Arbuscular Mycorrhizal Fungi Alter Arsenic Translocation Characteristics of Iris tectorum Maxim.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Iris tectorum Maxim.
2.2. Mycorrhizal Colonization Analysis of Pre-Inoculated Seedlings
2.3. Short-Term As Uptake Kinetics of Iris tectorum Maxim.
2.4. As Efflux from Iris tectorum Maxim.
2.5. Time-Dependent As Accumulation Potential of Iris tectorum Maxim.
2.6. As Concentrations and As Speciation Measurement
2.7. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization Status between R. irregularis and I. tectorum
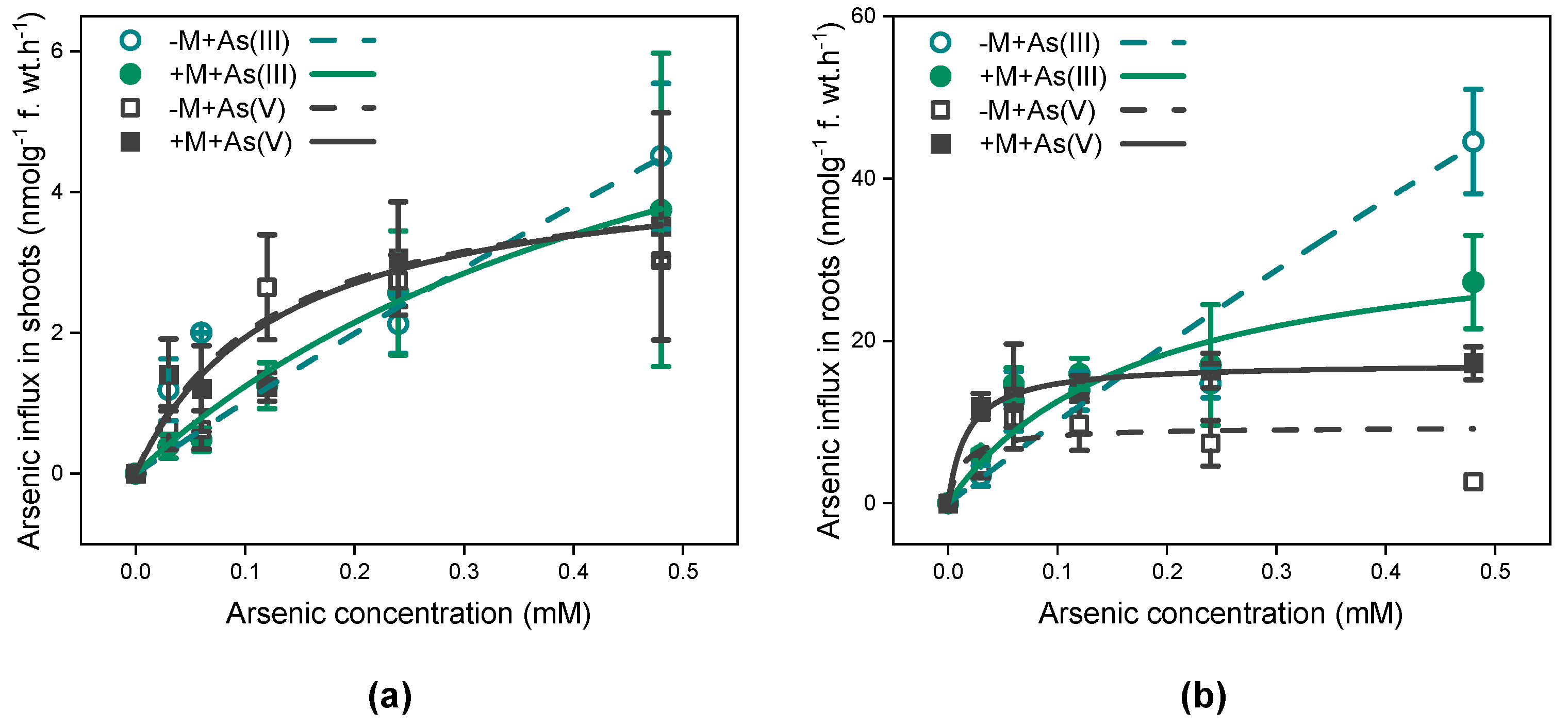
3.2. Short-Term Concentration-Dependent As Influx Kinetics in I. tectorum
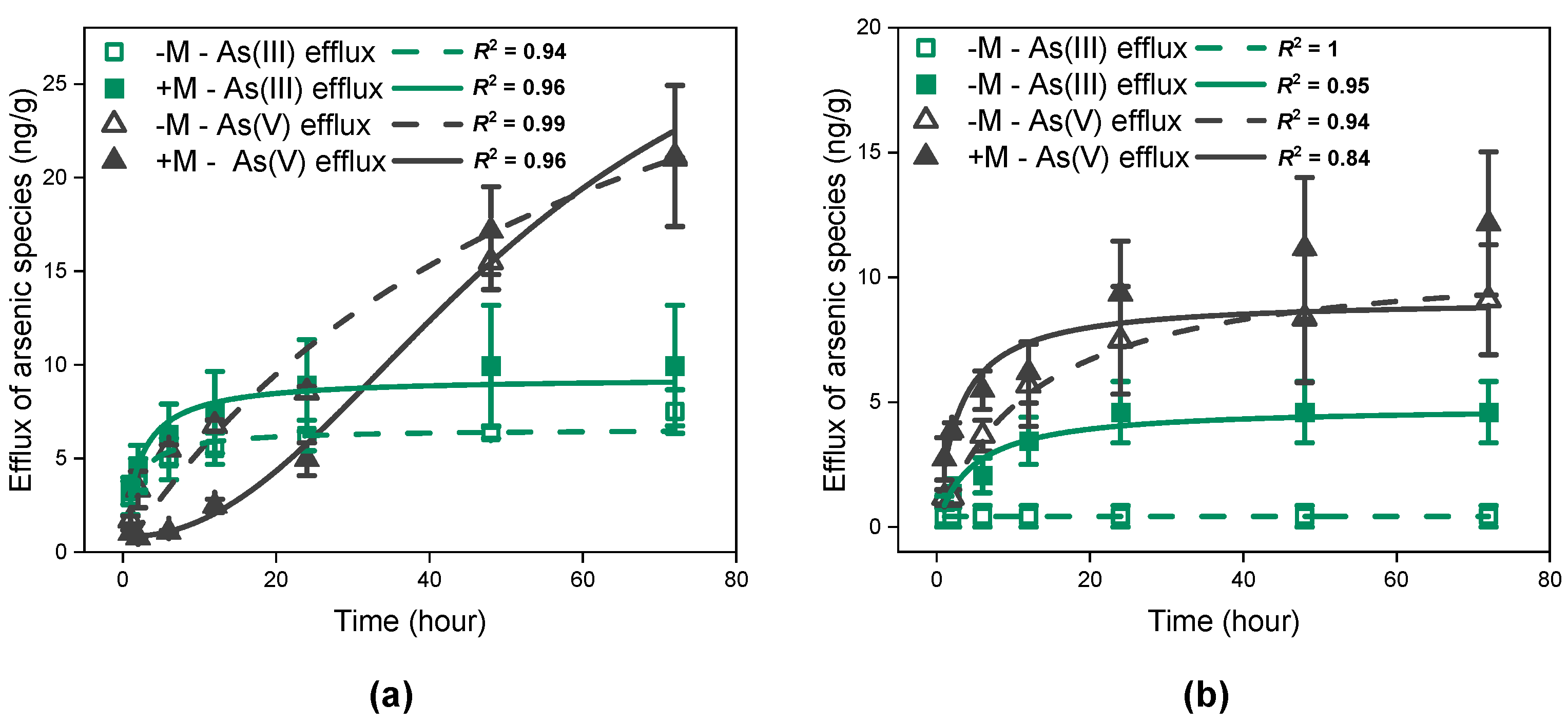
3.3. Arsenic Efflux from I. tectorum
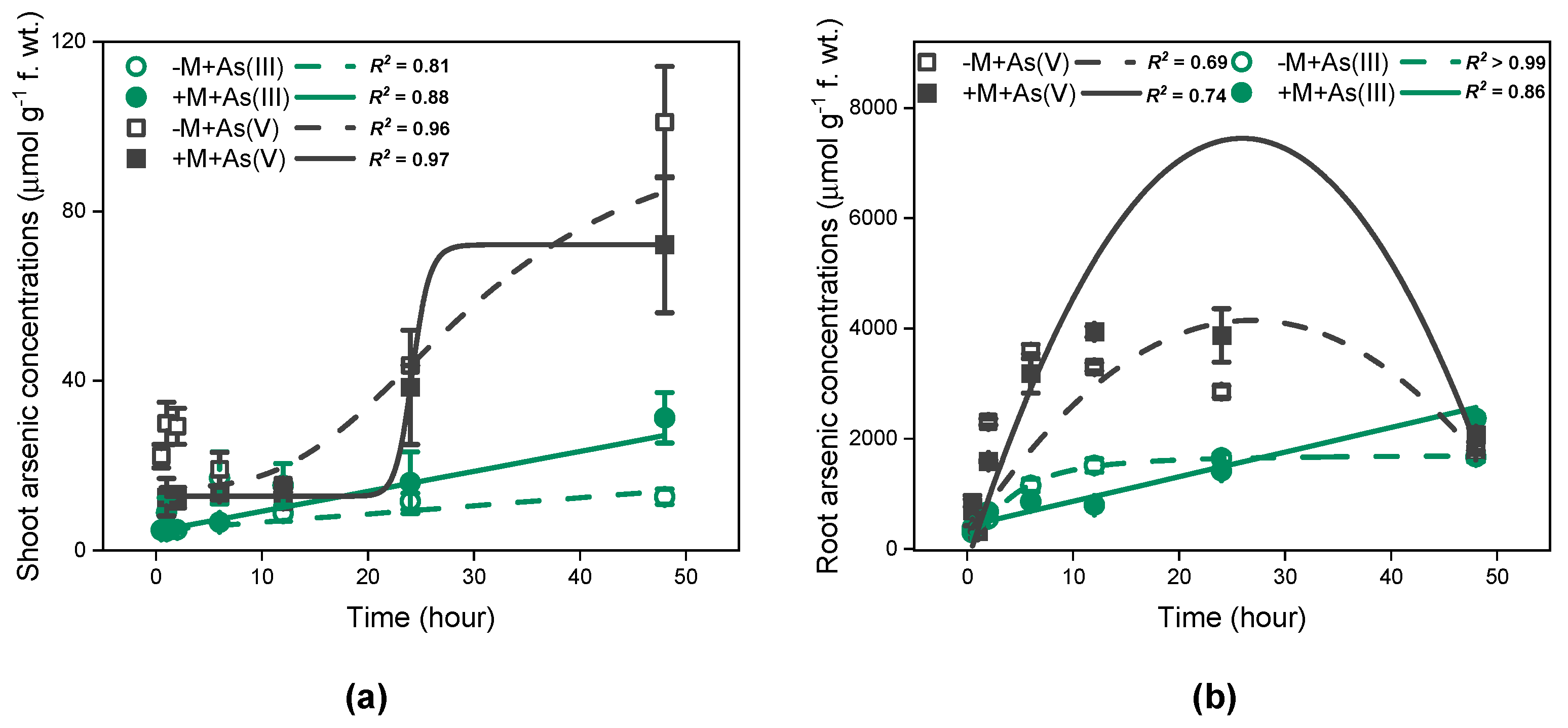
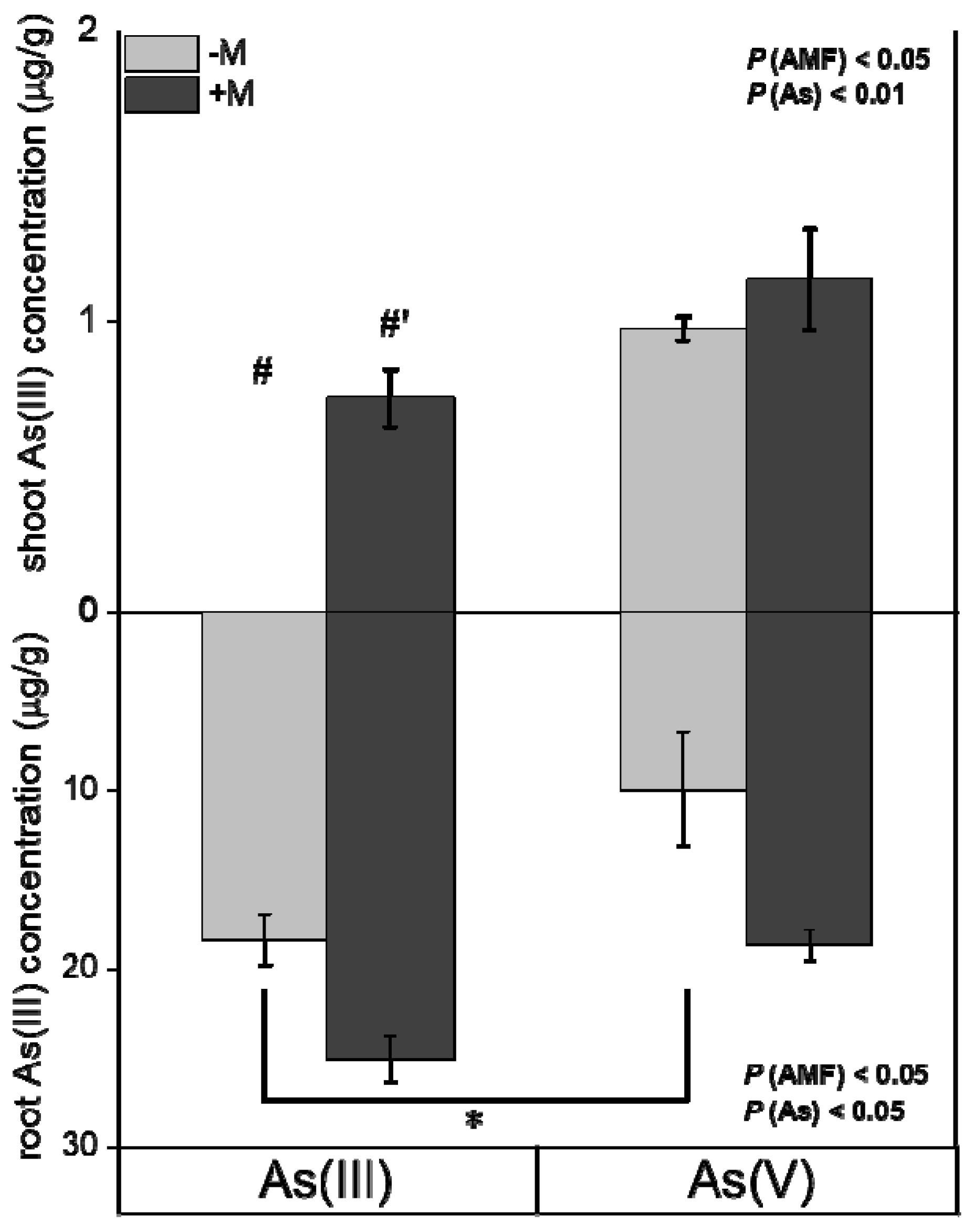
3.4. Time-Dependent Kinetics of As and As Speciation in I. tectorum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langner, P.; Mikutta, C.; Kretzschmar, R. Arsenic sequestration by organic sulphur in peat. Nat. Geosci. 2012, 5, 66–73. [Google Scholar]
- Li, X.W.; Yu, X.B.; Jiang, L.G.; Li, W.Y.; Liu, Y.; Hou, X.Y. How important are the wetlands in the middle-lower Yangtze River region: An ecosystem service valuation approach. Ecosyst. Serv. 2014, 10, 54–60. [Google Scholar]
- An, J.; Kim, J.Y.; Kim, K.W.; Park, J.Y.; Lee, J.S.; Jang, M. Natural attenuation of arsenic in the wetland system around abandoned mining area. Environ. Geochem. Health 2011, 33, 71–80. [Google Scholar]
- Wang, X.D.; Sun, Y.F.; Li, S.Y.; Wang, H.X. Spatial distribution and ecological risk assessment of heavy metals in soil from the Raoyanghe Wetland, China. PLoS ONE 2019, 14, e0220409. [Google Scholar]
- Craw, D.; Chappell, D.; Reay, A.; Walls, D. Mobilisation and attenuation of arsenic around gold mines, east Otago, New Zealand. N. Z. J. Geol. Geophys. 2000, 43, 373–383. [Google Scholar] [CrossRef]
- Simpson, S.; Sherriff, B.L.; Gulck, J.V.; Khozhina, E.; Londry, K.; Sidenko, N. Source, attenuation and potential mobility of arsenic at New Britannia mine, Snow Lake, Manitoba. Appl. Geochem. 2011, 26, 1843–1854. [Google Scholar]
- Carbonell, A.A.; Aarabi, M.A.; DeLaune, R.D.; Gambrell, R.P.; Patrick, W.H., Jr. Arsenic in wetland vegetation: Availability, phytotoxicity, uptake and effects on plant growth and nutrition. Sci. Total Environ. 1998, 217, 189–199. [Google Scholar] [CrossRef]
- Wang, J.J.; Bai, J.H.; Gao, Z.Q.; Lu, Q.Q.; Zhao, Q.Q. Soil As levels and bioaccumulation in Suaeda salsa and Phragmites australis wetlands of the Yellow River estuary, China. BioMed Res. Int. 2015, 2015, e301898. [Google Scholar]
- Munford, K.E.; Gilbert-Parkes, S.; Mykytczuk, N.C.S.; Basiliko, N.; Yakimovich, K.M.; Poulain, A.; Watmough, S.A. How arsenic contamination influences downslope wetland plant and microbial community structure and function. Sci. Total Environ. 2023, 876, 162839. [Google Scholar] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.L.M.; de Souza, A.F.; Hernández, A.H.; Elias, L.P.; Eismann, C.E.; Rezende-Filho, A.T.; Barbiero, L.; Menegario, A.A.; Fostier, A.H. In situ arsenic speciation at the soil/water interface of saline-alkaline lakes of the Pantanal, Brazil: A DGT-based approach. Sci. Total Environ. 2021, 804, 150113. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar]
- Jia, Y.; Huang, H.; Chen, Z.; Zhu, Y.G. Arsenic uptake by rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere. Environ. Sci. Technol. 2014, 48, 1001–1007. [Google Scholar]
- Chen, Y.S.; Sun, S.K.; Tang, Z.; Liu, G.; Moore, K.L.; Maathuis, F.J.M.; Miller, A.J.; McGrath, S.P.; Zhao, F.J. The Nodulin 26-like intrinsic membrane protein OsNIP3;2 is involved in arsenite uptake by lateral roots in rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [PubMed]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.Y.; Liu, X.; Sun, S.B.; Xu, G.H.; Liu, Y.G.; Chen, Y.S.; Ma, L.Q. Knocking out OsPT4 gene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [PubMed]
- Duan, G.L.; Zhou, Y.; Tong, Y.P.; Mukhopadhyay, R.; Rosen, B.P.; Zhu, Y.G. A CDC25 homologue from rice functions as an arsenate reductase. New Phytol. 2007, 174, 311–321. [Google Scholar] [CrossRef]
- Smith, S.E.; Christophersen, H.M.; Pope, S.; Smith, F.A. Arsenic uptake and toxicity in plants: Integrating mycorrhizal influences. Plant Soil 2010, 327, 1–21. [Google Scholar]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Lin, G.; Chen, B.D. Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 2015, 119, 224–230. [Google Scholar]
- Li, J.L.; Chen, B.D.; Zhang, X.; Hao, Z.P.; Zhang, X.; Zhu, Y.G. Arsenic transformation and volatilization by arbuscular mycorrhizal symbiosis under axenic conditions. J. Hazard. Mater. 2021, 413, 125390. [Google Scholar]
- Gonzalez-Chavez, C.; Harris, P.J.; Dodd, J.; Meharg, A.A. Arbuscular mycorrhizal fungi confer enhanced arsenate resistance on Holcus lanatus. New Phytol. 2002, 155, 163–171. [Google Scholar]
- Spagnoletti, F.N.; Balestrasse, K.; Lavado, R.S.; Giacometti, R. Arbuscular mycorrhiza detoxifying response against arsenic and pathogenic fungus in soybean. Ecotoxicol. Environ. Saf. 2016, 133, 47–56. [Google Scholar] [PubMed]
- Wang, Z.H.; Zhang, J.L.; Christie, P.; Li, X.L. Influence of inoculation with Glomus mosseae or Acaulospora morrowiae on arsenic uptake and translocation by maize. Plant Soil. 2008, 311, 235–244. [Google Scholar]
- Sun, D.; Zhang, X.; Liao, D.H.; Yan, S.; Feng, H.Y.; Tang, Y.T.; Cao, Y.; Qiu, R.L.; Ma, L.Q. Novel mycorrhiza-specific P transporter PvPht1;6 contributes to As accumulation at the symbiotic interface of As-hyperaccumulator Pteris vittate. Environ. Sci. Technol. 2022, 56, 14178–14187. [Google Scholar]
- Hu, S.S.; Chen, Z.B.; Vosátka, M.; Vymazal, J. Arbuscular mycorrhizal fungi colonization and physiological functions toward wetland plants under different water regimes. Sci. Total Environ. 2020, 716, 137040. [Google Scholar]
- Wang, X.Q.; Wang, Y.H.; Song, Y.B.; Dong, M. Formation and functions of arbuscular mycorrhizae in coastal wetland ecosystems: A review. Ecosyst. Health Sustain. 2022, 8, 2144465. [Google Scholar]
- Wu, F.Y.; Liu, X.P.; Wu, S.C.; Wong, M.H. Effects of mycorrhizal inoculation of upland rice on uptake kinetics of arsenate and arsenite. J. Plant Nutr. Soil Sci. 2015, 178, 333–338. [Google Scholar]
- Li, H.; Chen, X.W.; Wong, M.H. Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chemosphere 2016, 145, 224–230. [Google Scholar] [PubMed]
- Abedin, M.J.; Feldmann, J.; Meharg, A.A. Uptake kinetics of arsenic species in rice plants. Plant Physiol. 2002, 128, 1120–1128. [Google Scholar] [PubMed]
- Li, H.; Wu, C.; Ye, Z.H.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Uptake kinetics of different arsenic species in lowland and upland rice colonized with Glomus intraradices. J. Hazard. Mater. 2011, 194, 414–421. [Google Scholar]
- Zhang, J.; Liu, J.; Zheng, F.; Yu, M.; Shabala, S.; Song, W.Y. Comparative analysis of arsenic transport and tolerance mechanisms: Evolution from prokaryote to higher plants. Cells 2022, 11, 2741. [Google Scholar]
- Chan, W.F.; Li, W.C.; Wong, M.H. Uptake kinetics of arsenic in upland rice cultivar Zhonghan 221 inoculated with arbuscular mycorrhizal fungi. Int. J. Phytoremediation 2015, 17, 1073–1080. [Google Scholar]
- Naing, A.H.; Park, D.Y.; Park, H.C.; Kim, C.K. Removal of heavy metals using Iris species: A potential approach for reclamation of heavy metal-polluted sites and environmental beautification. Environ. Sci. Pollut. R. 2023, 30, 78004–78016. [Google Scholar]
- Leng, Y.L.; Lu, M.Y.; Li, F.L.; Yang, B.X.; Hu, Z.T. Citric acid-assisted phytoextraction of trace elements in composted municipal sludge by garden plants. Environ. Pollut. 2021, 288, 117699. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Chen, B.D.; Li, R.J. Rhizophagus irregularis influences As and P uptake by alfafa and the neighboring non-host pepperweed growing in an As-contaminated soil. J. Environ. Sci. 2018, 67, 36–44. [Google Scholar]
- Li, J.L.; Sun, Y.Q.; Jiang, X.L.; Chen, B.D.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [PubMed]
- Liu, C.W.; Chen, Y.Y.; Kao, Y.H.; Maji, S.K. Bioaccumulation and translocation of arsenic in the ecosystem of the Guandu wetland, Taiwan. Wetlands 2014, 34, 129–140. [Google Scholar]
- Tan, Q.Y.; Guo, Q.J.; Wei, R.F.; Zhu, G.X.; Du, C.J.; Hu, H.Y. Influence of arbuscular mycorrhizal fungi on bioaccumulation and bioavailability of As and Cd: A meta-analysis. Environ. Pollut. 2023, 316, 120619. [Google Scholar] [PubMed]
- Yu, Y.; Zhang, S.Z.; Huang, H.L.; Luo, L.; Wen, B. Arsenic accumulation and speciation in maize as affected by inoculation with arbuscular mycorrhizal fungus Glomus mosseae. J. Agric. Food Chem. 2009, 57, 3695–3701. [Google Scholar]
- González-Chávez, M.D.A.; Miller, B.; Maldonado-Mendoza, I.E.; Scheckel, K.; Carrillo-González, R. Arsenate induces the expression of fungal genes involved in As transport in arbuscular mycorrhiza. Fungal Biol. 2011, 115, 1197–1209. [Google Scholar]
- Xu, X.Y.; McGrath, S.P.; Zhao, F.J. Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol. 2007, 176, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ago, Y.; Mitani, N.; Li, R.Y.; Su, Y.H.; Yamaji, N.; McGrath, S.P.; Ma, J.F. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010, 186, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Isner, J.C.; Isayenkov, S.V.; Liu, W.J.; Zhao, F.J.; Maathuis, F.J.M. Heterologous expression of the yeast arsenite efflux system ACR3 improves Arabidopsis thaliana tolerance to arsenic stress. New Phytol. 2012, 194, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Liu, P.; Wang, K.; Yang, Z.G.; Wang, L. Influence of different arsenic species on uptake, speciation and efflux of arsenic in hydroponic rice plants. Ecotoxicol. Environ. Saf. 2019, 186, 109791. [Google Scholar]
- González-Chávez, M.D.A.; Miller, B.; Maldonado-Mendoza, I.E.; Scheckel, K.; Carrillo-González, R. Localization and speciation of arsenic in Glomus intraradices by synchrotron radiation spectroscopic analysis. Fungal Biol. 2014, 118, 444–452. [Google Scholar]
| Treatments | ||||
|---|---|---|---|---|
| As(III) | As(V) | |||
| −M | +M | −M | +M | |
| F% | 0 | 88.10 ± 2.01 | 0 | 88.55 ± 3.98 |
| M% | 0 | 19.94 ± 4.11 | 0 | 16.73 ± 4.79 |
| m% | 0 | 16.73 ± 4.79 | 0 | 12.22 ± 5.19 |
| a% | 0 | 50.09 ± 6.90 | 0 | 43.16 ± 3.02 |
| A% | 0 | 10.83 ± 3.19 | 0 | 6.20 ± 2.78 |
| Treatments | Vmax | Km | R2 | ||||
|---|---|---|---|---|---|---|---|
| nmol g−1 Fresh Weight | mM | ||||||
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| As(III) | −M | 42.74 ± 180.21 | 395.20 ± 1275.13 * | 4.09 ± 19.16 | 3.83 ± 13.80 | 0.91 | 0.93 |
| +M | 8.11 ± 1.11 | 34.61 ± 8.73 | 0.56 ± 0.13 | 0.18 ± 0.12 | 0.99 | 0.72 | |
| As(V) | −M | 4.44 ± 1.59 | 9.44 ± 1.75 * | 0.12 ± 0.10 | 0.01 ± 0.02 | 0.77 | 0.24 |
| +M | 4.50 ± 0.63 | 17.32 ± 0.63 | 0.13 ± 0.06 | 0.02 ± 0.00 | 0.90 | 0.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, S.; Zhang, K.; Hao, Z.; Zhang, X.; Chen, B. Arbuscular Mycorrhizal Fungi Alter Arsenic Translocation Characteristics of Iris tectorum Maxim. J. Fungi 2023, 9, 998. https://doi.org/10.3390/jof9100998
Xing S, Zhang K, Hao Z, Zhang X, Chen B. Arbuscular Mycorrhizal Fungi Alter Arsenic Translocation Characteristics of Iris tectorum Maxim. Journal of Fungi. 2023; 9(10):998. https://doi.org/10.3390/jof9100998
Chicago/Turabian StyleXing, Shuping, Kangxu Zhang, Zhipeng Hao, Xin Zhang, and Baodong Chen. 2023. "Arbuscular Mycorrhizal Fungi Alter Arsenic Translocation Characteristics of Iris tectorum Maxim." Journal of Fungi 9, no. 10: 998. https://doi.org/10.3390/jof9100998
APA StyleXing, S., Zhang, K., Hao, Z., Zhang, X., & Chen, B. (2023). Arbuscular Mycorrhizal Fungi Alter Arsenic Translocation Characteristics of Iris tectorum Maxim. Journal of Fungi, 9(10), 998. https://doi.org/10.3390/jof9100998








