Abstract
In this study, four new species of Russula subg. Brevipedum collected from China are described based on morphological characteristics and molecular evidence. Russula brevispora has a white body whose whole parts often stain brownish-orange or grayish-orange, extremely crowded lamellae with the presence of lamellulae, basidiospores with low warts and an inamyloid suprahilar spot, and clavate or lageniform hymenial cystidia often with a papillary or submoniliform appendage. Russula flavescens is characterized by a white pileus often turning yellowish brown when touched, white lamellae turning brown or light orange, basidiospores with an inamyloid suprahilar spot, and fusiform hymenial cystidia often with an appendage. Russula longicollis is morphologically characterized by a white pileus, turning grayish orange when bruised, white lamellae turning pale yellow when bruised, basidiospores with isolated warts and an amyloid suprahilar spot, and fusiform hymenial cystidia usually with a long appendage. Russula pseudojaponica has a yellowish-brown pileus center, yellowish lamellae unchanging when bruised, basidiospores with low warts and an inamyloid suprahilar spot, clavate hymenial cystidia often with a papillary appendage, and clavate pileocystidia with granulose contents. Phylogenetic analyses showed that R. flavescens, R. brevispora, and R. pseudojaponica are members of the subsect. Pallidosporinae, whereas R. longicollis belongs to subsect. Lactarioideae, and is somewhat related to R. leucocarpa.
1. Introduction
Russula Pers. is a genus with a great diversity of mushroom-forming fungi species, predictably containing at least 2000 species within the genus [1,2]. The members of this genus are important plant root symbionts in forest ecosystems, occurring across a wide range of habitats, from the arctic tundra to tropical forests [3]. Recently, Russula has been classified into eight subgenera: Russula subg. Archaea (Buyck and V. Hofst.), R. subg. Brevipedum (Buyck and V. Hofst.), R. subg. Compactae (Fr.) (Bon), R. subg. Crassotunicata (Buyck and V. Hofst.), R. subg. Glutinosae (Buyck and X.H. Wang), R. subg. Heterophyllidiae (Romagn.), R. subg. Malodorae (Buyck and V. Hofst.), and R. subg. Russula [4,5].
The Russula subg. Brevipedum, typified by R. brevipes Peck, was originally described in 2015 as R. subg. Brevipes; however, this was an invalid name and was changed to Brevipedum in 2020 [5]. The members of this subgenus mostly have a medium to very large basidiomata, which often stains yellowish-brown to reddish-brown, regularly unequal lamellae, a distinct smell or acrid to strongly acrid taste, a whitish to yellow spore print, and mucronate to obtuse-rounded cystidia in all parts of the fruiting body [4]. The species of the R. subg. Brevipedum was initially divided into two subsections: R. subsect. Lactarioideae Maire (R. subsect. Delicinae Bat), and R. subsect. Pallidosporinae Bon, a division subsequently supported by DNA analyses [6]. The Russula subsect. Lactarioideae (type R. delica Fr.) was once mistaken for the genus Lactifluus (Pers.) Roussel because of its white milky pileus [7]. In fact, it is distinguished without difficulty by the combination of thick fleshy basidiocarps, a whitish pileus and stipe staining yellowish-brown when mature, and the regularly unequal, whitish to greenish-white or pale yellowish lamellae [8,9]. Russula subsect. Pallidosporinae was originally mentioned by Bon [10]. It is characterized by an ochraceous cream to yellow spore print, yellowish lamellae, basidiospores with inamyloid suprahilar, and a distinctly thicker pileipellis. This subgenus has a cosmopolitan distribution, in Europe [10,11,12], North and South America [8,13,14,15,16], Australia [17,18], Africa [19], and Asia [20,21,22,23]. In China, a total of 13 taxa of R. subg. Brevipedum have been reported [9,24,25,26,27].
Russula japonica Hongo is a poisonous species in R. subg. Brevipedum originally described from Japan with a white body, crowded lamellae, and a stumpy stipe [28]. In the past, some Chinese Russula specimens with similar characteristics were reported as R. japonica, especially specimens with gastrointestinal toxicity [29,30,31,32,33,34,35]. However, morphological characteristics and molecular evidence showed that these specimens were different from R. japonica. Based on comparative studies of available collections, four new species of R. subg. Brevipedum are proposed, with detailed morphological descriptions coupled with illustrations and phylogenetic analysis.
2. Materials and Methods
2.1. Morphological Study
Fresh basidiomata from five provinces of China were collected and photographed. The samples were dried at 50 °C and deposited in the herbarium of the Research Institute of Tropical Forestry, Chinese Academy of Forestry (RITF). Macromorphological descriptions were based on detailed notes and photographs. Color codes and terms follow the Methuen Handbook of Colour [36]. Micromorphological characteristics were observed using a ZEISS Imager M2 (Carl Zeiss AG; Oberkohen, Germany) with oil-immersion lenses at a magnification of 1000×. Macroscopic characters were observed in 5% KOH, 1% phloxin, and 1% ammoniacal Congo red. Tissues were mounted in cresyl blue [37], sulfovanillin [1], and treated with carbolfuchsin [38] to observe the presence and color changes of the incrustations and cystidium contents. Basidiospores were observed in Melzer’s reagent and measured in lateral view excluding the height of ornamentations. Basidiospores measurements are represented as (Min–)AV-SD–AV–AV+SD(–Max), where Min is the minimum value, Max is the maximum value, AV is the average value, SD is the standard deviation, and Q represents the length/width ratio of the basidiospores [2]. Basidium length excludes the length of the sterigmata. Hyphal terminations and pileocystidia were observed both near the pileus margin and in the pileus center. Scanning electron microscopy (SEMJEOL JSM-6510) was used to photograph the shape and ornamentation of the basidiospores.
2.2. DNA Extraction, PCR, and Sequencing
Total genomic DNA was extracted from the dry specimens following an improved CTAB protocol [39]. The ITS region of rDNA was amplified with the primers ITS1 and ITS4 [40]. The amplification protocol consists of a 5 min initial denaturation at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 53 °C, and 2 min at 72 °C, with a final extension of 10 min at 72 °C. The remaining three loci (nrLSU, RPB2, and mtSSU) were amplified using the primers and protocols complying with Buyck [4]. The PCR products were purified using a TaKaRa MiniBEST Agarose Gel DNA Extraction Kit according to the operation manual. The amplified PCR products were subsequently sequenced on an ABI 3730 DNA analyzer using an ABI BigDye Terminator v3.1 Cycle Sequencing Kit (Shanghai Songon Biological Engineering Technology and Services Co., Ltd., Shanghai, China). The newly generated sequences were submitted to the GenBank database (https://www.ncbi.nlm.nih.gov/genbank, URL (29/9/23) Table 1).

Table 1.
Taxa, vouchers, and GenBank accession numbers of sequences analyzed in this study.
2.3. Sequence Alignment and Molecular Phylogenetic Analyses
All the available sequences of species in the subg. Brevipedum from GenBank were included for phylogenetic analyses. The original bidirectional sequences were assembled with the help of Contigexpress software [41]. The sequences were aligned using the MAFFT 7.0 online version [42] and manually adjusted in BioEdit 7.0.9 [43]. The four datasets were concatenated using Phyutility v2.2 for further analysis [44]. The final aligned result was submitted to TreeBase (S29992). Maximum likelihood (ML) analysis was carried out in RAxML 7.0.3 [45]. All parameters were kept at the default settings, except the models set as GTRGAMMA, and statistical support was obtained using nonparametric bootstrapping with 1000 replicates. Bootstrap support (BS) greater than 75% was considered significant. Bayesian inference (BI) analysis was conducted in MrBayes 3.2.6 [46]. The best-fit model was estimated with MrModelTest v. 2.3 [47] using the Akaike information criterion (AIC). Four chains were run for 5 million generations sampling from the posterior distribution every 100 generations. Other parameters were kept at the default settings. The analysis was terminated when the average standard deviation of split frequencies was stable below 0.01. The parameters and tree samples were then summarized, and the Bayesian posterior probabilities (BPPs) were calculated after discarding the first 25% of the samples as the burn-in. BPPs over 0.95 were considered significant.
The combined dataset included sequences from 73 specimens representing 35 taxa. The dataset had an aligned length of 3018 characters including gaps, of which ITS contains 772 characters, nrLSU contains 896 characters, mtSSU contains 564 characters, and RPB2 contains 786 characters. The best model for the Bayesian analysis of the nrLSU and mtSSU sequence datasets was GTR+I+G; for ITS, the best model was HKY+I+G, and for RPB2 it was SYM+I. Six partitions were implemented for further phylogenetic analyses (ITS, nrLSU, mtSSU, RPB2 1st, RPB2 2nd, and RPB2 3rd).
3. Results
3.1. Phylogeny
The phylogenetic trees generated from the RAxML and Bayesian analyses were similar in topology; thus, only the ML tree is shown in Figure 1. The phylogenetic analyses confirmed that R. subg. Brevipedum formed an independent clade with strong support (BS/BPP = 100/1), and can be divided into two lineages, subsect. Pallidosporinae and subsect. Lactarioideae.
The sequences of the four new species, R. brevispora, R. flavescens, R. longicollis, and R. pseudojaponica each formed a strongly supported clade, and were clearly distinct from other known and sequenced species of the subg. Brevipedum. Russula brevispora, R. flavescens, and R. pseudojaponica clustered together with R. vesicatoria Murrill and R. japonica Hongo (BS/BPP = 100%/1. Figure 1). Another species, R. longicollis, was close to R. leucocarpa (G.J. Li and C.Y. Deng).
3.2. Taxonomy
3.2.1. Russula brevispora (Y.L. Chen and J.F. Liang sp. nov.)
Mycobank. MB 846486
Diagnosis. Differs from all known members (except R. japonica) of the subsect. Pallidosporinae in shorter basidiospores. It differs from R. japonica in its lageniform hymenial cystidia and thinner basidia.
Holotype. CHINA, Guizhou Province, Tongren City, Fanjingshan National Nature Reserve, 27°54′43.25″ N, 108°39′25.62″ E, 1950 m asl., 1 August 2019, C.Y. Deng (HGAS-MF009915), GenBank: MN648956 (ITS); OP850852 (nrLSU); OP856542 (mtSSU).
Etymology. “brevispora” refers to shorter basidiospores.
Basidiomata (Figure 2A,B) medium- to large-sized; pileus 60–105 mm in diameter; hemispherical when young, then convex, applanate with a shallowly depressed center when mature; surface smooth, dry, white (1A1), often staining orange white (5A2) or grayish orange (6B5) to brown (6D7); margin not cracked, nonstriate. Lamellae adnate to slightly decurrent, 24–26 pieces at 1 cm near the pileus margin, white (1A1) to cream, often staining grayish-orange (5B4) to brownish-orange (6C8) when bruised; lamellulae frequently present and irregular in length, with furcations absent; edge entire and concolorous. Stipe central, 15–45 × 20–35 mm, cylindrical, slightly tapering towards the base, smooth, white (1A1), solid. Context white (1A1), staining grayish-orange (6B5) when bruised, compact; taste slightly acid, odor inconspicuous. Spore print cream.
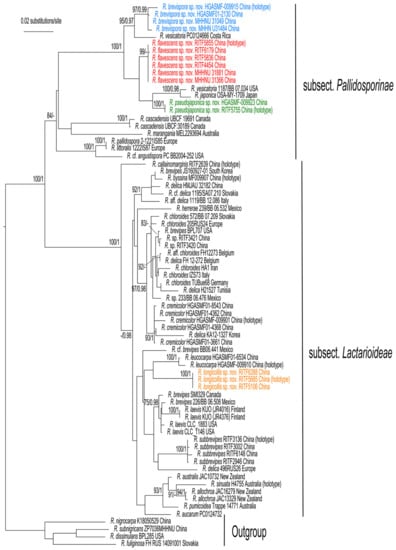
Figure 1.
Phylogenetic tree of R. subg. Brevipedum based on the ITS-nrLSU-RPB2-mtSSU dataset. Bootstrap support (BS) ≥ 75% and Bayesian posterior probabilities (PP) ≥ 0.95 are shown. Four species of R. subg. Compactae were selected as the outgroup. New species are marked with colored characters.
Figure 1.
Phylogenetic tree of R. subg. Brevipedum based on the ITS-nrLSU-RPB2-mtSSU dataset. Bootstrap support (BS) ≥ 75% and Bayesian posterior probabilities (PP) ≥ 0.95 are shown. Four species of R. subg. Compactae were selected as the outgroup. New species are marked with colored characters.
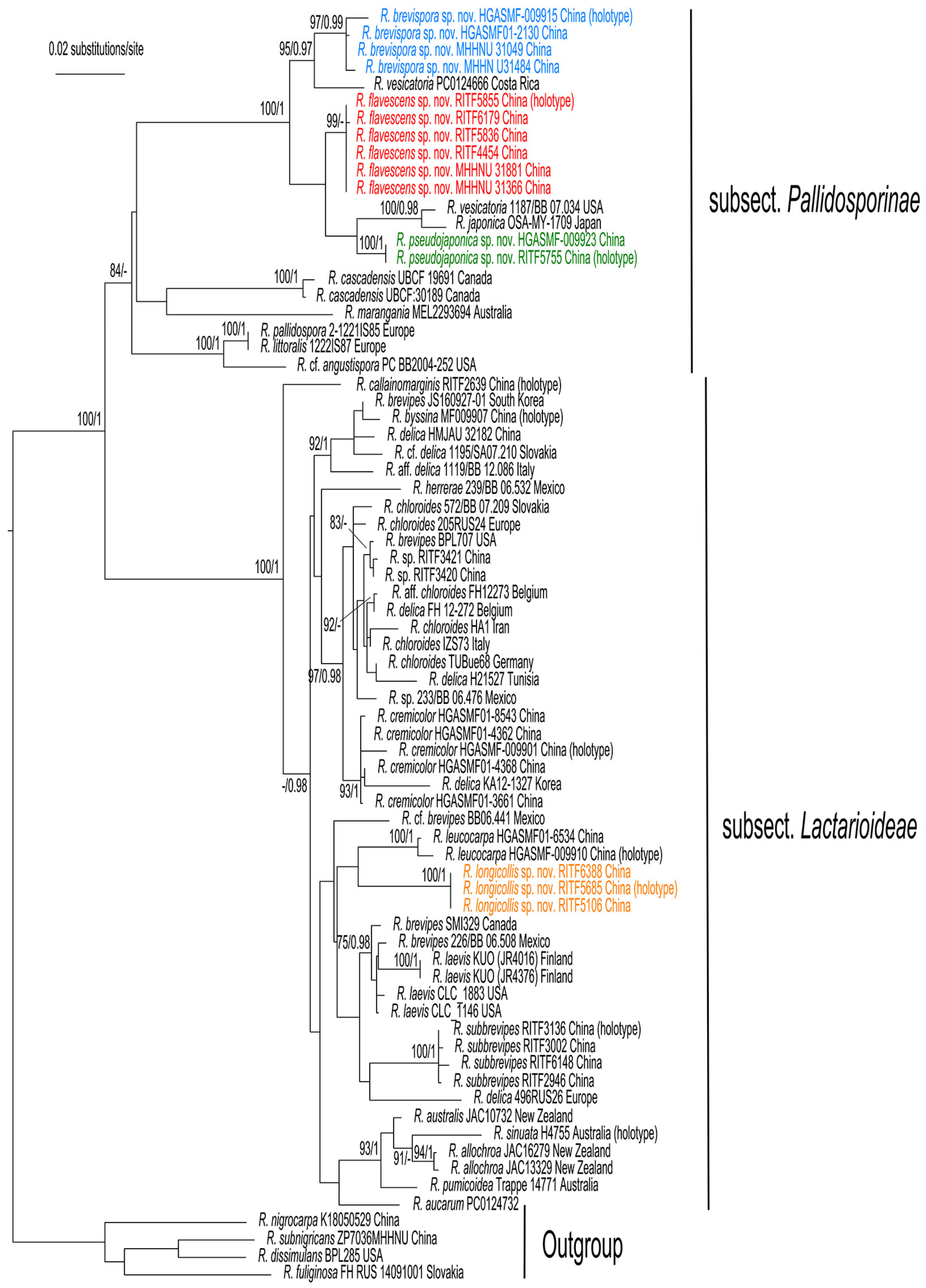

Figure 2.
Fruiting bodies of four new species: (A) R. brevispora (MHHNU 31049, photo by Z.H. Chen); (B) R. brevispora (MHHNU 31484, photo by Z.H. Chen) (C) R. flavescens (RITF6179, photo by W.J. Li); (D) R. flavescens (RITF5855, photo by W.J. Li); (E) R. longicollis (RITF5685, holotype, photo by Y.L. Chen); (F) R. longicollis (RITF6388, photo by X.L. Gao); (G) R. pseudojaponica (RITF5755, holotype, photo by Y.L. Chen); (H) R. pseudojaponica (HGASMF-009923, dry material, photo by Y.L. Chen). Scale bars = 20 mm.
Figure 2.
Fruiting bodies of four new species: (A) R. brevispora (MHHNU 31049, photo by Z.H. Chen); (B) R. brevispora (MHHNU 31484, photo by Z.H. Chen) (C) R. flavescens (RITF6179, photo by W.J. Li); (D) R. flavescens (RITF5855, photo by W.J. Li); (E) R. longicollis (RITF5685, holotype, photo by Y.L. Chen); (F) R. longicollis (RITF6388, photo by X.L. Gao); (G) R. pseudojaponica (RITF5755, holotype, photo by Y.L. Chen); (H) R. pseudojaponica (HGASMF-009923, dry material, photo by Y.L. Chen). Scale bars = 20 mm.
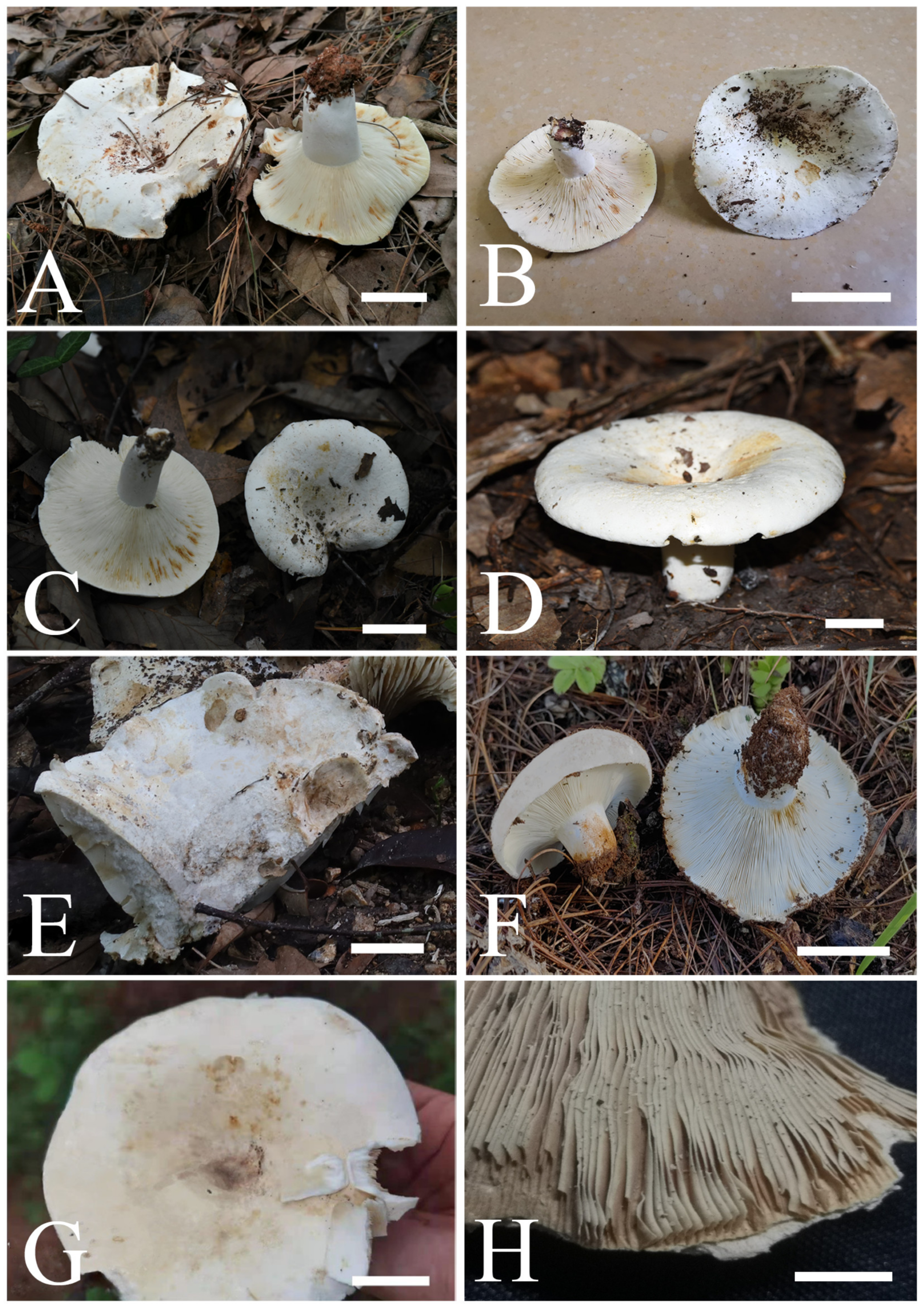
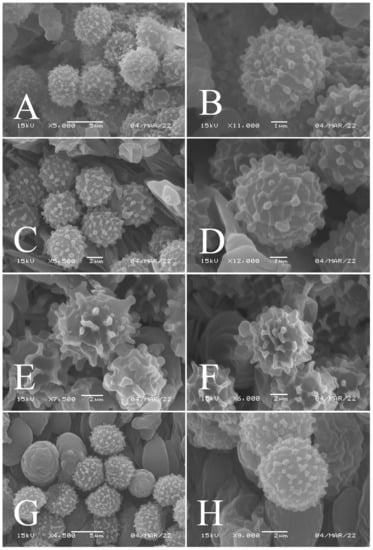
Figure 3.
Basidiospores of four new species in SEM: (A,B) R. brevispora (HGASMF-009915, holotype); (C,D) R. flavescens (RITF5855, holotype); (E,F) R. longicollis (RITF5685, holotype); (G,H) R. pseudojaponica (RITF5755, holotype).
Figure 3.
Basidiospores of four new species in SEM: (A,B) R. brevispora (HGASMF-009915, holotype); (C,D) R. flavescens (RITF5855, holotype); (E,F) R. longicollis (RITF5685, holotype); (G,H) R. pseudojaponica (RITF5755, holotype).
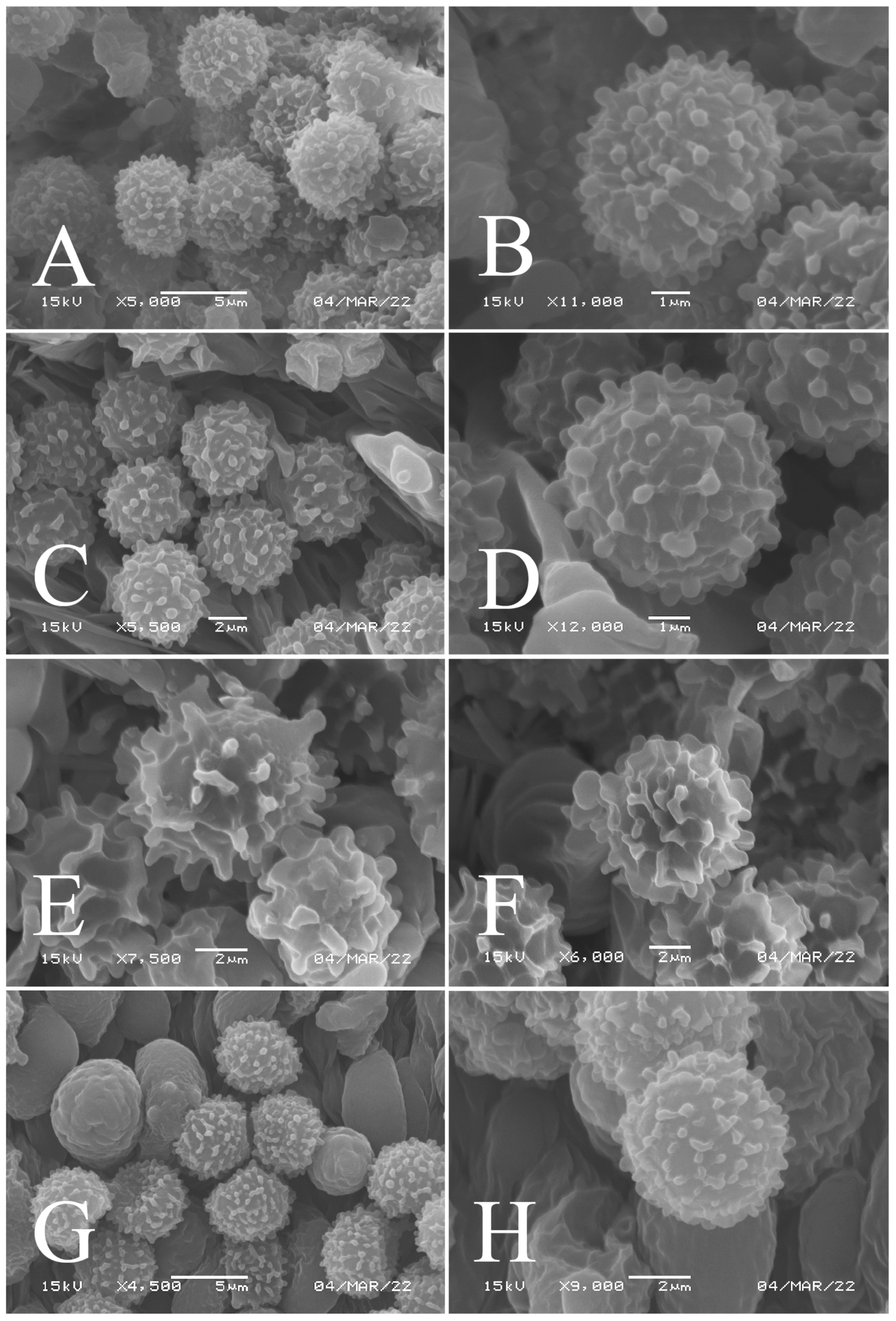
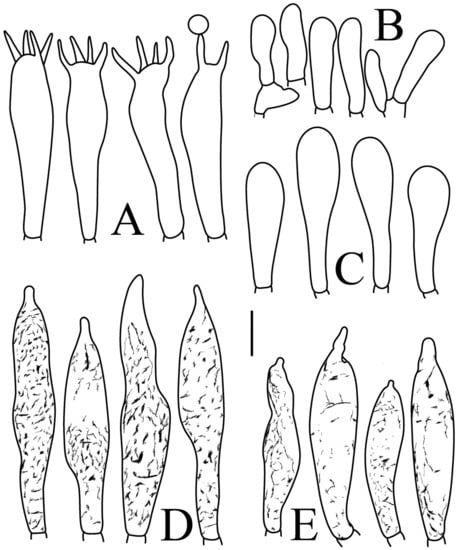
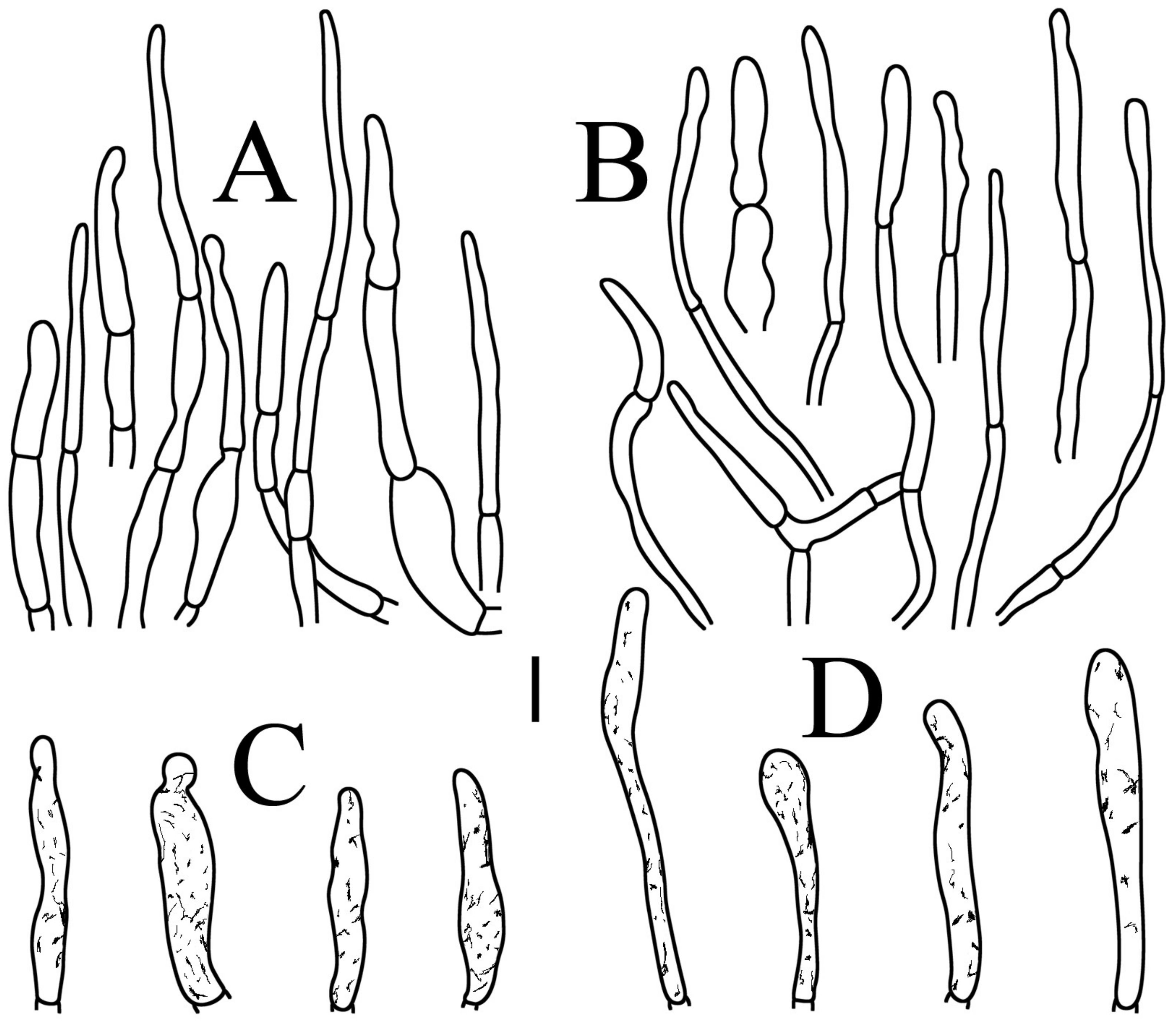
Figure 4.
Russula brevispora. (HGASMF-009915, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars: 10 μm.
Figure 4.
Russula brevispora. (HGASMF-009915, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars: 10 μm.
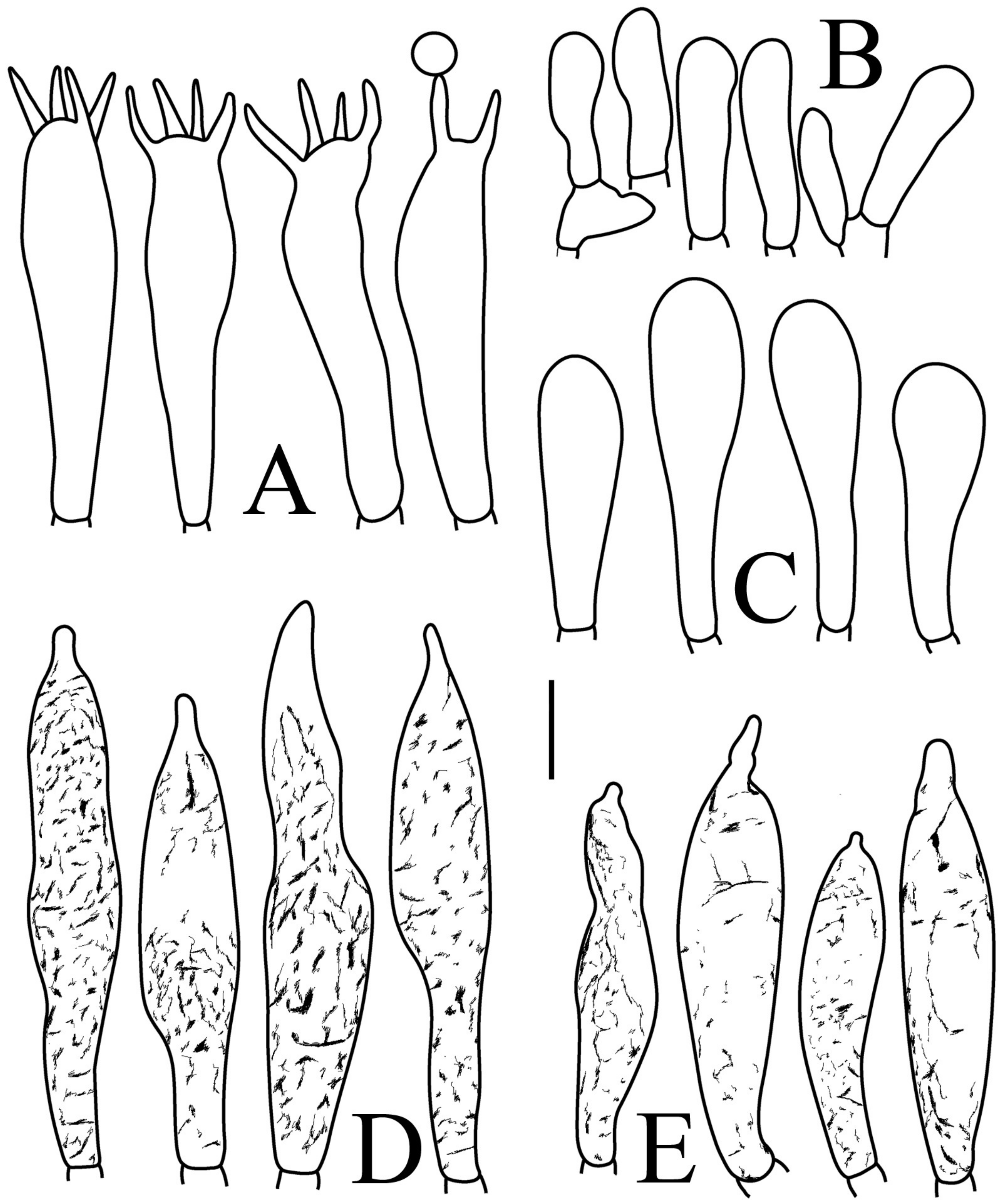
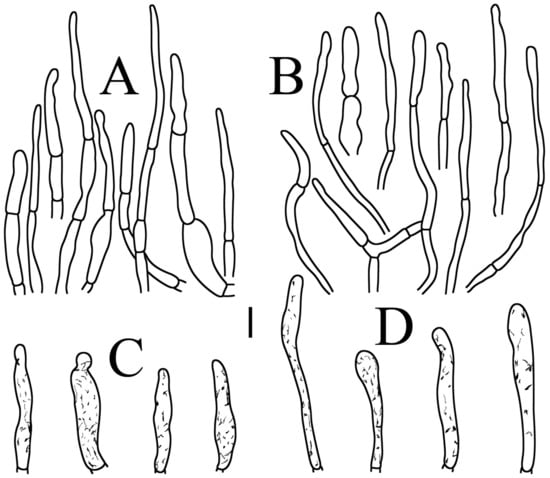
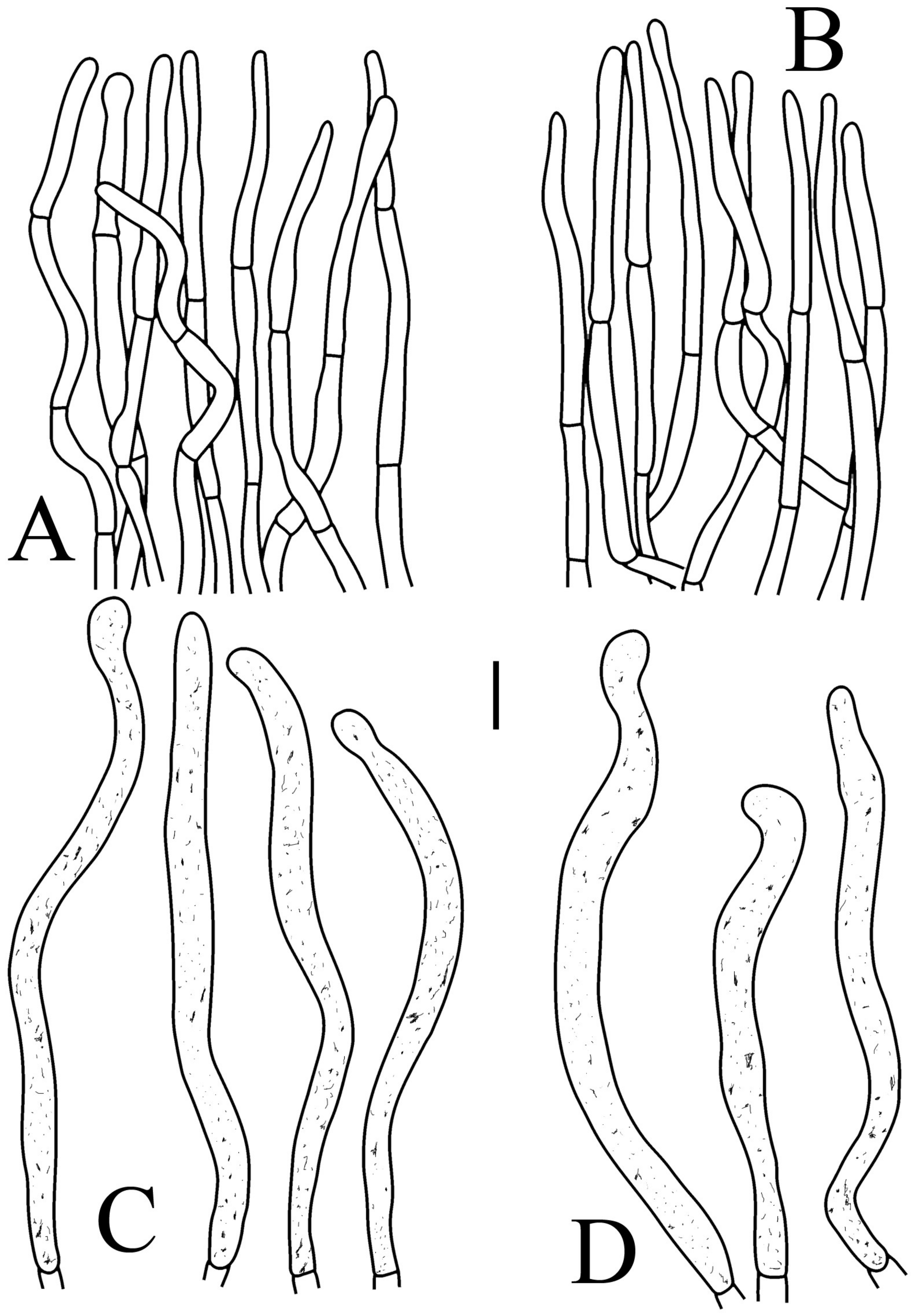
Figure 5.
Russula brevispora. (HGASMF-009915, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
Figure 5.
Russula brevispora. (HGASMF-009915, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
Basidiospores (Figure 3A,B) (5.3–)5.6–5.9–6.2(–6.6) × (4.8–)5–5.3–5.6(–6) µm, Q = (1.01–)1.05–1.11–1.17(–1.28), mainly subglobose, sometimes broadly ellipsoid and globose; ornamentation of small to medium-sized, dense to very dense (6–10 in a 3 µm diameter circle) amyloid conical warts, 0.4–0.7 µm high, subreticulate, never fused by fusions, often connected by fine line connections (2–4 in the circle); suprahilar spot small-sized, inamyloid. Basidia (Figure 4A) (31–)34–38–42(–45) × (7.3–)8–8.7–9.3(–9.9) µm, 2–4-spored, narrowly clavate, thin-walled; sterigmata up to 8.1 µm long; basidiola (Figure 4C) clavate, ca. 7.1–9.7 µm wide. Hymenial cystidia on lamellae sides (Figure 4D) moderately numerous, ca. 1200/mm2, (39.5–)42.7–51.4–60(–67.5) × (7.4–)8.4–9.3–10.2(–11.3) µm, subfusiform or lageniform, occasionally clavate, apically acute, occasionally obtuse, often with a papillary or coracoid appendage, thin-walled; contents granulose-crystalline or banded, no reaction in SV. Hymenial cystidia on lamellae edges (Figure 4E) dispersed to moderately numerous, ca. 500–900/mm2, (37.5–)41–47–53(–59.5) × (7.6–)8.3–9.2–10.1(–11.5) µm, lageniform or clavate, apically obtuse or acute, often with a papillary or submoniliform appendage, thin-walled; contents granulose-crystalline or banded, no reaction in SV. Marginal cells (Figure 4B) (15.3–)16.6–18.2–19.9(–20.2) × (4.8–)5–5.6–6.1(–6.4) µm, subclavate or cylindrical, colorless. Pileipellis orthochromatic in cresyl blue, sharply delimited from the underlying context, two-layered, composed of suprapellis and subpellis; suprapellis 53–100 µm deep, composed of prostrate to ascending hyphae; subpellis composed of closely interweaved colorless hyphae 1.7–4.3 µm wide. Hyphal terminations near the pileus margin (Figure 5A) unbranched, sometimes flexuous, thin-walled, two forms: the first form is usually shorter and broader, terminal cells (17.4–)21.6–26.3–31 (–34.6) × (3–)3.5–4.1–4.7(–5), cylindrical, sometimes apically constricted; the second form is longer and slender, terminal cells (30.2–)34.2–42.7–51.2(–62.8) × (2.4–)3.5–3.1–3.9(–5.1), subconical, cylindrical and apically constricted; the subterminal cells are cylindrical, often expanded or inflated, ca. 3–6.3 µm wide, unbranched. Hyphal terminations near the pileus center (Figure 5B) are sometimes flexuous, occasionally branched, thin-walled, similarly two forms; one-form terminal cells (21.3–)22–24.3–26.5(–28.6) × (3.6–)3.7–4.4–5.2(–5.7) µm, cylindrical, apically obtuse; other form (27.4–)34.9–43.9–52.8(–60) × (2.3–)2.7–3.3–4(–4.7) µm, conical, cylindrical, and apically constricted and attenuated; terminal cells cylindrical or inflated, ca. 2.5–5.6 µm wide, unbranched. Pileocystidia near the pileus margin (Figure 5C) always one-celled, dispersed, (36.3–)38.1–42.3–44.5(–48.1) × 5.1–6.5–8(–9.4) µm, lageniform or fusiform, apically obtuse, sometimes with a papillary appendage, thin-walled; contents granulose-crystalline, no reaction in SV. Pileocystidia near the pileus center (Figure 5D) longer than those near the margin, 40–49.4–57.8(–63.1) × 5.5–6.6–7.7(–8.7) µm, clavate, lageniform or fusiform, apically obtuse, thin-walled; contents granulose-crystalline, no reaction in SV. Cystidioid hyphae dispersed in subpellis and context with granulose contents, oleiferous hyphae frequent in subpellis and context with refringent contents.
Habitat. On the ground in a mixed forest of Pinus massoniana Lamb. and Quercus sp.
Additional specimens examined. CHINA, Guizhou Province, Qiannan Buyei and Miao Autonomous Prefecture, Pingtang County, Zhangbu Town, Kala Village, 25°55′41.60″ N, 107°5′46.78″ E, 1000 m asl., 20 September 2019, C.Y. Deng (HGASMF01-2130). Hunan Province, Zhuzhou City, You County, Shangyunqiao Town, 27°2′20.68″ N, 113°21′34.67″ E, 90 m asl., 9 June 2017, Z.H. Chen (MHHNU 31049, epitype); Ningxiang City, Batang Town, Niaoming Village, 28°7′34.49″ N, 112°27′51.34″ E, 90 m asl., 20 July 2019, Z.H. Chen (MHHNU 31484).
Note. Molecular phylogenetical analysis showed that R. brevispora belongs to the subsect. Pallidosporinae, and has a relationship with R. vesicatoria Murrill from Costa Rica. However, R. vesicatoria can be easily distinguished by its unchanging context, larger basidiospores (7.3–7.9 × 6–6.7 µm), longer basidia (41–50 × 8–10.5 µm), and longer and more slender hyphal terminations (37–67 × 2–3 µm) [8,15]. In the field, R. brevispora was often misidentified as R. japonica owing to a white basidiomata, crowded lamellae, and stumpy stipe. However, our molecular phylogenetical results showed that R. brevispora is a new species distinguished from R. japonica. Morphologically, R. japonica differs with a longer stipe (50–60 mm in length), wider basidia (33–37 × 9.5–11.5 µm) and cylindric fusiform to long clavate hymenial cystidia [28].
3.2.2. Russula flavescens (Y.L. Chen and J.F. Liang, sp. nov.)
MycoBank. MB 843373
Diagnosis. Russula flavescens is similar to R. japonica and R. pallidospora J. Blum ex Romagn. However, it differs from R. japonica in its longer hymenial cystidia and from R. pallidospora in its longer basidiospores.
Etymology. “flavescens” means “becoming yellow” in Latin.
Holotype. CHINA, Jiangsu Province, Nanjing City, Zhongshan Botanical Garden, 32°3′31.56″ N, 118°49′53.81″ E, 65 m asl., 17 July 2021, Y.L. Chen (RITF5855), GenBank: ON010532 (ITS); ON751756 (nrLSU); ON751748 (mtSSU).
Basidiomata (Figure 2C,D) is small-to-medium-sized; pileus 35–80 mm in diameter; initially convex when young, then concave to infundibuliform when mature, obviously depressed at the center; surface smooth, dry, pure white, turning light yellow (4A5) to light brown (6D5–7D5) when touched or bruised, pellicle broken up into small patches; margin first inrolled, then expanded, rarely cracked, nonstriate. Lamellae adnate to slightly decurrent, 18–20 pieces at 1 cm near the pileus margin, white, staining light brown (7D5) or light orange (5A4) when bruised or touched; edge entire and concolor, lamellulae often present and irregular in length. Stipe central, 23–45 × 18–23 mm, subcylindrical, often tapering towards the base, smooth, white, staining yellowish (2A2) or grayish brown (6B6), solid. Context white, unchanging or changing grayish red (7B4) when bruised, compact; taste mild, odor slightly smelly. Spore print cream.
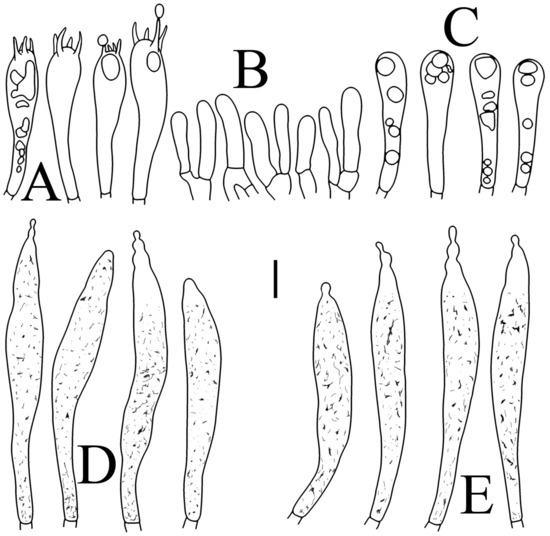
Figure 6.
Russula flavescens (RITF5855, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars = 10 μm.
Figure 6.
Russula flavescens (RITF5855, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars = 10 μm.
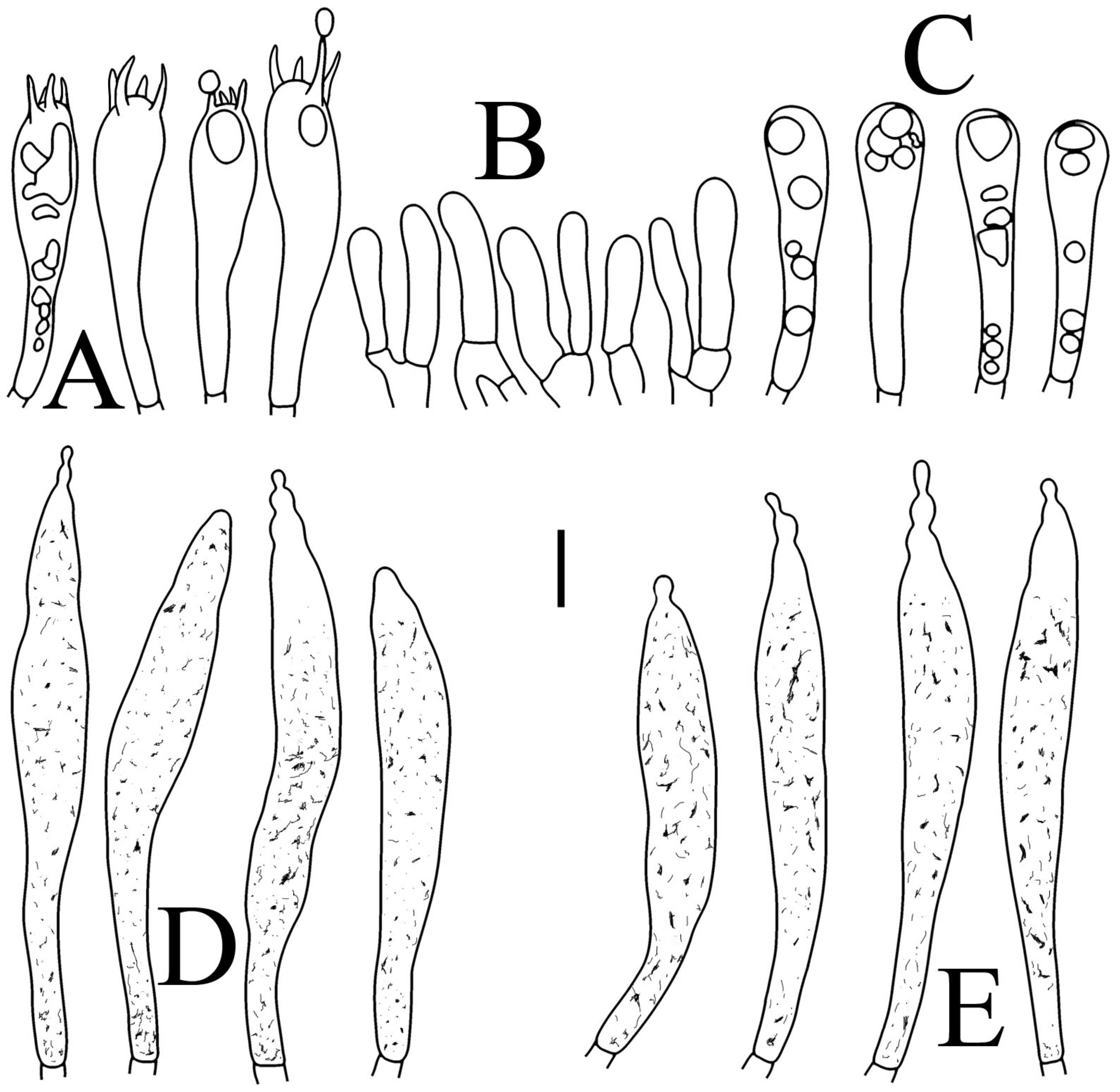
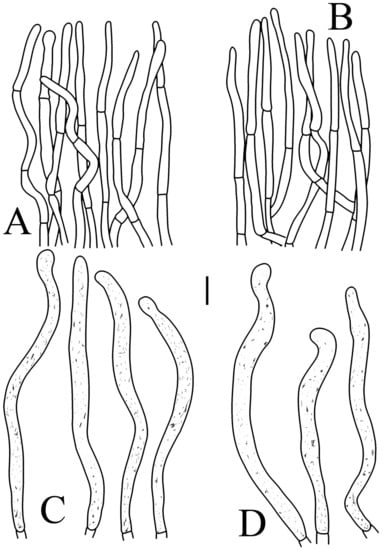
Figure 7.
Russula flavescens (RITF5855, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars = 10 μm.
Figure 7.
Russula flavescens (RITF5855, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars = 10 μm.
Basidiospores (Figure 3C–D) (5.3–)5.6–5.8–6(–6.4) × (4.9–)5.1–5.4–5.6(–6) µm, Q = (1.01–)1.03–1.08–1.13(–1.19), globose to subglobose, sometimes broadly ellipsoid; ornamentation of small- to medium-sized, moderately distant to dense (5–7 in a 3 µm diameter circle) amyloid warts and spines, 0.6–0.9 µm high, subreticulate, rarely fused (0–1 in the circle), often connected by line connections [(0)1–4 in the circle]; suprahilar spot small-sized, inamyloid. Basidia (Figure 6A) (34.5–)36.5–39.5–42.5(–46) × (7–)8.1–8.8–9.5(–10) µm, 4-spored, rarely 2 or 3-spored, clavate; sterigmata up to 7.8 µm long; basidiola (Figure 6C) clavate, ca. 7.8–9.5 µm wide. Hymenial cystidia on lamellae sides (Figure 6D) widely dispersed to dispersed, ca. 200–500/mm2, (55.5–)61.5–69.5–78(–85) × (7.5–)8.5–9.5–10.5(–12.5) µm, fusiform, apically obtuse or rounded, occasionally acute, often with a submoniliform appendage, thin-walled; contents granulose or striated, no reaction in SV. Hymenial cystidia on lamellae edges (Figure 6E) dispersed, ca. 400–700/mm2, (55–)60–68.3–76.5(–88.5) × (8.4–)8.6–9.2–9.8(–11) µm, fusiform, apically obtuse, always with a papillary or submoniliform appendage, thin-walled; contents granulose, no reaction in SV. Marginal cells (Figure 6B) (10.5–)14.5–18–21.5(–23.5) × (3.5–)4.1–4.7–5.3(–5.5) µm, subclavate or cylindrical. Pileipellis orthochromatic in cresyl blue, not sharply delimited from the underlying context, single-layered, 186.8–383 µm deep, composed of dense, hyaline, horizontally oriented, 1.5–5.2 µm wide hyphae. Hyphal terminations near the pileus margin (Figure 7A) unbranched, thin-walled; terminal cells (21.2–)30–40.5–51.5(–59) × (2.9–)3–3.4–3.9(–4.6) µm, narrowly cylindrical, apically obtuse; subterminal cells cylindrical, ca. 1.8–6.1 µm wide, unbranched. Hyphal terminations near the pileus center (Figure 7B) slightly thinner than those near the pileus margin; terminal cells (28.6–)29.2–36–43(–46.5) × (2.7–)3–3.3–3.5(–3.6) µm, mainly cylindrical, apically obtuse; subterminal cells cylindrical, ca. 2.5–5.8 µm wide, unbranched. Pileocystidia near the pileus margin (Figure 7C) one-celled, (64.5–)80.5–93.5–106(–117) × (5.7–)5.9–6.5–7(–7.5) µm, clavate, apically obtuse, thin-walled, often flexuous; contents granulose, no reaction in SV. Pileocystidia near the pileus center (Figure 7D) one-celled, (75.5–)86.5–100–115(–126) × (4–)5.5–6.4–7.3(–8.1) µm, clavate, apically obtuse, thin-walled; contents granulose, no reaction in SV. Cystidioid hyphae frequent in context, with granulose contents; oleiferous hyphae frequent in context, with yellowish pigments.
Habitat. On the ground in broad-leaved forest of Quercus variabilis Blume.
Additional specimens examined. China, Jiangsu Province, Nanjing City, Zhongshan Botanical Garden, 32°3′31.39″N, 118°49′53.89″E, 70 m asl., 31 July 2021, Y.L. Chen (RITF5836); same place, 7 July 2022, W.J. Li (RITF6179). Yunnan Province, Chuxiong Autonomous Prefecture, Nanhua County, Yingwushan Park, 25°12′26.5″N, 101°15′29.61″E, 1930 m asl., 26 August 2018, F.C. Yang (RITF4454).
Notes. Phylogenetical analyses indicated that the new species belonged to subsect. Pallidosporinae. The ITS sequences of specimens MHHNU 31,366 (GenBank: OM760677) and MHHNU 31,881 (GenBank: OM760763) were submitted to the NCBI as R. japonica by Long [48]. However, close inspection of these collections indicated that they were different from R. japonica. Russula japonica can be easily distinguished by its shorter basidia (33–37 × 9.5–11.5 µm) and shorter hymenial cystidia (31–48 × 7.5–10.5 µm) [28]. Morphologically, R. flavescens also shares the same cream spore print, small basidiospores, and crowded lamellae with R. brevispora, R. inopina Shaffer, R. pallidospora, and R. vesicatoria in subsect. Pallidosporinae. However, R. brevispora has moderately numerous hymenial cystidia on lamellae sides and longer pileocystidia. Russula inopina, originally described from North America, is distinct in its longer and ellipsoid spores (6.9–7.5 × 5.2–5.7 µm), longer basidia (54–66 × 7.2–10 µm), and clavate hymenial cystidia [7,8]. Russula pallidospora differs in its longer stipe (40–45 × 18–28 mm), larger basidiospore (7.5–8.7 × 6–7 µm), longer basidia (48–62 × 8–11 µm), and hymenial cystidia (65–165 µm) [11,38], whereas R. vesicatoria is distinguished by its strong odor, bitter-acrid taste, larger basidiospores (7.3–7.9 × 6–6.7 µm), and moderately numerous hymenial cystidia [7,8].
3.2.3. Russula longicollis (Y.L. Chen and J.F. Liang, sp. nov.)
MycoBank. MB 843374
Diagnosis. Russula longicollis is distinguished from other known species in subsect. Lactarioideae by its mainly subglobose basidiospores (Q ≤ 1.15) and longer appendage of hymenial cystidia. It is similar to R. leucocarpa but differs in larger basidiospores.
Holotype. CHINA, Guangdong Province, Huizhou City, Boluo County, Luofu Mountain Provincial Nature Reserve, 23°16′5.64″ N, 114°3′24.92″ E, 100 m asl., 27 July 2021, Y.L. Chen (RITF5685), GenBank: ON010527 (ITS); ON751752 (nrLSU); ON745448 (RPB2); ON751744 (mtSSU).
Etymology. “longicollis” refers to the hymenial cystidia with a long neck.
Basidiomata (Figure 2E–F) medium- to large-sized; pileus 77–164 mm in diameter; initially convex to plano-applanate when young, then concave to shallowly infundibuliform, depressed at the center when mature; surface smooth, dry, white (1A1), staining yellowish-white (4A2), grayish-yellow (4B5) or grayish-orange (5B4) when bruised; margin rarely cracked, nonstriate. Lamellae adnate, 7–8 mm in height at the halfway, 12–15 pieces at 1 cm near the pileus margin, cream, staining pale yellow (4A3) when bruised; lamellulae frequently present and irregular in length, and furcations absent; edge entire and concolor. Stipe central, 30–42 × 16–30 mm, cylindrical, slightly narrow towards the base, smooth, white (1A1), staining pale yellow (4A3), solid. Context up to 10 mm thick at the pileus center, white (1A1), staining orange-white (5A2) when bruised, compact; taste slightly acrid, odor slightly putrid. Spore print cream.
Basidiospores (Figure 3E,F) (7.8–)8.1–8.5–8.6(–9.7) × (7.5–)7.7–8.0–8.3(–8.6) µm, Q = (1.01–)1.04–1.07–1.11(–1.15), mainly subglobose, sometimes globose; ornamentation of medium-sized, distant (3–6 in a 3 µm diameter circle), amyloid warts and ridges, up to 1.67 µm high, subreticulate, fused [(0)1–4(5) fusions in the circle], occasionally connected by fine line connections [(0)1–2 in the circle]; suprahilar spot medium-sized, amyloid. Basidia (Figure 8A) (47.5–)49.5–52.5–56(–58.5) × (10.2–)10.6–11.7–12.2(–12.9) µm, 2–4-spored, broadly clavate, colorless, thin to slightly thick-walled (1.09 µm); sterigmata up to 14.3 µm long; basidiola (Figure 8C) clavate, ca. 8.7–12.4 µm wide, colorless. Hymenial cystidia on lamellae sides (Figure 8E) dispersed to moderately numerous, ca. 600–900/mm2, (73–)77–83–89.5(–95.5) × (7.6–)8.5–9.1–9.7(–10) µm, fusiform or lageniform, apically obtuse, with a 6.5–14 µm long submoniliform and attenuate appendage, thin-walled, colorless; contents granulose-crystalline or banded, yellowish in SV. Hymenial cystidia on lamellae edges (Figure 8D) dispersed to moderately numerous, ca. 600–900/mm2, (61–)64–70.5–77(–84.5) × (7.8–)8.4–9.0–9.6(–10.4) µm, fusiform, apically obtuse, often with a 4.54–8.15 µm long submoniliform appendage, thin-walled, colorless; contents granulose or banded, yellowish-orange in SV. Marginal cells (Figure 8B) (13–)18.5–21.5–24.5(–26.5) × (5.8–)6.5–7.3–8.1(–8.8) µm, clavate or cylindrical, colorless. Pileipellis orthochromatic in cresyl blue, not sharply delimited from the underlying context, single-layered, 196–332 µm deep, composed of dense, horizontally oriented hyphae. Hyphal terminations near the pileus margin (Figure 9A) unbranched, rarely flexuous, thin-walled; terminal cells (21–)27–32.5–38(–41.5) × (3.1–)3.7–4.6–5.6(–7.1) µm, cylindrical or clavate, apically obtuse; subterminal cells cylindrical, ca. 2.6–5.3 µm wide, unbranched. Hyphal terminations near the pileus center (Figure 9B) thinner than those near the pileus margin; terminal cells (16–)20.5–26–31.5(–37.5) × (2.5–)3–3.5–4(–5.5) µm, mainly cylindrical, apically obtuse; subterminal cells cylindrical, ca. 2.5–4.2 µm wide, unbranched. Pileocystidia near the pileus margin (Figure 9C) always one-celled, (67–)75–90.5–109(–113) × (4–)4.5–5.5–6.5(–7.1) µm, subclavate or subcylindrical, apically obtuse, thin-walled, often flexuous; contents granulose, no reaction in SV. Pileocystidia near the pileus center (Figure 9D) always one-celled, (63.5–)69–80.5–91.5(–110) × (3.7–)3.9–4.7–5.5(–6.8) µm, subclavate or subcylindrical, apically obtuse, thin-walled; contents granulose, no reaction in SV. Cystidioid hyphae frequently present in context with granulose contents, and oleiferous hyphae in context present with yellowish pigments.
Habitat. On the ground in a broad-leaved forest dominated by Castanopsis chinensis Hance. or a mixed forest of Quercus sp. and Pinus yunnanensis Franch.
Additional specimens examined. CHINA, Yunnan Province, Dali Bai Autonomous Prefecture, Jianchuan County, 26°14′40.5″ N, 99°48′42.39″ E, 2190 m asl., 21 August 2020, J. Song (RITF5106); Binchuan County, 25°53′23.41″ N, 100°18′47.91″ E, 2450 m asl., 24 August 2022, X.L. Gao (RITF6388).
Notes. Phylogenetically, R. longicollis fell within subsect. Lactarioideae, and was close to R. leucocarpa. However, R. leucocarpa differs in its smaller basidiospores (5.4–6.7 × 4.5–5.9 µm), yellowish hymenial cystidia with granular contents, and habitat in coniferous forests [9]. Morphologically, R. longicollis resembles another three Chinese species R. cremicolor (G.J. Li and C.Y. Deng), R. luteolamellata (H. Zhou and C.L. Hou), and R. subbrevipes (J.F. Liang and J. Song). However, R. cremicolor can be distinguished by its subglobose to broadly ellipsoid (Q = 1.16 ± 0.07) basidiospores with completely reticulate ornamentation, basidia with shorter sterigmata (3−6 μm), and wider pileocystidia (6−8 μm) [9]. Russula luteolamellata (H. Zhou and C.L. Hou), a new species recently described from China easily differs in a yellowish to pale orange pileus, yellowish lamellae, and larger basidiospores (9–10.4 × 8–9.2 µm) [26]. Russula subbrevipes has a yellow ochre or yellowish-brown pileus and subglobose to broadly ellipsoid basidiospores [29]. Moreover, R. longicollis can be confused with other species within subsect. Lactarioideae. However, R. brevipes possesses subglobose to broadly ellipsoid basidiospores, larger basidia (55.5–68 × 9.5–14 µm), numerous hymenial cystidia, broader terminal hyphae, and weakly greying pileocystidia in SV [8]. Russula chloroides (Krombh.) Bres. differs from R. longicollis in a longer stipe (60−80 mm), greenish at the top of the stipe, longer basidiospores (8–11.2 × 7.2–8.8 µm), and larger hymenial cystidia with a mucronate to capitate appendage [10,11,38,49]. Russula delica Romagn. differs in its subdistant lamellae, longer basidiospores (8–11.5 × 6.5–8.7 µm) with lower ornamentation, longer hymenial cystidia (65–130 × 7.2–11.5 µm), and rare pileocystidia [7,10,11,38]. Russula laevis (Kälviäinen, Ruotsalainen and Taipale) has a smaller pileus (40–75 mm), slender stipe (12–20 mm), subglobose to broadly ellipsoid basidiospores, and longer hyphal terminations (36.5–71.5 × 5–7 µm) [2].
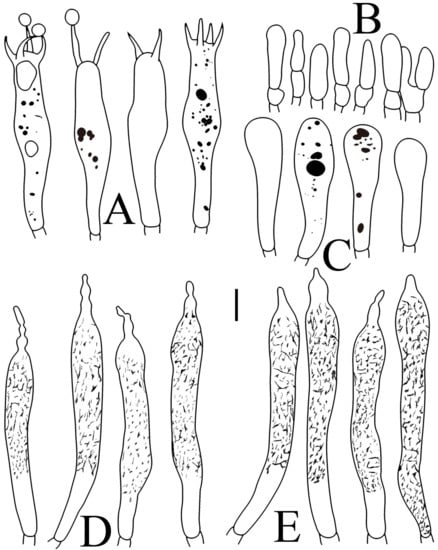
Figure 8.
Russula longicollis (RITF5685, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae edges; (E) hymenial cystidia on lamellae sides. Scale bars: 10 μm.
Figure 8.
Russula longicollis (RITF5685, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae edges; (E) hymenial cystidia on lamellae sides. Scale bars: 10 μm.
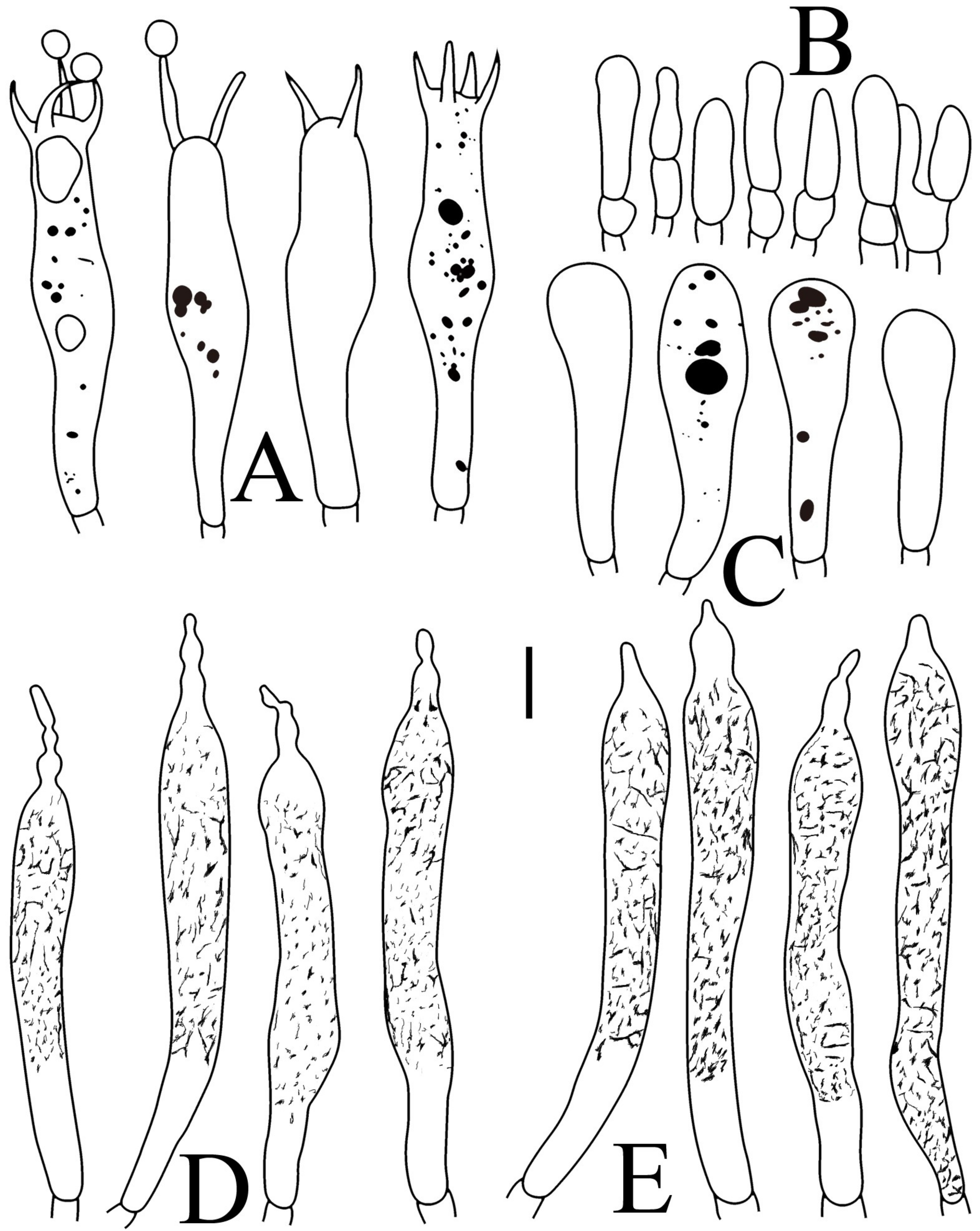
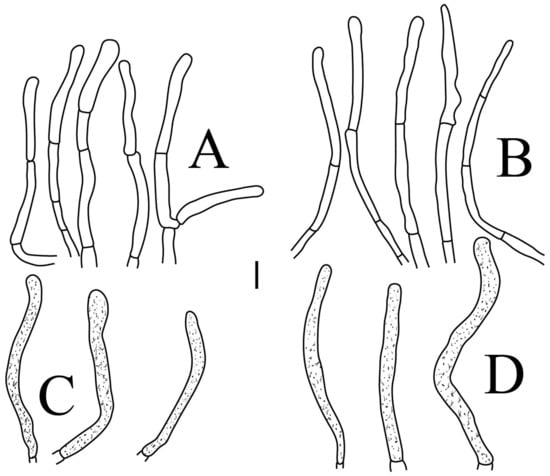
Figure 9.
Russula longicollis (RITF5685, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
Figure 9.
Russula longicollis (RITF5685, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
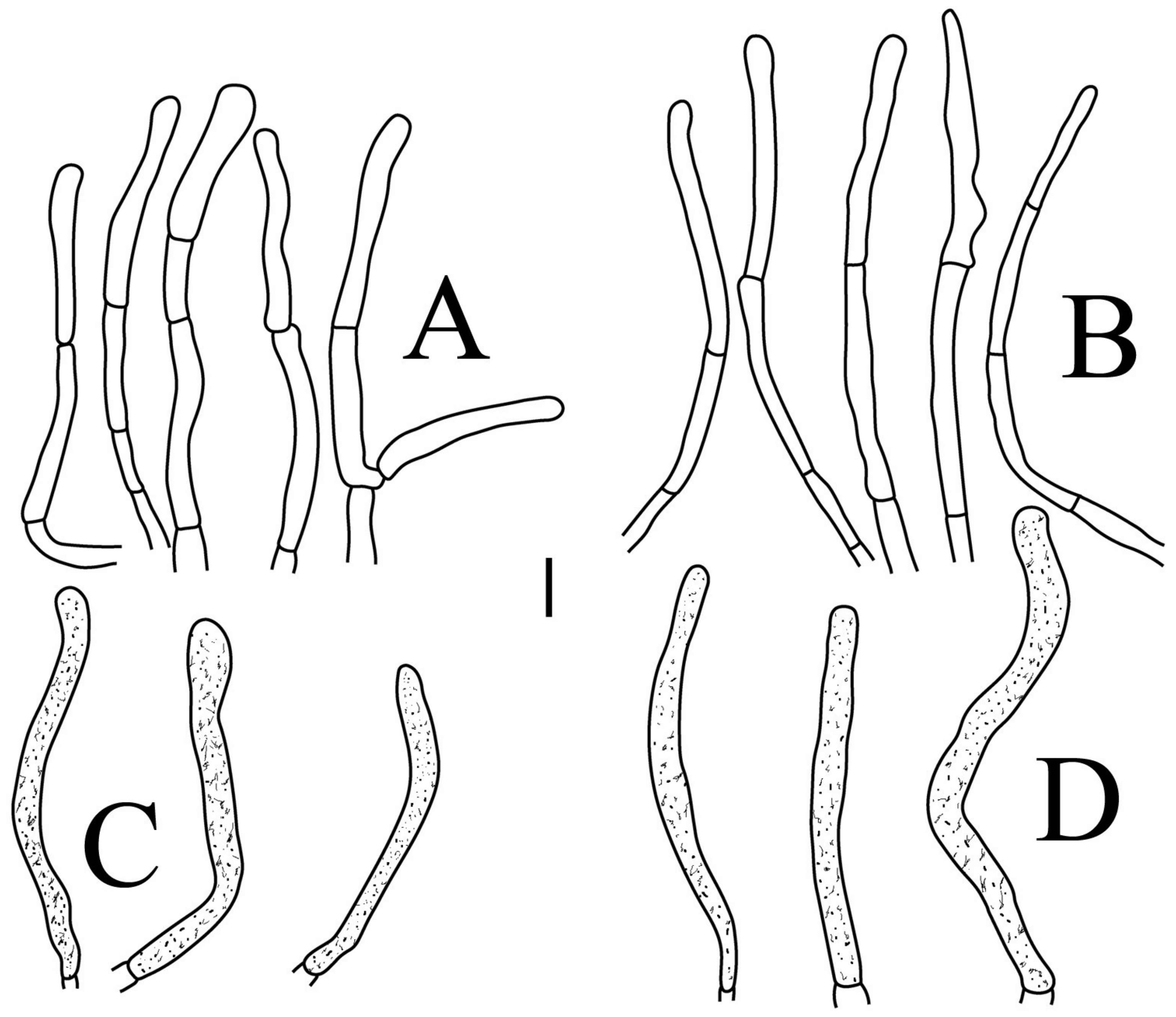
3.2.4. Russula pseudojaponica (Y.L. Chen and J.F. Liang, sp. Nov)
Mycobank. MB 843375
Diagnosis. Russula pseudojaponica is similar to R. japonica but differs in its longer hymenial cystidia and basidia.
Holotype. CHINA, Sichuan Province, Zigong City, Fushun County, Qingshan Village, 29°5′4.16″ N, 105°6′17.82″ E, 420 m asl., 16 July 2021, Y.L. Chen & M.Y. An (RITF5755), GenBank: ON010533 (ITS); ON751757 (nrLSU); ON751747 (mtSSU).
Etymology. “pseudojaponica” refers to the morphological similarity to R. japonica.
Basidiomata (Figure 2G,H) medium-sized; pileus 35–70 mm in diameter; initially convex when young, then concave to infundibuliform when mature; surface smooth, dry, white (1A1) or cream when young, staining yellowish (3A2) or grayish-orange (5B4) at the center; margin rarely cracked, nonstriate, white (1A1) or cream. Lamellae adnate to slightly decurrent, 18–20 pieces at 1 cm near the pileus margin, white (1A1) to cream, unchanging when bruised; lamellulae often present and irregular in length, and furcations absent; edge entire and concolorous. Stipe central, 15–20 × 10–15 mm, cylindrical, often tapering towards the base, smooth, white (1A1), staining yellowish (2A2) or grayish-brown (6B6), solid. Context white (1A1), unchanging when bruised, compact; taste mild, odor slightly smelly. Spore print cream.
Basidiospores (Figure 3G–H) (5.9–)6.2–6.6–6.9(–7.5) × (5.6–)5.8–6.1–6.4(–7) µm, Q = (1.01–)1.04–1.08–1.12(–1.16), globose to subglobose; ornamentation of small to medium-sized, moderately distant to dense [5–8(9) in a 3 µm diameter circle] amyloid warts, low (less than 0.5 µm high), subreticulate, rarely fused (0–2 in the circle), often connected by fine line connections (0–1 in the circle); suprahilar spot small-sized, inamyloid. Basidia (Figure 10A) (31–)33.5–36.5–40(–42.5) × (7.4–)7.9–8.6–9.3(–9.9) µm, 2–4-spored, clavate, thin-walled; sterigmata up to 6.9 µm long; basidiola (Figure 10C) clavate, ca. 7.2–9.9 µm wide. Hymenial cystidia on lamellae sides (Figure 10D) dispersed to moderately numerous, ca. 600–900/mm2, (56.5–)66–75–84(–89.5) × (7.2–)7.8–8.9–10 (–11.7) µm, clavate or subfusiform, apically obtuse, often with a papillary appendage, thin-walled; contents granulose-crystalline, no reaction in SV. Hymenial cystidia on lamellae edges (Figure 10E) dispersed to moderately numerous, ca. 600–900/mm2, smaller than those on lamellae sides, (39–)44–52–60(–71.5) × (7.5–)7.8–8.2–8.6(–9) µm, clavate, subfusiform or sublageniform, apically rounded or obtuse, occasionally with a papillary or submoniliform appendage, thin-walled; contents granulose, sometimes banded, no reaction in SV. Marginal cells (Figure 10B) (10.5–)15–18.5–22.5(–26) × (4.1–)4.2–4.7–5.2(–5.8) µm, subclavate or cylindrical. Pileipellis orthochromatic in cresyl blue, sharply delimited from the underlying context, two-layered, composed of suprapellis and subpellis; suprapellis a trichoderm, 160–267 µm deep, composed of thin-walled, narrowly cylindrical, colorless hyphae; subpellis a cutis, 131–310 µm deep, composed of gelatinized, interweaved colorless hyphae 2.5–5.4 µm wide, with sphaerocytes 12.8–39.9 µm wide. Hyphal terminations near the pileus margin (Figure 11A) unbranched, rarely flexuous, thin-walled; terminal cells (20.5–)24.5–33–41(–53) × (3–)3.2–3.5–3.9(–4.3), cylindrical or clavate, apically obtuse; subterminal cells cylindrical, ca. 2.6–4.5 µm wide, unbranched. Hyphal terminations near the pileus center (Figure 11B) similar to those near the pileus margin; terminal cells (20–)21–28.5–35.5(–43.5) × (2.6–)2.9–3.4–3.9(–4.5) µm, mainly cylindrical, apically obtuse; subterminal cells cylindrical, ca. 3–4.7 µm wide, unbranched. Pileocystidia near the pileus margin (Figure 11C) always one-celled, 51.5–70–109(–128) × (4.5–)5–6.2–7 (–8) µm, mainly cylindrical, apically obtuse, sometimes with a papillary appendage, thin-walled; contents granulose, no reaction in SV. Pileocystidia near the pileus center (Figure 11D) always one-celled, (50–)58–73–88(–106) × (5–)5.6–6.4–7.2(–7.7) µm, mainly cylindrical, apically obtuse, sometimes with a papillary appendage, thin-walled; contents granulose or banded, no reaction in SV. Cystidioid hyphae frequent in subpellis and context with granulose contents; oleiferous hyphae frequent in subpellis and context with yellowish pigments.
Habitat. On the ground in a mixed forest of P. massoniana and Quercus sp.
Additional specimens examined. CHINA, Guizhou Province, Tongren City, Fanjingshan National Nature Reserve, 27°54′43.25″ N, 108°39′25.62″ E, 1950 m asl., 1 August 2019, C.Y. Deng (HGASMF-009923).
Notes. Phylogenetically, R. pseudojaponica falls into subsect. Pallidosporinae, and is closely related to R. flavescens, R. japonica, R. brevispora, and R. vesicatoria. However, R. flavescens differs in its pure white basidiomata, which turn light yellow to light brown when touched or bruised, slightly larger basidiospores, and clavate hymenial cystidia on lamellae edges. Russula japonica is distinct from R. pseudojaponica in its larger fruiting bodies (60–140 mm), wider basidia (33–37 × 9.5–11.5 µm), and shorter hymenial cystidia (31–48 × 7.5–10.5 µm) [28]. Russula brevispora differs in lamellae and context staining grayish-orange, shorter hymenial cystidia and pileocystidia, and two forms of hyphal terminations in the pileipellis. Russula vesicatoria differs in its incurved pileus margin when mature, strong and pleasant odor, and astringent to bitter taste rapidly becoming very acrid [7,8]. Morphologically, R. pseudojaponica is also easily confused with R. littoralis Romagn., R. flavispora Romagn., R. pallidospora, and R. pseudodelica J.E. Lange. Russula littoralis, originally described from the Central America, differs in its longer basidia (46–56 × 8.5–10 µm) and its dispersed and slender hymenial cystidia (70–80 × 5–8.5 µm) [38]. The European R. flavispora differs in its larger pileus (60–120 mm), larger basidia (45–60 × 9.5–11 µm), longer cystidia (80–115 × 6–9 µm), and yellow spore print [11,38,49]. Russula pallidospora, originally described from Europe, has larger basidiospores (7.5–8.7 × 6–7 µm), longer basidia (48–62 × 8–11 µm), and fusiform and longer hymenial cystidia (65–160 µm) [11,38]. Russula pseudodelica differs in its larger stipe (30–60 × 20–30 mm), sparsely aculeate basidiospores, and absent pileocystidia [10,11].
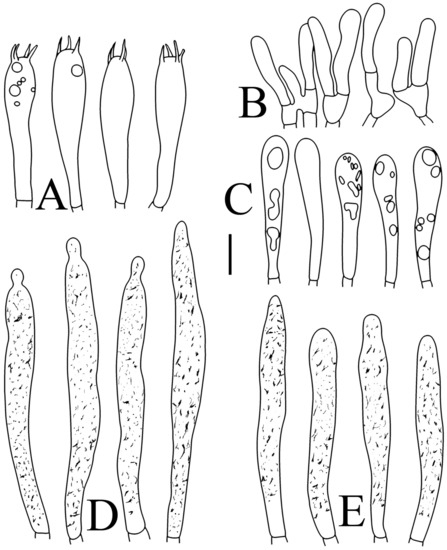
Figure 10.
Russula pseudojaponica (RITF5755, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars: 10 μm.
Figure 10.
Russula pseudojaponica (RITF5755, holotype): (A) basidia; (B) marginal cells; (C) basidiola; (D) hymenial cystidia on lamellae sides; (E) hymenial cystidia on lamellae edges. Scale bars: 10 μm.
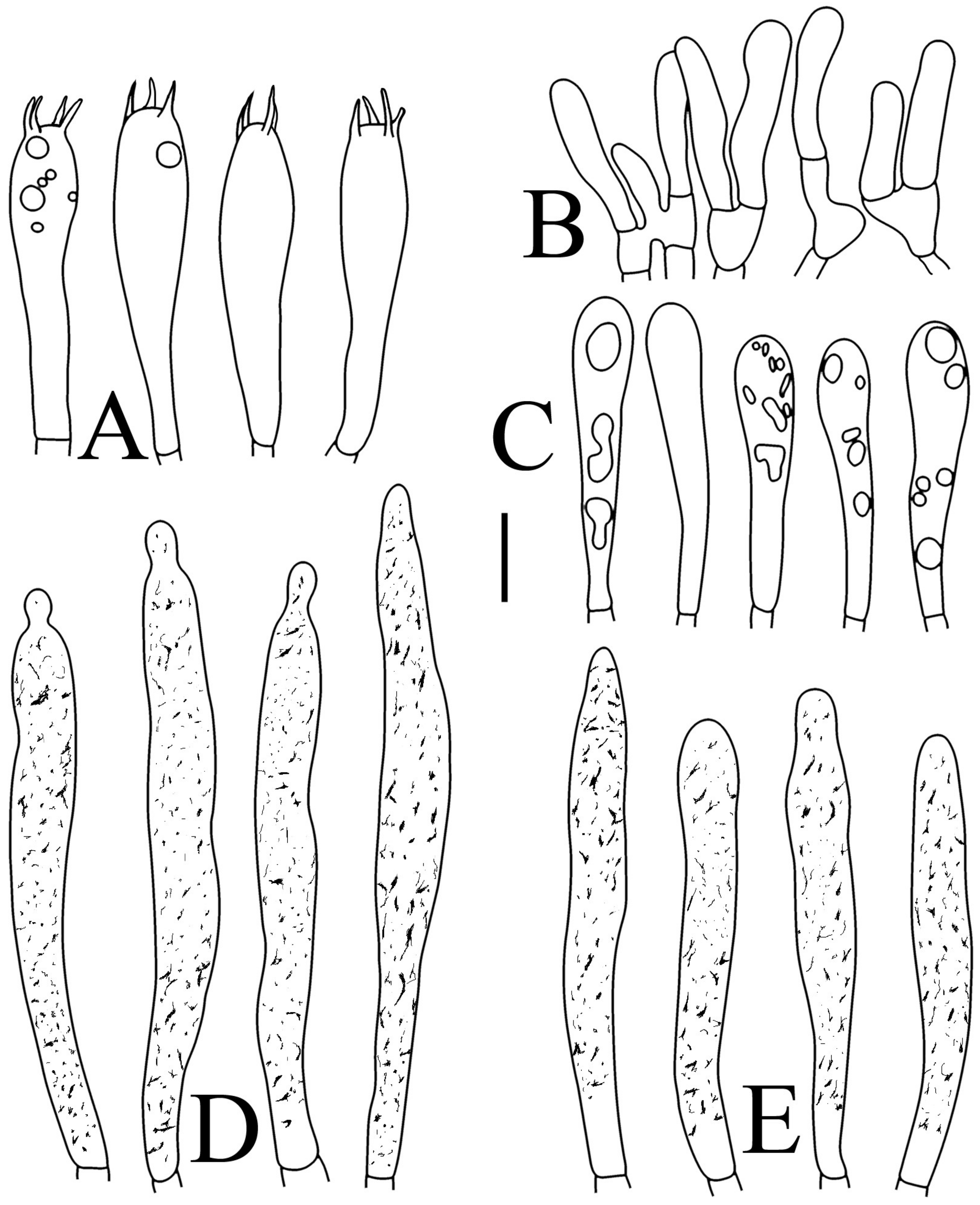
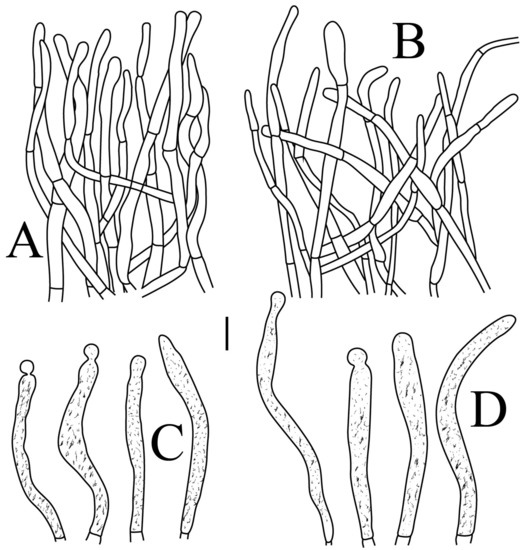
Figure 11.
Russula pseudojaponica (RITF5755, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
Figure 11.
Russula pseudojaponica (RITF5755, holotype): (A) hyphal terminations near the pileus margin; (B) hyphal terminations near the pileus center; (C) pileocystidia near the pileus margin; (D) pileocystidia near the pileus center. Scale bars: 10 μm.
4. Discussion
Russula subg. Brevipedum were formerly placed in R. subg. Compactae (Fr.) Bon as section Brevipes and elevated to subgenus in 2015 [5]. In China, 13 species of subg. Brevipedum were reported in the past, with six species—R. byssina (G.J. Li and C.Y. Deng), R. callainomarginis (J.F. Liang and J. Song), R. cremicolor, R. leucocarpa, R. luteolamellata, and R. subbrevipes—described as new in recent years [9,25,26]. The other species (R. chloroides, R. delica, R. pallidospora, R. pseudodelica, R. flavispora, R. japonica, and R. brevipes) were described in detail in various publications [24,27,30]. The species described in China were similar to those of Europe and North America in terms of macroscopical morphology, but there were obvious differences in microscopic characteristics. Moreover, most of the species reported in China lack support from molecular evidence.
Russula japonica was first described by Hongo [28], and was reported to be poisonous, causing gastroenteritis [33,34,35]. Over the past decades, most of the specimens with similar characteristics were recorded as R. japonica in China. Our morphological study results indicate that the Chinese species differ from R. japonica in their hymenial cystidia. Moreover, we selected a large ribosomal submit sequence from the location of type provided by Shimono [50] for molecular phylogenetical analyses. The molecular phylogeny results are consistent with the morphology.
In some other species, the differences between Chinese and North American and European specimens mainly appear in terms of the size of the basidiospores, hymenial cystidia, and basidia, the presence or absence of pileocystidia, and so on. For example, R. chloroides has longer basidiospores (7.5–8.9 × 5–6.7 μm in Li [9] and 8.3–11.4 × 7.7–10.3 μm in Liu [24] and shorter hymenial cystidia (34–67 μm in Li [9] and 55.3–91.1 μm in Liu [24]), but smaller basidiospores (6.5–8 × 6–6.7 μm) and longer hymenial cystidia (65–115 μm) are present in Romagnesi [30]. Russula brevipes has shorter basidia (17–46 × 5–12 μm in Li [9] and 37.8–48.2 × 11.8–13.6 μm in Liu [24]), shorter hymenial cystidia (29–64 × 5–12 μm in Li [9] and 56.3–81.5 × 6.9–12.6 μm in Liu [24]), and the absence of pileocystidia; however, there are longer basidia (55.5–68 × 9.5–14 μm) and hymenial cystidia (62–93 × 7.5–11 μm), along with the presence of pileocystidia, weakly greying in SV, in Buyck [8]. This indicates that these species reported from China might be misidentified. The main reason for this is that most descriptions of Russula species in the past have been incomplete. Besides being incomplete, the description style has not been consistent, with regional or author-specific patterns [2]. The combination of these factors makes comparing descriptions difficult, or even impossible. Therefore, we still need more reliable sequences as well as detailed macroscopic and microscopic descriptions to confirm the existence of these species in China.
In this study, we propose four new species, including three species that are closely related to R. japonica and one species that is close to R. leucocarpa from China based on molecular phylogeny and morphology data. It is worth noting that R. flavescens, R. pseudojaponica, and R. brevispora were recently reported to have gastrointestinal toxicity [34,35], whereas the toxicity of R. longicollis is still unknown.
Key to four new species and other closely related taxa within subg. Brevipedum
1 Basidiospores with inamyloid suprahilar spot………………………………..……….…….2
1 Basidiospores with amyloid suprahilar spot………………………………..………….…..9
2 Spore print yellow………………………………………….………………………R. flavispora
2 Spore print white to cream…………………………………………………….………..….…3
2 Spore print ochraceous………………………………………………………….………….….8
3 Hymenial cystidia on lamellae edges usually <50 µm in length……………….R. japonica
3 Hymenial cystidia on lamellae edges usually >50 µm in length………………..…….….4
4 Basidiospores on average < 7 µm in length……………………………………..……………5
4 Basidiospores on average > 7 µm in length……………………………….……….…..…….7
5 Lamellae unchanging when bruised………………………………………R. pseudojaponica
5 Lamellae turning yellowish or brownish when bruised……………………………………6
6 Moderately numerous hymenial cystidia on lamellae sides, 42.7–60 × 8.4–10.2 µm, no reaction in SV; comparatively longer pileocystidia……………………………..R. brevispora
6 Widely dispersed to dispersed hymenial cystidia on lamellae sides, 61.5–78 × 8.5–10.5 µm, grayish in SV; comparatively shorter pileocystidia………………..……….R. flavescens
7 Hymenial cystidia comparatively short, 70–80 µm……………………………...R. littoralis
7 Hymenial cystidia comparatively long, 65–160 µm…………………………R. pallidospora
8 Basidiospores comparatively short, 7.3–7.9 × 6–6.7 µm; Basidia comparatively small, 41–50 × 8–10.5 µm………….…………………………………….……………….…….R. vesicatoria
8 Basidiospores comparatively long, 6.9–9.3 × 6.3–7 µm; Basidia comparatively large, 48–62 × 9–11 µm…………………………………………..………………..………….R. pseudodelica
9 Pileus surface yellow-ochre or yellowish-brown……………………………….………….10
9 Pileus surface white to cream………………………………………………….……..….….11
10 Basidiospores comparatively large, 9–10.4 × 8–9.2 µm…………………..R. luteolamellata
10 Basidiospores comparatively small, 7.8–9 × 6.9–7.9 µm……………..……..R. subbrevipes
11 Basidiospores on average < 7 µm in length…….……………………………..R. leucocarpa
11 Basidiospores on average > 7 µm in length…………………………………….….……..12
12 Lamellae comparatively distant (usually less than 12 pieces/cm)……….………R. delica
12 Lamellae comparatively crowded (usually more than 12 pieces/cm)……………..…..13
13 Basidiospores globose to subglobose…………………………………….….…R. longicollis
13 Basidiospores subglobose to broadly ellipsoid………………………….……..……..….14
14 Basidiospores on average < 10 µm in length……………………………….……R. brevipes
14 Basidiospores on average > 10 µm in length……………………………..…….…….….15
15 Stipe comparatively broad (20–30 mm); hymenial cystidia comparatively wide (7–13 µm), more ellipsoid terminal cells in the pileipellis………………………………R. chloroides
15 Stipe comparatively slender (12–20 mm); hymenial cystidia comparatively narrow (7.5–8.5 µm), more clavate terminal cells in the pileipellis…………………………………R. laevis
Author Contributions
Writing—Original draft preparation, Y.C.; writing—review and editing, J.L. (Junfeng Liang); software, Y.C.; formal analysis, Y.C.; resources, M.A., J.L. (Jingying Liang), C.D., J.W., W.L. and Y.L.; funding acquisition, J.L. (Junfeng Liang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31770657), the National Non-profit Institute Research Fund of CAF (CAFYBB2021SY003 and CAFYBB2019ZA001), the Science and Technology Planning Project of Guizhou Province, China ((No. Qian Ke He Fu Qi (2019)4007), and the Project of Science and Technology Programs of Guizhou province ((2019)2451-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful for the help of Bin Chen who offered advice, suggestions and analytical assistance. We thank Zuohong Chen (MHHNU), Jie Song, Fucheng Yang, Guo-Jie Li (HMAS), and Xue-Lian Gao for providing specimens.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caboň, M.; Eberhardt, U.; Looney, B.; Hampe, F.; Kolařík, M.; Jančovičová, S.; Verbeken, A.; Adamčík, S. New insights in Russula subsect. Rubrinae: Phylogeny and the quest for synapomorphic characters. Mycol. Prog. 2017, 16, 877–892. [Google Scholar] [CrossRef]
- Adamčík, S.; Looney, B.; Caboň, M.; Jančovičová, S.; Adamčíková, K.; Avis, P.G.; Barajas, M.; Bhatt, R.P.; Corrales, A.; Das, K.; et al. The quest for a globally comprehensible Russula language. Fungal Divers. 2019, 99, 369–449. [Google Scholar] [CrossRef]
- Looney, B.P.; Meidl, P.; Piatek, M.J.; Miettinen, O.; Martin, F.M.; Matheny, P.B.; Labbe, J.L. Russulaceae: A new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. New Phytol. 2018, 218, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Buyck, B.; Zoller, S.; Hofstetter, V. Walking the thin line… ten years later: The dilemma of above- versus below-ground features to support phylogenies in the Russulaceae (Basidiomycota). Fungal Divers. 2018, 89, 267–292. [Google Scholar] [CrossRef]
- Buyck, B.; Wang, X.H.; Adamčíková, K.; Caboň, M.; Jančovičová, S.; Hofstetter, V.; Adamčík, S. One step closer to unravelling the origin of Russula: Subgenus Glutinosae subg. nov. Mycosphere 2020, 11, 285–304. [Google Scholar] [CrossRef]
- Miller, S.L.; Buyck, B. Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol. Res. 2002, 106, 259–276. [Google Scholar] [CrossRef]
- Shaffer, R.L. The Subsection Lactarioideae of Russula. Mycologia 1964, 56, 202–231. [Google Scholar] [CrossRef]
- Buyck, B.; Adamčík, S. Type Studies in Russula Subsection Lactarioideae from North America and a Tentative Key to North American Species. Cryptogam. Mycol. 2013, 34, 259–279. [Google Scholar] [CrossRef]
- Li, G.J.; Deng, C.Y.; Shi, L.Y.; Wang, J.; Meng, Q.F.; Li, S.M. Three new species of Russula subsect. Lactarioideae from China. . Mycosystema 2020, 39, 618–636. [Google Scholar] [CrossRef]
- Bon, M. Clé monographique des Russules d’Europe. Docum. Mycol. 1988, 18, 1–220. [Google Scholar]
- Sarnari, M. Monografia illustrata del Genere Russula in Europa; A.M.B. Fondazione Centro Studi Micologici: Vicenza, Italy, 1998; Volume 1, pp. 1–806. [Google Scholar]
- Vidal, J.M.; Alvarado, P.; Loizides, M.; Konstantinidis, G.; Chachula, P.; Mleczko, P.; Moreno, G.; Vizzini, A.; Krakhmalnyi, M.; Paz, A.; et al. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 2019, 42, 127–185. [Google Scholar] [CrossRef] [PubMed]
- Duque Barbosa, J.A. Análise filogenética de Russula pers. (Russulaceae, Russulales: Agaricomycetes). Master’s Thesis, Federal University of Santa Catarina, Florianopolis, Brazil, 2016. [Google Scholar]
- Kong, A.; Montoya, A.; Estrada-Torres, A. Russula herrerae, a new species with marginal veil from Mexico. Mycologia 2017, 94, 290–296. [Google Scholar] [CrossRef]
- Burlingham, G.S. Studies in North American Russulae. Mycologia 1944, 36, 104–120. [Google Scholar] [CrossRef]
- Noffsinger, C.; Cripps, C.L. Systematic analysis of Russula in the North American Rocky Mountain alpine zone. Mycologia 2020, 113, 1278–1315. [Google Scholar] [CrossRef]
- Lebel, T.; Tonkin, J.E. Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota). Aust. Syst. Bot. 2007, 20, 355–381. [Google Scholar] [CrossRef]
- McNabb, R.F.R. Russulaceae of New Zealand 2. Russula Pers. ex S. F. Gray. New Zeal. J. Bot. 2003, 11, 673–730. [Google Scholar] [CrossRef]
- Buyck, B.; Thoen, D.; Watling, R. Ectomycorrhizal fungi of the Guinea–Congo Region. Proc. Royal Soc. B 2011, 104, 313–333. [Google Scholar] [CrossRef]
- Kumar, R.; Tapwal, A.; Pandey, S.; Rishi, R.; Mishra, G.; Giri, K. Six unrecorded species of Russula (Russulales) from Nagaland, India and their nutrient composition. Nusant. Biosci. 1970, 6, 33–38. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, C.; Choi, S.Y.; Lee, H.B. Eight Previously Unreported Species of Macrofungi from Korea. Kor. J. Mycol. 2017, 45, 362–369. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Nuytinck, J.; Hyde, K.D.; Verbeken, A. Lactarius subgenus Russularia (Russulaceae) in Southeast Asia: 1. Species with very distant gills. Phytotaxa 2014, 158, 23–42. [Google Scholar] [CrossRef][Green Version]
- Kim, C.S.; Jo, J.W.; Kwag, Y.N.; Sung, G.H.; Lee, S.G.; Kim, S.Y.; Shin, C.H.; Han, S.K. Mushroom Flora of Ulleung-gun and a Newly Recorded Bovista Species in the Republic of Korea. Mycobiology 2015, 43, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y. The Study on Russula Specises on Taishan; Shandong Agricutural University: Taian, China, 2019. [Google Scholar]
- Song, J.; Li, H.J.; Wu, S.J.; Chen, Q.Q.; Yang, G.; Zhang, J.Y.; Liang, J.F.; Chen, B. Morphological and molecular evidence for two new species within Russula subgenus Brevipes from China. Diversity 2022, 14, 112. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, G.Q.; Hou, C.L. A new species, Russula luteolamellata (Russulaceae, Russulales) from China. Phytotaxa 2022, 556, 136–148. [Google Scholar] [CrossRef]
- Liu, X.L.; Bau, T.; Wang, X.H. Species diversity of Russula from the Greater and Lesser Hinggan Mountains in Northeast China. Mycosystema 2017, 36, 1355–1368. [Google Scholar] [CrossRef]
- Hongo, T. Two new agarics from omi. Acta. Phytotax. Geobot. 1954, 15, 102–104. [Google Scholar] [CrossRef]
- Song, B.; Li, T.H.; Wu, X.L.; Li, J.J.; Shen, Y.H.; Lin, Q.Y. Known Species of Russula from China and Their Distribution. J. Fungal Res. 2007, 5, 21–42. [Google Scholar]
- Li, G.J. The Taxonomy of Russula in China. Ph.D. Thesis, Chinese Academy of Sciences & University of Chinese Academy of Science, Beijing, China, 2014. [Google Scholar]
- Li, G.J.; Li, S.F.; Zhao, D.; Wen, H.A. Recent research progress of Russula (Russulales, Agaricomycetes): A review. Mycosystema 2015, 34, 821–848. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.H.; Yang, Z.L.; Bau, T.; Dai, Y.C. Atlas of Chinese Macrofungal Resources; Zhongyuan Farmers Publishing House: Zhengzhou, China, 2015. [Google Scholar]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Li, H.J.; Zhang, H.S.; Zhang, Y.Z.; Zhang, K.P.; Zhou, J.; Yu, Y.; Jiang, S.F.; Ma, P.B.; He, Q.; Zhang, Y.T.; et al. Mushroompoisoning outbreaks: China, 2019. China CDC Weekly 2020, 2, 19–27. [Google Scholar] [CrossRef]
- Li, H.J.; Zhang, H.S.; Zhang, Y.Z.; Zhou, J.; Yu, Y.; He, Q.; Jiang, S.F.; Wen, K.; Yuan, Y.; Lang, N.; et al. Mushroom poisoning outbreaks: China, 2020. China CDC Weekly 2021, 3, 41–50. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben, 3rd ed.; Muster-Schmidt Verlag: Göttingen, Germany, 1981. [Google Scholar]
- Buyck, B. New Taxa of Central African Russulaceae. Bull. Jard. Bot. Nat. Belg./Bull. Nat. Plantentuin Belg. 1989, 59, 241–253. [Google Scholar] [CrossRef]
- Romagnesi, H. Les Russules d’Europe et d’Afrique du Nord; Bordas: Paris, French, 1967. [Google Scholar]
- Zhou, L.L.; Liang, J.F. An improved protocol for extraction of DNA from macrofungi. Guangdong Forest Sci. Technol. 2011, 27, 13–16. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Lu, G.Q.; Moriyama, E.N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 2004, 5, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Smith, S.A.; Dunn, C.W. Phyutility: A phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 2008, 24, 715–716. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Long, P.; Jiang, Z.; He, Z.; Chen, Z. Development of a loop-mediated isothermal amplification assay for the rapid detection of Russula subnigricans and Russula japonica. Front Microbiol. 2022, 13, 918651–918665. [Google Scholar] [CrossRef]
- Llistosella, J.; Pérez-de-Gregori, M.À.; Llorens-Van-Waveren, I. Russula flavispora Romagn, una espÈcie rara trobada a Catalunya. Rev. Catalana Micol. 2008, 30, 101–106. [Google Scholar]
- Shimono, Y.; Kato, M.; Takamatsu, S. Molecular phylogeny of Russulaceae (Basidiomycetes; Russulales) inferred from the nucleotide sequences of nuclear large subunit rDNA. Mycoscience 2004, 45, 303–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
