acharige, M.J.T.; Koshy, S.; Ismail, N.; Aloum, O.; Jazaerly, M.; Astudillo, C.L.; Koo, S. Breath-based diagnosis of fungal infections. J. Breath Res. 2018, 12, 027108. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Dial, S.M.; Perrill, R.; Casement, R.; Galgiani, J.N. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect. Immun. 2008, 76, 5553–5564. [Google Scholar] [CrossRef]
- Lewis, E.R.G.; David, V.R.; Doyle, A.L.; Rajabi, K.; Kiefer, J.A.; Pirrotte, P.; Barker, B.M. Differences in Host Innate Responses among Coccidioides Isolates in a Murine Model of Pulmonary Coccidioidomycosis. Eukaryot. Cell 2015, 14, 1043–1053. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.N.; Bean, H.D. Predicting 624sil Retention Indices Using Nonpolar Values. In Proceedings of the 13th Multidimensional Chromatography Virtual Workshop, Virtual, 31 January–2 February 2022. [Google Scholar]
- Davis, T.J.; Karanjia, A.V.; Bhebhe, C.N.; West, S.B.; Richardson, M.; Bean, H.D. Pseudomonas aeruginosa volatilome characteristics and adaptations in chronic cystic fibrosis lung infections. mSphere 2020, 5, e00843-20. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.J.; Firzli, T.R.; Higgins Keppler, E.A.; Richardson, M.; Bean, H.D. Addressing Missing Data in GC × GC Metabolomics: Identifying Missingness Type and Evaluating the Impact of Imputation Methods on Experimental Replication. Anal. Chem. 2022, 94, 10912–10920. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect. Immun. 1983, 40, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Kennell, W.; Boncyk, L.; Murphy, J. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect. Immun. 1988, 56, 13–17. [Google Scholar] [CrossRef]
- Carlin, A.F.; Viriyakosol, S.; Okamoto, S.; Walls, L.; Fierer, J. Interleukin-8 Receptor 2 (IL-8R2)-Deficient Mice Are More Resistant to Pulmonary Coccidioidomycosis than Control Mice. Infect. Immun. 2020, 89, e00883-19. [Google Scholar] [CrossRef] [PubMed]
- Fierer, J. The Role of IL-10 in Genetic Susceptibility to Coccidioidomycosis on Mice. Ann. N. Y. Acad. Sci. 2007, 1111, 236–244. [Google Scholar] [CrossRef]
- Jimenez Mdel, P.; Walls, L.; Fierer, J. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect. Immun. 2006, 74, 3387–3395. [Google Scholar] [CrossRef]
- Higgins Keppler, E.A.; Mead, H.L.; Barker, B.M.; Bean, H.D. Life cycle dominates the volatilome character of dimorphic fungus Coccidioides spp. mSphere 2021, 6, e00040-21. [Google Scholar] [CrossRef]
- Wilkins, K.; Larsen, K.; Simkus, M. Volatile metabolites from mold growth on building materials and synthetic media. Chemosphere 2000, 41, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Verrall, S.; Hackett, C.A.; Wheatley, R.E. Identification of volatile organic compounds (VOCs) from bacteria and yeast causing growth inhibition of sapstain fungi. Holzforschung 2004, 58, 193–198. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol. 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Wheatley, R.; Hackett, C.; Bruce, A.; Kundzewicz, A. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Hertel, M.; Hartwig, S.; Schütte, E.; Gillissen, B.; Preissner, R.; Schmidt-Westhausen, A.M.; Paris, S.; Kastner, I.; Preissner, S. Identification of signature volatiles to discriminate Candida albicans, glabrata, krusei and tropicalis using gas chromatography and mass spectrometry. Mycoses 2016, 59, 117–126. [Google Scholar] [CrossRef]
- Fiedler, K.; Schütz, E.; Geh, S. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int. J. Hyg. Environ. Health 2001, 204, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Bezerra, A.R.; Almeida, A.; Rocha, S.M. Candida Species (Volatile) Metabotyping through Advanced Comprehensive Two-Dimensional Gas Chromatography. Microorganisms 2020, 8, 1911. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Nemčovič, M.; Jakubíková, L.; Víden, I.; Farkaš, V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol. Lett. 2008, 284, 231–236. [Google Scholar] [CrossRef]
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. Antagonistic activity of Aspergillus versicolor against Macrophomina phaseolina. Braz. J. Microbiol. 2022, 53, 1613–1621. [Google Scholar] [CrossRef]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of microbial volatile organic compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Buśko, M.; Kulik, T.; Ostrowska, A.; Góral, T.; Perkowski, J. Quantitative volatile compound profiles in fungal cultures of three different Fusarium graminearum chemotypes. FEMS Microbiol. Lett. 2014, 359, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Beer, R.; Sponring, A.; Filipiak, A.; Ager, C.; Schiefecker, A.; Lanthaler, S.; Helbok, R.; Nagl, M.; Troppmair, J. Breath analysis for in vivo detection of pathogens related to ventilator-associated pneumonia in intensive care patients: A prospective pilot study. J. Breath Res. 2015, 9, 016004. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 2003, 36, 263–267. [Google Scholar] [CrossRef]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Velazhahan, R.; Paranidharan, V. Volatiles of antagonistic soil yeasts inhibit growth and aflatoxin production of Aspergillus flavus. Microbiol. Res. 2022, 263, 127150. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Cheng, X.; Zhou, H.; Si, L. Hanseniaspora uvarum prolongs shelf life of strawberry via volatile production. Food Microbiol. 2017, 63, 205–212. [Google Scholar] [CrossRef]
- Rees, C.A.; Stefanuto, P.-H.; Beattie, S.R.; Bultman, K.M.; Cramer, R.A.; Hill, J.E. Sniffing out the hypoxia volatile metabolic signature of Aspergillus fumigatus. J. Breath Res. 2017, 11, 036003. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kimura, T.; Tanaka, H.; Kaneko, S.; Ichii, S.; Kiuchi, M.; Suzuki, T. Analysis of volatile metabolites emitted by soil-derived fungi using head space solid-phase microextraction/gas chromatography/mass spectrometry: I. Aspergillus fumigatus, Aspergillus nidulans, Fusarium solani and Penicillium paneum. Surf. Interface Anal. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; Malysheva, S.V.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; Picco, A.M.; De Kimpe, N. Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 2012, 116, 941–953. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.P.J.; Evans, P.; Ewen, R.J.; Gunson, H.E.; Jones, P.R.H.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 2001, 50, 489–496. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, H.; Yang, S.; Liu, H. Changes of volatile organic compounds and bioactivity of Alternaria brassicae GL07 in different ages. J. Basic Microbiol. 2019, 59, 713–722. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum Conidia Is Regulated by 1-Octen-3-ol, a Volatile Self-Inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823. [Google Scholar] [CrossRef] [PubMed]
- Pavirhra, R.; Lalitha, S. Tetradecane producing biocontrol agent, Trichoderma spp. against Fusarium oxysporum in tomato (Solanum lycopersicum L.). Int. J. Agric. Technol. 2020, 16, 1475–1492. [Google Scholar]
- Skanda, S.; Vijayakumar, B.S. Antioxidant and antibacterial potential of crude extract of soil fungus Periconia sp. (SSS-8). Arab. J. Sci. Eng. 2022, 47, 6707–6714. [Google Scholar] [CrossRef]
- Mead, H.L.; Roe, C.C.; Higgins Keppler, E.A.; Caballero Van Dyke, M.C.; Laux, K.L.; Funke, A.L.; Miller, K.J.; Bean, H.D.; Sahl, J.W.; Barker, B.M. Defining critical genes during spherule remodeling and endospore development in the fungal pathogen, Coccidioides posadasii. Front. Genet. 2020, 11, 483. [Google Scholar] [CrossRef]
- Gao, J.; Zou, Y.; Wang, Y.; Wang, F.; Lang, L.; Wang, P.; Zhou, Y.; Ying, K. Breath analysis for noninvasively differentiating Acinetobacter baumannii ventilator-associated pneumonia from its respiratory tract colonization of ventilated patients. J. Breath Res. 2016, 10, 027102. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Danaher, P.J.; Devadiga, A.; Legendre, D.A.; Nail, K.L.; Schmitt, P.; Wai, J. Effect of influenza vaccination on oxidative stress products in breath. J. Breath Res. 2010, 4, 026001. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Stajich, J.E.; Park, D.J.; Whiston, E.; Hung, C.Y.; McMahan, C.; White, J.; Sykes, S.; et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Hsu, A.P.; Holland, S.M.; Fierer, J. A review of innate and adaptive immunity to coccidioidomycosis. Med. Mycol. 2019, 57, S85–S92. [Google Scholar] [CrossRef] [PubMed]
- Donovan, F.M.; Shubitz, L.; Powell, D.; Orbach, M.; Frelinger, J.; Galgiani, J.N. Early Events in Coccidioidomycosis. Clin. Microbiol. Rev. 2019, 33, e00112-19. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef] [PubMed]

| VOC | Compound | Fungal Taxa * | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| 10 | isopropyl alcohol | x | x | [33,34,35] | |||||||
| 18 | 2-methylpentane | x | [36] | ||||||||
| 21 | 2-methyl-2-propanol | ||||||||||
| 34 | 2-methyl-1-pentene | ||||||||||
| 37 | hexane | x | x | [36,37] | |||||||
| 54 | 2-ethoxy-2-methylpropane | ||||||||||
| 60 | 2-butanone | x | x | x | x | [34,36,38,39,40,41,42] | |||||
| 70 | benzene | x | [43] | ||||||||
| 74 | 2-pentanone | x | x | x | x | x | x | [34,35,37,44,45] | |||
| 83 | 1,3,5-cycloheptatriene | ||||||||||
| 93 | cyclopentanone | x | x | [38,42] | |||||||
| 99 | p-xylene | x | x | x | x | x | x | [37,38,45,46,47,48] | |||
| 104 | 1,3,5,7-cyclooctatetraene | x | [49] | ||||||||
| 121 | 2,2-dimethyl-octane | ||||||||||
| 123 | benzaldehyde | x | x | x | x | x | x | [36,39,40,45,46,50,51] | |||
| 129 | octanal | x | x | x | [39,46,48,52] | ||||||
| 136 | 2-ethyl-1-hexanol | x | x | x | x | x | x | [38,39,44,53] | |||
| 142 | undecane | x | x | x | x | [34,35,38,43,45,47,52,54] | |||||
| 153 | nonanal | x | x | x | x | x | [35,36,39,45,46,48,52,53,54,55] | ||||
| 159 | (E)-4-dodecene | ||||||||||
| 160 | dodecane | x | x | [45,53] | |||||||
| 171 | 2,6-xylidine | ||||||||||
| 173 | decanal | x | x | x | x | x | [32,35,36,39,45,46,53,55,56] | ||||
| 188 | 2,5-dimethyl-benzaldehyde | x | [39,46] | ||||||||
| 270 | 2,4-di-tert-butylphenol | x | [57] | ||||||||
| 307 | diethyl phthalate | x | x | [45,54,56] | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins Keppler, E.A.; Van Dyke, M.C.C.; Mead, H.L.; Lake, D.F.; Magee, D.M.; Barker, B.M.; Bean, H.D. Volatile Metabolites in Lavage Fluid Are Correlated with Cytokine Production in a Valley Fever Murine Model. J. Fungi 2023, 9, 115. https://doi.org/10.3390/jof9010115
Higgins Keppler EA, Van Dyke MCC, Mead HL, Lake DF, Magee DM, Barker BM, Bean HD. Volatile Metabolites in Lavage Fluid Are Correlated with Cytokine Production in a Valley Fever Murine Model. Journal of Fungi. 2023; 9(1):115. https://doi.org/10.3390/jof9010115
Chicago/Turabian StyleHiggins Keppler, Emily A., Marley C. Caballero Van Dyke, Heather L. Mead, Douglas F. Lake, D. Mitchell Magee, Bridget M. Barker, and Heather D. Bean. 2023. "Volatile Metabolites in Lavage Fluid Are Correlated with Cytokine Production in a Valley Fever Murine Model" Journal of Fungi 9, no. 1: 115. https://doi.org/10.3390/jof9010115
APA StyleHiggins Keppler, E. A., Van Dyke, M. C. C., Mead, H. L., Lake, D. F., Magee, D. M., Barker, B. M., & Bean, H. D. (2023). Volatile Metabolites in Lavage Fluid Are Correlated with Cytokine Production in a Valley Fever Murine Model. Journal of Fungi, 9(1), 115. https://doi.org/10.3390/jof9010115







