1. Introduction
Histoplasmosis is the most widely distributed systemic respiratory mycosis in the American continent. It is acquired via inhalation of aerosolized infective mycelial morphotype (M-phase) propagules of the dimorphic fungus
Histoplasma capsulatum, mainly found in environments containing bat and bird guano, which favor M-phase growth. Clinical manifestations of the disease vary, ranging from a limited and mild respiratory infection to a life-threatening acute disseminated form, sometimes resulting in a fatal outcome. Under poorly defined circumstances, the disease evolves into a chronic form that develops tissue reactions represented by a well-organized cytoarchitecture known as a granuloma. The outcome of an
H. capsulatum infection depends on the immune status of a specific host, the infective propagules’ multiplicity, the virulence, and the phylogenetic species of the fungal strains [
1,
2,
3].
To date, the role of the innate immune response to
H. capsulatum infection has not been carefully explored [
1,
2]. In contrast, the activation of cellular immunity through the CD4+ Th1 lymphocytes of the adaptive arm of the immune response has been preferentially analyzed in several studies of this host–fungus interaction. This response is, undoubtedly, the main mechanism related to fungal clearance in host-infected tissues. Most of the knowledge generated about this issue has been associated with experimental assays using murine models inoculated with the yeast morphotype (Y-phase), which is the parasitic morphotype presenting the main multiple virulence factors of this fungus [
4,
5,
6,
7]. However, the use of the Y-phase to study the course of the immune response to a histoplasmosis infection can occasionally cause misinterpretations in the progression of the first step of the infection because it is not the natural infective morphotype of
H. capsulatum.
A few studies have processed the infective M-phase to explore the immune responses triggered by the host to this particular fungal morphotype, in the clinical evolution of histoplasmosis. New information regarding different sites and the time spent for the in vivo
H. capsulatum M-to-Y transition was reported by Suárez-Álvarez et al. [
8]. They detected the expression of phase-specific genes for M- and Y-phases using RT-PCR assays in tissue samples taken from the upper respiratory tract of infected mice. Thus, by utilizing this innovative genetic approach and by simulating a natural host infection, these authors found relevant data, which undoubtedly demonstrated that infective
Histoplasma M-phase propagules spent 2–3 h to convert in the parasitic Y-phase. Furthermore, they also demonstrated that this fungus can also utilize other extrapulmonary sites to initiate its M-to-Y transition, where the link between innate and adaptive immune responses occurs, such as the mucosal-associated lymphoid tissues in the upper respiratory tract [
8].
Sahaza et al. [
2] also used the infective M-phase to trigger the immune response in mice, demonstrating that the highest levels of several innate and adaptive proinflammatory cytokines, detected in pulmonary homogenates of adult male BALB/c mice infected with the fungal M-phase, were primarily associated with a particular
H. capsulatum strain isolated in Mexico (EH-46), which was first classified as a Latin American LAm A phylogenetic species, according to Kasuga et al. [
9], and later renamed LAm A2 by Teixeira et al. [
10]. As stated by Sahaza et al. [
2], these cytokine levels contrasted with those obtained using the G-217B
H. capsulatum strain isolated in the United States of America (USA), which belongs to the NAm 2 phylogenetic species and can also be cataloged as
Histoplasma ohiense sp. nov., according to Kasuga et al. [
9] and Sepúlveda et al. [
11], respectively. Thus, the morphotype and the phylogenetic species of
H. capsulatum are some of the major attributes that certainly participate in histoplasmosis manifestations and in the fate of this type of host–parasite interaction.
Durkin et al. [
12] documented the role of different fungal genotypes regarding the dissimilarities in the outcome of the disease and in the histopathological damage, which were triggered by
H. capsulatum strains from Latin America (earlier classified as classes 5 and 6) in mice infected intratracheally with the Y-phase, in contrast to those infected with a strain from North America (class 2). Concerning the virulence of distinct phylogenetic strains isolated in North America, Sepúlveda et al. [
3] reported differences in the fungal burden, disease progression, and cytokine responses in mice infected intranasally with yeast cells of
H. capsulatum.
In human histoplasmosis, Karimi et al. [
13] reported clinical differences between AIDS-associated histoplasmosis patients from the USA and those from Brazil, highlighting the most frequent skin lesions in Brazilian patients infected with classes 5 and 6 genotypes of
H. capsulatum strains, in contrast to patients from the USA infected with the class 2
H. capsulatum genotype. Mucocutaneous manifestations of histoplasmosis are frequently observed in Brazilian patients and are caused by specific strains with unique pathogenic characteristics within the phylogenetic species of
H. capsulatum from Latin America, which might explain its increased dermatotropism [
14]. Although in human patients, the host genetic characteristics could participate in the fate of the interaction with the fungus performance, in experimental models, these characteristics can usually be homogenized; also, the genetic attributes of the fungus involved in the infective process of the host should be considered as a relevant factor.
Currently, several authors emphasize the fact that
H. capsulatum realizes its dimorphic transition when it reaches alveoli, within their macrophages. However, our research group has found that the upper respiratory tract of the infected host, including the nasal-associated lymphoid tissue, plays an important role in the in vivo
H. capsulatum M-to-Y transition and in the immune response to this pathogen, taking part in the initial mechanisms activated by the defense of the hosts against airborne infective M-phase propagules [
8]. During the early stages of the innate immunity (probably up to 5 days postinfection), no real evidence of chronic inflammation characterized by the development of granulomatous reactions could be found [
2,
8]. These reactions are formed in histoplasmosis after the fungal dimorphic transition to the Y-phase has occurred in respiratory microenvironments of the host, once the adaptive chronic inflammatory reaction is established. The development of a granuloma throughout the histoplasmosis infectious process was monitored in an elegant study conducted by Heninger et al. [
15]. These authors evaluated granuloma formation at different times after intraperitoneal and intranasal infection of mice, using the Y-phase of the G-217B
H. capsulatum strain (NAm 2 phylogenetic species or
H. ohiense); after the seventh day postinfection, histopathological findings revealed the presence of granulomas containing yeast cells in the lungs and liver of the infected mice, irrespective of the infection route.
Considering all the abovementioned antecedents and the fact that H. capsulatum affects the lungs as a main target organ, the present study aimed to record the most exciting features in the lung’s cytoarchitecture during the inflammatory response associated with differential histopathological changes generated by the intranasal infection of mice with the M-phase propagules of two different phylogenetic strains of this fungal pathogen.
4. Discussion
The host inhalation route certainly participates in the initial recognition of the infective M-phase of
H. capsulatum by promoting the activation of multifaceted mechanisms triggered by the host–fungus interplay, which include the immune response of the host respiratory system and fungal strategies. Regarding the
H. capsulatum strategies, the fast in vivo morphotype transition to the parasitic-virulent Y-phase is necessary for the establishment of the infection and subsequent dissemination, via lymph drainage and lymph nodes. Notably, we have demonstrated that fungal dissemination could occur before it affects the lung as the main target organ [
8].
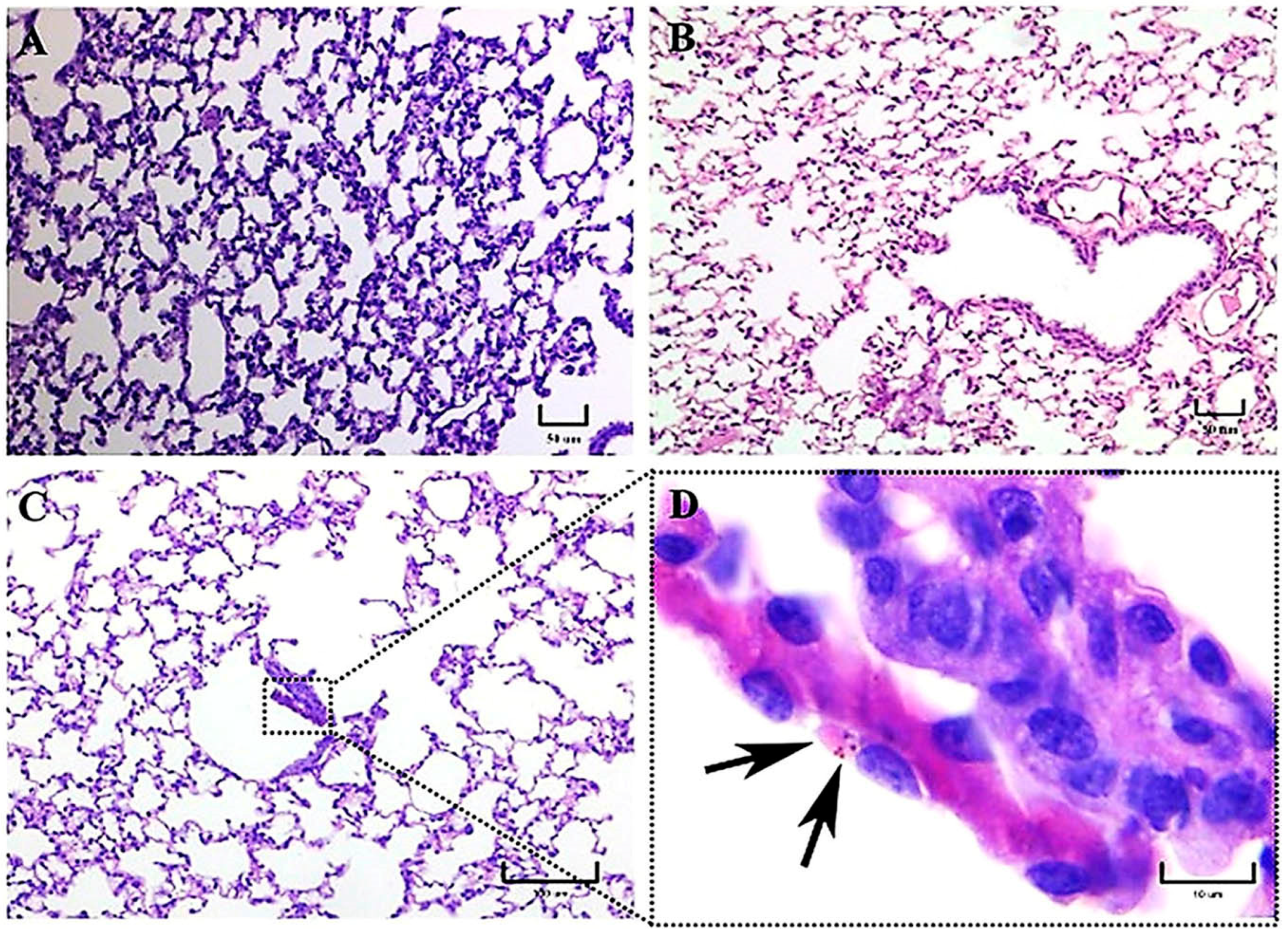
To investigate the inflammatory reaction generated by distinct genotypes from two H. capsulatum strains belonging to different phylogenetic species, LAm A2/EH-46 (Latin America) and NAm 2/G-217B (North America), we used an intranasal inoculum of fungal M-phase propagules in a murine model to simulate a natural infection. In this study, intracellular yeast cells were primarily observed after 3 h postinfection in macrophages of mice infected with the EH-46 strain, particularly in the bronchiole-alveolar lumen. However, macrophages with intracellular yeast cells were less frequent in mice infected with the G-217B strain, which was mainly found in the BALT.
Studies associated with human clinical cases and animal models have found differences in virulence and gene expression, as well as in the pathogenesis of the disease, among
H. capsulatum strains from different genotypes and phylogenetic species [
1,
2,
3,
12,
13,
14,
16].
Several steps of the host response are activated during the host–fungus interaction, involving various levels of complexity, where the inflammatory process is critical for the host defense; however, sometimes, this inflammatory response progresses into chronic inflammation. In this setting, during the progression of the lung chronic inflammatory reaction, the associated immunopathology is characterized by changes in tissue architecture and variable cellularity in pulmonary lesions with well-organized granulomas, with or without necrosis development [
12,
15]. In the present paper, important histopathological differences were described in the lung inflammatory response of mice infected intranasally with M-phase propagules of
H. capsulatum strains from distinct phylogenetic species, regarding the presence of pneumonia, the development of granulomas, and the BALT induction. The latter is an unexpected finding because BALT is not constitutive in mice [
17]. According to our histopathological observations, after 14 and 21 days postinfection, granuloma development was clearly associated with the EH-46 strain, whereas BALT induction was associated with a lesser diffuse inflammatory response related to the G-217B strain infection. These findings support the existence of relevant differences in the pulmonary histopathology produced by each of the two
H. capsulatum strains used in the present study, which could be crucial for the formation of granulomas in mice infected with the EH-46 strain. Overall, granuloma plays a protective role in the infected host by avoiding the progression of the fungal infection; however, depending on the course of some chronic infections, the granuloma could subvert the host defense, involving several organs and leading to tissue damage with fibrotic processes. Moreover, granulomas produced by
H. capsulatum infection develop hypoxic microenvironments, which favor the infectious process [
18]. To change this hypoxic microenvironment, host macrophages activate a transcription factor, the hypoxia-inducible factor (HIF)-1a, which abrogates the progression of
H. capsulatum infection by controlling IL-10 production and, consequently, circumvents the negative effect of this cytokine on the antimicrobial activity of macrophages [
19].
The presence of alveolar proteinosis (eosinophilic protein deposits) in the alveoli lumen was observed in a mouse infected with the EH-46 strain. Curiously, in human clinical cases, pulmonary alveolar proteinosis has also been documented in acute disseminated histoplasmosis [
20].
Initially, progressive changes in the pulmonary parenchyma with increased cellularity (macrophages and neutrophils) compatible with pneumonitis were observed in mice infected with both strains at 1 h postinfection. According to our observations,
H. capsulatum intracellular PAS-positive fungal cells, compatible with yeast- or microconidia-like cells, were first observed within alveolar macrophages at 1 h postinfection, whereas at 3 h postinfection, we recognized that all fungal cells were converted to yeast cells, as this time matched with the time required by this fungus to complete its dimorphic transition in vivo [
8]. PAS-positive structures like macroconidia of
H. capsulatum were only observed until 3 h postinfection. Therefore, from this time forward, all the fungal structures observed in lung histological sections were assumed as yeast cells, in accordance with the findings reported in a similar mice model by Suárez-Álvarez et al. [
8].
As stated in our results, during the first 3 h postinfection with the EH-46 strain, the scarce number of macrophages with intracellular yeast cells contrasts with the number found for the G-217B strain; however, after 24 h postinfection, this relation changed for each H. capsulatum strain.
At 5–7 days, mainly in mice infected with the EH-46 strain, persistent inflammatory infiltrates were observed in their lung samples, which could evolve toward granulomas in development. Well-structured lung granulomas containing several yeast cells, predominate at 14 and 21 days postinfection, in mice infected with the EH-46 H. capsulatum strain. However, in mice infected with the G-217B strain, induced BALT prevailed.
Durkin et al. [
12] showed that strains originating from Latin America (classes 5 and 6) caused more tissue damage and death in B6C3F mice infected intratracheally with sublethal doses of
H. capsulatum yeast cells than in mice infected with a strain (class 2) from North America. They also stated that yeast cells were more abundant in B6C3F mice infected with
H. capsulatum strains from Latin America. In addition, at 14 days postinfection, they showed the presence of granulomas and early caseous necrosis in mice infected with these strains, whereas mice infected with strain class 2 developed only lung inflammatory processes without evidence of granulomas or necrosis. No multinucleated giant cells were reported by these authors in the lung histopathology from mice infected with both strains. Our results using a LAm A2
H. capsulatum strain matched well with data reported by Durkin et al. [
12], supporting the presence of important changes in the host response due to the genetic characteristics of
H. capsulatum strains.
Regarding the time required for granuloma formation in an experimental histoplasmosis model, our findings are also consistent with those reported previously by Heninger et al. [
15], who suggested that during the development of granuloma at different postinfection times in C57BL/6 mice, the appearance of well-structured granulomas in the lung and liver begin at day 7 postinfection and persist through days 10 and 14 in both organs. Similar times were observed by our team for granuloma development when we used the EH-46
H. capsulatum strain.
A previous report by Sahaza et al. [
2], highlighted differences in granuloma formation based on the description of an original panoramic or a semi-microscopic lung section from mice infected with the LAm A2/EH-46 and NAm 2/G-217B strains; granulomas were well developed at days 14 and 21 postinfection, decreasing at day 28 after infection with the EH-46 strain. In contrast, mice infected with the G-217B strain did not show any images suggestive of granulomas. In our present study, we performed the same experimental infection conditions described by Sahaza et al. [
2]; however, unlike us, they did not conduct histopathological observations to discriminate between lung tissue injuries generated by the genetically distinct
H. capsulatum strains used.
Other authors have contributed to the description of the histoplasmosis course depending on the
Histoplasma species, such as Sepúlveda et al. [
3], who compared the disease progression using one
Histoplasma strain from the Panama lineage and two
Histoplasma strains representative of the NAm 1 and NAm 2 phylogenetic species to infect mice intranasally with low or high sublethal doses of
H. capsulatum yeast cells. They showed changes in clinical manifestations (weight loss), pathogenesis (lung inflammatory infiltrate), host response (cytokine production), and disease resolution, depending on the inoculum size, the virulence, and the
H. capsulatum phylogenetic group. Their results highlight that the lineage from Panama showed the highest virulence in mice exposed to a lower yeast cells inoculum, in contrast to the strains from the NAm 1 and NAm 2 groups. However, using the same experimental conditions, Jones et al. [
16] reported that the
Histoplasma strain from the NAm 2 phylogenetic group is mostly found within alveolar macrophages and produces progressive lung inflammation in infected mice.
Some findings observed during the present study should be discussed, such as the presence of intracellular
H. capsulatum yeast aggregates surrounding the infected macrophage nucleus, which started hours after the infection and persisted for several days postinfection. This yeast aggregate construction is compatible with previous observations reported by Pitangui et al. [
21] in cultured murine alveolar macrophages (AMJ2-C11 cell line) infected with
H. capsulatum yeast cells. These authors were the first to describe this intracellular conformational architecture of yeast cells in aggregation, and they proposed that this phenomenon may be related to injuries in the nuclei of the infected host cells, causing their DNA fragmentation and apoptosis. According to our results, this repetitive perinuclear arrangement of the
H. capsulatum Y-phase was primarily observed in macrophages of mice infected with the EH-46 strain. These yeast cell aggregates within lung macrophages emerged in mice inoculated with fungal M-phase propagules, and their presence was conspicuous after 3 h postinfection, corresponding to the time required to complete the M-to-Y transition under a simulated natural infection [
8]. Thus, this is the first report describing the
H. capsulatum Y-phase (parasitic phase) forming intracellular perinuclear organized yeast cell structures in macrophages of the pulmonary parenchyma of mice infected intranasally with the
H. capsulatum M-phase (infective phase).
In the human clinical course of histoplasmosis, the impact of fungal genetic diversity associated with
H. capsulatum strains from different phylogenetic species is still an emerging field to be explored in the study of this fungal disease. Reports by Damasceno et al. [
22,
23] referred to an unusual human coinfection with different
H. capsulatum genetic groups (Northeast BR1 and Northeast BR2), and they also showed differences in their mating types (MAT1-1 and MAT1-2) in the same AIDS–histoplasmosis patients. At this moment, no experimental information has explained the consequence of this type of coinfection; however, such coinfection can reasonably be speculated to interfere with the optimal host immune response and to trigger a more aggressive pathogenesis as well as a threatening outcome.