First Cases of Feline Sporotrichosis Caused by Sporothrix brasiliensis in Paraguay
Abstract
:1. Introduction
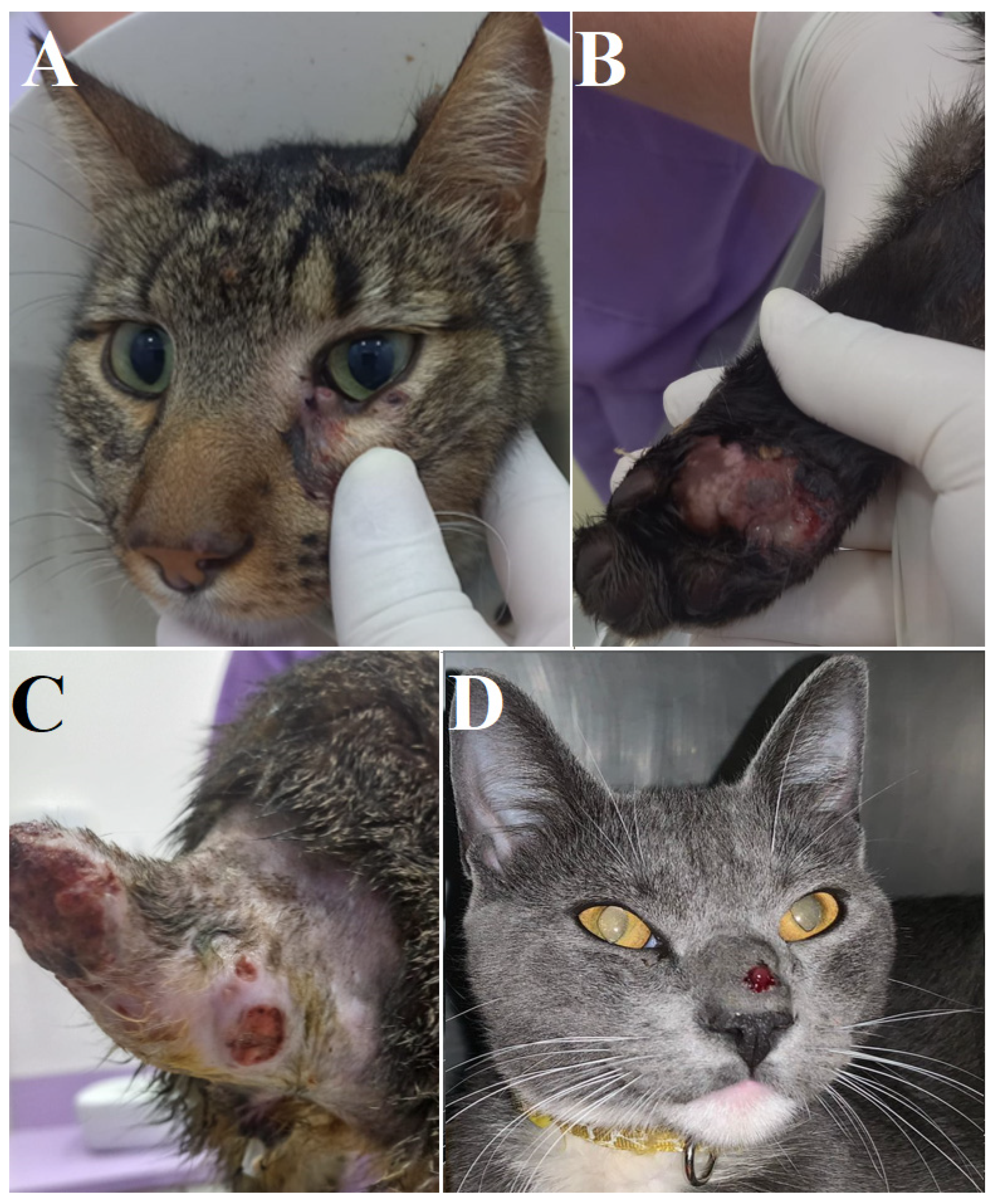
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Species | Isolate Code | CBS Code | Geographic Origin | Source | Genbank | Reference |
|---|---|---|---|---|---|---|
| Sporothrix_schenckii | Ss159 | CBS_132976 | Japan | Human | KF574464 | [28] |
| Sporothrix_schenckii | Ss141 | CBS_132975 | Brazil | Human | JQ041905 | [29] |
| Sporothrix_schenckii | - | CBS_359.36_(T) | NK | Human | AM117437 | [30] |
| Sporothrix_schenckii | - | CBS_211.61 | South Africa | Not Know | KP101393 | [31] |
| Sporothrix_pallida | - | CBS_302.73_(T) | United Kingdom | Soil | AM398396 | [32] |
| Sporothrix_pallida | - | CBS_131.56(T) | Japan | Soil | KX590811 | [33] |
| Sporothrix_mexicana | Ss 132 | CBS_132927 | Brazil | Human | JF811340 | [34] |
| Sporothrix_mexicana | FMR 9108 | CBS_120341_(T) | Mexico | Soil | AM398393 | [32] |
| Sporothrix_luriei | ATCC 18616 | CBS_937.72_(T) | South Africa | Human | AM747302 | [35] |
| Sporothrix_humicola | - | CBS_118129 | South Africa | Soil | KX590808 | [33] |
| Sporothrix_globosa | - | CBS_120340_(T) | Spain | Human | KP101459 | [31] |
| Sporothrix_chilensis | Ss469 | CBS_139891_(T) | Chile | Human | KP711815 | [36] |
| Sporothrix_chilensis | Ss470 | CBS_139890 | Chile | Soil | KP711816 | [36] |
| Sporothrix_brunneoviolacea | - | CBS_101570 | USA | Vegetal | KP017101 | [31] |
| Sporothrix_brasiliensis | Ss227 | CBS_133004 | Brazil | Canine | KC693876 | [37] |
| Sporothrix_brasiliensis | Ss226 | CBS_133003 | Brazil | Feline | KC693875 | [37] |
| Sporothrix_brasiliensis | Ss151 | CBS_132994 | Brazil | Canine | KC693864 | [37] |
| Sporothrix brasiliensis | 0090013908 | - | Brazil | Human | KJ769111 | [38] |
| Sporothrix brasiliensis | Ss05 | CBS 132985 | Brazil | Feline | KC693830 | [37] |
| Sporothrix brasiliensis | HUPE117298 | - | Brazil | Human | KC329494 | [39] |
| Sporothrix brasiliensis | HUPE114500 | - | Brazil | Human | KF048980 | [40] |
| Sporothrix brasiliensis | IPEC 16490 | CBS 120339 (T) | Brazil | Human | AM116899 | [32] |
| Sporothrix brasiliensis | CMRP5787 | - | Brazil | Feline | OR501574 | This study |
| Sporothrix brasiliensis | CMPRP5786 | - | Brazil | Feline | OR501573 | This study |
References
- Gonçalves, S.S.; da Cruz Bahiense Rocha, I.; Rediguieri, B.C.; de Carvalho, J.A.; Maifrede, S.B.; Kruschewsky, W.L.L.; Falqueto, A.; Rodrigues, A.M. Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species. J. Fungi 2023, 9, 831. [Google Scholar] [CrossRef]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Gremião, I.D.F.; Pereira, S.A. A One Health Approach to Combatting Sporothrix Brasiliensis: Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Cognialli, R.C.R.; Cáceres, D.H.; Bastos, F.d.A.G.D.; Cavassin, F.B.; Lustosa, B.P.R.; Vicente, V.A.; Breda, G.L.; Santos-Weiss, I.; Queiroz-Telles, F. Rising Incidence of Sporothrix Brasiliensis Infections, Curitiba, Brazil, 2011–2022. Emerg Infect. Dis. 2023, 29, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Mora-Montes, H.M. Current Progress in Sporothrix Brasiliensis Basic Aspects. J. Fungi 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Schechtman, R.C.; Falcão, E.M.M.; Carard, M.; García, M.S.C.; Mercado, D.S.; Hay, R.J. Sporotrichosis: Hyperendemic by Zoonotic Transmission, with Atypical Presentations, Hypersensitivity Reactions and Greater Severity. An. Bras. Dermatol. 2022, 97, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The Threat of Emerging and Re-Emerging Pathogenic Sporothrix Species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef]
- Bastos, F.; Farias, M.; Monti, F.; Cognialli, R.; Lopuch, L.; Gabriel, A.; Vicente, V.; Razzolini, E.; Wu, K.; Queiroz-Telles, F. Spread of Sporothrix Brasiliensis from the Sneeze of Infected Cats: A Potential Novel Route of Transmission. Med. Mycol. 2022, 60, 272. [Google Scholar] [CrossRef]
- Gremião ID, F.; Martins da Silva da Rocha, E.; Montenegro, H.; Carneiro, A.J.B.; Xavier, M.O.; de Farias, M.R.; Lopes-Bezerra, L.M. Guideline for the Management of Feline Sporotrichosis Caused by Sporothrix Brasiliensis and Literature Revision. Braz. J. Microbiol. 2021, 52, 107–124. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Gonçalves, S.S.; de Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; Camargo, Z.P. de Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends. J. Fungi 2022, 8, 776. [Google Scholar] [CrossRef]
- García Duarte, J.M.; Wattiez Acosta, V.R.; Fornerón Viera PM, L.; Aldama Caballero, A.; Gorostiaga Matiauda, G.A.; de Oddone, V.B.R.; Pereira Brunelli, J.G. Esporotricosis Trasmitida Por Gato Doméstico. Reporte de Un Caso Familiar. Revista del Nacional (Itauguá) 2017, 9, 67–76. [Google Scholar] [CrossRef]
- Kaadan, M.I.; Dennis, M.; Desai, N.; Yadavalli, G.; Lederer, P. One Health Education for Future Physicians: A Case Report of Cat-Transmitted Sporotrichosis. Open Forum Infect. Dis. 2020, 7, ofaa049. [Google Scholar] [CrossRef]
- Barnacle, J.R.; Chow, Y.J.; Borman, A.M.; Wyllie, S.; Dominguez, V.; Russell, K.; Roberts, H.; Armstrong-James, D.; Whittington, A.M. The First Three Reported Cases of Sporothrix Brasiliensis Cat-Transmitted Sporotrichosis Outside South America. Med. Mycol. Case Rep. 2023, 39, 14–17. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Sporothrix Brasiliensis, an Emerging Fungal Pathogen, Notable for Its Zoonotic Transmission and Epidemic Potential for Human and Animal Health in the Americas; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; del Río, C.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix Brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix Brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Voidaleski, M.F.; Queiroz-Telles, F.; Itikawa, H.T.; Müller, G.G.; Lima, B.J.F.S.; Trevisoli, L.E.; Cognialli, R.C.R.; Crispim, R.C.L.; Vicente, V.A. An Atypical Etiology of Fungal Keratitis Caused by Roussoella Neopustulans. J. Fungi 2022, 8, 507. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.2, a Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 May 2023).
- Prado, C.M.; Svoboda, W.K.; Chiyo, L.; Queiroz-Telles, F. Fundamentos de Saúde Única (One Health) e Planejamento Estratégico Situacional para Implementação de Política Pública de Saúde para Prevenção e Controle da Esporotricose na Região da Tríplice Fronteira (Brasil, Paraguai, Argentina). In Saúde Pública na Região da Fronteira Brasil-Paraguai-Argentina; Pedro & João Editores: São Carlos, Brasil, 2022; Volume 1, Chapter 5, p. 101. [Google Scholar]
- Lecca, L.O.; Paiva, M.T.; de Oliveira, C.S.F.; Morais, M.H.F.; de Azevedo, M.I.; Bastos, C.D.V.E.; Keller, K.M.; Ecco, R.; Alves, M.R.S.; Pais, G.C.T.; et al. Associated Factors and Spatial Patterns of the Epidemic Sporotrichosis in a High Density Human Populated Area: A Cross-Sectional Study from 2016 to 2018. Prev. Vet. Med. 2020, 176, 104939. [Google Scholar] [CrossRef]
- Maschio-Lima, T.; Marques, M.D.R.; Lemes, T.H.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; de Almeida, B.G.; Bianco, L.M.; Monteiro, R.C.; Rodrigues, A.M.; de Camargo, Z.P.; et al. Clinical and Epidemiological Aspects of Feline Sporotrichosis Caused by Sporothrix Brasiliensis and in Vitro Antifungal Susceptibility. Vet. Res. Commun. 2021, 45, 171–179. [Google Scholar] [CrossRef]
- Rabello, V.B.S.; Almeida, M.A.; Bernardes-Engemann, A.R.; Almeida-Paes, R.; de Macedo, P.M.; Zancopé-Oliveira, R.M. The Historical Burden of Sporotrichosis in Brazil: A Systematic Review of Cases Reported from 1907 to 2020. Braz. J. Microbiol. 2022, 53, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.E.; Valeriano, C.A.T.; Ferraz, C.E.; Neves, R.P.; Oliveira, M.M.E.; Silva, J.C.A.L.; Magalhães, V.; Lima-Neto, R.G. Epidemiological Features and Geographical Expansion of Sporotrichosis in the State of Pernambuco, Northeastern Brazil. Future Microbiol. 2021, 16, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Hartmann, K.; Pennisi, M.G.; Ferrer, L.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Sporotrichosis in Cats: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2013, 15, 619–623. [Google Scholar] [CrossRef]
- Taylor, S.; St Denis, K.; Collins, S.; Dowgray, N.; Ellis, S.L.H.; Heath, S.; Rodan, I.; Ryan, L. 2022 ISFM/AAFP Cat Friendly Veterinary Environment Guidelines. J Feline Med. Surg. 2022, 24, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.A.; Fernandes, G.F.; Rodrigues, A.M.; Lima, F.M.; Marini, M.M.; Feitosa, L.D.S.; De Melo Teixeira, M.; Felipe, M.S.S.; Da Silveira, J.F.; De Camargo, Z.P. Chromosomal Polymorphism in the Sporothrix Schenckii Complex. PLoS ONE 2014, 9, e86819. [Google Scholar] [CrossRef]
- Fernandes, G.F.; dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; de Camargo, Z.P. Characterization of Virulence Profile, Protein Secretion and Immunogenicity of Different Sporothrix Schenckii Sensu Stricto Isolates Compared with S. Globosa and S. Brasiliensis Species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef]
- Marimon, R.; Gené, J.; Cano, J.; Trilles, L.; Lazéra, M.D.S.; Guarro, J. Molecular Phylogeny of Sporothrix Schenckii. J. Clin. Microbiol. 2006, 44, 3251–3256. [Google Scholar] [CrossRef]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and Evolutionary Patterns in Sporothrix Spanning More than 14 000 Human and Animal Case Reports. Persoonia Mol. Phylogeny Evol. Fungi 2015, 35, 1–20. [Google Scholar] [CrossRef]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J.; Sporothrix Brasiliensis, S.G.; Mexicana, S. Three New Sporothrix Species of Clinical Interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef]
- de Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The Divorce of Sporothrix and Ophiostoma: Solution to a Problematic Relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; De Hoog, S.; De Camargo, Z.P. Emergence of Pathogenicity in the Sporothrix Schenckii Complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef]
- Marimon, R.; Genè, J.; Cano, J.; Guarro, J. Sporothrix Luriei: A Rare Fungus from Clinical Origin. Med. Mycol. 2008, 46, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Cruz Choappa, R.; Fernandes, G.F.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix Chilensis Sp. Nov. (Ascomycota: Ophiostomatales), a Soil-Borne Agent of Human Sporotrichosis with Mild-Pathogenic Potential to Mammals. Fungal Biol. 2016, 120, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Melo Teixeira, M.; de Hoog, G.S.; Schubach, T.M.P.; Pereira, S.A.; Fernandes, G.F.; Bezerra, L.M.L.; Felipe, M.S.; de Camargo, Z.P. Phylogenetic Analysis Reveals a High Prevalence of Sporothrix Brasiliensis in Feline Sporotrichosis Outbreaks. PLoS Negl. Trop. Dis. 2013, 7, e2281. [Google Scholar] [CrossRef] [PubMed]
- Marques De Macedo, P.; Sztajnbok, D.C.N.; Camargo, Z.P.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. Dacryocystitis due to Sporothrix brasiliensis: A case report of a successful clinical and serological outcome with low-dose potassium iodide treatment and oculoplastic surgery. Br. J. Dermatol. 2015, 172, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Unterstell, N.; Carlos Gripp, A.; De Macedo, P.M.; Brota, A.; Dias, E.; De Melo Teixeira, M.; Felipe, M.S.; Bernardes-Engemann, A.R.; Lopes-Bezerra, L.M. Pulmonary Cavitation and Skin Lesions Mimicking Tuberculosis in a HIV Negative Patient Caused by Sporothrix Brasiliensis. Med. Mycol. Case Rep. 2013, 2, 65–71. [Google Scholar] [CrossRef]
- Castro, R.A.; Kubitschek-Barreira, P.H.; Teixeira, P.A.C.; Sanches, G.F.; Teixeira, M.M.; Quintella, L.P.; Almeida, S.R.; Costa, R.O.; Camargo, Z.P.; Felipe, M.S.S.; et al. Differences in Cell Morphometry, Cell Wall Topography and Gp70 Expression Correlate with the Virulence of Sporothrix Brasiliensis Clinical Isolates. PLoS ONE 2013, 8, e75656. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Prado, C.M.; Razzolini, E.; Santacruz, G.; Ojeda, L.; Geraldo, M.R.; Segovia, N.; Pereira Brunelli, J.; Vicente, V.A.; Svoboda, W.K.; Queiroz-Telles, F. First Cases of Feline Sporotrichosis Caused by Sporothrix brasiliensis in Paraguay. J. Fungi 2023, 9, 972. https://doi.org/10.3390/jof9100972
do Prado CM, Razzolini E, Santacruz G, Ojeda L, Geraldo MR, Segovia N, Pereira Brunelli J, Vicente VA, Svoboda WK, Queiroz-Telles F. First Cases of Feline Sporotrichosis Caused by Sporothrix brasiliensis in Paraguay. Journal of Fungi. 2023; 9(10):972. https://doi.org/10.3390/jof9100972
Chicago/Turabian Styledo Prado, Carolina Melchior, Emanuel Razzolini, Gabriela Santacruz, Leticia Ojeda, Marlon Roger Geraldo, Nancy Segovia, José Pereira Brunelli, Vânia Aparecida Vicente, Walfrido Kühl Svoboda, and Flávio Queiroz-Telles. 2023. "First Cases of Feline Sporotrichosis Caused by Sporothrix brasiliensis in Paraguay" Journal of Fungi 9, no. 10: 972. https://doi.org/10.3390/jof9100972
APA Styledo Prado, C. M., Razzolini, E., Santacruz, G., Ojeda, L., Geraldo, M. R., Segovia, N., Pereira Brunelli, J., Vicente, V. A., Svoboda, W. K., & Queiroz-Telles, F. (2023). First Cases of Feline Sporotrichosis Caused by Sporothrix brasiliensis in Paraguay. Journal of Fungi, 9(10), 972. https://doi.org/10.3390/jof9100972








