Lichen Biodiversity and Near-Infrared Metabolomic Fingerprint as Diagnostic and Prognostic Complementary Tools for Biomonitoring: A Case Study in the Eastern Iberian Peninsula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomonitoring Network and Air Quality Monitoring Stations
2.2. Index of Atmospheric Purity
2.3. Lichen Damage Index (DI)
2.4. Statistical Analyses of IAP and DI
2.5. Acquisition of Visible–Near Infrared Spectra
2.6. Data Analysis of FT-NIRS Spectra
3. Results
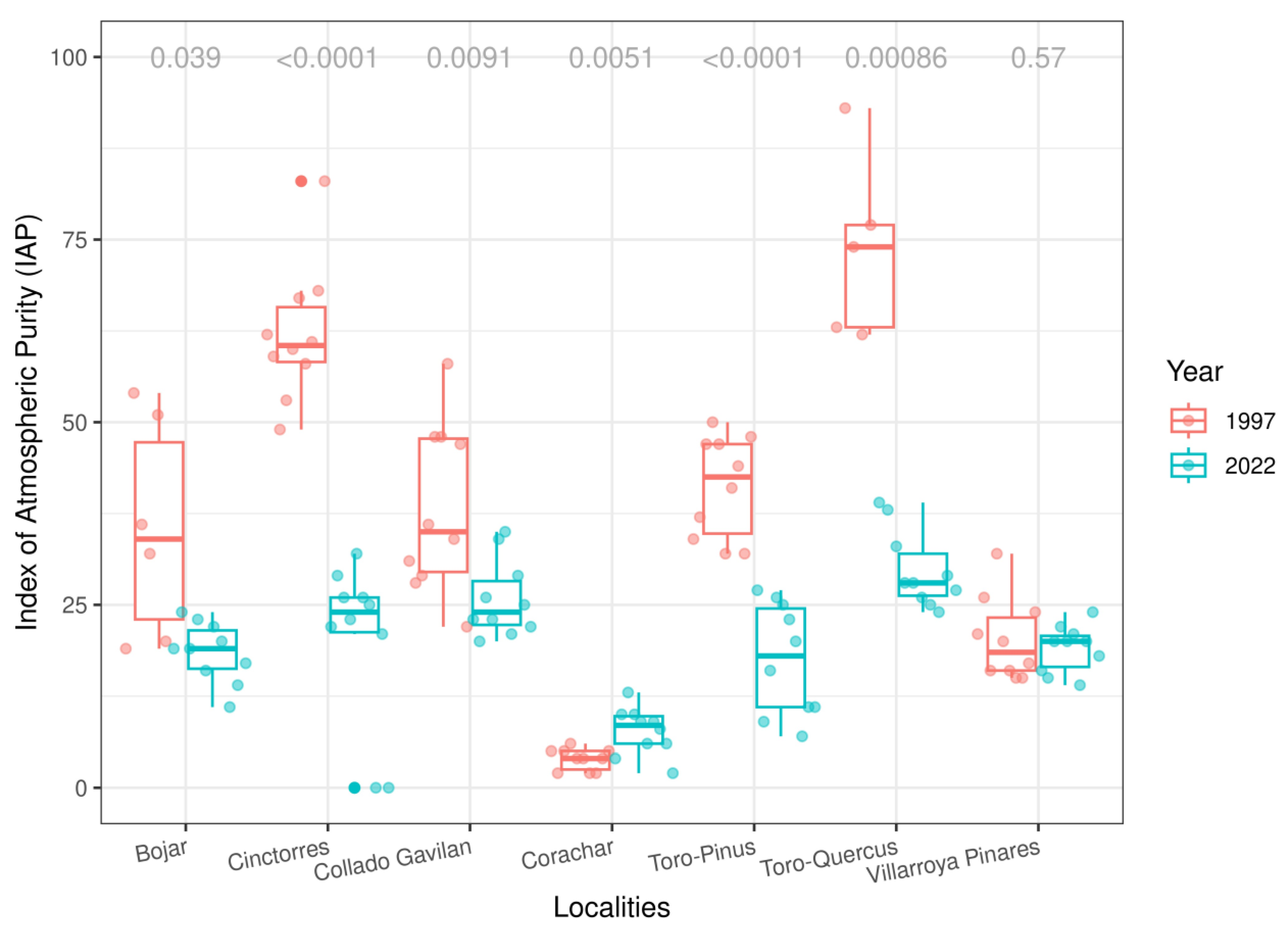
3.1. IAP
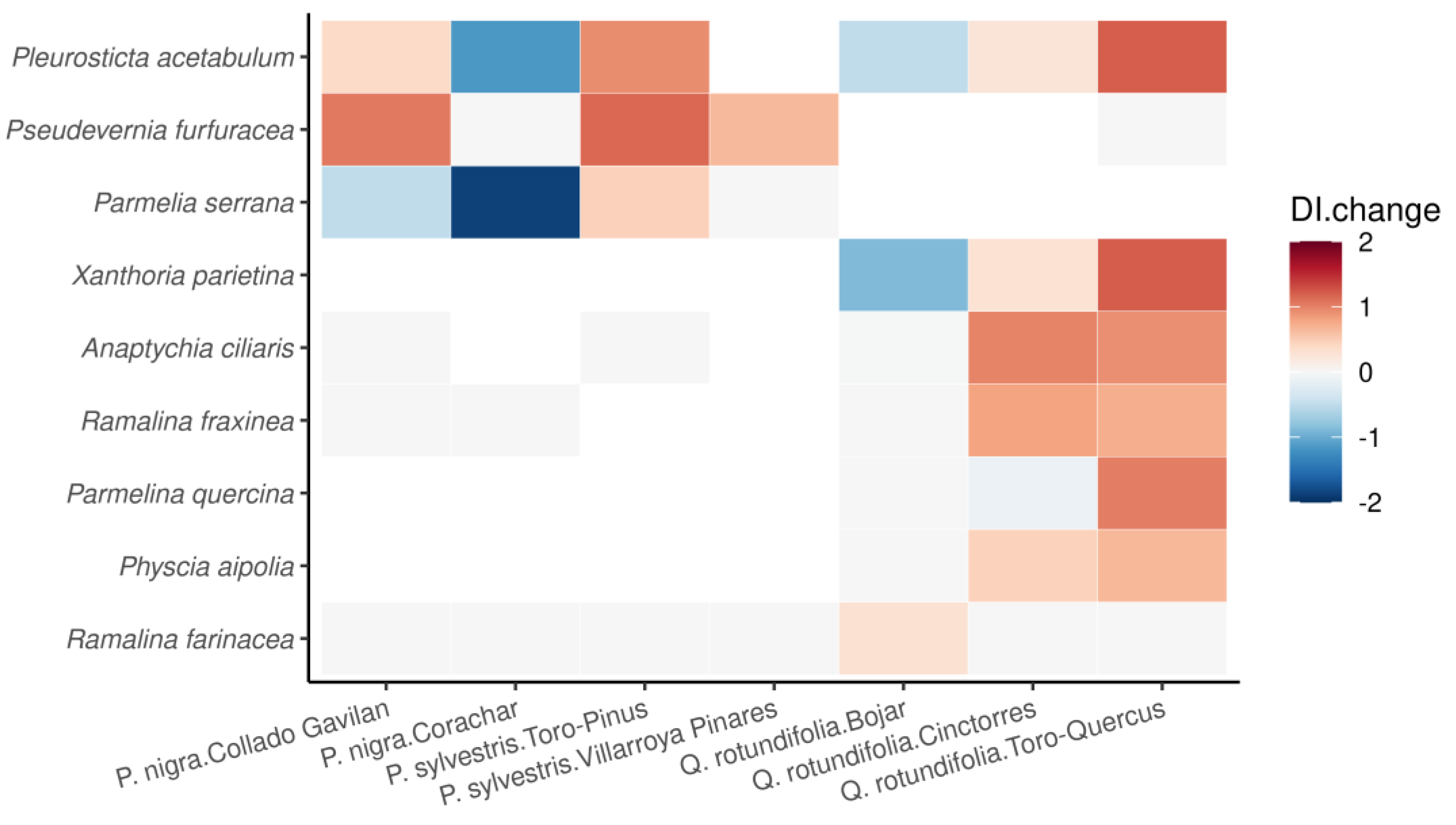
3.2. DI
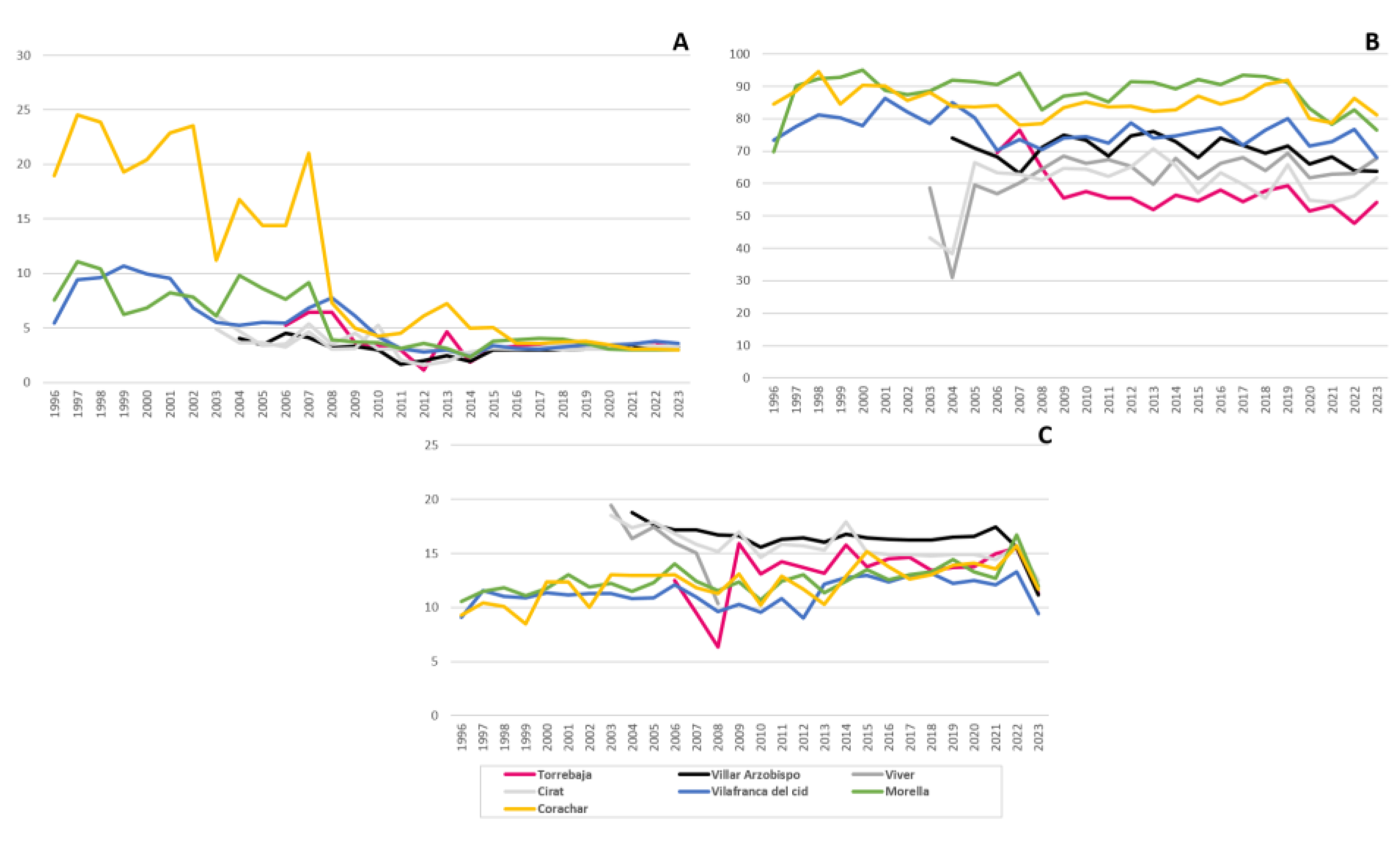
3.3. Air Quality Stations
3.4. Near-Infrared Aquaphotomics Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Honegger, R. The lichen thallus: A symbiotic phenotype of nutritionally specialized fungi and its response to gall producers. Syst. Assoc. Spec. Vol. 1994, 49, 77. [Google Scholar]
- Aschenbrenner, I.A.; Cardinale, M.; Berg, G.; Grube, M. Microbial Cargo: Do Bacteria on Symbiotic Propagules Reinforce the Microbiome of Lichens? Environ. Microbiol. 2014, 16, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Cernava, T.; Berg, G.; Grube, M. High Life Expectancy of Bacteria on Lichens. Microb. Ecol. 2016, 72, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Biosca, E.G.; Flores, R.; Santander, R.D.; Díez-Gil, J.L.; Barreno, E. Innovative approaches using lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLoS ONE 2016, 11, e0160328. [Google Scholar] [CrossRef]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 714. [Google Scholar] [CrossRef]
- Barreno, E. Life Is Symbiosis. In Once upon a Time Lynn Margulis: A Portrait of Lynn Margulis by Colleagues and Friends; Chica, C., Ed.; Septimus: Barcelona, Spain, 2013; pp. 56–60. [Google Scholar]
- Allen, J.L.; Lendemer, J.C. A call to reconceptualize lichen symbioses. Trends Ecol. Evol. 2022, 37, 582–589. [Google Scholar] [CrossRef]
- Honegger, R. The lichen symbiosis—What is so spectacular about it? Lichenologist 1998, 30, 193–212. [Google Scholar] [CrossRef]
- Margulis, L.; Barreno, E. Looking at lichens. Bioscience 2003, 53, 776. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi-species symbioses. Front. Microbiol. 2016, 7, 180. [Google Scholar] [CrossRef]
- Cernava, T.; Erlacher, A.; Aschenbrenner, I.A.; Krug, L.; Lassek, C.; Riedel, K.; Grube, M.; Berg, G. Deciphering functional diversification within the lichen microbiota by meta-omics. Microbiome 2017, 5, 82. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.H. Lichen Biology; Cambridge University Press: Leiden, UK, 2006; ISBN 978-0-511-41407-7. [Google Scholar]
- Giordani, P. Lichen diversity and biomonitoring: A special issue. Diversity 2019, 11, 171. [Google Scholar] [CrossRef]
- Frati, L.; Brunialti, G. Recent trends and future challenges for lichen biomonitoring in forests. Forests 2023, 14, 647. [Google Scholar] [CrossRef]
- Álvarez, R.; del Hoyo, A.; García-Breijo, F.; Reig-Armiñana, J.; del Campo, E.M.; Guéra, A.; Barreno, E.; Casano, L.M. Different strategies to achieve Pb-tolerance by the two Trebouxia algae coexisting in the lichen Ramalina farinacea. J. Plant. Physiol. 2012, 169, 1797–1806. [Google Scholar] [CrossRef]
- Van der Wat, L.; Forbes, P.B.C. Lichens as biomonitors for organic air pollutants. Trends Analyt. Chem. 2015, 64, 165–172. [Google Scholar] [CrossRef]
- Cecconi, E.; Fortuna, L.; Benesperi, R.; Bianchi, E.; Brunialti, G.; Contardo, T.; Di Nuzzo, L.; Frati, L.; Monaci, F.; Munzi, S.; et al. New interpretative scales for lichen bioaccumulation data: The Italian proposal. Atmosphere 2019, 10, 136. [Google Scholar] [CrossRef]
- Catalá, M.; Gasulla, F.; Del Real, A.P.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. The organic air pollutant cumene hydroperoxide interferes with NO antioxidant role in rehydrating lichen. Environ. Pollut. 2013, 179, 277–284. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Bioindication: Calibrated scales and their utility. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; NATO Science Series; Springer: Dordrecht, The Netherlands, 2002; Volume 7, pp. 11–20. [Google Scholar]
- Giordani, P.; Calatayud, V.; Stofer, S.; Seidling, W.; Granke, O.; Fischer, R. Detecting the nitrogen critical loads on European forests by means of epiphytic lichens. A signal-to-noise evaluation. For. Ecol. Manage. 2014, 311, 29–40. [Google Scholar] [CrossRef]
- Root, H.T.; Jovan, S.E.; Fenn, M.; Amacher, M.; Hall, J.; Shaw, J.D. Lichen bioindicators of nitrogen and sulfur deposition in dry forests of Utah and New Mexico, USA. Ecol. Indic. 2021, 127, 107727. [Google Scholar] [CrossRef]
- Barreno, E.; Manzanera, A. Los líquenes, grandes conquistadores de medios extremos que sucumben a los cambios ambientales. In La Biodiversidad Valenciana Ante el Reto del Cambio Global, Chapter 8; Barba, E., Ed.; Publicacions de la Universitat de València: Valencia, Spain, 2023; pp. 245–294. [Google Scholar]
- Ellis, C.J. Climate change, bioclimatic models and the risk to lichen diversity. Diversity 2019, 11, 54. [Google Scholar] [CrossRef]
- Nash, T.H. Lichen sensitivity to air pollution. In Lichen Biology, 2nd ed.; Nash, T.H., Ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Loppi, S. Lichens as sentinels for air pollution at remote alpine areas (Italy). Environ. Sci. Pollut. R. 2014, 21, 2563–2571. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Munzi, S.; Alonso, R.; Arróniz-Crespo, M.; Avila, A.; Bermejo, V.; Bobbink, R.; Branquinho, C.; Concostrina-Zubiri, L.; Cruz, C.; et al. Ecological impacts of atmospheric pollution and interactions with climate change in terrestrial ecosystems of the Mediterranean Basin: Current research and future directions. Environ. Pollut. 2017, 227, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McMullin, R.T.; Tripp, E.A.; Lendermen, J.C. Lichen conservation in North America: A review of current practices and research in Canada and the United States. Biodivers. Conserv. 2019, 28, 3103–3138. [Google Scholar]
- Mayer, A.L.; Vihermaa, L.; Nieminen, N.; Luomi, A.; Posch, M. Epiphytic macrolichen community correlates with modeled air pollutants and forest conditions. Ecol. Indic. 2009, 9, 992–1000. [Google Scholar] [CrossRef]
- Sancho, L.G.; Pintado, A.; Green, T.A. Antarctic studies show lichens to be excellent biomonitors of climate change. Diversity 2019, 11, 42. [Google Scholar] [CrossRef]
- Santos, A.M.D.; Vitorino, L.C.; Cruvinel, B.G.; Ávila, R.G.; Vasconcelos Filho, S.D.C.; Batista, P.F.; Bessa, L.A. Impacts of Cd pollution on the vitality, anatomy and physiology of two morphologically different lichen species of the genera Parmotrema and Usnea, evaluated under experimental conditions. Diversity 2022, 14, 926. [Google Scholar] [CrossRef]
- Calatayud, A.; Sanz, M.J.; Calvo, E.; Barreno, E.; del Valle-Tascon, S. Chlorophyll a fluorescence and chlorophyll content in Parmelia quercina thalli from a polluted region of northern Castellon (Spain). Lichenologist 1996, 28, 49–65. [Google Scholar] [CrossRef]
- Palharini, K.M.Z.; Vitorino, L.C.; Bessa, L.A.; Vasconcelos Filho, S.D.C.; Silva, F.G. Parmotrema tinctorum as an indicator of edge effect and air quality in forested areas bordered by intensive agriculture. Environ. Sci. Pollut. R. 2021, 28, 68997–69011. [Google Scholar] [CrossRef]
- Boonpragob, K.; Nash III, T.H. Physiological responses of the lichen Ramalina Menziessi Tayl. to the Los Angeles urban environment. Environ. Exp. Bot. 1990, 31, 229–238. [Google Scholar] [CrossRef]
- Tarhanen, S.; Holopainen, T.; Poikolainen, J.; Oksanen, J. Effect of industrial emissions on membrane permeability of epiphytic lichen in Northern Finland and the Kola Peninsula industrial areas. Water Air Soil Poll. 1996, 88, 189–201. [Google Scholar] [CrossRef]
- Arhoun, M.; Barreno, E.; Ramis-Ramos, G. Releasing rates of inorganic ions in lichen monitored by capillary zone electrophoresis as indicators of atmospheric pollution. Cryptog. Mycol. 2000, 21, 275–289. [Google Scholar] [CrossRef]
- Meyer, A.R.; Valentin, M.; Liulevicius, L.; McDonald, T.R.; Nelsen, M.P.; Pengra, J.; Smith, R.J.; Stanton, D. Climate warming causes photobiont degradation and carbon starvation in a boreal climate sentinel lichen. Am. J. Bot. 2023, 110, e16114. [Google Scholar] [CrossRef] [PubMed]
- Barreno, E.; Fos, S.; Calatayud, A. Seguimiento de la Calidad Atmosférica en la Comarca de Els Ports y Maestrazgo Mediante Bioindicadores Vegetales. Informe Final 1994–1997; Universitat de València: Valencia, Spain; Empresa Nacional de Electricidad (ENDESA): Madrid, Spain; Fundación Universidad Empresa (ADEIT): Valencia, Spain, 1998. [Google Scholar]
- Gasulla, F.; Herrero, J.; Esteban-Carrasco, A.; Ros-Barceló, A.; Barreno, E.; Zapata, J.M.; Guéra, A. Photosynthesis in Lichen: Light Reactions and Protective Mechanisms; InTech: Rijeka, Croatia, 2011; pp. 149–174. [Google Scholar]
- Gasulla, F.; Del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in understanding of desiccation tolerance of lichens and lichen-forming algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Belnap, J.; Harper, K.T. Effects of a coal-fi- red power plant on the rock lichen Rhizoplaca melanophthalma: Chlorophyll degradation and electrolyte leakage. Bryologist 1990, 93, 309–312. [Google Scholar] [CrossRef]
- Insarova, I.D.; Insarov, G.E.; Brakenhielms, S.; Hultengen, S.; Martinsson, P.Q.; Semenov, S.M. Lichen Sensitivity and Air Pollution. A Review of Literature Data. Swedish Environmental Protection Agency Report 4007; Swedish Environmental Protection Agency: Uppsala, Sweden, 1992. [Google Scholar]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P. Monitoring with Lichens-Monitoring Lichens. An Introduction. Monitoring with Lichens.—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academics: Dordrecht, The Netherlands, 2002; Volume 7, pp. 1–4. [Google Scholar]
- Leavitt, S.D.; St. Clair, L.L. Bio-monitoring in Western North America: What Can Lichens Tell Us About Ecological Disturbances? In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Upreti, D., Divakar, P., Shukla, V., Bajpai, R., Eds.; Springer: New Dheli, India, 2015; pp. 119–138. [Google Scholar]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Ind. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G. Sampling and interpreting lichen diversity data for biomonitoring purposes. In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Upreti, D., Divakar, P., Shukla, V., Bajpai, R., Eds.; Springer: New Dheli, India, 2015; pp. 19–46. [Google Scholar]
- Stofer, S.; Calatayud, V.; Giordani, P.; Neville, P. Part VII.2: Assessment of epiphytic lichen diversity. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016. [Google Scholar]
- García-Marzá, D.; Sarria, C.F.; Esteban, E.G. Across-the-border contamination, the Andorra power plant (Teruel): A business ethics case. J. Bus. Ethics 1999, 22, 261–271. [Google Scholar] [CrossRef]
- ENDESA. Gestión Ambiental de Endesa. In Razón y Controversia Sobre la Central Térmica Teruel, en Andorra; Martínez, C., Ed.; Fundación ENDESA: Madrid, Spain, 1994; pp. 167–172. [Google Scholar]
- Palau, J.L.; Krupa, S.V.; Calatayud, V.; Sanz, M.; Millán, M. Relating Source-Specific Atmospheric Sulfur Dioxide Inputs to Ecological Effects Assessment in a Complex Terrain. Dev. Environ. Sci. 2009, 9, 99–120. [Google Scholar]
- Ammann, K.; Herzig, R.; Liebendoerfer, L.; Urech, M. Multivariate correlation of deposition data of 8 different air pollutants to lichen data in a small town in Switzerland. In Advances in Aerobiology; Boehm, G., Leuschner, R.M., Eds.; Birkhäuser: Basel, Switzerland, 1987; Volume 51, pp. 401–406. [Google Scholar]
- Kricke, R.; Loppi, S. Bioindication: The I.A.P. approach. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; Volume 7, pp. 21–37. [Google Scholar]
- Das, P. Lichen-based index of atmospheric purity (IAP) for biomonitoring of air. In New Paradigms in Environmental Biomonitoring Using Plants; Tiwari, S., Agrawal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–26. ISBN 9780128243510. [Google Scholar]
- Muñoz, M.M.; Sanz, M.J.; Giménez, A.C.; Iglesias, E.M. Calidad del aire en la Comunidad Valenciana: Comarcas de Els Ports-Maestrat. In Ética y Ecología. La gestión Empresarial del Medio Ambiente; Servei de Comunicació i Publicacions: Valencia, Spain, 1999; pp. 67–103. [Google Scholar]
- Millán, M.; Sanz, M.; Calatayud, V.; Palau, J.; Diéguez, J.; Landa, G.P.; Mantilla, E.; Cerveró, J.; Chordá, J. La Calidad del Aire en las Comarcasde ElsPorts-Maestrat; Fundación CEAM: Paterna, Spain, 2004. [Google Scholar]
- Nimis, P.L.; Castello, M.; Perotti, M. Lichens as biomonitors of sulphur dioxide pollution in La Spezia (northen Italy). Lichenologist 1990, 22, 333–344. [Google Scholar] [CrossRef]
- Millán, M.M.; Sanz, M.J. La Contaminación Atmosférica en la Comunidad Valenciana: Estado de Conocimientos Sobre los Problemas en el Maestrazgo y Els Ports de Castellón. Informes CEAM93-1; Centro de Estudios Ambientales del Mediterráneo: Paterna, Spain, 1993. [Google Scholar]
- Millán, M.M.; Salvador, R.; Mantilla, E. Photooxidant dynamics in the Mediterranean basin in summer: Results from European research projects. J. Geoph. Res. 1997, 102, 8811–8823. [Google Scholar] [CrossRef]
- Fernández-Salegui, A.B.; Terrón, A.; Fos, S.; Barreno, E. Síntomas de daños por contaminantes atmosféricos en Parmelia sulcata Tayl. en la zona de La Robla (León, España). Lazaroa 2003, 23, 7–16. [Google Scholar]
- Fernández-Salegui, A.B.; Terrón, A.; Barreno, E. Bioindicadores de la calidad del aire en La Robla (León, noroeste de España) diez años después. Lazaroa 2006, 27, 29–41. [Google Scholar]
- Fernández-Salegui, A.B.; Terrón, A.; Barreno, E.; Nimis, P.L. Biomonitoring with cryptogams near the power station of La Robla (León, Spain). Bryologist 2009, 110, 723–737. [Google Scholar] [CrossRef]
- Barreno, E.; Atienza, V.; Sanz, M.J. Catálogo de líquenes epifitos y terrícolas de la Font Roja (Alicante, España). Cienc. Nat. 1990, 1, 85–99. [Google Scholar]
- Calvo, E.; Sanz, M.J. Líquenes como bioindicadores de la calidad ambiental en el Parque Natural de La Font Roja (Alicante, España). Ecologiía 2000, 14, 103–111. [Google Scholar]
- Sánchez-Bañares, A. Evaluación de Efectos de Contaminantes en la Sª de Espadán, Usando los Líquenes Como Bioindicadores; Universidad Politécnica de Valencia: Valencia, Spain, 2011. [Google Scholar]
- Heras, R.D.L.; Rodríguez-Gil, J.L.; Sauto, J.S.S.; Sánchez, P.S.; Catalá, M. Analysis of lipid peroxidation in animal and plant tissues as field-based biomarker in Mediterranean irrigated agroecosystems (Extremadura, Spain). J. Environ. Sci. Health 2018, 53, 567–579. [Google Scholar] [CrossRef]
- Casale, M.; Bagnasco, L.; Giordani, P.; Mariotti, M.G.; Malaspina, P. NIR Spectroscopy as a Tool for Discriminating between Lichens Exposed to Air Pollution. Chemosphere 2015, 134, 355–360. [Google Scholar] [CrossRef]
- Malaspina, P.; Casale, M.; Malegori, C.; Hooshyari, M.; Di Carro, M.; Magi, E.; Giordani, P. Combining spectroscopic techniques and chemometrics for the interpretation of lichen biomonitoring of air pollution. Chemosphere 2018, 198, 417–424. [Google Scholar] [CrossRef]
- Dufour, É. Principles of Infrared Spectroscopy. In Infrared Spectroscopy for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–27. ISBN 9780123741363. [Google Scholar]
- Pupeza, I.; Huber, M.; Trubetskov, M.; Schweinberger, W.; Hussain, S.A.; Hofer, C.; Fritsch, K.; Poetzlberger, M.; Vamos, L.; Fill, E.; et al. Field-Resolved Infrared Spectroscopy of Biological Systems. Nature 2020, 577, 52–59. [Google Scholar] [CrossRef]
- Bruñas Gómez, I.; Casale, M.; Barreno, E.; Catalá, M. Near-Infrared Metabolomic Fingerprinting Study of Lichen Thalli and Phycobionts in Culture: Aquaphotomics of Trebouxia Lynnae Dehydration. Microorganisms 2022, 10, 2444. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; Volume 12, pp. 29–80. [Google Scholar]
- LeBlanc, F.; De Sloover, J. Relation between industrialization and the distribution and growth of epiphytic lichens and mosses in Montreal. Can. Jo. Bot. 1970, 48, 1485–1496. [Google Scholar] [CrossRef]
- Nimis, P.L.; Lazzarin, G.; Gasparo, D. Lichens as bioindicators of air pollution by SO2 in the Veneto region NE Italy. Stud. Geobot. 1991, 11, 3–76. [Google Scholar]
- Kranner, I.; Beckett, R.; Varma, A. Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Nimis, P.L. ITALIC—The Information System on Italian Lichens, Version 7.0; Department of Biology, University of Trieste: Trieste, Italy, 2023; Available online: https://italic.units.it/ (accessed on 17 September 2023).
- Nascimbene, J.; Nimis, P.L.; Marini, L. Testing indicators of epiphytic lichen diversity: A case study in N Italy. Biodivers. Conserv. 2007, 16, 3377–3383. [Google Scholar] [CrossRef]
- Sigal, L.; Nash III, T.H. Lichen communities on conifers in Southern California mountains: An ecological survey relative to oxidant air pollution. Ecology 1983, 64, 1343–1354. [Google Scholar] [CrossRef]
- Nash III, T.H.; Sigal, L.L. Epiphytic lichens in the San Bernardino mountains in relation to oxidant gradients. In Oxidant Air Pollution Impacts in the Montane Forests of Southern California: A Case Study of the San Bernardino Mountains; Springer: Berlin/Heidelberg, Germany, 1999; pp. 223–234. [Google Scholar]
- Miller, P.R.; Stolte, K.W.; Duriscoe, D.M.; Pronos, J. Evaluating Ozone Air Pollution Effects on Pines in the Western United States; General Technical Report PSW-GTR-155; Pacific Southwest Research Station: Albany, CA, USA; Forest Service, U.S. Department of Agriculture: Washington, DC, USA, 1996. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 September 2023).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 1 September 2023).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 1 September 2023).
- Wickham, H.; Vaughan, D.; Girlich, M. Tidyr: Tidy Messy Data. 2023. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 1 September 2023).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2023. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 1 September 2023).
- Aphalo, P.J. Ggpmisc: Miscellaneous Extensions to Ggplot2. 2022. Available online: https://CRAN.R-project.org/package=ggpmisc (accessed on 1 September 2023).
- Patil, I. Ggstatsplot: Ggplot2 Based Plots with Statistical Details. 2023. Available online: https://CRAN.R-project.org/package=ggstatsplot (accessed on 1 September 2023).
- Kindt, R. BiodiversityR: Package for Community Ecology and Suitability Analysis. 2023. Available online: http://www.worldagroforestry.org/output/tree-diversity-analysis (accessed on 1 September 2023).
- Slowikowski, K. Ggrepel: Automatically Position Non-Overlapping Text Labels with Ggplot2. 2023. Available online: https://github.com/slowkow/ggrepel (accessed on 1 September 2023).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. 2022. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 1 September 2023).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. 2023. Available online: https://CRAN.R-project.org/package=readxl (accessed on 1 September 2023).
- Xie, Y. Knitr: A General-Purpose Package for Dynamic Report Generation in R. 2023. Available online: https://yihui.org/knitr/ (accessed on 1 September 2023).
- Allen, J.L. Lichen Conservation in Eastern North America: Population Genomics, Climate Change, and Translocations; City University of New York: New York, NY, USA, 2017. [Google Scholar]
- Matos, P.; Geiser, L.; Hardman, A.; Glavich, D.; Pinho, P.; Nunes, A.; Soares, A.M.; Branquinho, C. Tracking global change using lichen diversity: Towards a global-scale ecological indicator. Met. Ecol. Evol. 2017, 8, 788–798. [Google Scholar] [CrossRef]
- Hurtado, P.; Prieto, M.; de Bello, F.; Aragón, G.; López-Angulo, J.; Giordani, P.; Díaz-Peña, E.M.; Vicente, R.; Merinero, S.; Košuthová, A.; et al. Contrasting environmental drivers determine biodiversity patterns in epiphytic lichen communities along a European gradient. Microorganisms 2020, 8, 1913. [Google Scholar] [CrossRef] [PubMed]
- Aptroot, A.; Stapper, N.J.; Kosuthovaá, A.; van Herk, K. Lichens as an indicator of climate and global change. In Climate Change: Observed Impacts on Planet Earth; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 483–497. [Google Scholar]
- Gibson, M.D.; Heal, M.R.; Li, Z.; Kuchta, J.; King, G.H.; Hayes, A.; Lambert, S. The spatial and seasonal variation of nitrogen dioxide and sulfur dioxide in Cape Breton Highlands National Park, Canada, and the association with lichen abundance. Atmos. Environ. 2013, 64, 303–311. [Google Scholar] [CrossRef]
- Morillas, L.; Roales, J.; Cruz, C.; Munzi, S. Resilience of epiphytic lichens to combined effects of increasing nitrogen and solar radiation. J. Fungi 2021, 7, 333. [Google Scholar] [CrossRef]
- Geiser, L.H.; Root, H.T.; Smith, R.J.; Jovan, S.E.; St Clair, L.; Dillman, K.L. Lichen-based critical loads for deposition of nitrogen and sulfur in US forests. Environ. Pollut. 2021, 291, 118187. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Jaroszewicz, B.; Czortek, P. Shifts in lichen species and functional diversity in a primeval forest ecosystem as a response to environmental changes. Forests 2021, 12, 686. [Google Scholar] [CrossRef]
- Geiser, L.H.; Neitlich, P.N. Air pollution and climate gradients in western Oregon and Washington indicated by epiphytic macrolichens. Environ. Pollut. 2007, 145, 203–218. [Google Scholar] [CrossRef]
- Fenn, M.E.; Jovan, S.; Yuan, F.; Geiser, L.; Meixner, T.; Gimeno, B.S. Empirical and simulated critical loads for nitrogen deposition in California mixed conifer forests. Environ. Pollut. 2008, 155, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Pinho, P.; Augusto, S.; Martins-Loucāo, M.; Pereira, M.; Soares, A.; Máguas, C.; Branquinho, C. Causes of change in nitrophytic and oligotrophic lichen species in a Mediterranean climate: Impact of land cover and atmospheric pollutants. Environ. Pollut. 2008, 154, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Pinho, P.; Branquinho, C.; Cruz, C.; Tang, Y.S.; Dias, T.; Rosa, A.P.; Máguas, C.; Martins-Loução, M.-A.; Sutton, M.A. Assessment of critical levels of atmospheric ammonia for lichen diversity in cork-oak woodland, Portugal. Atmos. Ammon. 2009, 10, 109–119. [Google Scholar]
- Geiser, L.H.; Jovan, S.E.; Glavich, D.A.; Porter, M.K. Lichen-based critical loads for atmospheric nitrogen deposition in Western Oregon and Washington Forests, USA. Environ. Pollut. 2010, 158, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- SFS 5670; Ilmansuojelu. Bioindikaatio. Jäkäläkartoitus. Finnish Standards Association: Helsinki, Finland, 1990.
- Ribas, À.; Peñuelas, J. Temporal patterns of surface ozone levels in different habitats of the North Western Mediterranean basin. Atm. Environ. 2004, 38, 985–992. [Google Scholar] [CrossRef]
- Waldner, P.; Marchetto, A.; Thimonier, A.; Schmitt, M.; Rogora, M.; Granke, O.; Mues, V.; Hansen, K.; Karlsson, G.P.; Žlindra, D.; et al. Detection of temporal trends in atmospheric deposition of inorganic nitrogen and sulphate to forests in Europe. Atmos. Environ. 2014, 95, 363–374. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of Aquaphotomics and Its Chemometrics Approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef]
- Munćan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef]
- Malegori, C.; Muncan, J.; Mustorgi, E.; Tsenkova, R.; Oliveri, P. Analysing the water spectral pattern by near-infrared spectroscopy and chemometrics as a dynamic multidimensional biomarker in preservation: Rice germ storage monitoring. Spectrochimica 2022, 265, 120396. [Google Scholar] [CrossRef]






| PLSDA | CLASS | LVs | Sn (CV) | Sp (CV) | Er (CV) | Sn (Pred) | Sp (Pred) | Er (Pred) |
|---|---|---|---|---|---|---|---|---|
| Reproductive structures | Normal | 3 | 0.722 | 0.903 | 0.187 | 0.429 | 0.886 | 0.342 |
| Excessive | 0.903 | 0.722 | 0.187 | 0.886 | 0.429 | 0.342 | ||
| Growth form | Foliose | 7 | 0.916 | 0.933 | 0.075 | 0.941 | 0.750 | 0.154 |
| Fruticose | 0.933 | 0.916 | 0.075 | 0.750 | 0.941 | 0.154 | ||
| Reproduction strategy | Apotecia | 11 | 0.862 | 0.740 | 0.198 | 0.829 | 0.783 | 0.194 |
| Isidia | 0.865 | 0.920 | 0.107 | 0.737 | 0.821 | 0.221 | ||
| Soredia | 0.846 | 0.879 | 0.137 | 0.750 | 0.815 | 0.217 | ||
| Phorophyte | Pinus | 10 | 0.855 | 0.866 | 0.1398 | 0.677 | 0.889 | 0.216 |
| Quercus | 0.866 | 0.855 | 0.1398 | 0.889 | 0.677 | 0.216 | ||
| Bioclimatic belt | 22a | 8 | 0.911 | 0.902 | 0.093 | 0.721 | 0.933 | 0.172 |
| 19c | 0.902 | 0.911 | 0.093 | 0.933 | 0.721 | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, P.; Chiva, S.; Catalá, M.; Garmendia, A.; Casale, M.; Gomez, J.; Pazos, T.; Giordani, P.; Calatayud, V.; Barreno, E. Lichen Biodiversity and Near-Infrared Metabolomic Fingerprint as Diagnostic and Prognostic Complementary Tools for Biomonitoring: A Case Study in the Eastern Iberian Peninsula. J. Fungi 2023, 9, 1064. https://doi.org/10.3390/jof9111064
Moya P, Chiva S, Catalá M, Garmendia A, Casale M, Gomez J, Pazos T, Giordani P, Calatayud V, Barreno E. Lichen Biodiversity and Near-Infrared Metabolomic Fingerprint as Diagnostic and Prognostic Complementary Tools for Biomonitoring: A Case Study in the Eastern Iberian Peninsula. Journal of Fungi. 2023; 9(11):1064. https://doi.org/10.3390/jof9111064
Chicago/Turabian StyleMoya, Patricia, Salvador Chiva, Myriam Catalá, Alfonso Garmendia, Monica Casale, Jose Gomez, Tamara Pazos, Paolo Giordani, Vicent Calatayud, and Eva Barreno. 2023. "Lichen Biodiversity and Near-Infrared Metabolomic Fingerprint as Diagnostic and Prognostic Complementary Tools for Biomonitoring: A Case Study in the Eastern Iberian Peninsula" Journal of Fungi 9, no. 11: 1064. https://doi.org/10.3390/jof9111064
APA StyleMoya, P., Chiva, S., Catalá, M., Garmendia, A., Casale, M., Gomez, J., Pazos, T., Giordani, P., Calatayud, V., & Barreno, E. (2023). Lichen Biodiversity and Near-Infrared Metabolomic Fingerprint as Diagnostic and Prognostic Complementary Tools for Biomonitoring: A Case Study in the Eastern Iberian Peninsula. Journal of Fungi, 9(11), 1064. https://doi.org/10.3390/jof9111064









