Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches
Abstract
:1. Introduction
2. Pharmacological Activity of Lichen Depsides and Tridepsides
2.1. Depsides
2.1.1. Atranorin
2.1.2. Baeomycesic Acid
2.1.3. Barbatic Acid
2.1.4. Diffractaic Acid
2.1.5. Divaricatic Acid
2.1.6. Evernic Acid
2.1.7. Isolecanoric Acid
2.1.8. Lecanoric Acid
2.1.9. Methyl Evernate
2.1.10. Olivetoric Acid
2.1.11. Perlatolic Acid
2.1.12. Ramalic Acid/Obtusatic Acid
2.1.13. Sekikaic Acid
2.1.14. Squamatic Acid
2.1.15. Thamnolic Acid
| Depside | Botanical Origin | Type of Study | Experimental Model | Activities | Results | References |
|---|---|---|---|---|---|---|
| Atranorin | Parmotrema saccatilobum (Taylor) Hale | In vitro | Cyclooxygenase inhibition assay | Analgesic | Inhibition of COX-1 (IC50 45 μM). Inhibition (40%) of COX-2 ranging between 17 μg/mL and 0.17 μg/mL. | [40] |
| Cladina kalbi. (Ahti) | In vivo | Male Swiss mice | Analgesic | Acetic acid-induced writhing test—200 and 400 mg/kg (p.o.)—reduction (p < 0.05) abdominal writhing by 52.6 and 61.3%, compared to control. Formalin test—200 and 400 mg/kg (p.o.) inhibition inflammatory processes (second phase) dose dependently. | [42] | |
| Cladina kalbi. (Ahti) | In vivo | Male Swiss mice | Analgesic Anti-inflammatory | Inhibitory effect in formalin- and capsaicin-induced orofacial pain tests. Anti-inflammatory effects in the acute model of inflammation (leukocyte migration to the peritoneal cavity), carrageenan- and arachidonic acid-induced paw edema in rats. | [43] | |
| - | In vitro In silico | α-Glucosidase assay HEK293 (Human embryonic kidney cell line) Docking studies | Antidiabetic | N-substituted hydrazide derivatives of atranorin, more potent inhibition than the original. Weak or no cytotoxicity toward HEK293 cell line. | [44] | |
| Parmelia nepalensis (Taylor) | In vitro | Polymorphonuclear leukocytes | Anti-inflammatory | Inhibition of LTB4 biosynthesis via non-redox mechanism. | [41] | |
| Atranorin | Kigelia africana (Lam.) Benth | In vitro | Chloroquine-resistant W-2 and two field isolates (CAM10 and SHF4) of Plasmodium falciparum LLC/MK2 monkey kidney cells | Antimalarial | Good activity against all parasite strains (IC50 < 5 μM). Cytotoxicity at high concentrations. | [36] |
| Kigelia africana (Lam.) Benth | In vitro | Multidrug-resistant W2mef strain of Plasmodium falciparum | Antimalarial | Parasite lactate dehydrogenase assay (IC50 1.78 μM). Synergistic effects with artemether. | [37] | |
| Homalia trichomanoides (Hedw.) B. S. G. | In vitro | Candida albicans | Antimicrobial | Minimum inhibitory doses of 2.0 µg. | [29] | |
| Parmelia reticulata (Taylor) | In vitro | Sclerotium rolfsii, Rhizoctonia solani, R. bataticola, Fusarium udum, Pythium aphanidermatum and Pythium debaryanum | Antimicrobial | Maximum antifungal activity against Sclerotium rolfsii (ED50: 39.70 µg/mL). | [30] | |
| Cladonia foliacea (Huds.) Willd | In vitro | Gram-positive bacteria: Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Streptococcus faecalis, Listeria monocytogenes Gram-negative bacteria: Proteus vulgaris, Aeromonas hydrophila. Fungi: Candida albicans, Candida glabrata | Antimicrobial | Low activity with high MIC values (15.6 µg to 500 µg per disk against 107 cells). | [27] | |
| Parmotrema dilatatum (Vain.) Hale, Parmotrema tinctorum (Nyl.) Hale | In vitro | Mycobacterium tuberculosis | Antimicrobial | Low-activity compound (MIC value 250 μg/mL). | [31] | |
| Atranorin | - | In vitro | Methicillin-resistant Staphylococcus aureus strains | Antimicrobial | Effective in counteracting adhesion to polystyrene, against biofilm formation and against MRSA. | [28] |
| Stereocaulon alpinum Laurer. | In vitro | Mycobacterium aurum strains | Antimicrobial | Low-activity MIC values >/= 125 µg/mL. | [32] | |
| Usnea laevis Nyl. | In vitro | Mycobacterium tuberculosis Mycobacterial multidrug-resistant (MDR) strains (MDR-A8, MDR-V791, MDR-R, MDR-40) | Antimicrobial | Inactive against mycobacterial strains MIC values ≥ 200 µg/mL. | [33] | |
| Cladina kalbii Ahti | In vitro | TRAP, TAR, TBARS, hydroxyl radical scavenging activity, nitric oxide scavenging activity, CAT- SOD-like activity. SH-SY5Y neuroblastoma cell line | Antioxidant | TRAP assay: 1–100 μg/mL significant antioxidant effects (dose-dependent). TAR assay: 100 μg/mL significant antioxidant capacity. TBARS: 0.1 to 100 μg/mL AAPH-induced lipoperoxidation. No hydroxyl radical/nitric oxide scavenging activity. Increase (↑) H2O2 formation in vitro ↑ superoxide degradation. | [47] | |
| Parmotrema austrosinense (Zahlbr.) Hale | In vitro | DPPH assay Anti-linoleic acid peroxidation activity | Antioxidant | IC50: 100 µg/mL. IC50: 116 µg/mL. | [34] | |
| Hypotrachyna revoluta (Flörke) Hale | In vitro | Hydroxyl radical-scavenging activity | Antioxidant | Metabolite (11.8 mg) same activity as Trolox (1 mg). | [46] | |
| Parmotrema stuppeum (Taylor) Hale | In vitro | Beta-carotene-linoleate model system | Antioxidant | 14% of antioxidant activity at 200 µg/mL. | [48] | |
| Atranorin | - | In vitro | Piroplasm parasites: Babesia. bovis, Babesia bigemina, Babesia divergens, Babesia caballi, and Theileria equi Hosts of piroplasm parasites: human foreskin fibroblasts (HFF), mouse embryonic fibroblast (NIH/3T3) Madin–Darby bovine kidney (MDBK) | Anti-parasitic | Suppression of multiplication: IC 50 (B. bovis): 98.4 µM, IC50 (B. bigemina): 64.5 µM, IC50 (B. divergens): 45.2 µM, IC50 (B. caballi): 46.6 µM, IC50 (T. equi): 71.3 µM. Reduce (↓) Cell viability. | [38] |
| - | In vivo | BALB/c mice infected by B. microti | Anti-parasitic | ↓ B. microti multiplication in mice by 68.17%. | [38] | |
| - | In vivo | Normal mammary epithelial NMuMG cells BALB/c mice with T1-induced cancer disease | Antitumoral | ↓ Clonogenic ability of carcinoma. ↑ Apoptosis associated with the activation of caspase-3 and PARP cleavage in 4T1 cells. ↑ Depletion of Bcl-xL protein in 4T1 cells. Longer survival time, reduced tumor size, and higher numbers of apoptotic 4T1 cells. Normal NMuMG cells are less sensitive to ATR. | [57] | |
| Stereospermum acuminatissimum K. Schum. | In vitro | Urease inhibition assay Chymotrypsin inhibition assay | Antiulcerogenic | Excellent urease inhibition IC50 (18.2 µM). No α-chymotrypsin inhibitory effect. | [45] | |
| Stereocaulon evolutum Graewe. | In vitro | HCV grown in Huh-7.5.1 human hepatic cell line | Antiviral | Interferes with the lifecycle of hepatitis C virus (HCV), inhibiting only viral entry (IC50: 22.3 µM). | [35] | |
| Parmotrema rampoddense (Nyl.) Hale | In silico In vitro | Docking studies with breast cancer oncoproteins MDA MB-231 and MCF-7 (breast cancer cell lines) | Cytotoxic | Molecular docking studies interaction: Akt > Bax, Bcl-xL and Bcl-2 > Bcl-w proteins. IC50 (MDA MB-231) = 5.36 μM; IC50 (MCF-7) = 7.55 μM. | [49] | |
| Atranorin | Everniastrum vexans (Zahlbr. ex W.L. Culb. and C.F. Culb.) | In vitro | A549 (human lung cancer cell line) | Cytotoxic | ↓ Lung cancer cell motility and tumorigenesis by affecting AP-1, Wnt, and STAT signaling and suppressing RhoGTPase activity. | [50] |
| Stereocaulon caespitosum Redinger | In vitro | SKHep1 and Huh-7 (epithelial carcinoma cell line) SNU-182 (primary cancer cell line) | Cytotoxic | ↓ Cell growth at 80 µg/mL in all cell lines Cell cycle attenuated. ↑ Cell death through necrosis. ↓ Metastatic potential by suppression of cell migration and invasion. | [51] | |
| - | In vitro | HTB-140 (melanoma cell line) DU-145 and PC-3 (prostate cancers) normal human skin fibroblasts PNT2 (prostate epithelial cell line) | Cytotoxic | ↓ Cancer cell proliferation, migration, and actin cytoskeleton organization. | [52] | |
| Hypogymnia physodes (L.) Nyl | In vitro | Human lymphocytes- cytochalasin-B blocked micronucleus (CBMN) assay. | Cytotoxic | No significant clastogenic and antiproliferative effects on selected concentrations. | [16] | |
| - | In vitro | A2780 (human ovarian cancer cell line) HT-29 (human colon cancer cell line) | Cytotoxic | Loss in the mitochondrial membrane potential. ↑ caspase-3 activation (only in HT-29 cells) and phosphatidylserine externalization. ↑ ROS/RNS. ↑ PARP, p53, Bcl-2/Bcl-xL, Bax, p38, pp38. | [56] | |
| Atranorin | - | In vitro | A2780 (human ovarian carcinoma) HCT-116 p53+/+ and HCT-116 p53−/− (human colon carcinoma) HeLa (human cervix adenocarcinoma) SK-BR-3 (human breast adenocarcinoma) HL-60 (human promyelocytic leukemia) HT-29 (human colon adenocarcinoma) Jurkat (human T cells lymphocyte leukemia) MCF-7 (human breast adenocarcinoma) | Cytotoxic | Cytotoxicity against all cell lines except against HeLa (especially effective against HL-60 cells (50 μM). Clonogenic inhibition ability of all tested tumor cells. Accumulation in S-phase at expense of G1/G0-phase. Lower incidence in p53-deficient cells. | [55] |
| Atranorin SPION | - | In vitro | GCSCs (gastric cancer stem cells) | Cytotoxic | Inhibition proliferation, invasion, angiogenesis, and tumorigenicity of CD44+/CD24+. ↑ Oxidative stress. ↑ Fe2+ accumulation/ferroptosis. Increase mRNA encoding apoptosis factors, COX-2 levels. Inhibition GCSC markers and GPX4, NCOA4.BRF2, CD98. Downregulation mRNA hm5C modification levels. | [54] |
| - | In vivo | NOD-scid mice | Cytotoxic Antitumor | Smaller tumors in weight and volume. Inhibition GPX4 and SLC7A11. | [54] | |
| Atranorin | Bacidia stipata I. M. Lamb. | In vitro | A375 (melanoma cancer cell line) | Cytotoxic | Low inhibition (only high concentrations) | [55] |
| Parmotrema dilatatum (Vain.) Hale | In vitro | UACC-62 and B16-F10 (melanoma cells) 3T3 (normal cells) | Cytotoxic | IC50: 250 µg/mL. Low cytotoxic effects on all the cell lines. | [59] | |
| Bacidia stipata I. M. Lamb. | In vitro | Androgen-sensitive (LNCaP) and androgen-insensitive (DU-145) human prostate cancer cells. | Cytotoxic | Lower activity inhibiting cancer cells only at higher concentrations (25 and 50 μM). | [60] | |
| Ramalina glaucescens Kremp. | In vitro | P388 murine leukemia cell line | Cytotoxic | Moderate activity against (IC50 of >33 µM). | [53] | |
| Usnea laevis Nyl. | In vitro | Human acute monocytic leukemia cell line (THP-1) | Cytotoxic | IC50: 286.13 µg/mL. Low cytotoxic effects on macrophages. | [33] | |
| - | In vitro | Calf thymus DNA | DNA-interacting agents | ATR acts as effective DNA-interacting agent. No inhibitory effect on Topo isomerase I. | [61] | |
| - | In vitro | Second and third instar larvae of the mosquito Culiseta longiareolata | Larvicidal activity | LC (50) values: 0.52 ppm. LC (90) values: 5.93 ppm. | [39] | |
| Usnea articulata (L.) Hoffm. | In vitro Ex vivo | Neuro2A (mouse neuroblastoma) cell line Primary neural stem or progenitor cells | Neuroprotective | Neurotrophic activity (131.73 μm at 5 μM). Gene expression of BDNF and NGF modulation. | [62] | |
| Umbilicaria antarctica Frey and I. M. Lamb. | In vitro | Red cell suspension | Photohemolytic | Significant hemolysis in a red cell suspension after irradiation of atranorin with 366 nm light. Higher in presence of nitrogen. | [64] | |
| Umbilicaria antarctica Frey and I. M. Lamb. | In vitro | Inhibition of 8-MOP-human serum albumin (HSA) photobinding. | Photoprotective | Atranorin (10 mM) and irradiation (360 nm) inhibited photobinding to HSA by 20.1%. | [63] | |
| Atranorin | Parmotrema austrosinense (Zahlbr.) Hale | In vitro | Bacterial strain Lactobacillus casei | Probiotic bacteria | Moderate growth stimulating activity in terms of increased dry matter of biomass (41.1 mg) of L. casei. | [34] |
| Baeomycesic acid | Thamnolia subuliformis (Ehrh.) W. Culb. | In vitro | Porcine leucocytes Sheep seminal vesicle microsomes | Cytotoxic | Potent 5-lipoxygenase inhibitor (IC50 = 8.3 µM). Inactive against COX. | [15] |
| Thamnolia vermicularis (Sw.) Schaer. | In vitro | Human platelets | Cytotoxic | Weak 12(S)-LOX inhibitor (14.7 +/− 2.76%). | [66] | |
| Thamnolia vermicularis (Sw.) Schaer. | In vitro | AGS (stomach cancer cell line) Capan-1, Capan-2 and PANC-1 (pancreas cell lines) HL-60, K-562 and JURKAT (blood cancer cell lines) NCI-H1417 (lung cancer cell line) NIH: OVCAR-3 (ovary cancer line) PC-3 (prostate cancer cell line) T47-D (breast cancer line) WiDr (colorectal cancer cell line) | Cytotoxic | Slight anti-proliferative activity. Selective 5-LOX inhibitor. | [65] | |
| Barbatic acid | Cladonia borealis Stenroos | In vitro | Staphylococcus. aureus NEWP0023 Enterococcus. faecalis (NEWP0012) Escherichia. coli (NEWP 0022) | Antimicrobial | MIC values: S. aureus (NEWP0023) = 31.3 µg/mL; S. aureus (clinic) = 31.3 µg/mL; E. faecalis (NEWP0012) = 7.8 µg/mL; E. faecalis (clinic) = 31.3 µg/mL; E. coli = nt. | [68] |
| Cladia longissima (Sw.) Nyl. | In vitro | Adult worms of Schistosoma mansoni | Antiparasitic | Schistosomicidal effect (death, tegumentary damages, and changes in mobility). | [18] | |
| Cladia longissima (Sw.) Nyl. | In vitro | Adult mollusks of Biomphalaria glabrata Cercariae of Schistosoma mansoni | Antiparasitic Antimolluscal | Molluscicidal activity against B. glabrata at 20 and 25 µg/mL. Schistosomicidal effect against the parasite S. mansoni at the second larval stage (1 µg/mL after 60 min of exposure). | [67] | |
| Barbatic acid | Usnea longissima Ach. | In vitro | A549 (lung cancer cell line) | Cytotoxic | Pro-apoptotic effect (G0/G1 accumulation and poly ADP-ribose polymerase cleavage). | [69] |
| Usnea longissima Ach | In vitro | Tissue culture | Cytotoxic | Slight inhibitor of tumor promoter-induced Epstein–Barr virus (EBV) activation. | [70] | |
| Pyrrosia petiolosa (Christ) Ching. | In silico | With-no-lysine 1 (WNK1) kinase | Diuretic | Weak diuretic potential. | [71] | |
| Diffractaic acid | Usnea diffracta Vain. | In vivo | Male ddY mice Lipopolysaccharide (LPS)-induced (hyperthermia model) Acetic acid-induced writhing and tail-pressure method (analgesic model) | Analgesic and antipyretic | Hypothermic effect (dose of 200 mg/kg) on normal body temperature. Analgesic effect (dose of 200 mg/kg). | [14] |
| Parmelia nepalensis (Taylor) Parmelia tinctorum Despr. Ex Nyl | In vitro | Polymorphonuclear leukocytes | Anti-inflammatory | Inhibition of LTB4 biosynthesis by specific enzyme interaction. | [41] | |
| Usnea blepharea Motyka | In vitro | Gram-positive bacteria: Staphylococcus aureus, Gram-negative bacteria: Escherichia coli | Antimicrobial | Antibacterial. Strong inhibition at 750 and 1000 ppm concentration. | [72] | |
| - | In vitro | Fusarium fujokuroi | Antimicrobial | Antifungal. MIC 16 × 10−3 mg/mL. Similar to amphotericin B, isovuconzole, terbafine, voriconazole. | [73] | |
| Usnea subcavata (Motyka) | In vitro | Mycobacterium tuberculosis | Antimicrobial | Anti-tubercular activity. High active compound (MIC value 15.6 μg/mL). | [31] | |
| Diffractaic acid | Usnea longissima Ach | In vivo | Albino Wistar rats Indomethacin-induced gastric lesions | Antiulcerogenic | Significant gastroprotective effect. ↑ SOD and GPx activities and GSH levels ↓ lipid peroxidation ↓ myeloperoxidase and inducible NOS (iNOS) activities ↑ constitutive NOS (cNOS) activity. | [74] |
| - | In vitro | U87MG (glioblastoma multiforme cell line) PRCC cells (neurons from Sprague Dawley® rats) | Cytotoxic | IC50 value (PRCC) = 122.26 mg/L. IC50 value (U87MG) = 35.67 mg/L. High antioxidant capacity on PRCC cells (10 mg/L). | [75] | |
| Parmelia nepalensis (Taylor) Parmelia tinctorum Despr. ex Nyl | In vitro | HaCaT (human keratinocyte cell line) | Cytotoxic | Inhibition cell growth (IC50 values of 2.6 mM). No changes on LDH activity, cytostatic effects. | [76] | |
| Usnea aciculifera Vain. | In vitro | HeLa (human epithelial carcinoma cell line) NCI-H460 (human lung cancer cell line) MCF-7 (human breast cancer cell line) | Cytotoxic | Strong cytotoxic activity against all cell lines (100 μg/mL). | [77] | |
| Protousnea magellanica (Mont.) Krog | In vitro | MCF-7 (breast adenocarcinoma cell line) HeLa (cervix adenocarcinoma cell line) HCT-116 (colon carcinoma cell line) | Cytotoxic | Cytotoxic effects in a concentration-dependent manner (2.5–100 μM). No increase intracellular ROS level. No prevention of oxidative injury induced by t-butylhydroperoxide in HeLa cells. | [78] | |
| Diffractaic acid | - | In vitro | Mitochondrial TrxR purified from rat lung | Cytotoxic | Moderate inhibitory effect on Thioredoxin reductase (TrxR). | [79] |
| Usnea longissima Ach | In vitro | Tissue culture | Cytotoxic | Slight inhibitor of tumor promoter-induced Epstein–Barr virus (EBV) activation. | [14] | |
| Usnea longissima Ach | In vivo | Titanium-implanted rabbits | Proapoptotic agent | ↑ Caspase-2, Csp-8, Csp-9, and Csp-3 activation. ↑ Strong myeloperoxidase and inducible nitric oxide synthase activities. ↓ SOD activity and total glutathione level. | [116] | |
| Divaricatic acid | Evernia mesomorpha Nyl. | In vitro | Gram-positive bacteria: Staphylococcus aureus, Enterococcus faecium, Bacillus subtilis, Micrococcus luteus, Streptococcus epidermidis, Streptococcus mutans Gram-negative bacteria: Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhimurium, Vibrio vulnificus Fungi: Candida albicans | Antimicrobial | Effective against Gram+ bacteria (MIC values ranging from 7.0 to 64.0 μg/mL) and Candida albicans. | [81] |
| - | In vitro | Gram-positive bacteria: Staphylococcus aureus Gram-negative bacteria: Escherichia coli, Mycobacteria: Mycobacterium tuberculosis Protozoan: Plasmodium berghei liver stage (LS) parasites, Plasmodium falciparum blood stage (BS) parasites | Antimicrobial Antiplasmodial | No antibacterial/antimycobacterial activity. Low antiplasmodial activity. Low LS activity (IC50 = 77.3 μM), high BS potential (IC50 = 142.1 μM). Plasmodial FAS-II enzyme (PfFabI and PfFabZ) inhibition. | [86] | |
| Divaricatic acid | - | In vitro | Pseudomonas aeruginosa | Antimicrobial | ↓ Pseudomonas aeruginosa virulence factors expression by inhibiting quorum sensing. | [23] |
| Ramalina aspera Räsänen | In vitro | Mollusk Biomphalaria glabrata Cercariae of the helminth Schistosoma mansoni | Molluscicidal and cercaricide | High toxicity against: adult snails (5 μg/mL) and embryos (20 μg/mL after 6 h of exposure) cercariae (10 μg/mL after 30 min of exposure). | [82] | |
| Dirinaria aspera Hasanen | In vitro | UACC-62 and B16-F10 (human and murine melanoma cells) 3T3 normal cells | Cytotoxic | Cytotoxic against both lines (LC50 50.2 μM (UACC-62) LC50 643.7 Μm (B16-F10). More selective against melanoma cells than normal cells. | [59] | |
| Canoparmelia texana | In vitro | PBMCs (peripheral blood mononuclear cell) | Cytotoxic | No cytotoxicity (IC50 > 200 μM). | [82] | |
| Cetraria ornata Müll.Arg. | In vitro | Tissue culture | Cytotoxic | Moderate inhibitor of tumor promoter-induced Epstein–Barr virus (EBV) activation. | [70] | |
| Evernic acid | Evernia prunastri (L.) Ach. | In vitro | Gram-positive bacteria: Staphylococcus aureus Gram-negative bacteria: Pseudomonas aeruginosa, Escherichia coli Fungi: Candida albicans | Antimicrobial | Inhibition of the growth of all tested microorganisms (MIC values = from 0.98 to 125 µg/mL). | [84] |
| Evernia prunastri (L.) Ach. | In silico | Prediction of toxicity risk based on fragment-based toxicity estimation | Toxicity | No mutagenic, no tumorigenic, no reproductive alterations and no irritant effects. | [84] | |
| Evernia prunastri (L.) Ach. | In vitro | Candida albicans biofilms | Antimicrobial | Slow maturation and reduction in biofilms with MBIC50 ≤ 12.5 µg/mL. | [85] | |
| Evernia prunastri (L.) Ach. | In vitro | HeLa (Human epithelial cervical cancer) | Cytotoxic | Strong cytotoxic and antiproliferative effects (25 and 50 µg/mL). | [91] | |
| Evernic acid | Evernia prunastri (L.) Pseudoevernia furfuraceae (L.) Zopf. | In vitro | A549 (human lung cancer cells) HUVEC (umbilical vein endothelial cells) | Cytotoxic | No significant effects in healthy cells. Decrease in proliferation in cancer cells (12.5–100 µg/mL). | [90] |
| Evernia prunastri (L.) Ach. | In vitro | Glioblastoma multiforme cell line: A-172 and T98G cell lines. | Cytotoxic | Reduction A-172 cell viability at 10 µM. Mildly cytotoxic on T98G cell line. Anti-IDO1 (32.8 % inhibition). Anti-COX-2 (50.7%) inhibition. Anti-hyaluronidase activity (IC50 600 µg/mL). Weak antioxidant properties (DPPH (750 µg/mL) CUPRAC (250 µg/mL)) (21.2 % SOD and 20 % GPx inhibition). Inhibition of BChE. (85.9 %) No AchE inhibition. BBB Permeability (8.6 × 10−6 (cm/s) at 4 h. | [92] | |
| Evernia prunastri (L.) Ach. | In vitro | U373-MG (human glioblastoma astrocytoma cell line) SH-SY5Y (human neuroblastoma cell line) | Neuroprotective | ↑ Cell viability; GSH/GSSG ratio; antioxidant enzymes expression. ↓ ROS; lipid peroxidation; protein carbonyls; Caspase-3 activity; Nrf2 pathway activation. | [87] | |
| - | In vitro | Primary neurons | Neuroprotective | Suppression/inhibition MPP+ induced: - Apoptosis (↑ Bcl-2/↓ Bax/Caspase-3) - Mitochondrial Dysfunction - Astrocyte Activation (GFAP expression) - Oxidative stress (↓ ROS production) - NF-κB Signaling Pathway. | [88] | |
| Evernic acid | - | In vivo | MPTP-induced mouse model C57BL/6 mice Rotarod | Neuroprotective | Attenuation of motor dysfunction Reduction in dopaminergic neuronal death and astroglial activation. | [88] |
| Evernia prunastri (L.) Ach. | In vitro | MM98 (malignant mesothelioma cell line) A431 (vulvar carcinoma cell line) HaCaT (human keratinocyte cell line) | Wound healing | No wound closure effects. | [89] | |
| Isolecanoric acid | Glarea lozoyensis | In vitro | SH-SY5Y (human dopaminergic neuroblastoma cell line) L-BMAA for amyotrophic lateral sclerosis (ALS) model and rotenone for Parkinson’s disease (PD) model | Neuroprotective | GSK3β and CK1 inhibition. ↓ Oxidative stress, mitochondrial damage, apoptosis, and cell death. | [93] |
| Lecanoric acid | - | In vitro | α-Glucosidase | Antidiabetic | Active against α-glucosidase (85.9% of inhibition; IC50 value of 350 µM) | [98] |
| Umbilicaria ntárctica Frey and I. M. Lamb. | In vitro | PTP1B enzyme activity and kinetic analysis | Antidiabetic Antiobesity | Moderate inhibition PTP1B activity IC50 31 μM. | [99] | |
| Melanelia subaurifera (Nyl.) Melanelia fuliginosa (Fr. Ex Duby) Ess | In vitro | Gram-positive bacteria: Bacillus cereus, Bacillus subtilis, Staphylococcus aureus. Gram-negative bacteria: Escherichia coli, Proteus mirabilis Fungi: Aspergillus flavus, Candida albicans, Mucor. mucedo, Trichoderma viride, Cladosporium cladosporioides, Fusarium oxysporum | Antimicrobial | Antimicrobial activity against all tested bacteria and fungi with MIC values of 0.5 to 1 mg/mL. | [24] | |
| Lecanoric acid | Parmelia cetrata Ach. | In vitro | Gram-negative bacteria: Aliivibrio fischeri Nematode Caenorhabditis elegans | Antimicrobial Antihelmintic | Antibacterial activity (100% inhibition at 100 µM). Antihelmintic effect (80% mortality at 100 µg/mL). | [4] |
| Melanelia subaurifera (Nyl.) Melanelia fuliginosa (Fr. ex Duby) Ess | In vitro | DPPH assay | Antioxidant | Slight DPPH scavenging activity (IC50 value of 424.5 μg/mL) and reducing power (0.0165 at 125 μg/mL). | [24] | |
| Parmotrema grayanum (Hue) Hale. | In vitro | Superoxide radical (SOR) Nitric oxide radical DPPH assay | Antioxidant | Good antioxidant activity: SOR assay (IC50 value = 91.5 µmol), DPPH (IC50 value = 34 µmol), NOR assay (IC50 value = 53.5 µmol). | [94] | |
| Parmotrema stuppeum (Nyl.) Hale | In vitro | Beta-carotene-linoleate model system | Antioxidant | Thirty-six percent of antioxidant activity at 500 µg/ml. | [48] | |
| Hypocenomyce scalaris (Ach. ex. Lilj | In vitro | Colorectal cancer cells (HCT116 and DLD-1) Human keratinocytes HaCaT cell line | Cytotoxic | Moderate cytotoxic effects against colon HCT116 cells. ↓ Slight Axin2 expression in HCT116 cells. | [95] | |
| Parmotrema tinctorum (Despr. ex Nyl.) Hale. | In vitro | Hep-2 (human larynx carcinoma cells) MCF7 (human breast carcinoma cells) 786-0 (human kidney carcinoma cells) B16-F10 (murine melanoma cells) | Cytotoxic | Slight activity against all tested cancer cell lines (IC50 values > 50 µg/mL). | [97] | |
| Melanelia subaurifera (Nyl.) Melanelia fuliginosa (Fr. ex Duby) Ess | In vitro | Hela (human epithelial carcinoma cells) A549 (human lung carcinoma cells) LS174 (human colon carcinoma cells) | Cytotoxic | Weak cytotoxic activity against Hela cells (IC50 value of 124 μg/mL) and against A549 and LS174 cells (IC50 value of 200 μg/mL). | [24] | |
| Lecanoric acid | - | In vitro | HCT-116 (human colon cancer cell line) | Cytotoxic | Inhibition cell colony formation already at 0.03 μg/mL. Induction of a G2 cell cycle block. Arrest of cells in the M phase. Upregulated expression of cyclin B1 and pH3. Inactive CDK1. More cell death in cancer cells than in primary human immune and endothelial cells. | [96] |
| - | In vitro | Mitochondrial TrxR from rat lung | Cytotoxic | High inhibitory effect on Thioredoxin reductase (TrxR). | [79] | |
| Methyl evernate | Ramalina fastigiata (Pers.) Ach. | In vitro | Gram-positive bacteria: Bacillus cereus, Staphylococcus aureus. Gram-negative bacteria: Escherichia coli, Proteus mirabilis Fungi: Aspergillus flavus, Candida albicans, Mucor mucedo, Trichoderma viride, Cladosporium cladosporioides, Fusarium oxysporum, Alternaria alternata, Penicillium expansum | Antimicrobial | Inhibition against all tested microorganisms. MIC values (from 0.125 to 1 mg/mL). | [24] |
| Ramalina fastigiata (Pers.) Ach. | In vitro | DPPH assay Reducing power assay | Antioxidant | Low DPPH radical scavenging activity (IC50 value of 391.57 μg/mL). Isolated components showed higher reducing power than lichen extracts. | [24] | |
| Ramalina fastigiata (Pers.) Ach. | In vitro | Hela (human epithelial carcinoma cells) A549 (human lung carcinoma cell line) LS174 (human colon carcinoma cells) | Cytotoxic | IC50 values of 46.45 μg/mL (Hela cell line), 76.84 μg/mL (A549 cell line), and 161.37 μg/mL (LS174 cell line). | [24] | |
| Olivetoric acid | Pseudevernia furfuracea var. ceratea (Ach.) D. Hawksw. | In vitro | RATECs (rat adipose tissue endothelial cells) | Anti-angiogenic | ↓ Proliferation. Disruption of endothelial tube formation. Depolymerization effects on F-actin stress fibers. | [104] |
| Pseudevernia furfuracea var. ceratea (Ach.) D. Hawksw. | In vitro | Gram-positive bacteria: Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Streptococcus faecalis, Listeria monocytogenes. Gram-negative bacteria: Escherichia coli, Pseudomonas aeruginosa, Pseudomonas vulgaris, Yersinia enterocolitica, Aeromonas hydrophila, Pseudomonas syringae, Klebsiella pneumoniae, Salmonella typhimurium. Fungi: Aspergillus niger, Penicillium notatum, Fusarium solani, Fusarium moniliforme, Fusarium oxysporum, Fusarium. culmorum, Candida albicans, C.glabrata, Alternaria. tenuissima, A. citri, A. alternata, Gaeumannomyces graminis. | Antimicrobial | Active against all bacteria and yeast except K. pneumoniae, P. aeruginosa, and P. syringae. Active against all tested fungi except A. citri, A. tenuissima, A.niger, and G. graminis. | [100] | |
| Pseudevernia furfuracea (L.) Zopf | In vitro | Cultured human amnion fibroblasts | Antioxidant | ↓ Cell viability (IC50 values of 571.27 mg/mL) <50 mg/L no oxidative stress and genotoxicity. | [101] | |
| Pseudevernia furfuracea (L.) Zopf | In vitro | HLs (cultured human lymphocytes) | Antioxidant | ↑ Total antioxidant capacity. | [16] | |
| Olivetoric acid | Pseudevernia furfuracea (L.) Zopf | In vitro | U87MG (glioblastoma multiforme cell line) PRCC cells (neurons from Sprague Dawley® rats) | Cytotoxic | ↓ Cell viability (IC50 values of 125.71 mg/mL, for PRCC cells and 17.55 mg/L for U87MG cells). ↑ 8-OH-dG levels. LDH activity and oxidative DNA damage. | [102] |
| Pseudevernia furfuracea (L.) Zopf | In vitro | HepG2 (human hepatocellular carcinoma cells) | Cytotoxic | Cytotoxicity with 100–400 mg/L. Upregulation of pro-apoptotic genes (BAK, CASP6, CASP7, CASP8, FADD, FAS, FASLG). | [103] | |
| Perlatolic acid | - | In silico | Microsomal prostaglandin E2 synthase 1 | Anti-inflammatory | Potent inhibitor of microsomal prostaglandin E2 synthase-1 (IC50 = 0.43 µM). | [106] |
| Cetrelia monachorum (Zahlbr.) W.L. Culb. and C.F. Culb. | In vitro In vivo | Stimulated A549 lung epithelial adenocarcinoma cells Stimulated HEK-293 cells Thioglycollate-induced C57BL/6J male murine peritonitis model | Anti-inflammatory | Microsomal prostaglandin E2 synthase-1 inhibition (IC50 = 0.4 µM), 5-Lipoxygenase inhibition (IC50 = 1.8 µM for cell-based assay and IC50 = 0.4 µM for purified enzyme). Tumor necrosis factor alpha-induced NF-kB (IC50 = 7 µM). Inhibition of leukocyte recruitment. | [107] | |
| Stereocaulon sp. | In vitro | Methicillin-resistant Staphylococcus aureus strains | Antimicrobial | MIC90 value of 32 µg/mL. Synergic action with gentamicin and antagonism action with levofloxacin. | [105] | |
| Cladina confusa (Sant.). Folmm and Ahti | In vitro | Cultures of peritoneal macrophage cells from mice | Immune modulating | ↑ Hydrogen peroxide release (10.48 nmol). Slight NO release activity. | [108] | |
| Cladonia portentosa (Dufour) Coem. | In vitro Ex vivo | Neuro2A (mouse neuroblastoma) cell line Primary neural stem or progenitor cells | Neuroprotective | Neurotrophic activity (125.34 μm at 0.5 μM). AChE inhibition activity (IC50 = 6.8 μM). Potent proneurogenic activity. Gene expression of BDNF and NGF modulation. ↑ Acetyl H3 and H4 protein levels. | [63] | |
| Ramalic acid/Obtusatic acid | Ramalina fraxinea (L.) Ach. Ramalina fastigiata (Pers.) Ach. | In vitro | Gram-positive bacteria: Bacillus cereus, Bacillus subtilis, Staphylococcus aureus. Gram-negative bacteria: Escherichia coli, Proteus mirabilis Fungi: Aspergillus flavus, Aspergillus niger, Candida albicans, Mucor mucedo, Trichoderma viride, Cladosporium cladosporioides. | Antimicrobial | Inhibition against all tested microorganisms. MIC values (from 0.125 to 1 mg/mL). | [24] |
| Ramalic acid/Obtusatic acid | Ramalina fraxinea (L.) Ach. Ramalina fastigiata (Pers.) Ach. | In vitro | DPPH assay Reducing power assay | Antioxidant | Slight to moderate antioxidant activity (DPPH radical scavenging activity with IC50 value of 324.61 μg/mL and reducing power of 0.0142 at 125 µg/mL). Isolated components showed higher reducing power than lichen extracts. | [24] |
| - | In vitro | HaCaT (human keratinocyte cell line) | Cytotoxic | No significant inhibitory activity against LTB (4) production via non-mediation by redox reactions. No cytotoxic activity. | [76] | |
| Ramalina fraxinea (L.) Ach. Ramalina fastigiata (Pers.) Ach. | In vitro | Hela (human epithelial carcinoma cell line) A549 (human lung carcinoma cell line) LS174 (human colon carcinoma cell line) | Cytotoxic | IC50 value (Hela) 43.24 μg/mL; IC50 value (A549) 93.98 μg/mL; IC50 value (LS174) 74.28 μg/mL. | [24] | |
| Sekikaic acid | Dirinaria consimilis (Stirt.) D. D. Awasthi | In vivo | STZ-induced type 2 diabetic albino rat model | Antidiabetic | ↑ α-glucosidase and α-amylase inhibition. ↓ Plasma glucose levels (44.17%), low-density. lipoprotein, total cholesterol, and total glycerides. | [111] |
| Ramalina roesleri Nyl | In vitro | Gram-positive bacteria: Bacillus subtilis, Staphylococcus aureus, Streptomyces viridochromogenes, Streptococcus mutans. Gram-negative bacteria: Escherichia coli. | Antimicrobial | Maximum antimicrobial activity against E. coli (78% inhibition), moderate against S. mutans, S. aureus, and S. viridochromogenes (60%, 50% and 55% inhibition, respectively), and low against B. subtilis (15% inhibition). | [109] | |
| Sekikaic acid | Ramalina farinacea (L.) Ach | In vitro | Respiratory syncytial virus | Antimicrobial | Potent antiviral action against a recombinant strain rg respiratory syncytial virus (IC50 5.69 µg/mL) and respiratory syncytial virus A2 strain (IC50 7.73 µg/mL). | [110] |
| Ramalina roesleri Nyl | In vitro | DPPH assay | Antioxidant | Good antioxidant activity: DPPH radical assay (IC50 value = 11.24 µg/mL). | [109] | |
| Heterodermia obscurata (Nyl.) Trevisan | In vitro | Superoxide radical (SOR) Nitric oxide radical DPPH assay | Antioxidant | Good antioxidant activity: SOR assay (IC50 value = 82.0 µmol), DPPH (IC50 value = 32.6 µmol). No nitric oxide radical activity. | [94] | |
| Dirinaria consimilis (Stirt.) D. D. Awasthi | In vitro | Ferric ion reducing power and hydroxyl radical assay. | Antioxidant | Good antioxidant activity: hydroxyl radical assay (IC50 value = 41.5 µg/mL) and ferric ion assay (IC50 value = 42.0 µg/mL). | [33] | |
| Niebla homalea (Ach.) Rundel and Bowler | In vitro | MCF-7 (human hormone-dependent breast) A2780 (ovarian cancer cell) | Cytotoxic | No antiproliferative activity. | [112] | |
| Squamatic acid | Cladonia uncialis (L.) F. H. Wigg. | In vitro | Gram-positive bacteria: Staphylococcus aureus Gram negative bacteria: Escherichia coli, Fungi: Candida albicans | Antimicrobial | Weak antibacterial activity (MIC = 1250.0 mg/mL against S. aureus). | [113] |
| Thamnolia vermicularis (Sw.) Schaer | In vitro | PC-3 (prostate cancer cells) | Cytotoxic | Weak antiproliferative effect. | [114] | |
| Thamnolic acid | Usnea florida (L.) F.H. Wigg | In vitro | Gram-positive bacteria: Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, Staphylococcus aureus, Enterococcus faecalis, Enterobacter aerogenes, Micrococcus luteus. Gram-negative bacteria: Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Salmonella typhimurium, Yersinia enterocolitica. Mycobacteria: Mycobacterium tuberculosis. Fungi: Candida parapsilosis, Candida albicans, Candida globrata, Aspergillus niger, Aspergillus flavus, Fusarium moniliforme, Rhizopus sp., Alternaria brassicola, Sclerotium rolfsii, Fusarium solani | Antimicrobial | Antifungal: Alternaria alternate, Aspergillus fumigatus and Sclerotium rolfsii with MIC values of 400, 400, and 200 µg/mL, respectively. Anti-yeast: Candida krusei with MIC value of 400 µg/mL. Antibacterial: Bacillus cereus, Bacillus subtilis, and Proteus vulgaris with MIC value of 400 µg/mL and Listeria monocytogenes and Micrococcus luteus with MIC value of 200 µg/mL. | [115] |
| Thamnolia vermicularis (Sw.) Schaer. | In vitro | PC-3 (prostate cancer cells) | Cytotoxic | Weak antiproliferative effect. | [114] |
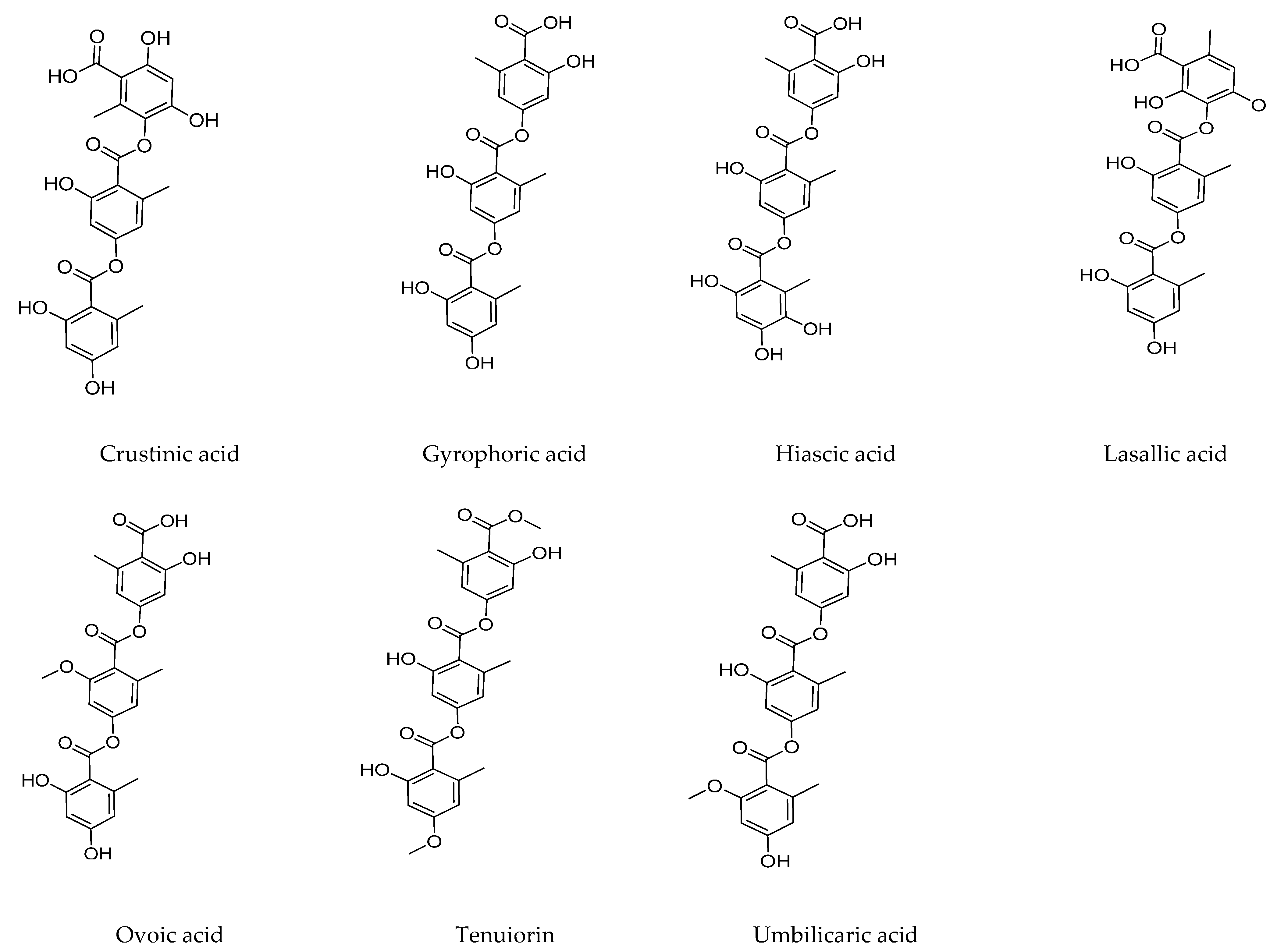
2.2. Tridepsides
2.2.1. Gyrophoric Acid
2.2.2. Tenuiorin Acid
2.2.3. Trivaric Acid
| Tridepside | Botanical Origin | Type of Study | Experimental Model | Activities | Results | References |
|---|---|---|---|---|---|---|
| Gyrophoric acid | Umbilicaria antarctica Frey and I. M. Lamb. | In vitro | PTP1B enzyme activity and kinetic analysis | Antidiabetic Antiobesity | Inhibition PTP1B activity IC50: 3.6 μM in a non-competitive manner. | [99] |
| Parmelia saxatilis (L.) Ach. | In vitro In silico | Angiotensin II type-1 receptor (AT1) interaction | Antihypertensive | AT1 antagonist. Calcium influx assay (IC50 29.76 μM). | [123] | |
| Acarospora fuscata (Nyl.) Th.Fr. | In vitro | Gram-positive bacteria: Bacillus mycoides, Bacillus subtilis Staphylococcus aureus. Gram-negative bacteria: Escherichia coli, Klebsiella pneumoniae. Fungi: Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Penicillium purpurescens and Penicillium verrucosum. | Antimicrobial | Minimum inhibitory concentration values ranging from 0.019 to 1.25 mg/mL. | [117] | |
| Xanthoparmelia pokornyi (Körb.) O.Blanco, A.Crespo, Elix, D.Hawksw. and Lumbsch | In vitro | Gram-positive bacteria: Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, Staphylococcus aureus. Streptococcus faecalis. Gram-negative bacteria: Aeromonas hydrophila, Proteus vulgaris, Yersinia enterocolitica. Fungi: Candida albicans and Candida glabrata. | Antimicrobial | Active against some bacteria and fungi (A. hydrophila, B. cereus, B. subtilis, L. monocytogenes, P. vulgaris, S. aureus, S. faecalis, Y. enterocolitica, C. albicans and C. glabrata) | [118] | |
| Gyrophoric acid | Acarospora fuscata (Nyl.) Th.Fr. | In vitro | DPPH Superoxide anion radical-scavenging reducing power | Antioxidant | DPPH (IC50 105.75 µg/mL). Superoxide anion radical (IC50 196.62 µg/mL). Reducing power (1.32 at 1000 µg/mL, 1.12 at 500 µg/mL, 0.71 at 250 µg/mL, 0.39 at 125 µg/mL, and 0.20 at 62.5 µg/mL). | [117] |
| Parmelia nepalensis Tayl. Parmelia tinctorum Nyl. | In vitro | HaCaT (human keratinocyte cell line) | Antiproliferative | Antiproliferative activity (IC50 value of 1.7 µM). Cytostatic mechanism. | [76] | |
| Umbilicaria hirsuta (Sw. ex Westr.) Hoffm | In vitro | A2780 (human ovarian carcinoma) HCT-116 p53+/+ and HCT-116 p53−/− (human colon carcinoma) HeLa (human cervix adenocarcinoma) SK-BR-3 (human breast adenocarcinoma) HL-60 (human promyelocytic leukemia) HT-29 (human colon adenocarcinoma) Jurkat (Human T cells lymphocyte leukemia) MCF-7 (Human breast adenocarcinoma) | Cytotoxic | Effective against A2780, HL-60, and Jurkat cells. Clonogenic ability inhibition of SK-BR-3 cells. A2780 cells accumulation in S-phase at expense of G1/G0-phase. | [55] | |
| Gyrophoric acid | Umbilicaria hirsuta (Sw. ex Westr.) Hoffm | In vitro | HeLa (human cervix carcinoma) | Cytotoxic | Oxidative stress pathway: ↑ ROS level, DNA oxidation and activity changes of stress/survival proteins as p38MAPK, Erk1/2 and Akt. Apoptosis pathway: ↑ caspase-3 activation, PARP cleavage, PS externalization, and cell cycle changes. | [17] |
| Acarospora fuscata (Nyl.) Th.Fr. | In vitro | A549 (human lung carcinoma cell line), Fem-x (malignant melanoma cell line), K562 (chronic myelogeneous leukemia cell line) LS174 (human colon carcinoma cell line) | Cytotoxic | Weak activity against A549 and LS174 (IC50 151.51 and 151.65 µg/mL). Moderate cytotoxic effect against Fem-x and K562 cells (IC50 64.01 and 78.45 µg/mL). Apoptosis of sub-G1 phase in malignant cells. Reduction percentage of cells in G0/G1 and S-G2/Mphases of the cell cycle. | [117] | |
| Ochrolechia deceptionis Hue. | In vitro | A375 (melanoma cancer cell line) | Cytotoxic | Low activity. | [58] | |
| - | In vitro | Primary cultures of rat hepatocytes | Cytotoxic | Inactive. | [119] | |
| Umbilicaria hirsuta (Sw. ex Westr.) Hoffm | In vitro | Calf thymus DNA | DNA-interacting agents | Topoisomerase I inhibition (25 μM). | [61] | |
| - | In vitro | Second and third instar larvae of the mosquito Culiseta longiareolata | Larvicidal activity | LC (50) values: 0.41 ppm, LC (90) values: 1.93 ppm. | [39] | |
| Xanthoparmelia pokornyi (Körb.) O.Blanco, A.Crespo, Elix, D.Hawksw. and Lumbsch | In vitro | HaCaT (human keratinocyte cell line) | Photoprotective | Prevention of cytotoxic, apoptotic, and cytoskeleton alterative effects of 2.5 J/cm2 UVB. | [121] | |
| Gyrophoric acid | Lasallia pustulata (L.) Méra | In vitro | DPPH assay NBT assay Human keratinocytes HaCaT cell line | Photoprotective | DPPH (IC50 25 µg/mL) Good PF-UVA candidate (SPF > 5). | [120] |
| - | In vitro | (UVA)-treated dermal fibroblasts | Photoprotective | Anti-aging effects. Upregulated mRNA levels of COL1A1/COL3A1/SOD2 genes and type I collagen protein levels. ↓MMP1 mRNA and protein expression levels. | [122] | |
| Lasallia pustulata (L.) Méra | In vitro | MM98 (Malignant mesothelioma cell line) A431 (vulvar carcinoma cell line) HaCaT (human keratinocyte cell line) | Wound healing | Strong wound closure effects. Better results combined with (+)-usnic acid. | [89] | |
| Tenuiorin acid | Peltigera leucophlebia (Nyl.) Gyeln. | In vitro | Calcium-stimulated porcine leucocytes. T-47D (human cancer breast cell line) WIDR (human cancer colon cell line) PANC-1 (human cancer pancreas cell line) | Antiproliferative | Moderate 5-lipoxygenase inhibition (IC50 values of 41.6 μM). Moderate/weak antiproliferative effects on PANC-1 and WIDR cells (ED50 87.9 and 95.9 μM, respectively) and weak activity against T-47D cells (ED50 152.6 μM). | [22] |
| Umbilicaria antarctica Frey and I. M. Lamb. | In vitro In silico | ThT fluorescence assay Docking studies | Neuroprotective | Tau inhibitor (IC50 100 µM). | [124] | |
| Trivaric acid | - | In silico | Docking and ITC studies | Antidiabetic | PTP1b inhibition by blocking its active site. | [126] |
| - | In vitro | PTP1b inhibition assay Human liver HepG2 cancer cell line | Antidiabetic | ↑ PTP1B inhibitory activity. IR/IRS/Akt/GLUT2 pathway stimulation. ↑ Glucose consumption | [125] | |
| Trivaric acid | - | In vivo | Diabetic mice model | Antidiabetic | ↓ insulin resistance ↓ leptin resistance. Improve lipid profile and weight control. | [125] |
| - | In vitro | Human leukocyte elastase assay | Anti-inflammatory | Potent human leukocyte elastase inhibitory activity (IC50 of 1.8 µM). | [127] |
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding Microbial Multi-Species Symbioses. Front. Microbiol. 2016, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 623839. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Untari, L.F.; Laub, A.; Porzel, A.; Franke, K.; Wessjohann, L.A. Anthelmintic and antimicrobial activities of three new depsides and ten known depsides and phenols from Indonesian lichen: Parmelia cetrata Ach. Nat. Prod. Res. 2020, 35, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Adenubi, O.T.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N. Lichens: An update on their ethnopharmacological uses and potential as sources of drug leads. J. Ethnopharmacol. 2022, 298, 115657. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Y.; Yue, P.; Li, H.; Wu, Y.; Hao, X.; Peng, F. Structure, stability, antioxidant activity, and controlled-release of selenium nanoparticles decorated with lichenan from Usnea longissima. Carbohydr. Polym. 2023, 299, 120219. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Baláž, M.; Goga, M.; Hegedüs, M.; Daneu, N.; Kováčová, M.; Tkáčiková, L.; Balážová, L.; Bačkor, M. Biomechanochemical Solid-State Synthesis of Silver Nanoparticles with Antibacterial Activity Using Lichens. ACS Sustain. Chem. Eng. 2020, 8, 13945–13955. [Google Scholar] [CrossRef]
- Olivier-Jimenez, D.; Chollet-Krugler, M.; Rondeau, D.; Beniddir, M.A.; Ferron, S.; Delhaye, T.; Allard, P.M.; Wolfender, J.L.; Sipman, H.J.M.; Lücking, R.; et al. A database of high-resolution MS/MS spectra for lichen metabolites. Sci. Data 2019, 28, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef]
- Okuyama, E.; Umeyama, K.; Yamazaki, M.; Kinoshita, Y.; Yamamoto, Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995, 61, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttir, K.; Wiedemann, B.; Birgisdóttir, M.; Nenninger, A.; Jónsdóttir, S.; Wagner, H. Inhibitory effects of baeomycesic acid from the lichen Thamnolia subuliformis on 5-lipoxygenase in vitro. Phytomedicine 1997, 4, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Emsen, B.; Togar, B.; Turkez, H.; Aslan, A. Effects of two lichen acids isolated from Pseudevernia furfuracea (L.) Zopf in cultured human lymphocytes. Z. Naturforsch. C J. Biosci. 2018, 73, 303–312. [Google Scholar] [CrossRef]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative stress mediated by gyrophoric acid from the lichen Umbilicaria hirsuta affected apoptosis and stress/survival pathways in HeLa cells. BMC Complement. Altern. Med. 2019, 19, 221. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.A.M.F.; Aires, A.L.; Soares, C.L.R.; Sá, J.L.F.; Martins, M.C.B.; Albuquerque, M.C.P.A.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Melo, A.M.M.A.; et al. Barbatic acid from Cladia aggregata (lichen): Cytotoxicity and in vitro schistosomicidal evaluation and ultrastructural analysis against adult worms of Schistosoma mansoni. Toxicol. In Vitro 2020, 65, 104771. [Google Scholar] [CrossRef]
- Karunaratne, V.; Bombuwela, K.; Kathirgamanathar, S.; Thadhani, V.M. Lichens: A chemically important biota. J. Natl. Sci. Found. Sri Lanka 2005, 33, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Leavitt, S.D.; Hawksworth, D.L. Species in lichen-forming fungi: Balancing between conceptual and practical considerations, and between phenotype and phylogenomics. Fungal Divers. 2021, 109, 99–154. [Google Scholar] [CrossRef]
- Singh, G.; Armaleo, D.; Dal Grande, F.; Schmitt, I. Depside and Depsidone Synthesis in Lichenized Fungi Comes into Focus through a Genome-Wide Comparison of the Olivetoric Acid and Physodic Acid Chemotypes of Pseudevernia furfuracea. Biomolecules 2021, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttir, K.; Gudmundsdóttir, G.F.; Ogmundsdóttir, H.M.; Paulus, K.; Haraldsdóttir, S.; Kristinsson, H.; Bauer, R. Effects of tenuiorin and methyl orsellinate from the lichen Peltigera leucophlebia on 5-/15-lipoxygenases and proliferation of malignant cell lines in vitro. Phytomedicine 2002, 9, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Gökalsın, B.; Sesal, N.C. Lichen secondary metabolite evernic acid as potential quorum sensing inhibitor against Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2016, 32, 150. [Google Scholar] [CrossRef]
- Ristic, S.; Rankovic, B.; Kosanić, M.; Stamenkovic, S.; Stanojković, T.; Sovrlić, M.; Manojlović, N. Biopharmaceutical Potential of Two Ramalina Lichens and their Metabolites. Curr. Pharm. Biotechnol. 2016, 17, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Nat. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef] [PubMed]
- Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Türk, A.O.; Tay, T.; Kivanç, M. The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (-)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z. Naturforsch. C J. Biosci. 2004, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Pomponio, S.; Di Vincenzo, V.; Crocetta, V.; Nicoletti, M.; Piovano, M.; Garbarino, J.A.; Di Bonaventura, G. Antimicrobial and antibiofilm activity of secondary metabolites of lichens against methicillin-resistant Staphylococcus aureus strains from cystic fibrosis patients. Future Microbiol. 2013, 8, 281–292. [Google Scholar] [CrossRef]
- Wang, X.N.; Yu, W.T.; Lou, H.X. Antifungal constituents from the Chinese moss Homalia trichomanoides. Chem. Biodivers. 2005, 2, 139–145. [Google Scholar] [CrossRef]
- Goel, M.; Dureja, P.; Rani, A.; Uniyal, P.L.; Laatsch, H. Isolation, characterization and antifungal activity of major constituents of the Himalayan lichen Parmelia reticulata Tayl. J. Agric. Food Chem. 2011, 59, 2299–2307. [Google Scholar] [CrossRef]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q. Antimycobacterial activity of lichen substances. Phytomedicine 2010, 17, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttir, K.; Chung, G.A.; Skúlason, V.G.; Gissurarson, S.R.; Vilhelmsdóttir, M. Antimycobacterial activity of lichen metabolites in vitro. Eur. J. Pharm. Sci. 1998, 6, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Tatipamula, V.B.; Annam, S.S.P. Antimycobacterial activity of acetone extract and isolated metabolites from folklore medicinal lichen Usnea laevis Nyl. against drug-sensitive and multidrug-resistant tuberculosis strains. J. Ethnopharmacol. 2022, 282, 114641. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Verma, N.; Sharma, B.O.; Behera, B.C. Growth promoting effects of some lichen metabolites on probiotic bacteria. J. Food Sci. Technol. 2014, 51, 2624–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, T.H.; Le Lamer, A.C.; Lalli, C.; Boustie, J.; Samson, M.; Lohézic-Le Dévéhat, F.; Le Seyec, J. Depsides: Lichen metabolites active against hepatitis C virus. PLoS ONE 2015, 10, e0120405. [Google Scholar] [CrossRef]
- Zofou, D.; Kengne, A.B.; Tene, M.; Ngemenya, M.N.; Tane, P.; Titanji, V.P. In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem bark of Kigelia africana (Lam.) Benth (Bignoniaceae). Parasitol. Res. 2011, 108, 1383–1390. [Google Scholar] [CrossRef]
- Zofou, D.; Tene, M.; Tane, P.; Titanji, V.P. Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol. Res. 2012, 110, 539–544. [Google Scholar] [CrossRef]
- Beshbishy, A.M.; Batiha, G.E.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic Effects of Atranorin towards the Proliferation of Babesia and Theileria Parasites. Pathogens 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Cetin, H.; Tufan-Cetin, O.; Turk, A.O.; Tay, T.; Candan, M.; Yanikoglu, A.; Sumbul, H. Larvicidal activity of some secondary lichen metabolites against the mosquito Culiseta longiareolata Macquart (Diptera: Culicidae). Nat. Prod. Res. 2012, 26, 350–355. [Google Scholar] [CrossRef]
- Bugni, T.S.; Andjelic, C.D.; Pole, A.R.; Rai, P.; Ireland, C.M.; Barrows, L.R. Biologically active components of a Papua New Guinea analgesic and anti-inflammatory lichen preparation. Fitoterapia 2009, 80, 270–273. [Google Scholar] [CrossRef]
- Kumar, K.C.; Müller, K. Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J. Nat. Prod. 1999, 62, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.G.; Araújo, A.A.; Rocha, C.P.; Almeida, E.M.; Siqueira, R.e.S.; Bonjardim, L.R.; Quintans, L.J. Purification, physicochemical properties, thermal analysis and antinociceptive effect of atranorin extracted from Cladina kalbii. Biol. Pharm. Bull. 2008, 31, 1977–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, R.S.; Bonjardim, L.R.; Araújo, A.A.; Araújo, B.E.; Melo, M.G.; Oliveira, M.G.; Gelain, D.P.; Silva, F.A.; DeSantana, J.M.; Albuquerque-Júnior, R.L.; et al. Antinociceptive activity of atranorin in mice orofacial nociception tests. Z. Naturforsch. C J. Biosci. 2010, 65, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Paramita Devi, A.; Tran, N.M.; Phan, H.V.; Huynh, N.V.; Sichaem, J.; Tran, H.D.; Alam, M.; Nguyen, T.P.; Nguyen, H.H.; et al. Synthesis, α-glucosidase inhibition, and molecular docking studies of novel N-substituted hydrazide derivatives of atranorin as antidiabetic agents. Bioorg. Med. Chem. Lett. 2020, 30, 127359. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, K.S.; Wafo, P.; Ali, Z.; Khan, A.; Oluyemisi, O.O.; Marasini, B.P.; Khan, I.A.; Bonaventure, N.T.; Choudhary, M.I.; Atta-ur-Rahman. Chemical constituents of Stereospermum acuminatissimum and their urease and α-chymotrypsin inhibitions. Fitoterapia 2012, 83, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, P.; Tzakou, O.; Vagias, C.; Kefalas, P.; Roussis, V. Beta-orcinol metabolites from the lichen Hypotrachyna revoluta. Molecules 2007, 12, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.G.; dos Santos, J.P.; Serafini, M.R.; Caregnato, F.F.; Pasquali, M.A.; Rabelo, T.K.; da Rocha, R.F.; Quintans, L.; Araújo, A.A.; da Silva, F.A.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. In Vitro 2011, 25, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasha, G.K.; Rao, L.J. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z. Naturforsch. C J. Biosci. 2000, 55, 1018–1022. [Google Scholar] [CrossRef]
- Harikrishnan, A.; Veena, V.; Lakshmi, B.; Shanmugavalli, R.; Theres, S.; Prashantha, C.N.; Shah, T.; Oshin, K.; Togam, R.; Nandi, S. Atranorin, an antimicrobial metabolite from lichen Parmotrema rampoddense exhibited in vitro anti-breast cancer activity through interaction with Akt activity. J. Biomol. Struct. Dyn. 2021, 39, 1248–1258. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Y.; Park, S.Y.; Nguyen, T.T.; Seo, Y.W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.S.; Kim, H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep. 2017, 7, 8136. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, S.; Kim, J.H.; Youn, U.J.; Suh, S.S. The Comprehensive Roles of Atranorin, A Secondary Metabolite from the Antarctic Lichen Stereocaulon caespitosum, in HCC Tumorigenesis. Molecules 2019, 24, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Węgrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyż, J.; et al. Usnic acid and atranorin exert selective cytostatic and anti-invasive effects on human prostate and melanoma cancer cells. Toxicol. In Vitro 2017, 40, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S. Phytochemical investigation of the Australian lichens Ramalina glaucescens and Xanthoria parietina. Nat. Prod. Commun. 2009, 4, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Nie, X.; Zhang, H.; Wang, L.; Geng, Z.; Du, X.; Qian, H.; Liu, W.; Liu, T. Atranorin driven by nano materials SPION lead to ferroptosis of gastric cancer stem cells by weakening the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis and its expression. Int. J. Med. Sci. 2022, 19, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef]
- Solár, P.; Hrčková, G.; Koptašíková, L.; Velebný, S.; Solárová, Z.; Bačkor, M. Murine breast carcinoma 4T1 cells are more sensitive to atranorin than normal epithelial NMuMG cells in vitro: Anticancer and hepatoprotective effects of atranorin in vivo. Chem. Biol. Interact. 2016, 250, 27–37. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- Brandão, L.F.; Alcantara, G.B.; Matos, M.e.F.; Bogo, D.; Freitas, D.o.S.; Oyama, N.M.; Honda, N.K. Cytotoxic evaluation of phenolic compounds from lichens against melanoma cells. Chem. Pharm. Bull. 2013, 61, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Caggia, S.; Piovano, M.; Garbarino, J.; Cardile, V. Effect of vicanicin and protolichesterinic acid on human prostate cancer cells: Role of Hsp70 protein. Chem.-Biol. Interact. 2012, 195, 1–10. [Google Scholar] [CrossRef]
- Plsíkova, J.; Stepankova, J.; Kasparkova, J.; Brabec, V.; Backor, M.; Kozurkova, M. Lichen secondary metabolites as DNA-interacting agents. Toxicol. In Vitro 2014, 28, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Dévéhat, F.L.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Reyes, A.; Hidalgo, M.E.; Quilhot, W. Photoprotector capacity of lichen metabolites assessed through the inhibition of the 8-methoxypsoralen photobinding to protein. J. Photochem. Photobiol. B 1998, 42, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.E.; Fernández, E.; Quilhot, W.; Lissi, E.A. Photohemolytic activity of lichen metabolites. J. Photochem. Photobiol. B 1993, 21, 37–40. [Google Scholar] [CrossRef]
- Haraldsdóttir, S.; Guolaugsdóttir, E.; Ingólfsdóttir, K.; Ogmundsdóttir, H.M. Anti-proliferative effects of lichen-derived lipoxygenase inhibitors on twelve human cancer cell lines of different tissue origin in vitro. Planta Med. 2004, 70, 1098–1100. [Google Scholar] [CrossRef]
- Bucar, F.; Schneider, I.; Ogmundsdóttir, H.; Ingólfsdóttir, K. Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine 2004, 11, 602–606. [Google Scholar] [CrossRef]
- Martins, M.C.; Silva, M.C.; Silva, H.A.; Silva, L.R.; Albuquerque, M.C.; Aires, A.L.; Falcão, E.P.; Pereira, E.C.; de Melo, A.M.; da Silva, N.H. Barbatic Acid Offers a New Possibility for Control of Biomphalaria Glabrata and Schistosomiasis. Molecules 2017, 22, 568. [Google Scholar] [CrossRef] [Green Version]
- Micheletti, A.C.; Honda, N.K.; Ravaglia, L.M.; Matayoshi, T.; Spielmann, A.A. Antibacterial potencial of 12 Lichen species. An. Acad. Bras. Cienc. 2021, 93, S0001-37652021000700904. [Google Scholar] [CrossRef]
- Reddy, S.D.; Siva, B.; Kumar, K.; Babu, V.S.P.; Sravanthi, V.; Boustie, J.; Nayak, V.L.; Tiwari, A.K.; Rao, C.H.V.; Sridhar, B.; et al. Comprehensive Analysis of Secondary Metabolites in Usnea longissima (Lichenized Ascomycetes, Parmeliaceae) Using UPLC-ESI-QTOF-MS/MS and Pro-Apoptotic Activity of Barbatic Acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Miura, Y.; Kinoshita, Y.; Higuchi, M.; Yamada, Y.; Murakami, A.; Ohigashi, H.; Koshimizu, K. Screening of tissue cultures and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced Epstein-Barr virus activation. Chem. Pharm. Bull. 1995, 43, 1388–1390. [Google Scholar] [CrossRef]
- Lang, T.Q.; Zhang, Y.; Chen, F.; Luo, G.Y.; Yang, W.D. Characterization of chemical components with diuretic potential from Pyrrosia petiolosa. J. Asian Nat. Prod. Res. 2021, 23, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Maulidiyah Maulidiyah, M.N.; Nazila, W.; Musdalifah, A.; Salim, L.O.A.; Nurdin, M. Isolation and antibacterial activity of diffractic acid compound from lichen Usnea blepharea Motyka. J. Appl. Pharm. Sci. 2021, 11, 121–130. [Google Scholar] [CrossRef]
- Furmanek, Ł.; Czarnota, P.; Seaward, M.R.D. A review of the potential of lichen substances as antifungal agents: The effects of extracts and lichen secondary metabolites on Fusarium fungi. Arch. Microbiol. 2022, 204, 523. [Google Scholar] [CrossRef] [PubMed]
- Bayir, Y.; Odabasoglu, F.; Cakir, A.; Aslan, A.; Suleyman, H.; Halici, M.; Kazaz, C. The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine 2006, 13, 584–590. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Turkez, H.; Taghizadehghalehjoughi, A.; Kaya, A. The anti-cancer efficacies of diffractaic, lobaric, and usnic acid: In vitro inhibition of glioma. J. Cancer Res. Ther. 2018, 14, 941–951. [Google Scholar] [CrossRef]
- Kumar, K.C.; Müller, K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J. Nat. Prod. 1999, 62, 821–823. [Google Scholar] [CrossRef]
- Truong, T.L.; Nga, V.T.; Huy, D.T.; Chi, H.B.; Phung, N.K. A new depside from Usnea aciculifera growing in Vietnam. Nat. Prod. Commun. 2014, 9, 1179–1180. [Google Scholar] [CrossRef] [Green Version]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic activity and antioxidant capacity of purified lichen metabolites: An in vitro study. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef]
- Ozgencli, I.; Budak, H.; Ciftci, M.; Anar, M. Lichen Acids May Be Used as a Potential Drug for Cancer Therapy; by Inhibiting Mitochondrial Thioredoxin Reductase Purified from Rat Lung. Anticancer Agents Med. Chem. 2018, 18, 1599–1605. [Google Scholar] [CrossRef]
- Silva, C.V.N.S.; Barbosa, J.A.P.; Ferraz, M.S.; Silva, N.H.; Honda, N.K.; Rabello, M.M.; Hernandes, M.Z.; Bezerra, B.P.; Cavalcanti, I.M.F.; Ayala, A.P.; et al. Molecular modeling and cytotoxicity of diffractaic acid: HP-β-CD inclusion complex encapsulated in microspheres. Int. J. Biol. Macromol. 2016, 92, 494–503. [Google Scholar] [CrossRef]
- Oh, J.M.; Kim, Y.J.; Gang, H.S.; Han, J.; Ha, H.H.; Kim, H. Antimicrobial Activity of Divaricatic Acid Isolated from the Lichen Evernia mesomorpha against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H.A.M.F.; Siqueira, W.N.; Sá, J.L.F.; Silva, L.R.S.; Martins, M.C.B.; Aires, A.L.; Amâncio, F.F.; Pereira, E.C.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; et al. Laboratory assessment of divaricatic acid against Biomphalaria glabrata and Schistosoma mansoni cercariae. Acta Trop. 2018, 178, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Aires, A.L.; Soares, C.L.R.; Siqueira, W.N.; Lima, M.V.; Martins, M.C.B.; Albuquerque, M.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; et al. Schistosomicidal effect of divaricatic acid from Canoparmelia texana (Lichen): In vitro evaluation and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2021, 222, 106044. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, A.; Strömstedt, A.A.; Göransson, U.; Gnezdilov, O.; Turanov, A.; Boldbaatar, D.; Kochkin, D.; Ulrich-Merzenich, G.; Koptina, A. Antimicrobial and antioxidant activity of Evernia prunastri extracts and their isolates. World J. Microbiol. Biotechnol. 2021, 37, 129. [Google Scholar] [CrossRef]
- Girardot, M.; Millot, M.; Hamion, G.; Billard, J.L.; Juin, C.; Ntoutoume, G.; Sol, V.; Mambu, L.; Imbert, C. Lichen Polyphenolic Compounds for the Eradication of Candida albicans Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 698883. [Google Scholar] [CrossRef]
- Lauinger, I.L.; Vivas, L.; Perozzo, R.; Stairiker, C.; Tarun, A.; Zloh, M.; Zhang, X.; Xu, H.; Tonge, P.J.; Franzblau, S.G.; et al. Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target. J. Nat. Prod. 2013, 76, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food Chem. Toxicol. 2017, 105, 262–277. [Google Scholar] [CrossRef]
- Lee, S.; Suh, Y.J.; Yang, S.; Hong, D.G.; Ishigami, A.; Kim, H.; Hur, J.S.; Chang, S.C.; Lee, J. Neuroprotective and Anti-Inflammatory Effects of Evernic Acid in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2021, 22, 2098. [Google Scholar] [CrossRef]
- Burlando, B.; Ranzato, E.; Volante, A.; Appendino, G.; Pollastro, F.; Verotta, L. Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Med. 2009, 75, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Kizil, H.E.; Agar, G.; Anar, M. Antiproliferative effects of Evernic acid on A549 and healthy human cells: An in vitro study. J. Biotechnol. 2015, 208, S28. [Google Scholar] [CrossRef]
- Kizil, H.E.; Ağar, G.; Anar, M. Cytotoxic and antiproliferative effects of evernic acid on HeLa cell lines: A candidate anticancer drug. J. Biotechnol. 2014, 185, S29. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, N.; Cantizani, J.; Ortiz-López, F.J.; González-Menéndez, V.; Cautain, B.; Rodríguez, L.; Bills, G.F.; Reyes, F.; Genilloud, O.; Vicente, F. Protective effects of isolecanoric acid on neurodegenerative in vitro models. Neuropharmacology 2016, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, L.A.; Erkoc, P.; Ingelfinger, R.; Henke, M.; Ulshöfer, T.; Schneider, A.-K.; Laux, V.; Geisslinger, G.; Schmitt, I.; Fürst, R.; et al. Lecanoric acid mediates anti-proliferative effects by an M phase arrest in colon cancer cells. Biomed. Pharmacother. 2022, 148, 112734. [Google Scholar] [CrossRef] [PubMed]
- Bogo, D.; de Matos, M.F.; Honda, N.K.; Pontes, E.C.; Oguma, P.M.; da Santos, E.C.; de Carvalho, J.E.; Nomizo, A. In vitro antitumour activity of orsellinates. Z. Naturforsch. C J. Biosci. 2010, 65, 43–48. [Google Scholar] [CrossRef]
- Rama Krishna, B.; Ramakrishna, S.; Rajendra, S.; Madhusudana, K.; Mallavadhani, U.V. Synthesis of some novel orsellinates and lecanoric acid related depsides as α-glucosidase inhibitors. J. Asian Nat. Prod. Res. 2019, 21, 1013–1027. [Google Scholar] [CrossRef]
- Seo, C.; Sohn, J.H.; Ahn, J.S.; Yim, J.H.; Lee, H.K.; Oh, H. Protein tyrosine phosphatase 1B inhibitory effects of depsidone and pseudodepsidone metabolites from the Antarctic lichen Stereocaulon alpinum. Bioorg. Med. Chem. Lett. 2009, 19, 2801–2803. [Google Scholar] [CrossRef]
- Türk, H.; Yilmaz, M.; Tay, T.; Türk, A.O.; Kivanç, M. Antimicrobial activity of extracts of chemical races of the lichen Pseudevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetoric acid constituents. Z. Naturforsch. C J. Biosci. 2006, 61, 499–507. [Google Scholar] [CrossRef]
- Emsen, B.; Turkez, H.; Togar, B.; Aslan, A. Evaluation of antioxidant and cytotoxic effects of olivetoric and physodic acid in cultured human amnion fibroblasts. Hum. Exp. Toxicol. 2017, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Emsen, B.; Aslan, A.; Togar, B.; Turkez, H. In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm. Biol. 2016, 54, 1748–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsen, B.; Sadi, G.; Bostanci, A.; Gursoy, N.; Emsen, A.; Aslan, A. Evaluation of the biological activities of olivetoric acid, a lichen-derived molecule, in human hepatocellular carcinoma cells. Rend. Lincei. Sci. Fis. E Nat. 2021, 32, 135–148. [Google Scholar] [CrossRef]
- Koparal, A.T.; Ulus, G.; Zeytinoğlu, M.; Tay, T.; Türk, A.O. Angiogenesis inhibition by a lichen compound olivetoric acid. Phytother. Res. 2010, 24, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Segatore, B.; Mancini, A.; Di Pietro, L.; Bottoni, C.; Sabatini, A.; Brisdelli, F.; Piovano, M.; Nicoletti, M.; Amicosante, G.; et al. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine 2015, 22, 223–230. [Google Scholar] [CrossRef]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. ChemMedChem 2012, 7, 2077–2081. [Google Scholar] [CrossRef]
- Oettl, S.K.; Gerstmeier, J.; Khan, S.Y.; Wiechmann, K.; Bauer, J.; Atanasov, A.G.; Malainer, C.; Awad, E.M.; Uhrin, P.; Heiss, E.H.; et al. Imbricaric acid and perlatolic acid: Multi-targeting anti-inflammatory depsides from Cetrelia monachorum. PLoS ONE 2013, 8, e76929. [Google Scholar] [CrossRef] [Green Version]
- Carlos, I.Z.; Quilles, M.B.; Carli, C.B.; Maia, D.C.; Benzatti, F.P.; Lopes, T.I.; Gianini, A.S.; Brum, R.L.; Vilegas, W.; dos Santos, L.C.; et al. Lichen metabolites modulate hydrogen peroxide and nitric oxide in mouse macrophages. Z. Naturforsch. C J. Biosci. 2009, 64, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Sisodia, R.; Geol, M.; Verma, S.; Rani, A.; Dureja, P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat. Prod. Res. 2013, 27, 2235–2239. [Google Scholar] [CrossRef]
- Lai, D.; Odimegwu, D.C.; Esimone, C.; Grunwald, T.; Proksch, P. Phenolic compounds with in vitro activity against respiratory syncytial virus from the Nigerian lichen Ramalina farinacea. Planta Med. 2013, 79, 1440–1446. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Annam, S.S.P.; Nguyen, H.T.; Polimati, H.; Yejella, R.P. Sekikaic acid modulates pancreatic β-cells in streptozotocin-induced type 2 diabetic rats by inhibiting digestive enzymes. Nat. Prod. Res. 2021, 35, 5420–5424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, C.Y.; Spjut, R.W.; Fuchs, J.R.; Kinghorn, A.D.; Rakotondraibe, L.H. Specialized metabolites of the United States lichen Niebla homalea and their antiproliferative activities. Phytochemistry 2020, 180, 112521. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Hołderna-Kędzia, E.; Galanty, A.; Bylka, W.; Kacprzak, K.; Ćwiklińska, K. In vitro antimicrobial activity of extracts and compounds isolated from Cladonia uncialis. Nat. Prod. Res. 2015, 29, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Z.L.; Wang, A.L.; Liu, X.Q.; Wang, J.; Guo, X.; Jing, Y.K.; Hua, H.M. Three new phenolic compounds from the lichen Thamnolia vermicularis and their antiproliferative effects in prostate cancer cells. Planta Med. 2011, 77, 2042–2046. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, M.; Sarıözlü, N.Y.; Candan, M.; Tay, N. Screening of antibacterial, antituberculosis and antifungal effects of lichen Usnea florida and its thamnolic acid constituent. Biomed. Res. 2017, 28, 3108–3113. [Google Scholar]
- Odabasoglu, F.; Yildirim, O.S.; Aygun, H.; Halici, Z.; Halici, M.; Erdogan, F.; Cadirci, E.; Cakir, A.; Okumus, Z.; Aksakal, B.; et al. Diffractaic acid, a novel proapoptotic agent, induces with olive oil both apoptosis and antioxidative systems in Ti-implanted rabbits. Eur. J. Pharmacol. 2012, 674, 171–178. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities and chemical composition of lichens from Serbia. EXCLI J. 2014, 13, 1226–1238. [Google Scholar]
- Candan, M.; Yilmaz, M.; Tay, T.; Kivanç, M.; Türk, H. Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. Z. Naturforsch. C J. Biosci. 2006, 61, 319–323. [Google Scholar] [CrossRef]
- Correché, E.R.; Enriz, R.D.; Piovano, M.; Garbarino, J.; Gómez-Lechón, M.J. Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens. Altern. Lab. Anim. 2004, 32, 605–615. [Google Scholar] [CrossRef]
- Lohézic-Le Dévéhat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B 2013, 120, 17–28. [Google Scholar] [CrossRef]
- Varol, M.; Türk, A.; Candan, M.; Tay, T.; Koparal, A.T. Photoprotective Activity of Vulpinic and Gyrophoric Acids toward Ultraviolet B-Induced Damage in Human Keratinocytes. Phytother. Res. 2016, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H. Anti-Aging Effects of Gyrophoric Acid on UVA-Irradiated Normal Human Dermal Fibroblasts. Nat. Prod. Commun. 2020, 15, 1934578X20919545. [Google Scholar] [CrossRef]
- Huo, X.; Qiao, L.; Chen, Y.; Chen, X.; He, Y.; Zhang, Y. Discovery of Novel Multi-target Inhibitor of angiotensin type 1 receptor and neprilysin inhibitors from Traditional Chinese Medicine. Sci. Rep. 2019, 9, 16205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, F.; Caballero, J.; Vargas, R.; Cornejo, A.; Areche, C. Continental and Antarctic Lichens: Isolation, identification and molecular modeling of the depside tenuiorin from the Antarctic lichen Umbilicaria antarctica as tau protein inhibitor. Nat. Prod. Res. 2020, 34, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, B.; Zheng, H.; Zhuang, C.; Li, X.; Lu, X.; Quan, C.; Dong, Y.; Zheng, Z.; Xiu, Z. Trivaric acid, a new inhibitor of PTP1b with potent beneficial effect on diabetes. Life Sci. 2017, 169, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhuang, C.; Li, X.; Zhang, B.; Lu, X.; Zheng, Z.; Dong, Y. Varic acid analogues from fungus as PTP1B inhibitors: Biological evaluation and structure-activity relationships. Bioorg. Med. Chem. Lett. 2017, 27, 3382–3385. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Lu, X.; Ma, Y.; Fan, Y.; Shi, Y.; Dong, A.; Duan, B. Trivaric acid, a potent depside human leukocyte elastase inhibitor. Biol. Pharm. Bull. 2012, 35, 2247–2251. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. J. Fungi 2023, 9, 116. https://doi.org/10.3390/jof9010116
Ureña-Vacas I, González-Burgos E, Divakar PK, Gómez-Serranillos MP. Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. Journal of Fungi. 2023; 9(1):116. https://doi.org/10.3390/jof9010116
Chicago/Turabian StyleUreña-Vacas, Isabel, Elena González-Burgos, Pradeep Kumar Divakar, and María Pilar Gómez-Serranillos. 2023. "Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches" Journal of Fungi 9, no. 1: 116. https://doi.org/10.3390/jof9010116
APA StyleUreña-Vacas, I., González-Burgos, E., Divakar, P. K., & Gómez-Serranillos, M. P. (2023). Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. Journal of Fungi, 9(1), 116. https://doi.org/10.3390/jof9010116









