Cell-Free Supernatant of Bacillus subtilis Reduces Kiwifruit Rot Caused by Botryosphaeria dothidea through Inducing Oxidative Stress in the Pathogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Materials
2.2. Preparation of the Cell-Free Supernatant (CFS)
2.3. Fruits
2.4. Effectiveness of the CFS against Kiwifruit Soft Rot Pathogen In Vitro and In Vivo
2.5. Ultrastructural Morphology of Pathogens Treated with the CFS
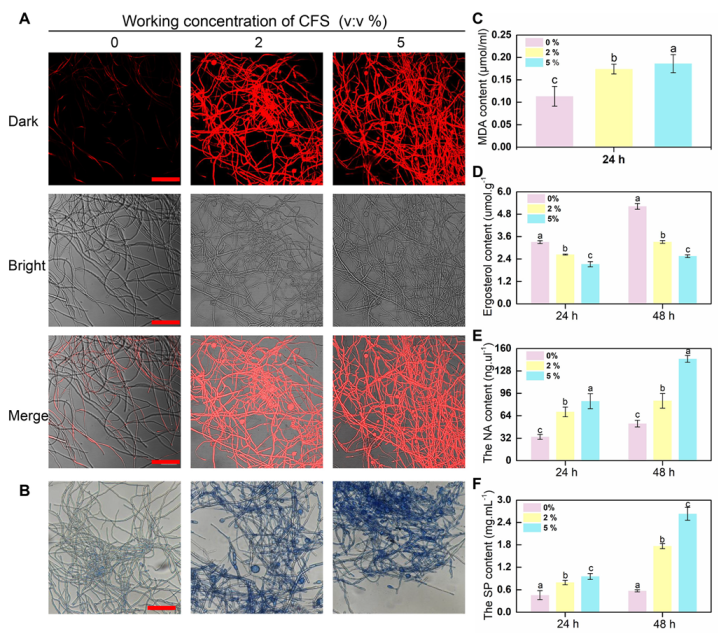
2.6. Effect of the CFS on Cell Death of Pathogenic Mycelium
2.7. Determination of the Integrity of Cell Membranes
2.8. Determination of Ergosterol, Determination of the Leakage of Cellular Contents
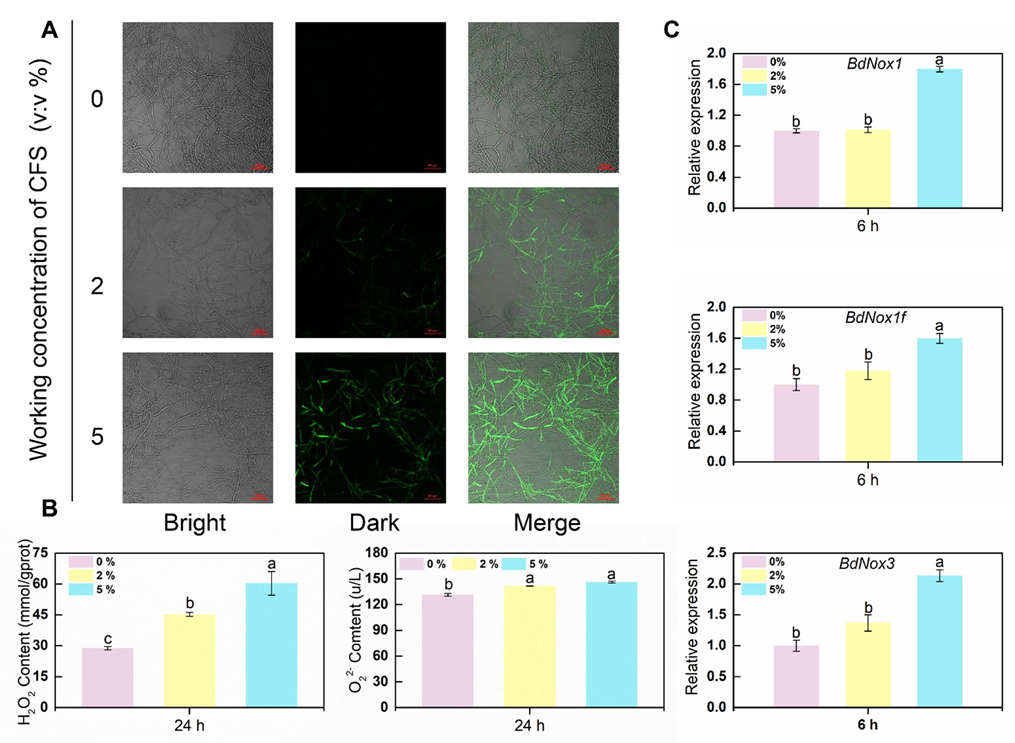
2.9. The CFS induces Malondialdehyde (MDA) Production and Reactive Oxygen Species (ROS) Accumulation
2.10. Enzyme Activity Assay and Real-Time Fluorescence Quantitative PCR Assay
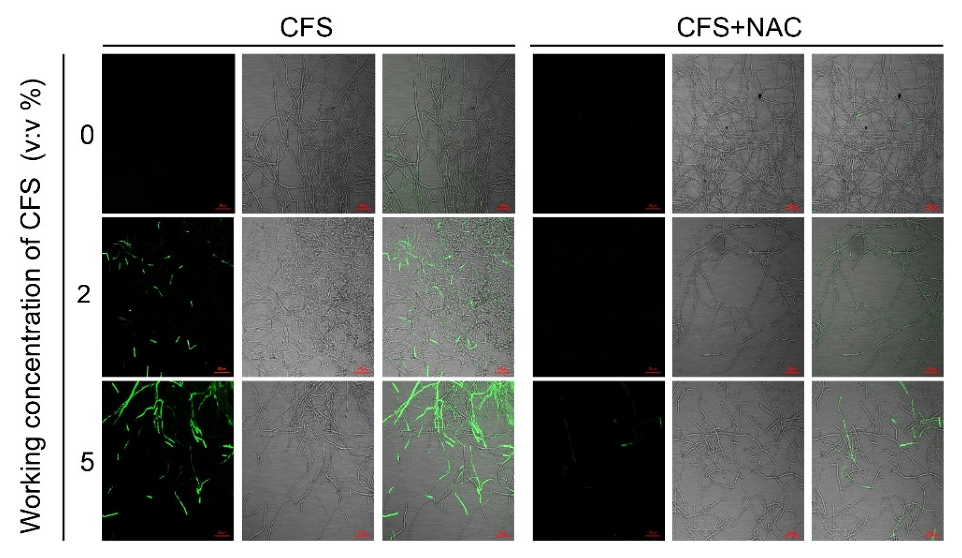
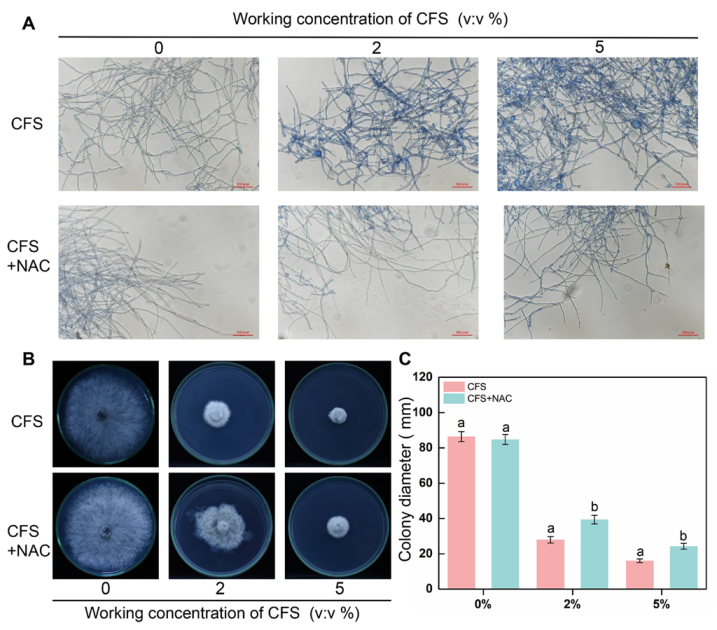
2.11. Effect of N-Acetylcysteine (NAC) on the Recovery of Mycelial Growth
2.11.1. Plate Recovery Experiments
2.11.2. Mycelial ROS Accumulation and Mycelial Cell Damage/Integrity Recovery Test
2.12. Statistical Analysis
3. Result
3.1. The CFS of Bacillus Subtilis BS-1 Inhibits Vegetative Growth and Pathogenicity of Botryosphaeria Dothidea
3.2. The CFS Damages and Ruptures the Hyphae of B. dothidea
3.3. The CFS Destroys the Hyphal Cytomembrane and Cause Hyphae death of B. dothidea In Vitro
3.4. The CFS Promotes the Explosion of Reactive Oxygen Species in Hyphal Cells of B. dothidea
3.5. Defense-Related Enzyme Activity and Corresponding Defense Enzyme Synthesis-Related Gene Expression in B. dothidea Mycelial Cells
3.6. N-Acetylcysteine Alleviated the Burst of Reactive Oxygen Species in Hyphae of B. dothidea Induced by CFS
3.7. N-Acetylcysteine (NAC) Reduces Cell Death and Recovers the Weak Growth of B. dothidea Caused by CFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleki, S.; Maleki-Zanjani, B.; Gallego, P.P. Kiwifruit status in Iran: Management and production. Acta Hortic. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Meng, F.B.; Gou, Z.Z.; Li, Y.C.; Zou, L.H.; Chen, W.J.; Liu, D.Y. The Efficiency of Lemon Essential Oil-Based Nanoemulsions on the Inhibition of Phomopsis sp. and Reduction of Postharvest Decay of Kiwifruit. Foods 2022, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.T.; Wu, X.M.; Su, Y.; Long, Y.Y. Co-Application of Tetramycin and Chitosan in Controlling Leaf Spot Disease of Kiwifruit and Enhancing Its Resistance, Photosynthesis, Quality and Amino Acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Characterization of Phenolics in Rejected Kiwifruit and Their Antioxidant Potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Chen, M.Y.; Zhang, S.J.; Zhong, C.H. First Report of Nigrospora oryzae Causing Brown/Black Spot Disease of Kiwifruit in China. Plant Dis. 2018, 102, 243. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, C.; Wu, X.M.; Long, Y.H.; Su, Y. Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef]
- Hur, J.; Oh, S.; Lim, K.; Jung, J.S.; Kim, J.; Koh, Y.J. Novel effects of TiO2 photocatalytic ozonation on control of postharvest fungal spoilage of kiwifruit. Postharvest Biol. Technol. 2005, 35, 109–113. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Mo, F.; Shu, R.; Yin, X.; Wu, X.; Zhang, R.; Zhang, Z.; He, L.; Chen, T.; et al. Antifungal Activity and Biocontrol Mechanism of Fusicolla violacea J-1 against Soft Rot in Kiwifruit Caused by Alternaria alternata. J. Fungi 2021, 7, 937. [Google Scholar] [CrossRef]
- Koh, Y.J.; Hur, J.; Jung, J.S. Postharvest fruit rots of kiwifruit (Actinidia deliciosa) in Korea. N. Z. J. Crop Hortic. Sci. 2005, 33, 303–310. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Chen, M.Y.; Zhang, S.J.; Zhong, C.H. Isolation and identification of pathogenic fungi causing postharvest fruit rot of kiwifruit (Actinidia chinensis) in China. J. Phytopathol. 2017, 165, 782–790. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Li, J.H.; Li, M.; Xing, D.K.; An, H.M.; Wu, X.M.; Wu, Y.Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, G.S.; Cui, Y.L.; Zhang, D.X.; Chang, X.L.; Hu, R.P.; Liu, N.; Sun, X.F. Identification of Botryosphaeriaceae Species Causing Kiwifruit Rot in Sichuan Province, China. Plant Dis. 2015, 99, 699–708. [Google Scholar] [CrossRef]
- Wang, L.; Hou, H.; Zhou, Z.; Tu, H.; Yuan, H. Identification and Detection of Botryosphaeria dothidea from Kiwifruit (Actinidia chinensis) in China. Plants 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Zhu, H.; Zhong, J.; Liao, X.; Zhou, Q. Molecular characterization of a novel mitovirus from the plant-pathogenic fungus Botryosphaeria dothidea. Arch. Virol. 2021, 166, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Yang, M.; Zhang, M.; Hong, N.; Wang, G. Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea. Viruses 2019, 11, 266. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin against Various Kiwifruit Diseases. Antibiotics 2021, 10, 589. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Yuan, Z.; Chen, P. Biological prevention and control of pitaya fruit canker disease using endophytic fungi isolated from papaya. Arch. Microbiol. 2021, 203, 4033–4040. [Google Scholar] [CrossRef]
- Choi, H.W.; Ahsan, S.M. Biocontrol Activity of Aspergillus terreus ANU-301 against Two Distinct Plant Diseases, Tomato Fusarium Wilt and Potato Soft Rot. Plant Pathol. J. 2022, 38, 33–45. [Google Scholar] [CrossRef]
- Ling, L.; Zhao, Y.; Tu, Y.; Yang, C.; Ma, W.; Feng, S.; Lu, L.; Zhang, J. The inhibitory effect of volatile organic compounds produced by Bacillus subtilis CL2 on pathogenic fungi of wolfberry. J. Basic Microbiol. 2021, 61, 110–121. [Google Scholar] [CrossRef]
- Bu, S.W.; Munir, S.H.; He, P.F.; Li, Y.M.; Wu, Y.X.; Li, X.Y.; Kong, B.H.; He, P.B.; He, Y.Q. Bacillus subtilis L1-21 as a biocontrol agent for postharvest gray mold of tomato caused by Botrytis cinerea. Biol. Control 2021, 157, 104568. [Google Scholar] [CrossRef]
- Hong, H.A.; Huang, J.M.; Khaneja, R.; Hiep, L.V.; Urdaci, M.C.; Cutting, S.M. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.M.; Ma, D.Y.; Liu, X.Y.; Zhang, Z.Q.; Li, B.Q.; Xu, Y.; Chen, T.; Tian, S.P. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Kilani-Feki, O.; Dammak, M.; Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Tounsi, S. Efficacy of Bacillus subtilis V26 as a biological control agent against Rhizoctonia solani on potato. Comptes Rendus Biol. 2015, 338, 784–792. [Google Scholar] [CrossRef]
- Yan, Y.F.; Yang, C.J.; Shang, X.F.; Zhao, Z.M.; Liu, Y.Q.; Zhou, R.; Liu, H.; Wu, T.L.; Zhao, W.B.; Wang, Y.L.; et al. Bioassay-guided isolation of two antifungal compounds from Magnolia officinalis, and the mechanism of action of honokiol. Pestic. Biochem. Physiol. 2020, 170, 104705. [Google Scholar] [CrossRef]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal efficacy of ursolic acid in control of Alternaria alternata causing black spot rot on apple fruit and possible mechanisms involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Cai, J.H.; Chen, J.; Lu, G.B.; Zhao, Y.M.; Tian, S.P.; Qin, G.Z. Control of brown rot on jujube and peach fruits by trisodium phosphate. Postharvest Biol. Technol. 2015, 99, 93–98. [Google Scholar] [CrossRef]
- Kai, K.; Bi, W.L.; Sui, Y.; Hua, C.Y.; Liu, Y.S.; Zhang, D.F. Curcumin inhibits Diaporthe phaseolorum and reduces postharvest decay in kiwifruit. Sci. Hortic. 2020, 259, 108860. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.S.; Wei, X.; Liu, J.; Xu, Y.; Luo, Z. Elevated CO(2) delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.Y.; Zhang, X.Y.; Zhang, H.Y. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol. Technol. 2021, 171, 111346. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Hua, C.Y.; Kai, K.; Bi, W.L.; Shi, W.; Liu, Y.S.; Zhang, D.F. Curcumin Induces Oxidative Stress in Botrytis cinerea, Resulting in a Reduction in Gray Mold Decay in Kiwifruit. J. Agric. Food Chem. 2019, 67, 7968–7976. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K.; Rodriguez, G.; Tirajoh, A. Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 2016, 84, 98–104. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Ashengroph, M.; Bahramnejad, B.; Mozafari, A.A. Biocontrol of strawberry anthracnose disease caused by Colletotrichum nymphaeae using Bacillus atrophaeus strain DM6120 with multiple mechanisms. Trop. Plant Pathol. 2021, 47, 245–259. [Google Scholar] [CrossRef]
- Jiang, C.; Shi, J.; Liu, Y.; Zhu, C. Inhibition of Aspergillus carbonarius and fungal contamination in table grapes using Bacillus subtilis. Food Control 2014, 35, 41–48. [Google Scholar] [CrossRef]
- Suryawanshi, K.T.; Sawant, I.S.; Sawant, S.D.; Shabeer, T.P.A.; Saha, S.; Pudale, A.; Dantre, R.K. Field evaluation of the bio-efficacy of Bacillus subtilis DR-39 formulation for enhancing pesticide degradation in grapes and optimisation of application dose. Indian Phytopathol. 2018, 71, 571–577. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, C.Y.; Wu, L.F.; Wang, L.; Gao, W.B.; Jiang, J.Z.; Wu, Y.Q. Inhibitory effect of Bacillus subtilis WL-2 and its IturinA lipopeptides against Phytophthora infestans. bioRxiv 2019. [Google Scholar] [CrossRef]
- Li, W.S.; Yuan, S.Z.; Li, Q.Q.; Sang, W.N.; Cao, J.K.; Jiang, W.B. Methyl p-coumarate inhibits black spot rot on jujube fruit through membrane damage and oxidative stress against Alternaria alternata. Postharvest Biol. Technol. 2018, 145, 230–238. [Google Scholar] [CrossRef]
- Song, M.; Yun, H.Y.; Kim, Y.H. Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J. Ginseng Res. 2014, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Toro, J.J.; Mosquera, S.; Villegas-Escobar, V. Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol. Control 2017, 114, 195–200. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Dunlap, C.A.; Bowman, M.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol. Res. 2016, 192, 30–36. [Google Scholar] [CrossRef]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yang, C.; Wei, L.; Cui, L.; Osei, R.; Cai, F.; Ma, T.; Wang, Y. Transcriptome analysis of Stipa purpurea interacted with endophytic Bacillus subtilis in response to temperature and ultraviolet stress. Plant Growth Regul. 2022, 98, 205–218. [Google Scholar] [CrossRef]
- Xiang, Y.-Z.; Li, X.-Y.; Zheng, H.-L.; Chen, J.-Y.; Lin, L.-B.; Zhang, Q.-L. Purification and antibacterial properties of a novel bacteriocin against Escherichia coli from Bacillus subtilis isolated from blueberry ferments. LWT 2021, 146, 111456. [Google Scholar] [CrossRef]
- Haddoudi, I.; Cabrefiga, J.; Mora, I.l.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of Fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 104671. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.Q.; Li, D.; Wu, X.W.; Zhang, X.C.; Chen, Y.P.; Li, L.; Luo, Z.S. Potential epigenetic regulation of RNA 5’-terminal NAD decapping associated with cellular energy status of postharvest Fragaria × ananassa in response to Botrytis cinerea invasion. Postharvest Biol. Technol. 2022, 186, 111840. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Veronneau, P.Y.; Roussel, D.; Rolland, D. Ultraviolet-C priming of strawberry leaves against subsequent Mycosphaerella fragariae infection involves the action of reactive oxygen species, plant hormones, and terpenes. Plant Cell Environ. 2019, 42, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.R.; Krapp, A.R.; Bisaro, F.; Maiale, S.J.; Pieckenstain, F.L.; Carrillo, N. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. 2017, 92, 761–773. [Google Scholar] [CrossRef]
- Zhao, H.L.; Liu, K.; Fan, Y.Z.; Cao, J.C.; Li, H.H.; Song, W.; Liu, Y.S.; Miao, M. Cell-free supernatant of Bacillus velezensis suppresses mycelial growth and reduces virulence of Botrytis cinerea by inducing oxidative stress. Front. Microbiol. 2022, 13, 980022. [Google Scholar] [CrossRef]
- Ji, D.C.; Chen, T.; Ma, D.Y.; Liu, J.L.; Xu, Y.; Tian, S.P. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Kim, H.J. Exploitation of reactive oxygen species by fungi: Roles in host-fungus interaction and fungal development. J. Microbiol. Biotechnol. 2014, 24, 1455–1463. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of Endophytic Bacillus Subtilis and Salicylic Acid on Postharvest Diseases (Phytophthora infestans, Fusarium oxysporum) Development in Stored Potato Tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Ma, D.T.; He, X.; Wang, F.; Liu, Y.; Jiao, J.Y.; Deng, J. Bacillus subtilis KLBC BS6 induces resistance and defence-related response against Botrytis cinerea in blueberry fruit. Physiol. Mol. Plant Pathol. 2021, 114, 101599. [Google Scholar] [CrossRef]



| Gene Name | Accession Number | Primer Name | Primer Sequences (5′→3′) |
|---|---|---|---|
| Nox1 | WWBZ02000033.1 | BdNox1-F1 | CGAGTCGATATGGTTCCACAG |
| BdNox1-R1 | GCCTGGGTATAGTGAATCTGG | ||
| Nox1f | WWBZ02000073.1 | BdNox1f-F1 | TGGCATCTACCTTTTCGAGC |
| BdNox1f-R1 | CCAGGCTTGTACTTCATCGAG | ||
| Nox3 | WWBZ02000020.1 | BdNox3-F1 | CGTTTCCAAGGTTATTCAGCAC |
| BdNox3-R1 | TTGTGAGAGTGAAAGGGTGG | ||
| Sod | WWBZ02000033.1 | BdSod-F1 | GTCGGTGACAACTCTGGC |
| BdSod-R1 | GGTAATGGATCACGATGCTG | ||
| Pod | WWBZ02000001.1 | BdPod-F1 | TCTCGGCTACAAGATCGGAG |
| BdPod-R1 | TCACAGAATCCGGCAAGC | ||
| Cat | WWBZ02000022.1 | BdCat-F1 | TATTCCTCAGGCACGATCTTG |
| BdCat-R1 | GTCTATTGAGAAGGGTCGGTTC | ||
| Actin | WWBZ02000009.1 | BdActin-F1 | GGTTCAACTACCACCTCAAGAATG |
| BdActin-R1 | GCCGTGGGCGTCAGAAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Liu, K.; Lu, R.; Gao, J.; Song, W.; Zhu, H.; Tang, X.; Liu, Y.; Miao, M. Cell-Free Supernatant of Bacillus subtilis Reduces Kiwifruit Rot Caused by Botryosphaeria dothidea through Inducing Oxidative Stress in the Pathogen. J. Fungi 2023, 9, 127. https://doi.org/10.3390/jof9010127
Fan Y, Liu K, Lu R, Gao J, Song W, Zhu H, Tang X, Liu Y, Miao M. Cell-Free Supernatant of Bacillus subtilis Reduces Kiwifruit Rot Caused by Botryosphaeria dothidea through Inducing Oxidative Stress in the Pathogen. Journal of Fungi. 2023; 9(1):127. https://doi.org/10.3390/jof9010127
Chicago/Turabian StyleFan, Yezhen, Kui Liu, Ruoxi Lu, Jieyu Gao, Wu Song, Hongyan Zhu, Xiaofeng Tang, Yongsheng Liu, and Min Miao. 2023. "Cell-Free Supernatant of Bacillus subtilis Reduces Kiwifruit Rot Caused by Botryosphaeria dothidea through Inducing Oxidative Stress in the Pathogen" Journal of Fungi 9, no. 1: 127. https://doi.org/10.3390/jof9010127
APA StyleFan, Y., Liu, K., Lu, R., Gao, J., Song, W., Zhu, H., Tang, X., Liu, Y., & Miao, M. (2023). Cell-Free Supernatant of Bacillus subtilis Reduces Kiwifruit Rot Caused by Botryosphaeria dothidea through Inducing Oxidative Stress in the Pathogen. Journal of Fungi, 9(1), 127. https://doi.org/10.3390/jof9010127







