The Th2 Response and Alternative Activation of Macrophages Triggered by Strongyloides venezuelensis Is Linked to Increased Morbidity and Mortality Due to Cryptococcosis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Preparation of S. venezuelensis Antigens
2.3. Mice
2.4. Phagocytosis Assay
2.5. Coinfection Protocol: Mouse Survival and Behavior
2.5.1. Fungal Burden, Differential Leukocyte Counting, and Histopathology
2.5.2. Inflammatory Response of Coinfected Mice
2.6. Statistical Analysis
3. Results
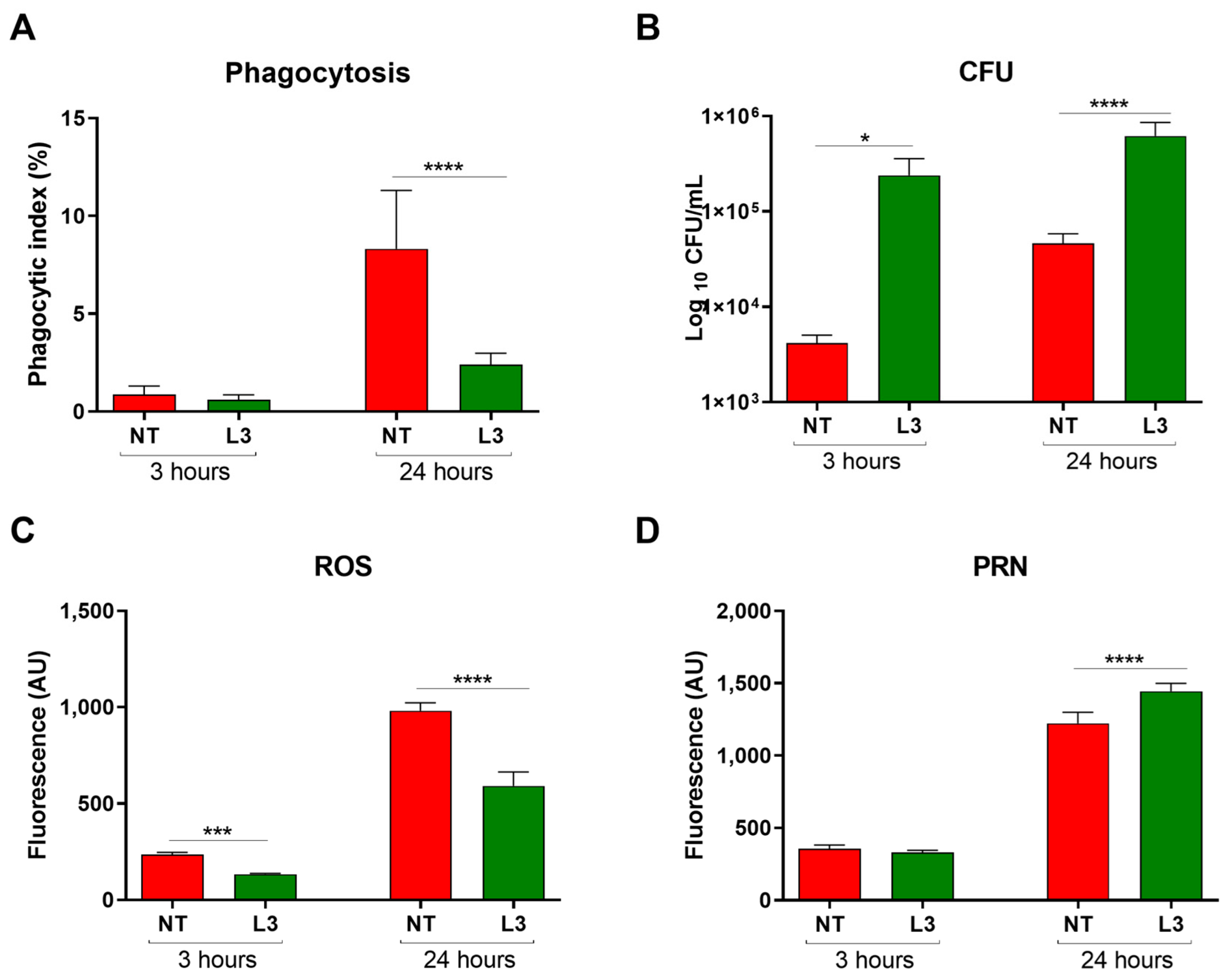
3.1. Sv Impairs Cg Phagocytosis, Alters Macrophage’s Oxidative Response, and Increases Fungal Intracellular Proliferation Rate
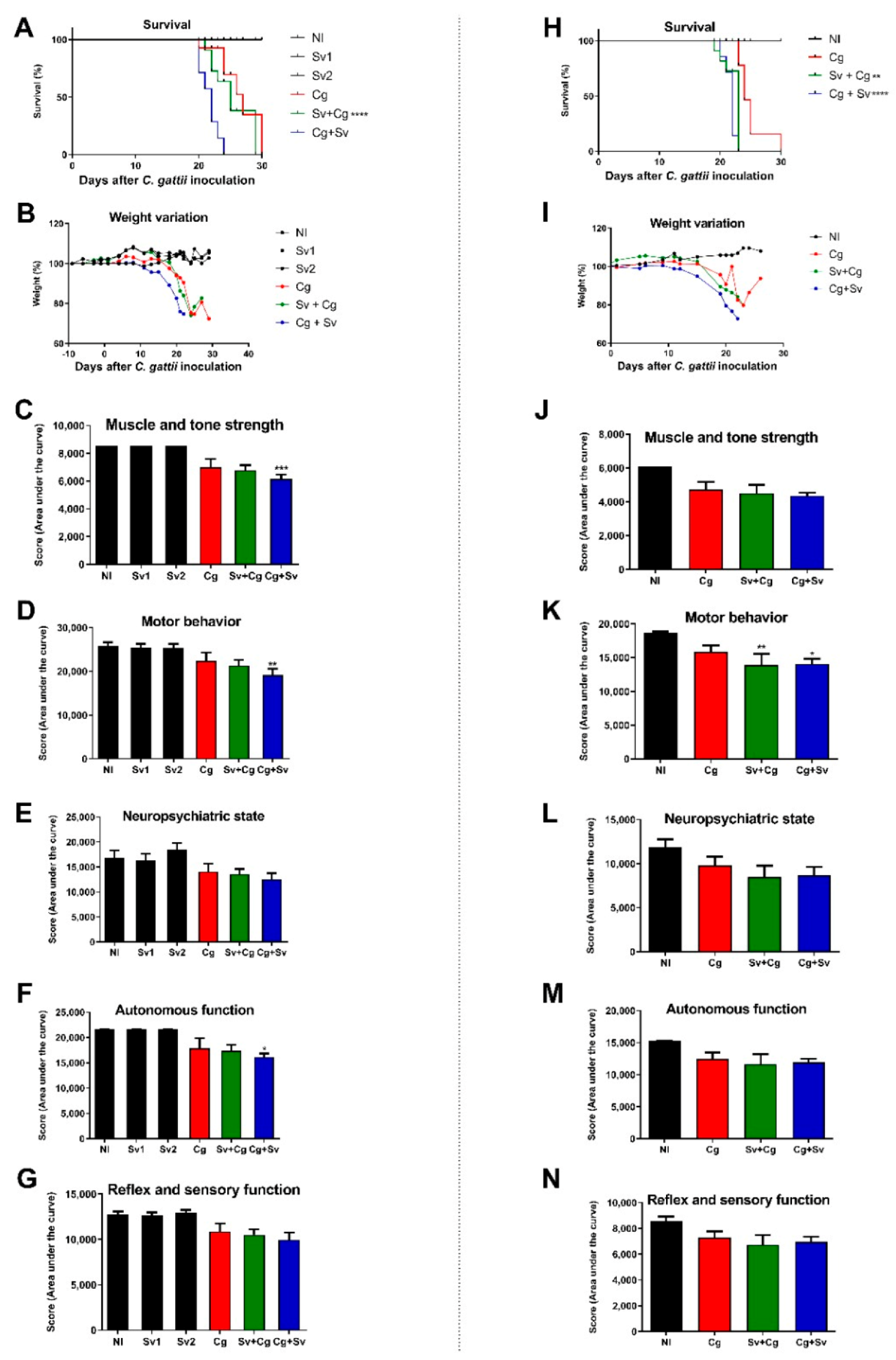
3.2. Sv Infection Increases Morbidity and Mortality in Cg-Infected Mice
3.3. Previous Infections with Sv Increase the Fungal Burden in the Organs
3.4. Sv–Cg Coinfection Increases Recruitment of Eosinophils and CD206+ Arginase-1+ Macrophages and Production of IL-4 in the Lungs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From Environmental Saprophyte to Global Pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Cogliati, M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientifica 2013, 2013, 675213. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The Global Burden of HIV-Associated Cryptococcal Infection in Adults in 2020: A Modelling Analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Bahn, Y.-S.; Jung, K.-W. Stress Signaling Pathways for the Pathogenicity of Cryptococcus. Eukaryot. Cell 2013, 12, 1564–1577. [Google Scholar] [CrossRef]
- Prado, M.; da Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality Due to Systemic Mycoses as a Primary Cause of Death or in Association with AIDS in Brazil: A Review from 1996 to 2006. Memórias Inst. Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef]
- Ellis, J.; Cresswell, F.V.; Rhein, J.; Ssebambulidde, K.; Boulware, D.R. Cryptococcal Meningitis and Tuberculous Meningitis Coinfection in HIV-Infected Ugandan Adults. Open Forum Infect. Dis. 2018, 5, ofy193. [Google Scholar] [CrossRef]
- Asif, S.; Bennett, J.; Pauly, R.R. A Unique Case of Cryptococcus and Histoplasmosis Coinfection in an HIV-Negative Male on Chronic Steroid Therapy. Cureus 2019, 11, e4654. [Google Scholar] [CrossRef]
- He, S.; Lv, D.; Xu, Y.; Wu, X.; Lin, L. Concurrent Infection with Talaromyces marneffei and Cryptococcus neoformans in a Patient without HIV Infection. Exp. Ther. Med. 2019, 19, 160–164. [Google Scholar] [CrossRef]
- Shi, Y.-F.; Wang, Y.-K.; Wang, Y.-H.; Liu, H.; Shi, X.-H.; Li, X.-J.; Wu, B.-Q. Metastatic Infection Caused by Hypervirulent Klebsiella pneumonia and Coinfection with Cryptococcus Meningitis: A Case Report. World J. Clin. Cases 2019, 7, 3812–3820. [Google Scholar] [CrossRef]
- Awari, D.W.; Shah, A.S.; Sexton, A.M.; Sexton, M.A. Coinfection of Aspergillus and Cryptococcus in Immunocompromised Host: A Case Report and Review of Literature. Case Rep. Infect. Dis. 2020, 2020, 8888270. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, H.; Takahashi, K.; Tashiro, H.; Ogusu, S.; Haraguchi, T.; Nakashima, C.; Nakamura, T.; Sueoka-Aragane, N. A Rare Case of Trichosporon mycotoxinivorans and Cryptococcus neoformans Coinfection in Lung. J. Infect. Chemother. 2020, 26, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Valente-Acosta, B.; Padua-Garcia, J.; Tame-Elorduy, A. Pulmonary Coinfection by Pneumocystis jirovecii and Cryptococcus Species in a Patient with Undiagnosed Advanced HIV. BMJ Case Rep. 2020, 13, e233607. [Google Scholar] [CrossRef] [PubMed]
- Peres-Emidio, E.C.; Freitas, G.J.C.; Costa, M.C.; Gouveia-Eufrasio, L.; Silva, L.M.V.; Santos, A.P.N.; Carmo, P.H.F.; Brito, C.B.; Arifa, R.D.N.; Bastos, R.W.; et al. Pseudomonas aeruginosa Infection Modulates the Immune Response and Increases Mice Resistance to Cryptococcus gattii. Front. Cell. Infect. Microbiol. 2022, 12, 811474. [Google Scholar] [CrossRef]
- Murphy, L.; Pathak, A.K.; Cattadori, I.M. A Coinfection with Two Gastrointestinal Nematodes Alters Host Immune Responses and Only Partially Parasite Dynamics. Parasite Immunol. 2013, 35, 421–432. [Google Scholar] [CrossRef]
- Lello, J.; McClure, S.J.; Tyrrell, K.; Viney, M.E. Predicting the Effects of Parasite Coinfection across Species Boundaries. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172610. [Google Scholar] [CrossRef]
- Mendes, T.; Minori, K.; Ueta, M.; Miguel, D.C.; Allegretti, S.M. Strongyloidiasis Current Status with Emphasis in Diagnosis and Drug Research. J. Parasitol. Res. 2017, 2017, 5056314. [Google Scholar] [CrossRef]
- Viney, M.; Kikuchi, T. Strongyloides ratti and S. venezuelensis—Rodent Models of Strongyloides Infection. Parasitology 2017, 144, 285–294. [Google Scholar] [CrossRef]
- Viney, M. Strongyloides. Parasitology 2017, 144, 259–262. [Google Scholar] [CrossRef]
- Varatharajalu, R.; Kakuturu, R. Strongyloides stercoralis: Current Perspectives. Rep. Parasitol. 2016, 23, 23–33. [Google Scholar] [CrossRef]
- Toledo, R.; Muñoz-Antoli, C.; Esteban, J.-G. Strongyloidiasis with Emphasis on Human Infections and Its Different Clinical Forms. Adv. Parasitol. 2015, 88, 165–241. [Google Scholar]
- World Health Organization. Strongyloidiasis; World Health Organization: Geneva, Switzerland.
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A.; et al. The Global Prevalence of Strongyloides stercoralis Infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef]
- Araujo, E.S.; de Jesus Pereira, C.A.; de Moura Pereira, A.T.; Moreira, J.M.P.; de Rezende, M.C.; Rodrigues, J.L.; Teixeira, M.M.; Negrão-Corrêa, D. The Role of IL-33/ST2, IL-4, and Eosinophils on the Airway Hyperresponsiveness Induced by Strongyloides venezuelensis in BALB/c Mice. Parasitol. Res. 2016, 115, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.A.; Holanda, R.A.; Frases, S.; Bravim, M.; Araujo, G.d.S.; Santos, P.C.; Costa, M.C.; Ribeiro, M.J.A.; Ferreira, G.F.; Baltazar, L.M.; et al. Fluconazole Alters the Polysaccharide Capsule of Cryptococcus gattii and Leads to Distinct Behaviors in Murine Cryptococcosis. PLoS ONE 2014, 9, e112669. [Google Scholar] [CrossRef]
- Negrão-Corrêa, D.; Pinho, V.; Souza, D.G.; Pereira, A.T.M.; Fernandes, A.; Scheuermann, K.; Souza, A.L.S.; Teixeira, M.M. Expression of IL-4 Receptor on Non-Bone Marrow-Derived Cells Is Necessary for the Timely Elimination of Strongyloides venezuelensis in Mice, but Not for Intestinal IL-4 Production. Int. J. Parasitol. 2006, 36, 1185–1195. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- de Rezende, M.C.; Araújo, E.S.; Moreira, J.M.P.; Rodrigues, V.F.; Rodrigues, J.L.; de Jesus Pereira, C.A.; Negrão-Corrêa, D. Effect of Different Stages of Schistosoma mansoni Infection on the Parasite Burden and Immune Response to Strongyloides venezuelensis in Co-Infected Mice. Parasitol. Res. 2015, 114, 4601–4616. [Google Scholar] [CrossRef] [PubMed]
- Weischenfeldt, J.; Porse, B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot5080. [Google Scholar] [CrossRef]
- Costa, M.C.; Barros Fernandes, H.; Gonçalves, G.K.N.; Santos, A.P.N.; Ferreira, G.F.; Freitas, G.J.C.; Carmo, P.H.F.; Hubner, J.; Emídio, E.C.P.; Santos, J.R.A.; et al. 17-β-Estradiol Increases Macrophage Activity through Activation of the G-protein-coupled Estrogen Receptor and Improves the Response of Female Mice to Cryptococcus gattii. Cell Microbiol. 2020, 22, e13179. [Google Scholar] [CrossRef]
- Ma, H.; May, R.C. Chapter 5 Virulence in Cryptococcus Species. Adv. Appl. Microbiol. 2009, 67, 131–190. [Google Scholar]
- Soares, B.M.; da Silva, D.L.; Sousa, G.R.; Amorim, J.C.F.; de Resende, M.A.; Pinotti, M.; Cisalpino, P.S. In Vitro Photodynamic Inactivation of Candida spp. Growth and Adhesion to Buccal Epithelial Cells. J. Photochem. Photobiol. B 2009, 94, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.C.; Fisher, E.M.C.; Brown, S.D.M.; Peters, J.; Hunter, A.J.; Martin, J.E. Behavioral and Functional Analysis of Mouse Phenotype: SHIRPA, a Proposed Protocol for Comprehensive Phenotype Assessment. Mamm. Genome 1997, 8, 711–713. [Google Scholar] [CrossRef]
- Ivey, C.L.; Williams, F.M.; Collins, P.D.; Jose, P.J.; Williams, T.J. Neutrophil Chemoattractants Generated in Two Phases during Reperfusion of Ischemic Myocardium in the Rabbit. Evidence for a Role for C5a and Interleukin-8. J. Clin. Investig. 1995, 95, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Green, A.P.; Mangan, F.; Ormerod, J.E. Induction of Cell Infiltration and Acid Hydrolase Release into the Peritoneal Cavity of Mice. Inflammation 1980, 4, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, L.S.; Talvani, A.; Teixeira, A.S.; Vieira, L.Q.; Cassali, G.D.; Andrade, S.P.; Teixeira, M.M. Impaired Inflammatory Angiogenesis, but Not Leukocyte Influx, in Mice Lacking TNFR1. J. Leukoc. Biol. 2005, 78, 352–358. [Google Scholar] [CrossRef]
- Strath, M.; Warren, D.J.; Sanderson, C.J. Detection of Eosinophils Using an Eosinophil Peroxidase Assay. Its Use as an Assay for Eosinophil Differentiation Factors. J. Immunol. Methods 1985, 83, 209–215. [Google Scholar] [CrossRef]
- Hesse, M.; Modolell, M.; la Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines In Vivo: Granulomatous Pathology Is Shaped by the Pattern of L-Arginine Metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Bahia, M.P.S.; Cândido, N.R.; Moreira, J.M.P.; Oliveira, V.G.; Araújo, E.S.; Rodrigues Oliveira, J.L.; de Carvalho Rezende, M.; Correa, A.; Negrão-Corrêa, D. Acute Infection with Strongyloides venezuelensis Increases Intestine Production IL-10, Reduces Th1/Th2/Th17 Induction in Colon and Attenuates Dextran Sulfate Sodium-Induced Colitis in BALB/c Mice. Cytokine 2018, 111, 72–83. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of Nitrite and Nitrate in Biological Fluids by Assays Based on the Griess Reaction: Appraisal of the Griess Reaction in the l-Arginine/Nitric Oxide Area of Research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.S.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Costa, M.C.; Magalhães, T.F.F.; Bastos, R.W.; Santos, P.C.; Carneiro, H.C.S.; Ribeiro, N.Q.; Ferreira, G.F.; Ribeiro, L.S.; Gonçalves, A.P.F.; et al. Influenza A Virus as a Predisposing Factor for Cryptococcosis. Front. Cell. Infect. Microbiol. 2017, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Bonne-Année, S.; Kerepesi, L.A.; Hess, J.A.; O’Connell, A.E.; Lok, J.B.; Nolan, T.J.; Abraham, D. Human and Mouse Macrophages Collaborate with Neutrophils To Kill Larval Strongyloides stercoralis. Infect. Immun. 2013, 81, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Bonne-Annee, S.; O’Connell, A.; Hess, J.; Abraham, D. Alternatively Activated Macrophages Kill the Parasitic Nematode Strongyloides stercoralis in Conjunction with Neutrophils through Two Unique Mechanisms. (37.44). J. Immunol. 2010, 184, 37–44. [Google Scholar] [CrossRef]
- Johnston, S.A.; May, R.C. Cryptococcus Interactions with Macrophages: Evasion and Manipulation of the Phagosome by a Fungal Pathogen. Cell. Microbiol. 2013, 15, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Stenzel, W.; Köhler, G.; Werner, C.; Polte, T.; Hansen, G.; Schütze, N.; Straubinger, R.K.; Blessing, M.; McKenzie, A.N.J.; et al. IL-13 Induces Disease-Promoting Type 2 Cytokines, Alternatively Activated Macrophages and Allergic Inflammation during Pulmonary Infection of Mice with Cryptococcus neoformans. J. Immunol. 2007, 179, 5367–5377. [Google Scholar] [CrossRef]
- Hardison, S.E.; Ravi, S.; Wozniak, K.L.; Young, M.L.; Olszewski, M.A.; Wormley, F.L. Pulmonary Infection with an Interferon-γ-Producing Cryptococcus neoformans Strain Results in Classical Macrophage Activation and Protection. Am. J. Pathol. 2010, 176, 774–785. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L. Cryptococcus and Phagocytes: Complex Interactions That Influence Disease Outcome. Front. Microbiol. 2016, 7, 105. [Google Scholar] [CrossRef]
- Zaragoza, O.; Rodrigues, M.L.; de Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. Chapter 4 The Capsule of the Fungal Pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar]
- García-Rodas, R.; Zaragoza, O. Catch Me If You Can: Phagocytosis and Killing Avoidance by Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 2012, 64, 147–161. [Google Scholar] [CrossRef]
- Takamure, A. Migration Route of Strongyloides venezuelensis in Rodents. Int. J. Parasitol. 1995, 25, 907–911. [Google Scholar] [CrossRef]
- Costa, F.S.; Rodrigues, V.F.; de Rezende, M.C.; Rodrigues-Oliveira, J.L.; Coelho, P.M.Z.; Negrão-Corrêa, D. The Effect of Maternal Strongyloides venezuelensis Infection on Mice Offspring Susceptibility and Immune Response. Vet. Parasitol. 2020, 278, 109037. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.C.; Juillard, P.-G.; Djordjevic, J.T.; Kaufman-Francis, K.; Dietmann, A.; Milonig, A.; Combes, V.; Grau, G.E.R. Cryptococcal Transmigration across a Model Brain Blood-Barrier: Evidence of the Trojan Horse Mechanism and Differences between Cryptococcus neoformans var. grubii Strain H99 and Cryptococcus gattii Strain R265. Microbes Infect. 2016, 18, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.Q.; Santos, A.P.N.; Emídio, E.C.P.; Costa, M.C.; Freitas, G.J.C.; Carmo, P.H.F.; Silva, M.F.; de Brito, C.B.; de Souza, D.G.; Paixão, T.A.; et al. Pioglitazone as an Adjuvant of Amphotericin B for the Treatment of Cryptococcosis. Int. J. Antimicrob. Agents 2019, 54, 301–308. [Google Scholar] [CrossRef]
- Oliveira-Brito, P.K.M.; Rezende, C.P.; Almeida, F.; Roque-Barreira, M.C.; da Silva, T.A. INOS/Arginase-1 Expression in the Pulmonary Tissue over Time during Cryptococcus gattii Infection. Innate Immun. 2020, 26, 117–129. [Google Scholar] [CrossRef]
- Chiapello, L.S.; Baronetti, J.L.; Garro, A.P.; Spesso, M.F.; Masih, D.T. Cryptococcus neoformans Glucuronoxylomannan Induces Macrophage Apoptosis Mediated by Nitric Oxide in a Caspase-Independent Pathway. Int. Immunol. 2008, 20, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Ruano, A.L.; López-Abán, J.; Fernández-Soto, P.; de Melo, A.L.; Muro, A. Treatment with Nitric Oxide Donors Diminishes Hyperinfection by Strongyloides venezuelensis in Mice Treated with Dexamethasone. Acta Trop. 2015, 152, 90–95. [Google Scholar] [CrossRef]
- Silveira, M.R.; Nunes, K.P.; Cara, D.C.; Souza, D.G.; Corrêa, A., Jr.; Teixeira, M.M.; Negrão-Corrêa, D. Infection with Strongyloides venezuelensis Induces Transient Airway Eosinophilic Inflammation, an Increase in Immunoglobulin E, and Hyperresponsiveness in Rats. Infect. Immun. 2002, 70, 6263–6272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaguchi, H.; Komase, Y.; Ikehara, M.; Yamamoto, T.; Shinagawa, T. Disseminated Cryptococcal Infection with Eosinophilia in a Healthy Person. J. Infect. Chemother. 2008, 14, 319–324. [Google Scholar] [CrossRef]
- Bassetti, M.; Mikulska, M.; Nicco, E.; Viscoli, C. Pulmonary Cryptococcosis with Severe Eosinophilia in an Immunocompetent Patient. J. Chemother. 2010, 22, 366–367. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kadowaki, M.; Yoshida, M.; Suzuki, K.; Komori, M.; Iwanaga, T. Disseminated Cryptococcosis with Marked Eosinophilia in a Postpartum Woman. Intern. Med. 2018, 57, 135–139. [Google Scholar] [CrossRef]
- Gao, L.-W.; Jiao, A.-X.; Wu, X.-R.; Zhao, S.-Y.; Ma, Y.; Liu, G.; Yin, J.; Xu, B.-P.; Shen, K.-L. Clinical Characteristics of Disseminated Cryptococcosis in Previously Healthy Children in China. BMC Infect. Dis. 2017, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Weatherhead, J.E.; Mejia, R. Immune Response to Infection with Strongyloides stercoralis in Patients with Infection and Hyperinfection. Curr. Trop. Med. Rep. 2014, 1, 229–233. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages As Well As Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Normile, T.G.; Bryan, A.M.; del Poeta, M. Animal Models of Cryptococcus neoformans in Identifying Immune Parameters Associated with Primary Infection and Reactivation of Latent Infection. Front. Immunol. 2020, 11, 581750. [Google Scholar] [CrossRef]
- Uicker, W.C.; Doyle, H.A.; Mccracken, J.P.; Langlois, M.; Buchanan, K.L. Cytokine and Chemokine Expression in the Central Nervous System Associated with Protective Cell-Mediated Immunity against Cryptococcus neoformans. Med. Mycol. 2005, 43, 27–38. [Google Scholar] [CrossRef]
- Piehler, D.; Stenzel, W.; Grahnert, A.; Held, J.; Richter, L.; Köhler, G.; Richter, T.; Eschke, M.; Alber, G.; Müller, U. Eosinophils Contribute to IL-4 Production and Shape the T-Helper Cytokine Profile and Inflammatory Response in Pulmonary Cryptococcosis. Am. J. Pathol. 2011, 179, 733–744. [Google Scholar] [CrossRef]
- Chiuso-Minicucci, F.; Marra, N.M.; Zorzella-Pezavento, S.F.G.; Franãa, T.G.D.; Ishikawa, L.L.W.; Amarante, M.R.V.; Amarante, A.F.T.; Sartori, A. Recovery from Strongyloides venezuelensis Infection in Lewis Rats Is Associated with a Strong Th2 Response. Parasite Immunol. 2010, 32, 74–78. [Google Scholar] [CrossRef]
- Yasuda, K.; Matsumoto, M.; Nakanishi, K. Importance of Both Innate Immunity and Acquired Immunity for Rapid Expulsion of S. venezuelensis. Front. Immunol. 2014, 5, 118. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia-Eufrasio, L.; de Freitas, G.J.C.; Costa, M.C.; Peres-Emidio, E.C.; Carmo, P.H.F.; Rodrigues, J.G.M.; de Rezende, M.C.; Rodrigues, V.F.; de Brito, C.B.; Miranda, G.S.; et al. The Th2 Response and Alternative Activation of Macrophages Triggered by Strongyloides venezuelensis Is Linked to Increased Morbidity and Mortality Due to Cryptococcosis in Mice. J. Fungi 2023, 9, 968. https://doi.org/10.3390/jof9100968
Gouveia-Eufrasio L, de Freitas GJC, Costa MC, Peres-Emidio EC, Carmo PHF, Rodrigues JGM, de Rezende MC, Rodrigues VF, de Brito CB, Miranda GS, et al. The Th2 Response and Alternative Activation of Macrophages Triggered by Strongyloides venezuelensis Is Linked to Increased Morbidity and Mortality Due to Cryptococcosis in Mice. Journal of Fungi. 2023; 9(10):968. https://doi.org/10.3390/jof9100968
Chicago/Turabian StyleGouveia-Eufrasio, Ludmila, Gustavo José Cota de Freitas, Marliete Carvalho Costa, Eluzia Castro Peres-Emidio, Paulo Henrique Fonseca Carmo, João Gustavo Mendes Rodrigues, Michelle Carvalho de Rezende, Vanessa Fernandes Rodrigues, Camila Bernardo de Brito, Guilherme Silva Miranda, and et al. 2023. "The Th2 Response and Alternative Activation of Macrophages Triggered by Strongyloides venezuelensis Is Linked to Increased Morbidity and Mortality Due to Cryptococcosis in Mice" Journal of Fungi 9, no. 10: 968. https://doi.org/10.3390/jof9100968
APA StyleGouveia-Eufrasio, L., de Freitas, G. J. C., Costa, M. C., Peres-Emidio, E. C., Carmo, P. H. F., Rodrigues, J. G. M., de Rezende, M. C., Rodrigues, V. F., de Brito, C. B., Miranda, G. S., de Lima, P. A., da Silva, L. M. V., Oliveira, J. B. S., da Paixão, T. A., da Glória de Souza, D., Fagundes, C. T., Peres, N. T. d. A., Negrão-Correa, D. A., & Santos, D. A. (2023). The Th2 Response and Alternative Activation of Macrophages Triggered by Strongyloides venezuelensis Is Linked to Increased Morbidity and Mortality Due to Cryptococcosis in Mice. Journal of Fungi, 9(10), 968. https://doi.org/10.3390/jof9100968







