Abstract
Floccularia luteovirens, a rare wild edible and medicinal fungus, is endemic to the Tibetan plateau. However, attempts to artificially domesticate this species have not been successful, resulting in extremely limited utilization of this valuable resource. This paper presents the geographical distribution of F. luteovirens, along with its ecological and biological characteristics. It explores population relations, symbiotic relationships, soil microbial community relations, fruiting body occurrence conditions, nutritional metabolism, and reproductive patterns. The cultivation techniques, as well as the edible and medicinal value of this mushroom, are also reviewed. Through an overall analysis of the physiological characteristics and current research status of F. luteovirens, the paper discusses its development prospects. The aim is to provide a reference for other researchers and promote its artificial domestication, resource development, and utilization.
1. Introduction
Edible fungi are a diverse group of large fungi with unique physiological characteristics and a wide range of forms. They are known for their delicious taste, rich nutrition, and diverse bioactive ingredients, making them a popular choice for healthy eating [1]. Among edible fungi, mycorrhizal fungi are a special type that form a symbiotic relationship with plants, relying on host plants to provide the necessary nutrients for their growth [2]. Research has shown that many mycorrhizal edible fungi have higher protein and trace element contents compared to saprophytic edible fungi [3,4,5]. Floccularia luteovirens (Alb. & Schwein.) Pouzar is a rare wild edible fungus found in the Qinghai–Tibet Plateau of China. It forms mycorrhizal symbiosis with Kokanica humilis and plays an important role in the alpine meadow or grassland ecosystem [6,7]. This mushroom is a local delicacy and has significant economic value. Additionally, it has been used as a traditional Tibetan medicine with notable effects in hypoglycemic, antioxidant, anti-tumor, and immune regulation [8]. Therefore, F. luteovirens holds great potential as a resource for functional food or medicine. However, as a local resource, the research on F. luteovirens is still very limited. Many aspects of this species remain unknown, such as the specific mechanism of fruiting body production, artificial culture conditions for mycelium, and the form of action of pharmacologically active ingredients. The inability to achieve commercial cultivation further restricts its processing and utilization. Additionally, environmental changes and overpicking contribute to the increasing scarcity of this resource. This review aims to synthesize the existing fragmented information about F. luteovirens and comprehensively analyze its ecological and biological characteristics, as well as its food and medicinal value, so as to raise awareness among researchers about this species and encourage further study on its conservation, domestication, and utilization.
2. Taxonomy of F. luteovirens
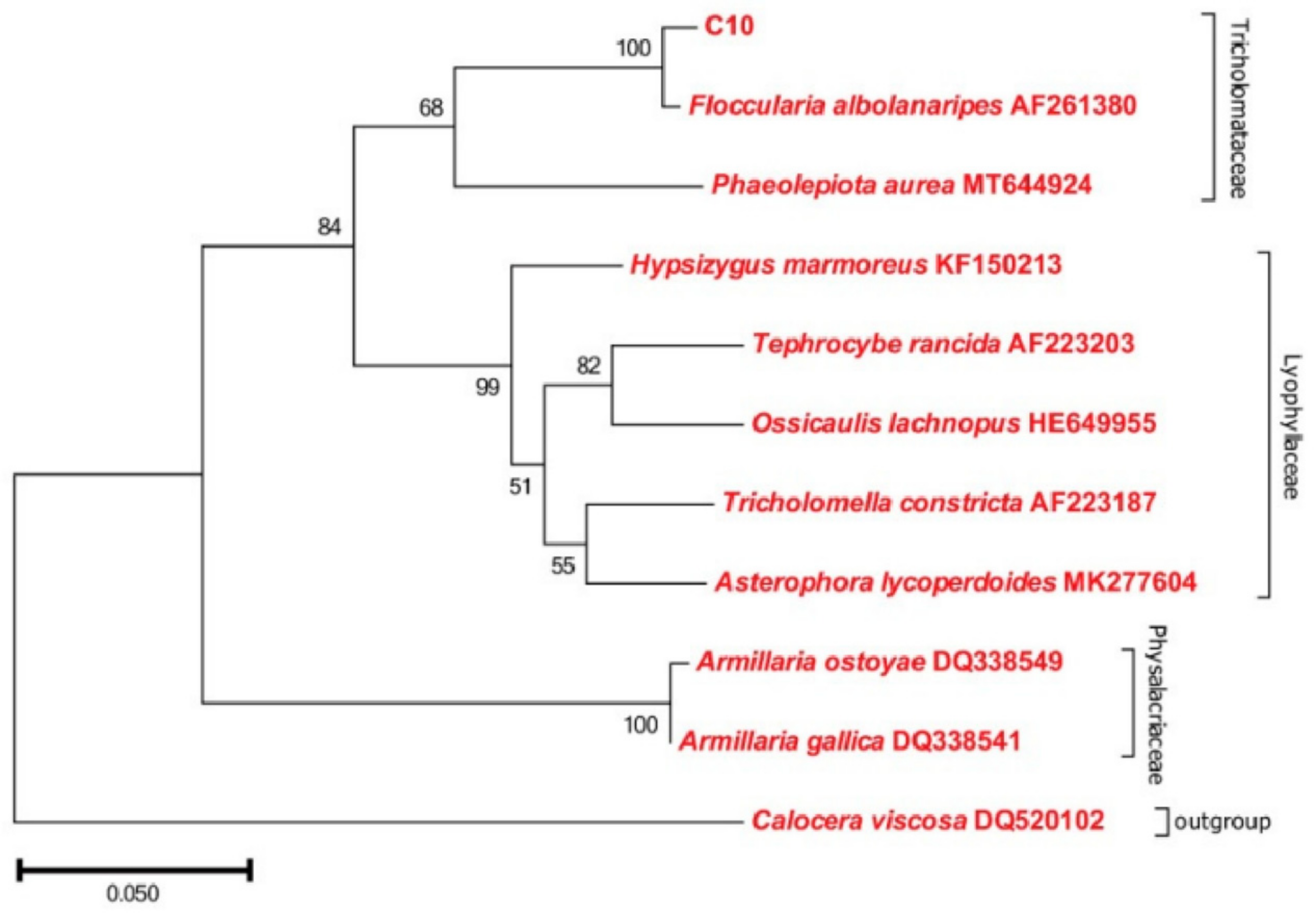
F. luteovirens is a member of the Basidiomycota phylum, belonging to the Agaricomycetes class, the Agaricales order, and the Agaricaceae family within the Floccularia genus [9]. This edible mushroom is commonly referred to as a golden mushroom or grassland yellow mushroom by local residents [10]. The taxonomic status of F. luteovirens has been controversial and misunderstood for a long time. It was previously thought to belong to the genus Armillaria, under the Physalacriaceae family, and was therefore referred to as Armillaria luteovirens in various studies [11], and is still used today. Gan et al. [12] assembled and annotated the genome of F. luteovirens. Through phylogenetic tree analysis, they determined that F. luteovirens belongs to the Agaricaceae family and that its species differentiation occurred approximately 170 million years ago. Liu et al. [13] conducted rDNA-ITS sequence determination on 36 F. luteovirens strains isolated from field collection in Qinghai Province, and then, combined with comparative genomic data, further confirmed that F. luteovirens should be classified under the genus Floccularia rather than the genus Armilaria. The NJ phylogenetic tree showed that the F. luteovirens C10 strain was closer to the species of Tricholomelaceae in a phylogenetic relationship and clustered with Floccularia albolanaripes with a bootstrap value of 99% (Figure 1). As a result, the NCBI database has also replaced the species Armillaria luteovirens with Floccularia luteovirens (NCBI: txid493452) in 2019.
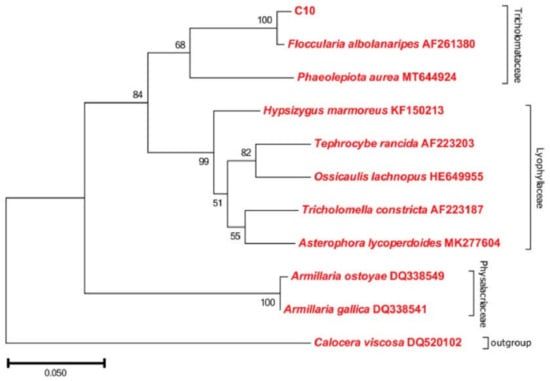
Figure 1.
Phylogenetic analysis of F. luteovirens C10 based on the ITS gene sequence. (LIU, 2021 [13]).
3. Ecological Characteristics of F. luteovirens
3.1. Geographical Distribution and Population Relationship of F. luteovirens
F. luteovirens is primarily found on the Qinghai–Tibet Plateau in China. Its geographical distribution range in China is approximately latitude 28°93′–37°69′ N and longitude 90°4′–102°1′ E [7]. It is most commonly found in the grasslands or alpine meadows of Qinghai and Tibet, with smaller populations in the western part of Sichuan and the southern part of Gansu [14]. This species has also been reported in the Altai Republic of Russia and Mexico [15,16]. According to reports [6,17,18], the altitude distribution range in Qinghai province is around 3000 to 4300 m, with the majority of sightings occurring in meadows between 3200 and 3800 m above sea level. The main areas of production include Haibei, Huangnan, Hainan, Guoluo, Yushu, and other regions. In Tibet, the altitude distribution ranges from about 3200 to 4600 m. The highest point in the Qomolangma region can reach up to 5000 m above sea level. Significant production areas for F. luteovirens include Lhasa, Dazi, Langkazi, Pali, Bangda, Dingri, and others. In Shiqu County, Sichuan Province, F. luteovirens mainly grows in the grasslands along the Yalong River at an altitude of 4000–4300 m. The geographical structure of F. luteovirens exhibits significant differences. A comprehensive analysis of 91 samples from 10 geographical groups in Qinghai, Tibet, and Sichuan provinces revealed that the population genotype comprises two branches. This study also found that population genetic variation primarily originates from within the population, with substantial genetic differentiation observed between populations [19]. Moreover, the genotype diversity (h) of F. luteovirens ranged from 0.385 to 0.876, indicating a moderate level of genetic diversity [20].
3.2. Symbiotic Relationship of F. luteovirens
F. luteovirens is a symbiotic mycorrhizal fungus that forms a partnership with the Kobresia plants. The hyphae of this fungus are distributed in the soil at a depth of 5–30 cm. During the fruiting bodiy harvest season, white mycelia can be observed on the roots of Kobresia humilis [6]. Although many scholars agree that F. luteovirens is an ectomycorrhizal fungus [20,21], there is a lack of anatomical or modern biological identification, and its mycorrhizal types need to be further clarified. Ectomycorrhiza symbiosis is a widespread association between the roots of woody plants and soil fungi in forest ecosystems [22]. Examples of mycorrhizal edible fungi include matsutake, boletus edulis, and truffle, which form symbiotic relationships with pine or oak trees [23]. However, the fruiting body of F. luteovirens occurs in alpine meadows, where the dominant vegetation consists of K. humilis and alpine weeds [24]. In Guo et al.’s study, they used a metagenomic method to determine the diversity of endophytic fungi in K. humilis. They found that F. luteovirens is one of the dominant endophytic fungi in K. humilis [25]. The habitat of F. luteovirens often forms a ribbon-like fairy ring, with a circle width of approximately 50 cm and a diameter of about 6 m [24]. According to Shantz and Piemeisel’s classification of fairy rings, the fairy ring formed by F. luteovirens falls under type II [26]. This means that the existence of the fairy rings stimulates the growth of plants, and there is only a green grass ring without a dry grass ring. Fairy rings are a well-known ecological landscape found in grasslands, and a similar phenomenon can be observed in the fairy ring formed by Leucocalocybe mongolica in the Mongolian Plateau [27]. Additionally, the plants within the F. luteovirens ring exhibit higher biomass, plant height, and plant density compared to those outside the ring [6,28]. The formation of a fairy ring is a distinctive occurrence resulting from the growth of fungal mycelium in the soil. Several studies have demonstrated [29,30,31] that Fairy Ring fungi can produce plant growth regulatory substances to stimulate plant growth, such as 2-azahypoxanthine (AHX) and imidazole-4-carboxamide (ICA), and regulate the activity of various enzymes in the soil, thereby improving soil characteristics and providing favorable conditions for plant growth. The plants on the fairy rings displayed a noticeable increase in greenness, and the abundance index and species diversity index of the plant communities on the rings were significantly higher. The promotion of plant growth by F. luteovirens is closely linked to the mycorrhizal system. On the one hand, plants are able to contribute to the decomposition and activation of mineral elements in the soil through a vast network of hyphae, particularly enhancing the absorption of organophosphorus and other nutrient elements. On the other hand, plants can also serve as a source of carbon for fungi in the form of sugar or fatty acids [32]. F. luteovirens can change minerals into usable organic forms by releasing organic acids. This makes roots absorb more minerals and improves the stress resistance of symbiotic plants. [33]. Physiological and metabolomic analysis has shown [34] that F. luteovirens can influence the accumulation of soil metabolites, regulate plant carbon/nitrogen metabolism, and enhance the growth of above-ground tissues in alpine meadow plants. Meanwhile, it may also reduce root growth to adjust the root-shoot ratio, consequently increasing nutrient accumulation in plants. In addition, the mycelium of F. luteovirens also produces volatile organic compounds (VOCs) that regulate the distribution of root auxin, thereby controlling the root development of plants. This, in turn, affects the growth, metabolism, and environmental adaptability of plants [35]. Andrea Polle et al. have also discovered that ECM fungi produce volatiles, particularly terpenoid derivatives, which can influence the development of host plants and their lateral roots [36]. This finding is similar to the role of F. luteovirens in regulating plant growth. The fairy ring effect, caused by the growth and metabolism of mycelia, has significant impacts on the plant-soil ecosystem in the habitat. It can alter the inter-specific competitive relationship of grassland communities and influence the composition and direction of plant community succession [37]. However, the growth of F. luteovirens has a minimal effect on the uniformity and succession of plant communities [24]. In conclusion, as an endemic species, F. luteovirens plays a positive role in regulating plant growth and maintaining ecosystem stability in the alpine meadows of the Qinghai–Tibet Plateau.
3.3. Soil and Soil Microbial Communities
The soil of the F. luteovirens habitat is dark, with a pH range of 6.8–7.2 and a humus layer measuring 10–20 cm. Below the humus of the soil is the parent material, containing gravel and grass roots [6,18]. The decomposition of fungal mycelia can enhance the mineralization of soil organic matter and release nutrients, thereby enriching the soil and significantly increasing the availability of nutrients. In the fairy ring area, the soil within the ring exhibits higher levels of nutrients and enzyme activities compared to both the inside and outside areas of the ring [38]. In the 0–10 cm soil layer, the soil water content, available phosphorus, nitrate nitrogen, and ammonia nitrogen content in the fairy ring formed by F. luteovirens were significantly higher than outside the circle [24,33]. Floccularia is the absolute dominant genus among soil microorganisms in F. luteovirens nest soil, with a relative abundance of 85.76%. The FunGuild function predicted that the soil fungi in the habitat of F. luteovirens were mainly symbiotic, followed by saprophytic [39]. This finding aligns with the mycorrhizal symbiotic nature of F. luteovirens. The presence of F. luteovirens in the soil has a regulatory effect on the microbial community. In the area where mycelia grow, the diversity of bacteria increases while the diversity of fungi decreases [33,40]. The possible reason is that the interspecific cooperation in mycorrhizal symbiosis between F. luteovirens and K. humilis enhances the competitive ability of F. luteovirens and inhibits the vitality of other fungal species in a limited ecological niche. Ectomycorrhizal (ECM) fungi play a crucial role in the rhizosphere community by interacting with various microorganisms. One of the main interactions involves the competition between different ECM fungi as they strive to occupy the root space of the host in order to obtain additional carbon sources [41]. A study on the fairy ring formed by F. luteovirens revealed interesting findings. The number of operational taxonomic units (OTUs) in the three regions was as follows: 300 in the IN region, 1107 in the ON region, and 14 in the OUT region. No other ECM fungi were detected in any of the three regions, with only a small amount of arbuscular mycorrhizal fungi being detected. These results suggest potential competition between F. luteovirens and other ECM species [33]. The presence of F. luteovirens in the soil has indirect effects on the composition and metabolism of soil microorganisms. This, in turn, leads to changes in the distribution and composition of metabolites in the soil. It is important to note that microorganisms in the soil have an impact on the development of mycelia, mycorrhizal synthesis, and fruiting bodies in F. luteovirens [40]. Soil bacteria and fungi play different roles in affecting soil nutrients, soil enzyme activity, and microbial activity [42]. At present, numerous comparative studies have already been conducted on soil microbial species in the vicinity of the F. luteovirens fairy ring. Further research could focus on the effects of enzymes secreted by fungi, particularly those originating from F. luteovirens, on the rhizosphere soil environment of plants.
4. Biological Characteristics of F. luteovirens
4.1. Morphological Characteristics of F. luteovirens
The fruiting bodies of F. luteovirens are moderately sized and lemon-yellow to sulfur-colored when fresh; when dried, they become practically white (Figure 2). The flesh of the fruiting bodies is thick, white to pale yellow, does not change color after damage, and has a peculiar mushroom odor. The pileus surface is dry and initially spherical but expands after maturity. It is covered with distinct, nearly concentrically arranged scales and measures 5.5–13 cm in diameter. The lamella of the fructification has sparse, yellow, and straight growth towards the pileus, with some curved growth as well. The stipe of the fructification is cylindrical, measuring 2–8 cm in length and 2–2.5 cm in diameter. It has a white to yellow color and is often covered with scales. The interior of the stipe is solid, and the base is frequently swollen. At the base of the stipe, there are spiral arrangements of curled hairs, along with remnants of the partial veil [6,7,43]. However, it is worth noting that there are significant differences in fruiting body morphology between F. luteovirens reported from Mexico and F. luteovirens reported from China and the Altai Republic of Russia. The population branches of F. luteovirens may have undergone different directions of evolution.

Figure 2.
The fruiting bodies of F. luteovirens are in different regions. (A) reported from Mexico (Arana-Gabriel 2020 [16]); (B) reported from the Altai Republic of Russia (Gorbunova 2017 [15]); (C) photographed in China (by the author).
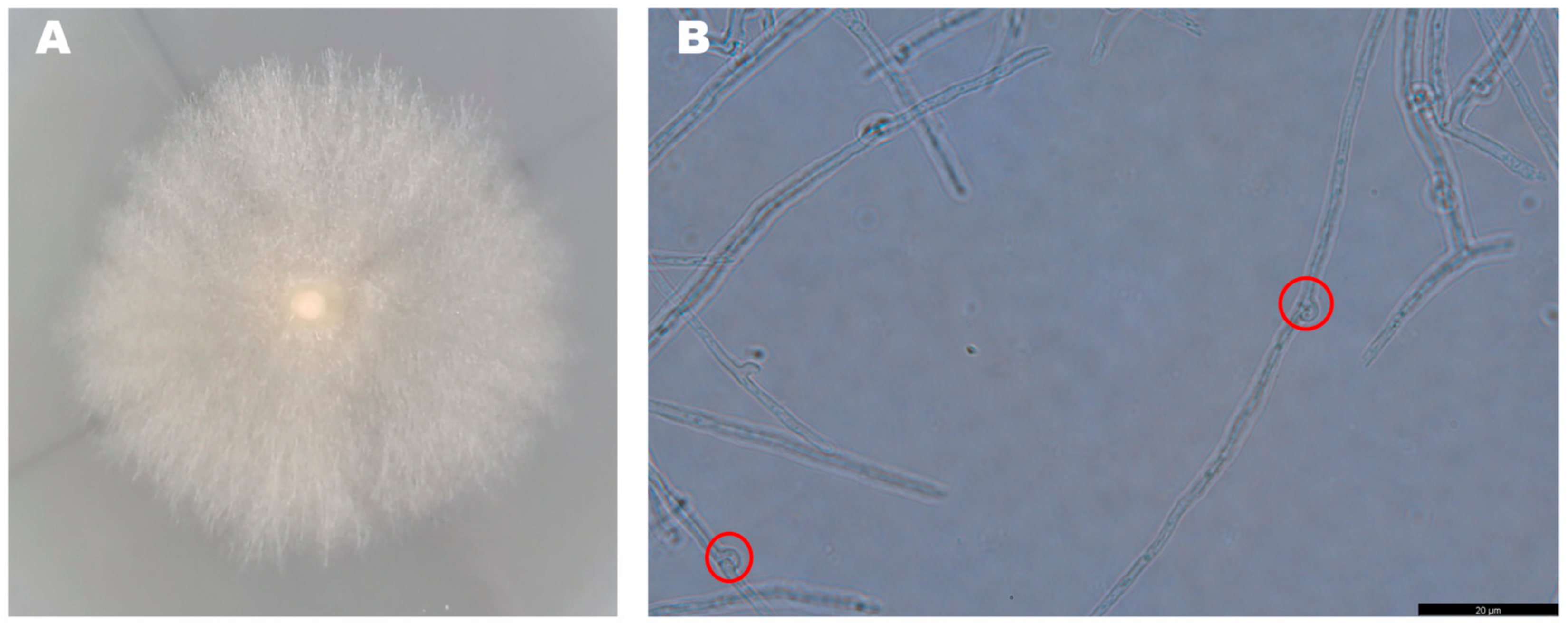
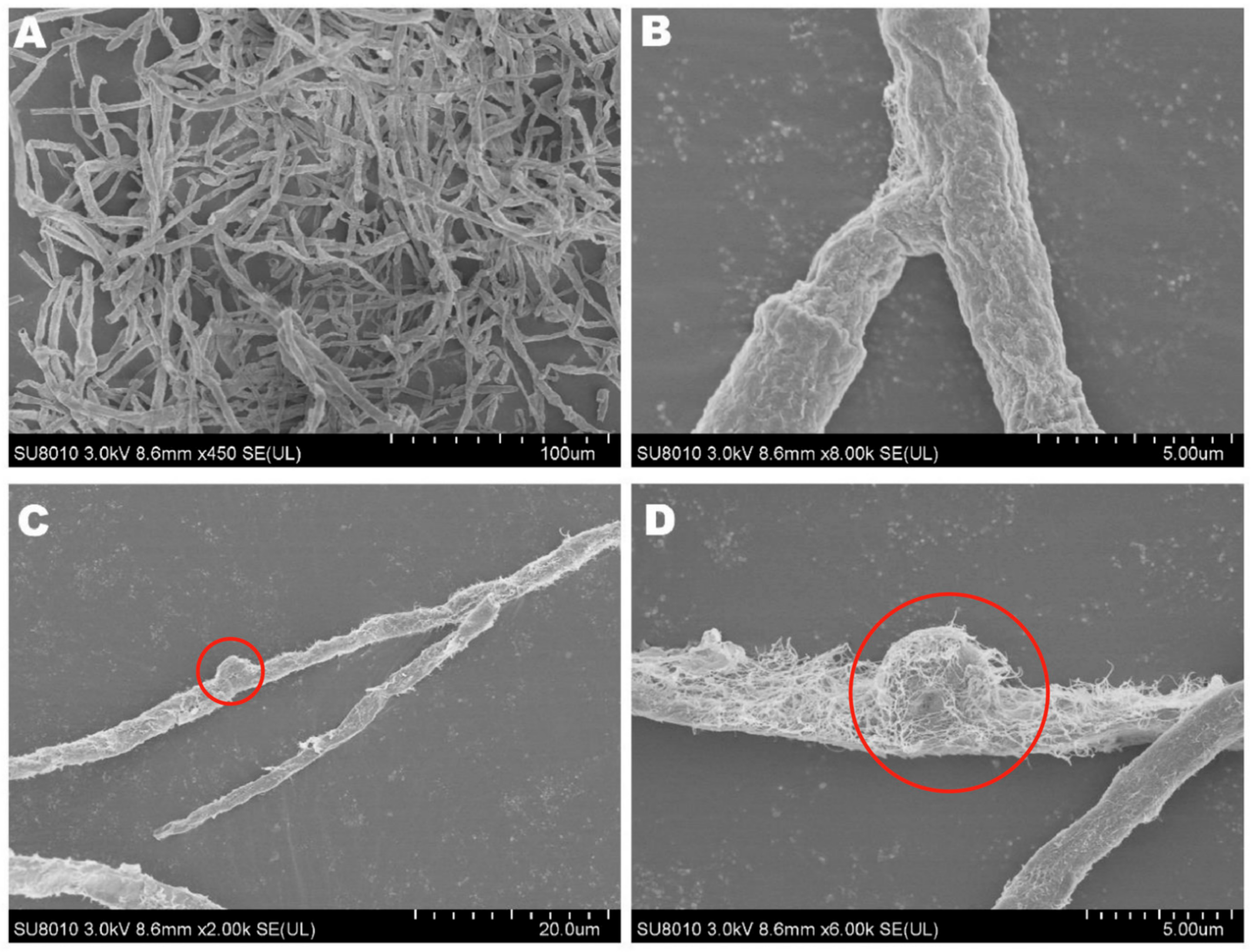
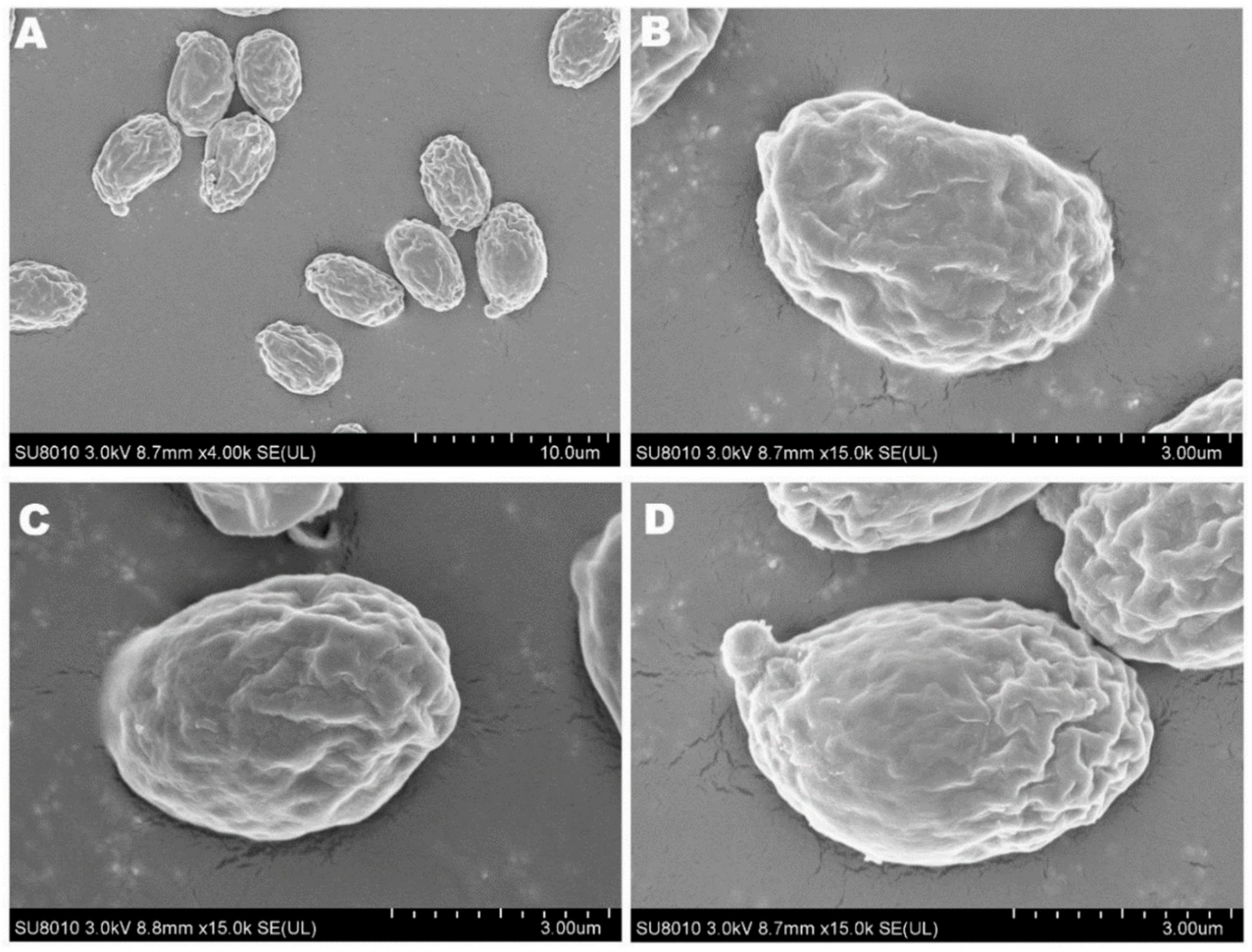
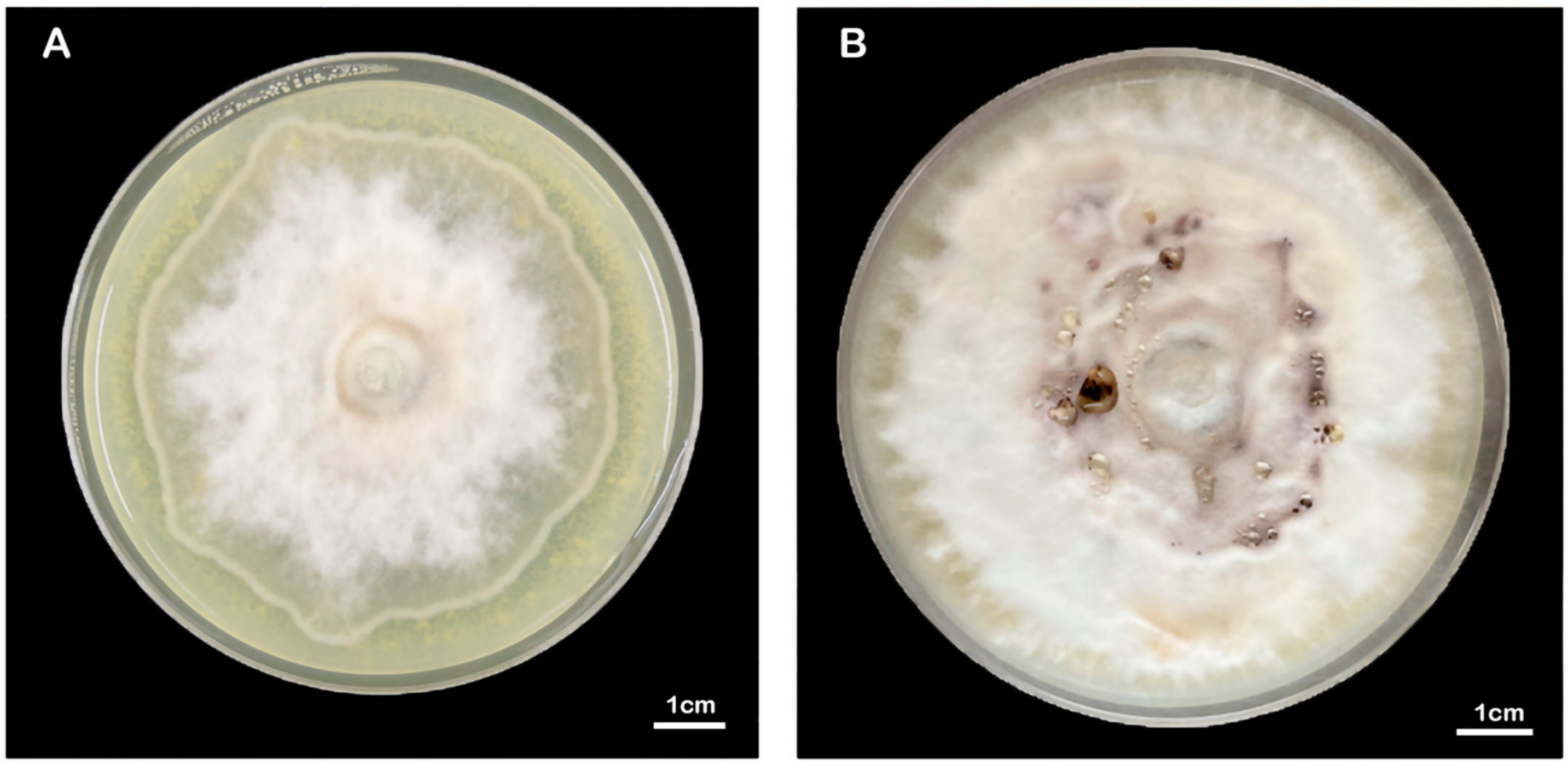
The mycelia of the strain isolated from F. luteovirens fruiting bodies grew slowly in PDA medium, and the mycelial color gradually transitioned to a yellowish-brown shade in the later stage, eventually ceasing further growth (Figure 3A). Under the light microscope, the structure of the clamp-connection can be distinctly seen (Figure 3B); this observation suggests that the mycelium of F. luteovirens is dikaryotic. Scanning electron microscopy showed that the surface of the hypha was smooth, with a thickness of 2–5 μm. The hypha grew straight without any curvature, and mycelia branches could be seen (Figure 4A,B). On the young mycelia, a clamp-connection structure was observed, and the diameter of the arc is approximately 2.5 μm (Figure 4C,D) [44].
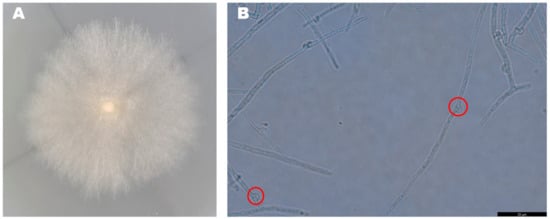
Figure 3.
Morphology of the F. luteovirens mycelia. (A) Colony of isolated F. luteovirens; (B) Morphology of F. luteovirens mycelia. Clamp-connections are indicated by red circles (Photograph by the author).
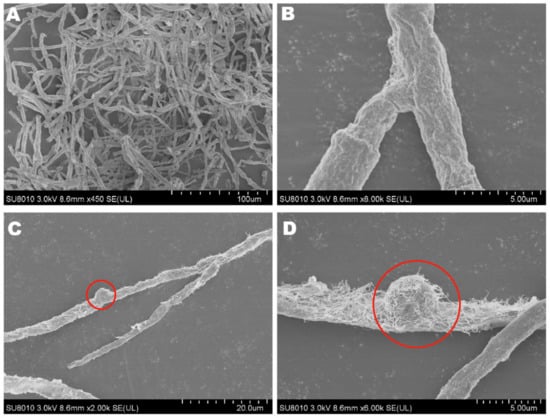
Figure 4.
The mycelial structures of F. luteovirens were observed with scanning electron microscopy. (A) Observation at 100 μm; (B,D) Observation at 5 μm; (C) Observation at 20 μm. Clamp connections are indicated by red circles (Liu 2020 [44]).
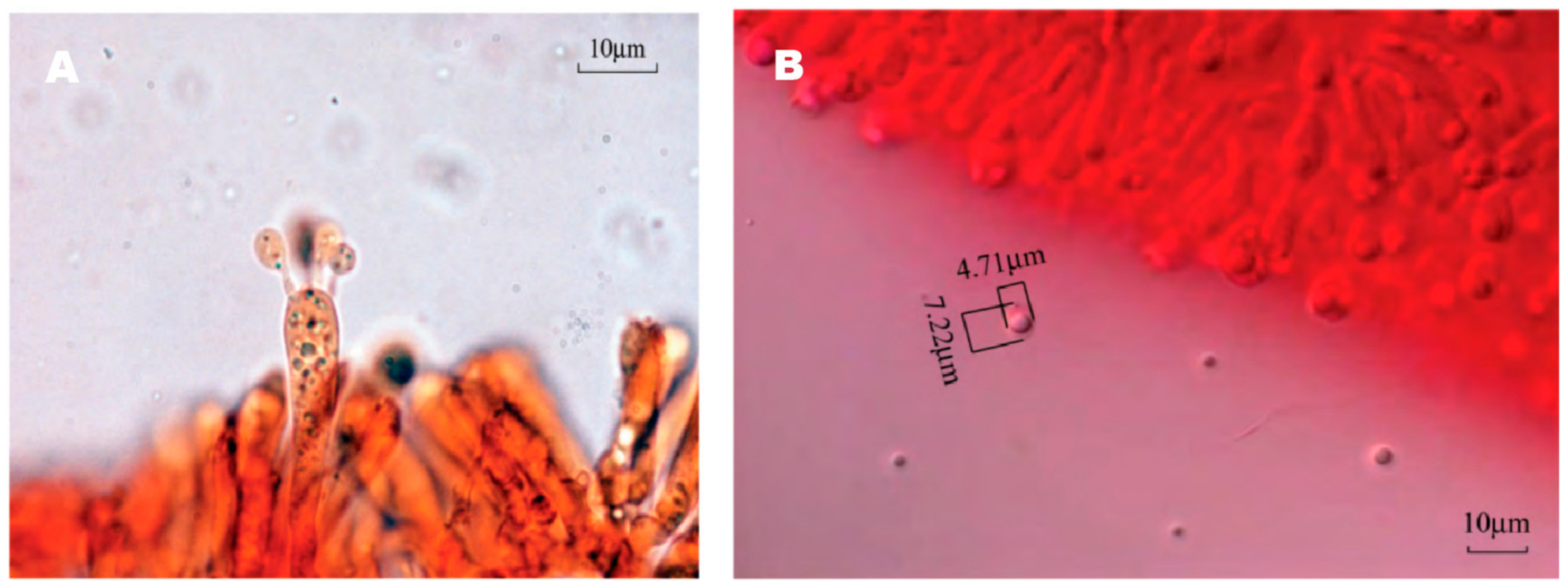
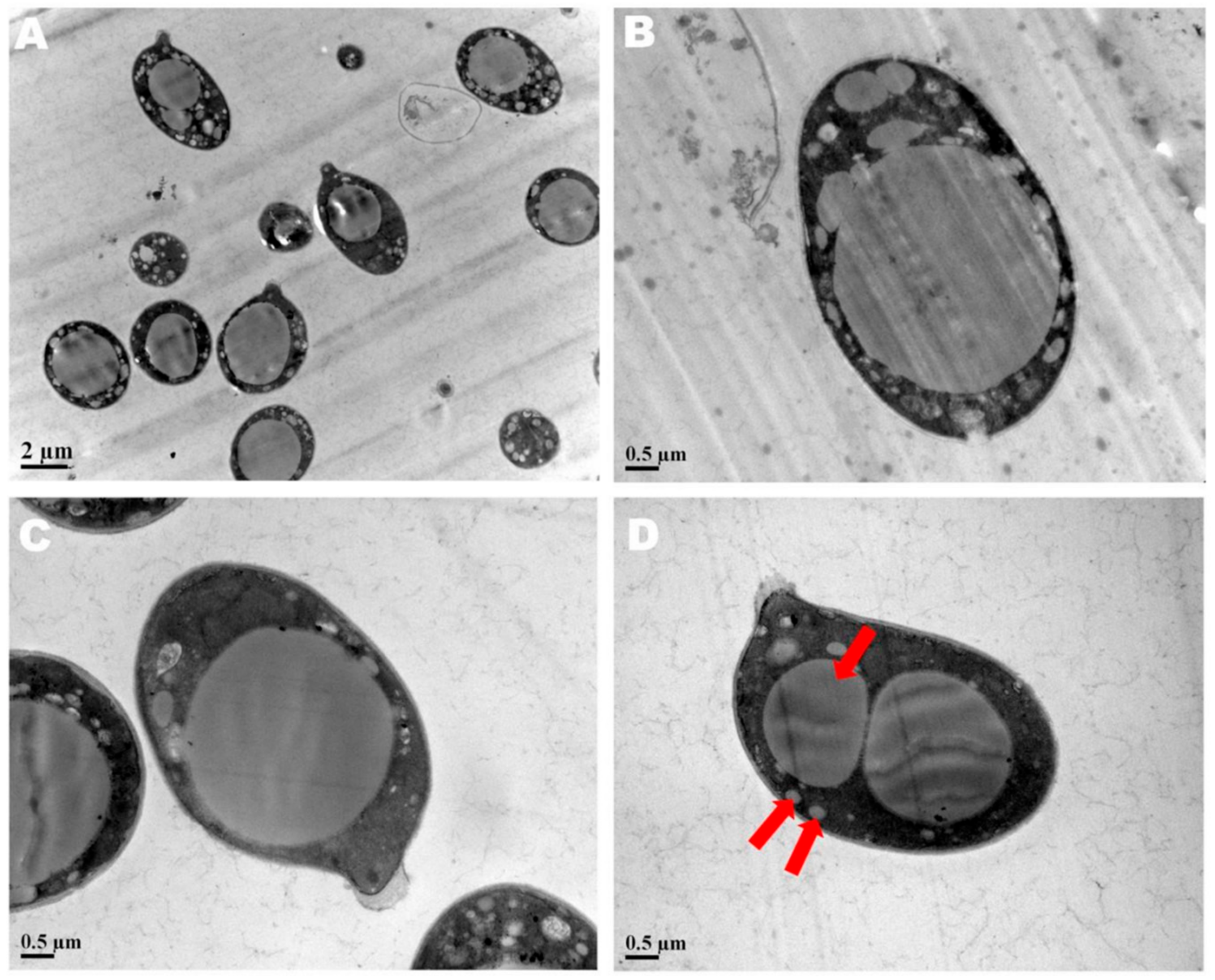
The spore print of F. luteovirens is white. Under the microscope, the basidiomata were rod-shaped, 12.5–16.8 × 4.0–4.8 μm in size, with 4 pedicles and occasionally 2 pedicles. Basidiospores were ellipsoid to spherical, 4.2–8.3 × 3.7–7.5 μm (Q = 1.1–2.2), smooth, and translucent (Figure 5) [7]. Under scanning electron microscopy, the spores of F. luteovirens were ellipsoid, with a size of 5.5~6.0 μm × 3.0~3.8 μm (Figure 6). There were some indentations on the surface, and at the base of some spores was an excipuliform appendage (Figure 6D). When the spores of F. luteovirens were observed under transmission electron microscopy (Figure 7), it could be seen that there were two layers of spore wall and that the thickness of the spore wall was uneven. Most spores have a vacuole that nearly fills the sporosomal cavity and a few lipid droplets of varying sizes (Figure 7B) [44].
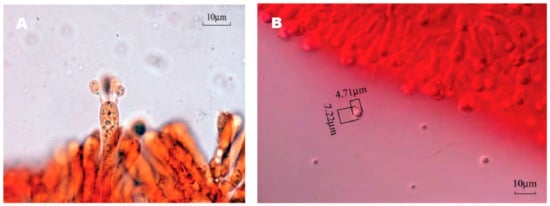
Figure 5.
Basidiomata (A) and basidiospores (B) of F. luteovirens (×1000) (Xie 2016 [7]).
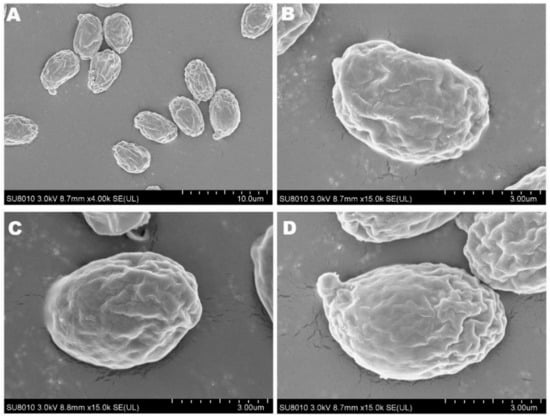
Figure 6.
The spores of F. luteovirens were observed with scanning electron microscopy. (A) Observation at 10 um; (B–D) Observation at 3 μm (Liu 2020 [44]).
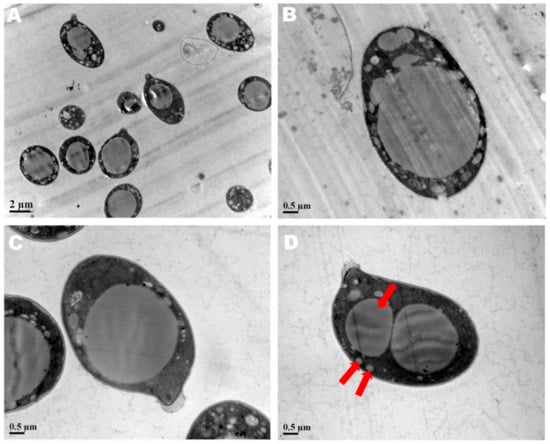
Figure 7.
The spores of F. luteovirens were observed with a transmission electron microscope. (A) Observation at 2 μm; (B–D) Observation at 0.5 μm. The red arrows indicate vacuoles and lipid droplets within the spore (Liu 2020 [44]).
4.2. Conditions for the Formation of the Fruiting Body
The fruit bodies of F. luteovirens are distributed in the grass, either scattered (solitary, scattered, clustered, or clumped) or on the fairy rings. The growth and development process can be divided into five stages: the primary base stage, the bud stage, the growth stage, the umbrella stage, and the decay stage. The growth cycle lasts for 18 to 22 days [43]. This mushroom is ripe from June to September each year, with a harvest period of about 1 to 1.5 months in the same area. The occurrence periods of varying regions differ due to differences in latitude and altitude, with specific times primarily dependent on ground temperature and humidity [17]. The hyphae of F. luteovirens could tolerate temperatures ranging from −17.4 °C to −3.8 °C, which is the average temperature of the coldest month. Both temperature and light intensity have an impact on the growth of F. luteovirens fruiting bodies throughout their entire growth period. The timing and amount of rainfall directly influence the timing and quantity of mushrooms. The more rainfall there is, the more abundant and robust the mushrooms are. Additionally, precipitation can promote the various growth stages of F. luteovirens [6]. According to Xie et al. [7], the fruiting bodies of F. luteovirens appear during the summer and autumn seasons, with an average relative humidity of 41~74%, an average temperature of 6.2~15.9 °C, an average annual evaporation of 1393.8~2441.4 mm, an annual precipitation of 344~574 mm, and a humidity coefficient of 0.42~0.78. The Tibetan Plateau is one of the regions with the highest ultraviolet radiation intensity in the world. To cope with this intense UV radiation, F. luteovirens produces riboflavin, which results in the mushrooms appearing brighter in high-UV environments [45].
4.3. Nutritional Metabolism and Reproductive Mode of F. luteovirens
As a mycorrhizal fungus, F. luteovirens does not rely on its own decomposition to obtain nutrients. Studies have shown that the genes related to lignin and cellulose degradation in this fungus are significantly fewer compared to white-rot fungi and brown-rot fungi [13,46,47]. The nutrient requirements of mycorrhizal fungi vary greatly among different species. Most ectomycorrhizal fungi utilize ammonium salts as a source of inorganic nitrogen, while a few can also use nitrates [48]. Current research suggests that F. luteovirens is capable of utilizing both ammonium salts and nitrates [49]. KEGG pathway analysis was conducted on transcripts from four major developmental stages of F. luteovirens: hyphal stage, primordium stage, immature fruiting body stage, and mature fruiting body stage. This study found significant differential expression of genes involved in cell cycle (ko04111), ribosome (ko03010), MAPK signaling (ko04011), and primary carbohydrate metabolism (ko00010) pathways during reproductive growth [13]. However, it should be noted that this study did not verify the key genes that influence the growth of F. luteovirens. In the fruiting body stage, F. luteovirens exhibits a complex range of nutrient types. A study utilizing transcriptome sequencing has identified a total of nine significantly different genes associated with carbohydrates. The expression of these genes leads to the breakdown of substances in the soil habitat into monosaccharides and glucose, ultimately completing energy metabolism through the glycolysis pathway and the tricarboxylic acid cycle [50]. At present, the nutritional types of F. luteovirens and their symbiotic relationship with plants remain incompletely understood. Further research is necessary to elucidate the adaptation mechanism between F. luteovirens and both biological and abiotic factors. Some scholars have studied the small-scale genetic diversity and gene exchange frequency of F. luteovirens, revealing that F. luteovirens exhibits a relatively large genome. Additionally, only a limited number of new genes were identified during the three-year study, indicating that this species heavily relies on vegetative growth and can persist underground as mycelia for extended periods [51]. The sexual reproductive mating types of edible fungi can be classified into homothallism and heterothallism [52]. However, there is currently no research available on the mating types of F. luteovirens. Accurately identifying mating types and genotypes is crucial for artificial domestication cultivation and hybridization breeding.
5. Current Status of Artificial Cultivation of F. luteovirens
5.1. Research on Mycelial Growth
Due to the lack of host plants and insufficient exploration of the necessary nutrient elements for the growth of F. luteovirens mycelia, the mycelia are unable to obtain enough nutrients under laboratory culture conditions, resulting in a slow growth rate of artificially cultured mycelia. F. luteovirens hyphae have a slow growth period from germination to 42 days and then only a fast growth period of about 5 days, which is one of the reasons for the slow growth of the strain. During the stagnant growth period, the hyphae change from their initial white color to a yellowish-brown color, although the mechanism behind this phenomenon is still unclear [53]. The growth temperature range of wild F. luteovirens hyphae is 15 °C to 30 °C, the optimum temperature range is 20 °C to 25 °C, and the suitable pH range is 5.5 to 6.5 [54]. Previous experiments have investigated the effects of different carbon and nitrogen sources, traditional Chinese herbal medicine, and growth regulators on the growth of F. luteovirens mycelia. Sucrose and peptone are considered more suitable carbon and nitrogen sources, with an optimal carbon-to-nitrogen ratio of 10:1. The most effective mineral nutrient for mycelial growth is MgSO4. Licorice and wolfberry extracts, triacontanol, inositol, and selenium have all been found to significantly enhance mycelial growth [55,56,57,58]. Other studies have shown that adding more plant species to the enriched medium improves mycelia growth, possibly due to the fact that mycelia growth depends on certain substances in the plant [50]. Arana-Gabriel et al. [16] conducted a culture screening test on a F. luteovirens strain isolated in Mexico and found that the strain reached a growth rate of 0.406 mm/d in a MEA (Malt Extract Agar) medium with a pH of 4. Although this growth rate was higher than previous reports, it was still slower compared to the growth rate of other cultivated or wild fungi. SHI et al. [49] conducted a single-factor experiment and an orthogonal experiment to identify an optimal culture condition. The culture medium consisted of 20% potato extract, 10% soybean sprout extract, 0.3% KH2PO4, and 1 mg/L VB1 + VB2. After 15 days, the mycelia dry weight could reach 6.5 mg/mL. Some studies have conducted statistical analyses on the number of clamp connections in F. luteovirens strains with excellent, good, and medium hyphae growth rates, and combined analysis indicates that F. luteovirens exhibits a faster hyphae growth rate and a greater average dry weight when there are fewer clamp connections [59]. Additionally, many fungi possess the ability to bioenrich selenium. Studies have shown that selenium can enhance the growth of F. luteovirens hyphae and significantly increase its biomass. The optimal concentration for selenium-enriched cultures is found to be 0.80 mmol/L [60]. To date, none of the aforementioned studies have successfully identified a comprehensive formula that is highly conducive to the growth of F. luteovirens mycelium. We are currently conducting research in this field and have successfully achieved dense growth of F. luteovirens mycelium on the composite medium (Figure 8). If we make more discoveries and progress, we will share the detailed results of this experiment with the public.
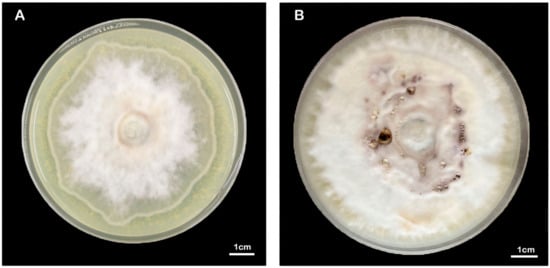
Figure 8.
Morphology of the F. luteovirens mycelia at different culture days. (A) Approximately 40 days, (B) 100 days.
5.2. Preliminary Study on Artificial Cultivation
In recent years, numerous researchers have attempted to domesticate F. luteovirens; however, no successful cases have been reported thus far. The growth of this particular type of mycorrhizal fungus necessitates a specialized culture medium and specific environmental conditions, including complex formulations, specific soil compositions, and nutrient requirements. In natural settings, mycorrhizal fungi rely on plant roots to obtain essential nutrient elements, transitioning from the vegetative stage to the reproductive stage and ultimately forming fruiting bodies [61]. This reliance on plant roots poses a challenge for artificial cultivation. It has previously been reported that a Japanese scholar processed the mycelia of F. luteovirens with trace nitrite ions, and the granular mycelia produced was incubated at a low temperature below 12 °C. When urea was added to the medium, a flat mushroom-like mycelium with a diameter of about 7 cm could be obtained in about 1 month [62]. LIU conducted the same experimental method as mentioned above. After adding potassium nitrite to the culture for a period of 6 months, the shaker bottle was filled with F. luteovirens mycelium, but no F. luteovirens fruiting bodies were observed [44]. We speculate that the success of the previous experiment was based on chance, or it could be attributed to inherent differences in the strains that led to the non-replicability of the experiments. WEI obtained the test strain by isolating the fruiting body of wild F. luteovirens [54]. In seed mycelium, it was observed that the pollution rate of the grain medium was high. The absorption and utilization of cottonseed shell medium were not ideal, while grass powder medium exhibited a relatively good growth rate in the later period. After soil-covering culture, the hyphae on the grass powder medium were able to form the primordia and yellow fruiting bodies. However, when the diameter of the pileus was about 1 cm, the fruiting bodies would appear to undergo autolysis and death. Insufficient temperature, light, water, and nutrients are all possible factors that adversely affect the normal growth of the hyphae. Currently, the key conditions for artificial cultivation of F. luteovirens have not been identified. The imitation of wild cultivation of F. luteovirens has been proposed [63]; however, the culture of the mycelium growth stage has not been successful yet, so this artificial domestication technology has not been attempted. Furthermore, there are limited reports on the cultivation of F. luteovirens, and no significant progress has been made in understanding its primordium and fruiting body formation.
6. Nutritional Components of F. luteovirens
6.1. Basic Components
The dry fruiting body of F. luteovirens contains 85.92% dry matter, 46% crude protein, 3.85% crude fat, 6.46% crude fiber, 18.63% soluble sugar, and 10.98% ash. The fruiting bodies contain 18 kinds of amino acids (including eight kinds of essential amino acids), vitamin B1, vitamin B2, vitamin C, and vitamin E, as well as P, K, Fe, Mg, Zn, and other minerals essential to the human body [6,64]. Li et al. compared the nutritional components of F. luteovirens with Lentinula edodes (L. edodes) and Agaricus bisporus (A. bisporus) (Table 1 and Table 2) and found that F. luteovirens had significantly higher protein and ash contents. It is a precious wild edible mushroom with a high protein and mineral content [64].

Table 1.
Comparison of physicochemical properties and chemical constituents of the dry fruiting bodies of F. luteovirens, L. dodes, and A. bisporus.

Table 2.
Comparison of amino acid content in the fruiting bodies of F. luteovirens, L. dodes, and A. bisporus.
6.2. Polysaccharide
The polysaccharide content in F. luteovirens is comparable to that in lentinus mushrooms, reaching 3.56~8.12 g/100 g [65]. Additionally, the polysaccharide content in mycelium water extract is approximately 31.21% [66]. Analysis reveals that the polysaccharides of F. luteovirens primarily consist of xylose and Arabic sugar [67], and they predominantly contain six sugar residues: (A)→4)-β-D-Manp-(1→; (B)→3)-α-L-Fucp-(1→; (C) α-L-Araf-(1→; (D)→6)-β-D-Galp-(1→; (E)→4)-α-D-GlcAp-(1→; (F)→3)-β-D-Glcp-(1→ [44].
6.3. Volatile Components
Analysis of the volatile fat-soluble components of F. luteovirens showed that the fruiting body was rich in fatty acids, esters, alkenes, and other compounds, with the highest relative content being linoleic acid (ω-6, 48.2%) [68]. Based on GC–MS analysis, a total of 25 fatty acid components and contents in the fruity bodies of F. luteovirens were identified, among which the relative contents of polyunsaturated fatty acid accounted for 10.6%, monounsaturated fatty acid accounted for 31.5%, and saturated fatty acid accounted for 56.9% [69]. Genomic analysis of F. luteovirens showed that there were 672 genes related to cell metabolism of terpenoids and polyketones, accounting for a large proportion, indicating that F. luteovirens is a potential resource species of terpenoids and polyketones [13].
6.4. Others
The selenium content in the fruiting body of F. luteovirens ranges from 0.57 to 2.53 mg/kg, which meets the standard for selenium-rich food. Additionally, various nucleosides (such as uridine, deoxyuridine, uracil, guanosine, inosine, and adenosine), esters, and sterols were isolated from the fruiting body [70,71].
7. Medicinal Value of F. luteovirens
F. luteovirens mushrooms are a traditional Tibetan herbal medicine. Historical records indicate that it has a sweet taste and provides benefits to the stomach and intestines. Regular consumption of these mushrooms could reduce blood cholesterol levels. Moreover, it is also used to alleviate symptoms such as dizziness, headache, neurasthenia, and insomnia and has been found to have preventive effects against viral infections and diseases like beriberi. It has a high content of iron and phosphorus and is also an ideal health food for benefiting qi and nourishing blood. Due to its rich nutritional value, it also has pharmacological effects on promoting children’s development and enhancing humans’ immunity [6,72,73].
7.1. Antioxidant and Anti-Aging Ability
Polysaccharides are significant components of edible fungi with both nutritional and medicinal value. Research has shown [74,75,76,77,78] that the scavenging ability of F. luteovirens polysaccharides towards DPPH is superior to that of Lentinula edodes polysaccharides, and the scavenging ability towards ABTS is better than that of Hericium erinaceus polysaccharides. Moreover, it has a higher moisture absorption rate than Cordyceps sinensis polysaccharides and a higher moisture retention rate than Sargassum horneri polysaccharides. F. luteovirens polysaccharides after extraction and separation can effectively increase the activities of SOD, GSH-Px, and CAT in PC12 cells while decreasing the production of ROS and MDA, thus protecting PC12 cells from H2O2-induced oxidative stress [79]. The protoilludane sesquiterpene aryl esters extracted from the fruiting bodies of F. luteovirens can have good scavenging efficiency on DPPH free radicals [80]. A highly polar free radical inhibitor, L-(+)-Ergothioneine [81], has been successfully extracted from the methanolic extract of F. luteovirens. It is a natural antioxidant that offers significant cell protection and is non-toxic. Additionally, it has the ability to chelate heavy metal ions and safeguard red blood cells against free radical damage.
7.2. Anti-Tumor and Anticancer Potential
The exopolysaccharides of F. luteovirens have been found to inhibit tumor cell proliferation while having no side effects on normal cells [79]. Polysaccharides play a role in enhancing the immune function of the body by regulating humoral immunity and regulating the expression of immune-related genes [82,83]. This leads to the promotion of the proliferation and activity of immune cells, ultimately resulting in an anti-tumor effect. The small-molecule isolates from F. luteovirens exhibited potent antitumor activity by inducing apoptosis of lung cancer cells through the activation of caspase-3. This isolate consisted of six amino acids, two nucleosides, two glycosides, two terpenoids, one phenylpropanoid compound, one ester, and one alkaloid [84]. Previous studies have demonstrated that a lectin called ALL [85], which was isolated from F. luteovirens, has the ability to inhibit the proliferation of tumor cell lines L1210 (leukemia), MBL2 (leukemia), and HeLa (cervical). Furthermore, this lectin exhibited greater stability compared to typical mushroom lectins and plant lectins, as it retained its activity even at a temperature of 70 °C.
7.3. Anti-Diabetic Activity
The water extract of F. luteovirens has certain benefits in the treatment of diabetes; it can significantly improve the oral glucose tolerance of rats with type 2 diabetes, significantly increase the level of SOD in the serum of rats, and reduce the expression of MDA and four inflammatory factors [86]. Additionally, the polysaccharides in it can effectively improve and alleviate renal tissue damage caused by hyperglycemia [87].
7.4. Other Functions
The water extract of F. luteovirens also demonstrates a significant anti-inflammatory and analgesic effect on experimental migraine rats. This extract effectively reduces vasodilation and alleviates stimulation of the trigeminal nerve [88]. These findings provide a plausible explanation for the historical use of F. luteovirens mushrooms in ancient medical books to treat headaches.
8. Future Prospects
8.1. Strengthen Resource Protection
F. luteovirens, as an edible mushroom, is known for its delicious taste and rich nutrition. It has become increasingly popular among people, with the market price for fresh mushrooms reaching as high as six or seven hundred yuan per kilogram during the harvest season. Higher-quality mushrooms can even be sold for thousands of yuan. However, in their pursuit of economic benefits, local residents have been expanding the number and area of pickings, resulting in a constant reduction in the number of fruiting bodies [7]. Excessive picking can have detrimental effects on the grassland ecological system. To ensure the long-term survival of F. luteovirens, it is important to promote the concept of sustainable development among local residents. In cases where it is necessary, certain protected areas can be established to safeguard the population of F. luteovirens. While the majority of resources for researching the F. luteovirens species are currently derived from Qinghai and Tibet, it is important not to disregard the potential resources available in other regions such as Sichuan and Gansu. It may be necessary to establish F. luteovirens species banks in research institutes or universities to ensure the long-term preservation of essential germplasm resources and their genetic information. This would provide a foundation for future genetic breeding and artificial domestication research.
8.2. Promote the Process of Artificial Domestication
F. luteovirens, a rare edible mushroom resource in the Qinghai–Tibet Plateau, thrives in a distinctive growing environment and forms mycorrhizal symbiosis with K. humilis; artificial cultivation of this mushroom has not yet been achieved. The challenge of artificially domesticating edible mycorrhizal fungi lies in their separation from the symbiotic host plant. When placed under pure culture conditions, these fungi often exhibit slow or stunted growth, emphasizing the importance of the symbiotic system in addressing this issue. There have been successful cases in which Pinus armandii Franch seedlings were inoculated with spore suspensions of Tuber melanosporum Vittad for the synthesis of ectomycorrhizal seedings [89]. Mycorrhizal synthesis of Tricholoma matsutake and pine seedlings can harvest fruiting bodies in field experiments. Although the colonized T. matsutake may be replaced by the original dominant ECM fungi during the growth of pines [90], it is still feasible to artificially construct an efficient symbiotic system of mycorrhizal edible fungi. The artificial domestication study of F. luteovirens can also try to infect the roots of K. humilis with F. luteovirens to observe the potential formation of mycorrhizal seedlings. Another approach is to simulate wild cultivation by artificially sowing the cultivation medium covered with hyphae in the growth areas of K. humilis and other forages to observe the occurrence of hyphae. In China, mycorrhizal edible fungi account for approximately 70% of the total number of edible fungi [11]. Breaking through the challenges of artificially cultivating mycorrhizal edible fungi would not only advance cultivation technology but also have significant economic and practical value.
8.3. Development and Utilization of Edible and Medicinal Value
F. luteovirens has high food and medicinal value. In addition to supplementing nutrition and improving diet balance, it also has the effect of enhancing immunity, making it an ideal raw material for developing health foods. Currently, F. luteovirens fruiting bodies are mostly sold in dried form, and there are few processed products available. By processing the mushrooms into various flavored foods, consumers can have a wider range of choices. Since this mushroom is a local specialty and not well-known nationwide, effective brand building and commercial publicity are necessary to enhance its economic value. F. luteovirens contains a variety of active ingredients, particularly those with antioxidant and anti-tumor properties. If the active ingredients can be isolated and purified, it is expected to lead to the development of new cancer treatment drugs. The advancement of omics technology, including genomics and metabolomics, allows for a more comprehensive and systematic study of these active components. Some researchers have explored the extraction of biosynthetic genes and gene clusters from slow-growing basidiomycetes and transferring them to faster-growing host organisms [91]. This approach provides a new avenue for studying the diversity of metabolites in F. luteovirens and further advancing their development and utilization. Many mushroom strains can be grown in submerged liquid to produce a variety of bioactive substances such as proteins, carbohydrates, enzymes, lipids, etc. These substances find applications in the food industry and drug production [92]. Compared to direct culture of fruiting bodies, this controlled bioreactor culture method is more convenient and efficient. Although it is currently not feasible to artificially cultivate F. luteovirens for mushroom production, a promising approach is to concentrate on the submerged culture of F. luteovirens mycelium for functional components.
9. Conclusions
F. luteovirens is a rare edible mushroom resource growing at an altitude of 3000 m above sea level and forms a symbiotic relationship with K. humilis. It has a high nutritional value, provides a variety of essential nutrients for the human body, and can also regulate the body’s immune ability. Existing studies have demonstrated the antioxidant, anti-aging, and anti-tumor activity of F. luteovirens, as well as its potential in the treatment of diabetes and migraine. F. luteovirens cannot currently be artificially cultivated; thus, we must first safeguard this species to prevent overharvesting and harm to the biological habitat of alpine meadows or grasslands. At the same time, we still require more researchers to thoroughly examine the interaction between F. luteovirens and the grassland ecosystem, solve the puzzle of the fruiting body’s formation mechanism, and investigate the functional component in it in order to create new beneficial health products or medications.
Author Contributions
Conceptualization, X.Z. and Q.Z.; investigation, L.C. and W.L.; resources, Z.Z. and R.F.; data curation, Y.N. and Q.Z.; writing—original draft preparation, Y.N.; writing—review and editing, Y.N.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific and Technological Innovation Talents of Sichuan Province (No. 2022JDRC0034) and the Agricultural Science and Technology Innovation Program (ASTIP-IUA-2023005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article, as no new data was created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, H.-Y.; Lei, J.-Y.; Li, S.-L.; Guo, L.-Q.; Lin, J.-F.; Wu, G.-H.; Lu, J.; Ye, Z.-W. Progress in biological activities and biosynthesis of edible fungi terpenoids. Crit. Rev. Food Sci. 2023, 63, 7288–7310. [Google Scholar] [CrossRef] [PubMed]
- Delseny, M. How did mycorrhizal fungi appear? Comptes. Rendus. Biol. 2020, 343, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agr. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Diao, Z.M. Study on ecology characters and nutrient value of armillaria luteo-virens in Qinghai grassland. Edible Fungi China 1997, 16, 21–22. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhao, L.Z.; Lei, J.Q.; Zhang, F.M. The correlation of geographic distribution and ecologic al environment of endemic species Floccularia luteovirens on Qinghai-Tibet Plateau. Acta Ecol. Sin. 2016, 36, 2851–2857. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.Y. Progress of Research on Medicinal Values of Armillaria Luteo-virens. J. Lishui Univ. 2016, 38, 74–77. (In Chinese) [Google Scholar] [CrossRef]
- Yang, M.J.; Deqing, Y.J.; Zhang, C.X. Growth rule of Floccularia luteovirens. Jiangxi Agric. 2018, 02, 106. (In Chinese) [Google Scholar] [CrossRef]
- Liu, K.; Jiang, J.; Zheng, Q.P.; He, J. Research Progress on Flocularia luteoviren. Edible Fungi China 2019, 38, 1–5+12. (In Chinese) [Google Scholar]
- Dai, Y.C.; Zhou, L.W.; Yang, Z.L.; Wen, H.A.; Tu, L.G.E.; Li, T.H. A revised checklist of edible fungi in China. Mycosystema 2010, 29, 1–21. (In Chinese) [Google Scholar] [CrossRef]
- Gan, X.; Cao, D.; Zhang, Z.; Cheng, S.; Wei, L.; Li, S.; Liu, B. Draft Genome Assembly of Floccularia luteovirens, an Edible and Symbiotic Mushroom on Qinghai-Tibet Plateau. G3 Genes|Genomes|Genet. 2020, 10, 1167–1173. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, H.; Zhang, X.; Chen, Q. The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai–Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation. J. Fungi 2021, 7, 887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Xiong, H.Y.; Sheng, H.Y.; Jiao, Y.C.; Yang, C.J. Comparison of the ecological environment and strains in different ecological regions of wild Armillaria luteovirens in Qinghai Province. Edible Fungi 2007, 29, 9–10. (In Chinese) [Google Scholar]
- Central Siberian Botanical Garden of the Siberian Branch of RAS; Gorbunova, I.A. Tigirek State Nature Reserve New data on agaricoid fungi of the Katunsky State Nature Reserve and rare fungi of the Republic of Altai (Russia). Nat. Conserv. Res. 2017, 2, 43–55. [Google Scholar] [CrossRef][Green Version]
- Arana-Gabriel, Y.; Burrola-Aguilar, C.; Alcalá-Adán, A.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Mycelial growth of the edible wild mushrooms Floccularia luteovirens in different culture mediums and pH. Agro Prod. 2020, 13, 33–38. [Google Scholar] [CrossRef]
- Wang, Q.L.; Han, Y.J.; Feng, C.; Chen, H.; Zhang, L.P. Suitability zoning of Floccularia luteovirens in Shiqu, Sichuan, China. Acta Edulis Fungi 2019, 26, 106–112. (In Chinese) [Google Scholar] [CrossRef]
- Xie, H.M.; Diao, Z.M.; Deng, J. Study on the present resource situation and sustainable development of Armillaria luteo-virens in Qinghai-Tibet Plateau. J. Hanjiang Norm. Univ. 2005, 25, 67–70. (In Chinese) [Google Scholar]
- Xie, Z.L.; Tian, F.; Yu, J.; Nie, S.Y.; Zhao, L.Z.; Zhao, J.W.; Lei, Y.N.; Guo, J. The genetic structure analysis of Floccularia luteovirens using LUS and ITS assay. Mycosystema 2015, 34, 26–37. (In Chinese) [Google Scholar] [CrossRef]
- Xing, R.; Gao, Q.; Zhang, F.; Fu, P.; Wang, J.; Yan, H.; Chen, S. Genetic variation and phylogenetic relationships of the ectomycorrhizal Floccularia luteovirens on the Qinghai-Tibet Plateau. J. Microbiol. 2017, 55, 600–606. [Google Scholar] [CrossRef]
- Li, H.B.; Wu, X.Q.; Wang, L.W. Pure culture isolation, cultivation and molecular identification of Armillaria luteo-virens from Tibet Plateau. Mycostseyema 2008, 27, 873–883. (In Chinese) [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Martins, R.; Carvalho, P.; Miranda, D.; Gonçalves, M.T.; Portugal, A. Edible ectomycorrhizal fungi and Cistaceae. A study on compatibility and fungal ecological strategies. PLoS ONE 2019, 14, e0226849. [Google Scholar] [CrossRef]
- Wang, Q.L.; Jiang, W.B.; Chen, B. Effects of fairy ring growth of Armillaria luteo-virens on soil fertility and plant community. Chin. J. Ecol. 2005, 03, 269–272. (In Chinese) [Google Scholar]
- Guo, J.; Xie, Z.L.; Luo, T.; Xue, Z.F.; Guo, J.J.; Li, F.X.; Zhang, X.J. Comparative study on endophytic fungi diversity of Kobresia humilis in Floccularia luteovirens. Biotechnol. Bull. 2019, 35, 109–117. (In Chinese) [Google Scholar]
- Tong, X.; Fan, K.K.; Yan, Y.C.; Xin, X.P.; Wang, X. Advance inecological research of fairy rings in grassland ecosystem. Chin. J. Agric. Resour. Reg. Plan. 2022, 43, 222–229. (In Chinese) [Google Scholar]
- Duan, M.; Lu, J.; Yang, W.; Lu, M.; Wang, J.; Li, S.; Chen, Y.; Hu, L.; Wang, L. Metabarcoding and Metabolome Analyses Reveal Mechanisms of Leymus chinensis Growth Promotion by Fairy Ring of Leucocalocybe mongolica. J. Fungi 2022, 8, 944. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wang, Q.J.; Jiang, W.B.; Wang, G.; Ma, J.W. The growth of fairy rings of Armillaria luteo-virens and their effect upon grassland vegetation and soil. Acta Prataculturae Sin. 2004, 13, 34–38. (In Chinese) [Google Scholar]
- Choi, J.; Ohnishi, T.; Yamakawa, Y.; Takeda, S.; Sekiguchi, S.; Maruyama, W.; Yamashita, K.; Suzuki, T.; Morita, A.; Ikka, T.; et al. The Source of “Fairy Rings”: 2-Azahypoxanthine and its Metabolite Found in a Novel Purine Metabolic Pathway in Plants. Angew. Chem. Int. Ed. 2014, 53, 1552–1555. [Google Scholar] [CrossRef]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones? Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 29–38. [Google Scholar] [CrossRef]
- Duan, M.; Lu, M.; Lu, J.; Yang, W.; Li, B.; Ma, L.; Wang, L. Soil Chemical Properties, Metabolome, and Metabarcoding Give the New Insights into the Soil Transforming Process of Fairy Ring Fungi Leucocalocybe mongolica. J. Fungi 2022, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, B.; Zheng, S.; Zhang, X.; Wang, X.; Dong, W.; Xie, Q.; Wang, G.; Xiao, Y.; Chen, F.; et al. A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 2021, 184, 5527–5540.e18. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Yan, H.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Microbial communities inhabiting the fairy ring of Floccularia luteovirens and isolation of potential mycorrhiza helper bacteria. J. Basic. Microbiol. 2018, 58, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, F.; Sun, L.; Wang, Y.; Wan, J.; Wang, R.; Zhou, H.; Wang, W.; Xu, J. Floccularia luteovirens modulates the growth of alpine meadow plants and affects soil metabolite accumulation on the Qinghai-Tibet Plateau. Plant Soil. 2021, 459, 125–136. [Google Scholar] [CrossRef]
- Sun, L.; Cao, M.; Liu, F.; Wang, Y.; Wan, J.; Wang, R.; Zhou, H.; Wang, W.; Xu, J. The volatile organic compounds of Floccularia luteovirens modulate plant growth and metabolism in Arabidopsis thaliana. Plant Soil. 2020, 456, 207–221. [Google Scholar] [CrossRef]
- Werner, S.; Polle, A.; Brinkmann, N. Belowground communication: Impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biot. 2016, 100, 8651–8665. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhao, M.; Wei, B.; Hu, T.H.; Yu, Y.W. Effects of fairy ring formation on community vegetation structures and stability in alpine meadows. Acta Prataculturae Sin. 2018, 27, 1–9. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Han, S.; Wang, A. High-throughput sequencing reveals soil bacterial community structure and their interactions with environmental factors of the grassland fairy ring. Env. Microbiol. Rep. 2022, 14, 479–493. [Google Scholar] [CrossRef]
- Guo, J.; Ou, W.Y.; Tang, Y.P.; Zhen, X.J.; Xie, Z.L.; Meng, Q.; Peng, Q.Q.; Wang, B.; Yang, J.B. Effects of Floccularia luteovirens on the microbial community structure in its habitat soil. Mycosystema 2023, 42, 1063–1076. (In Chinese) [Google Scholar] [CrossRef]
- Ren, L.Y.; Pema, Y.; Tenzin, J.M.; Liu, X.L.; Zong, T.G.; Liu, S.Y.; Liu, X.Y.; Puma, D.J. Composition of soil microbial community in the habitat of Floccularia luteovirens in Tibet, southwest China. Mycosystema 2022, 41, 906–917. (In Chinese) [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Sci. Rep. 2017, 7, 43077. [Google Scholar] [CrossRef] [PubMed]
- Han, X.B.; Xie, Z.L. A preliminary study on the growth regularity of Floccularia luteovirens. Jiangsu Agric. Sci. 2015, 43, 219–222. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Z.J. Study on Physiological Characteristics, Important Bioactive Compounds, the Multi-Omics Elucidation and Genetic Characterization of Floccularia luteovirens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2020. (In Chinese). [Google Scholar] [CrossRef]
- Gan, X.; Bao, X.; Liu, B.; Li, Y.; Cao, D.; Zhang, H.; Zong, Y. Chemical Constituents and Molecular Mechanism of the Yellow Phenotype of Yellow Mushroom (Floccularia luteovirens). J. Fungi 2022, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Su, X.; Luo, H.; Ma, R.; Yao, B.; Ma, F. Deciphering lignocellulose deconstruction by the white rot fungus Irpex lacteus based on genomic and transcriptomic analyses. Biotechnol. Biofuels 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-Y.; Lee, S.-Y.; Ryu, S.-H.; Kim, M. Whole-genome de novo sequencing of wood rot fungus Fomitopsis palustris (ATCC62978) with both a cellulolytic and ligninolytic enzyme system. J. Biotechnol. 2017, 251, 156–159. [Google Scholar] [CrossRef]
- France, R.C.; Reid, C.P.P. Pure culture growth of ectomycorrhizal fungi on inorganic nitrogen sources. Microb. Ecol. 1984, 10, 187–195. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Dang, J.; Yan, X.; Yue, H.L.; Wang, Q.L. Study on the separation and domestication of Armillaria luteo-rivens strain. Sci. Technol. Food Ind. 2015, 36, 180–182. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Z.L.; Guo, J.; Meng, J.; Xu, H.Y.; Dai, D.R.; Zhao, Y. The Nutrition Type of Floccularia luteovirens. In Proceedings of the Colorful Fungi Beautiful China—China Mycological Society 2019 Annual Conference Paper Abstract; Mycological Society of China: Beijing, China, 2019; p. 294. (In Chinese) [Google Scholar]
- Xing, R.; Deng, Y.; Yao, Y.; Gao, Q.; Zhang, F.; Wang, J.; Liu, H.; Chen, S. Fine-scale genetic diversity and genet dynamics of the fairy ring fungus Floccularia luteovirens on the Qinghai–Tibet plateau. Fungal Ecol. 2022, 60, 101194. [Google Scholar] [CrossRef]
- Casselton, L.; Feldbrügge, M. Mating and Sexual Morphogenesis in Basidiomycete Fungi. In Cellular and Molecular Biology of Filamentous Fungi; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 536–555. ISBN 978-1-68367-129-9. [Google Scholar]
- Dai, D.R.; Xie, Z.L.; Guo, J.; Mao, Y.J.; Meng, Q. Strain screening and growth characteristics of Floccularia luteovirens. Acta Edulis Fungi 2020, 27, 115–119. (In Chinese) [Google Scholar] [CrossRef]
- Wei, Y.L. Preliminary study on artificial cultivation experiment of wild yellow mushroom. J. Qinghai Meteorol. 2002, 02, 19–24. (In Chinese) [Google Scholar]
- Cai, X.; Zhang, Y.; Wang, X.L. Study on medium optimization of yellow-green Armillaria luteo-virens and condition of artificial culture. Anhui Agric. Sci. Bull. 2013, 19, 33–34. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, L.Y.; Deng, X.W.; Zhu, L.L. Effects of Chinese herbal medicine extract on growth of Armillaria luteo-virens. North. Hortic 2010, 06, 207–208. (In Chinese) [Google Scholar]
- Zhou, J.S.; Jiao, Y.C.; Sheng, H.Y.; Xiong, H.Y.; Yang, C.J. Effect of triacontanol, culture solution pH, and temperature on the growth of Armillaria luteovirens mycelium. Edible fungi 2011, 33, 8–9. (In Chinese) [Google Scholar]
- Zhou, J.S.; Xiong, H.Y.; Sheng, H.Y.; Jiao, Y.C.; Yang, C.J. Effect of the Growth Regulators, Triacontanol and Inositol, on the Growth of Armillaria luteovirens Mycelium. Acta Edulis Fungi 2007, 14, 48–50. (In Chinese) [Google Scholar] [CrossRef]
- Wang, B.; Xie, Z.L.; Dai, D.R.; Mao, Y.J.; Wang, X.F.; Zhen, X.J. Correlation between mycelial growth and microstructure of Floccularia luteovirens. J. Qinghai Univ. 2022, 40, 39–44. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, L.Y.; Gengga, Q.Z.; Tang, D.H.; Mi, Q. Effect of selenium on mycelium biomass of Submerged cultivation of Armillaria flavescens. Edible Fungi 2018, 40, 12–14. (In Chinese) [Google Scholar]
- Hall, I.R.; Yun, W.; Amicucci, A. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 2003, 21, 433–438. [Google Scholar] [CrossRef]
- Huang, Q.H. Artificial culture of Armillaria luteo-rivens. Edible Fungi 1985, 01, 32. (In Chinese) [Google Scholar]
- Li, Y.Z. Cultivation techniques of Qinghai wild Armillaria luteo-rivens. J. Qinghai Norm. Univ. Nat. Sci. 2005, 1, 74–76. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, J.W.; He, L.; Liu, Y.Q.; Bai, J.; Wu, X.Q. Analysis and Evaluation of Nutritional Components of Floccularia luteovirens in Tibet Plateau. Sci. Silvae Sin. 2010, 46, 122–126. (In Chinese) [Google Scholar]
- Cao, Y.W.; Zhaxi, Q.D.; Zhu, X.X.; Wang, S.S.; Ni, Z. Determination and Analysis of Nutrient Ingredients and Antioxidant Capacity of Naqu Armillaria luteo-viren. Food Ind. 2022, 43, 332–335. (In Chinese) [Google Scholar]
- Dang, J.; Wang, Y.; Tao, Y.Z.; Shao, Y.; Mei, L.J.; Wang, Q.L. Determination of Polysaccharide in Armillaria luteo-rivens Mycelium Extract. Chin. J. Spectrosc. Lab. 2011, 28, 2836–2840. (In Chinese) [Google Scholar]
- Liu, W.; Yu, Y.H.; Mao, Y.J.; Meng, L.J.; Liu, H.L. Anaylsis on Isolation, Purification, Component and Structure of Armillariauluteo-virens. J. Chang. Univ. Sci. Technol. 2007, 2, 102–105. (In Chinese) [Google Scholar]
- Tang, C.C.; Zhang, Y.Z.; Dang, J. Determination of Liposoluble Constituents in Armillaria luteo-Virens Acetone Extract by SPE-GC/MS. J. Zhejiang Sci-Tech Univ. Nat. Sci. 2016, 35, 103–108. (In Chinese) [Google Scholar]
- Wang, W.E. GC-MS analysis of supercritical carbon dioxide extraction products from Floccularia luteovirens. Mycosystema 2015, 34, 321–327. (In Chinese) [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.Z.; Dang, J. Chemical constituents from water extract of Armillaria luteo-virens. J. China Pharm. Univ. 2016, 47, 291–293. (In Chinese) [Google Scholar] [CrossRef]
- Jiao, L.; Tao, Y.; Wang, W.; Mei, L.; Shao, Y.; Wang, Q.; Dang, J. Chemical Constituents of Fruit Body of Armillaria luteo-virens. Chem. Nat. Compd. 2019, 55, 373–375. [Google Scholar] [CrossRef]
- Dang, Z. A kind of nutrient-rich edible fungus—Armillaria luteo-virens. Edible Fungi 1992, 01, 42. (In Chinese) [Google Scholar]
- Chen, Q.L.; Diao, Z.M.; Han, Y.Y. The Economic Value and Sustainable Utilization of Armillaria luteo-virens. World Notes Antibiot. 2011, 32, 161–164. (In Chinese) [Google Scholar] [CrossRef]
- Wu, M.Y.; Xu, M.H.; Zhang, X.; Zhu, Q.Y.; Chen, Y.J.; Chen, Q.H.; Liu, Z.J. Optimization of Fermentation Conditions and Biological Activities of Exopolysaccharides from Floccularia luteovirens. J. Nucl. Agric. Sci. 2023, 37, 1598–1608. (In Chinese) [Google Scholar]
- Wang, J.; Zhou, Z.; Dan, D.; Hu, G. Physicochemical properties and bioactivities of Lentinula edodes polysaccharides at different development stages. Int. J. Biol. Macromol. 2020, 150, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Ding, Z.-C.; Gao, X.; Wang, Y.-Y.; Yang, Y.; Wu, D.; Zhang, H.-N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohydr. Polym. 2018, 193, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Siu, K.-C.; Cheung, Y.-C.; Wu, J.-Y. Structure and properties of a (1→3)-β-d-glucan from ultrasound-degraded exopolysaccharides of a medicinal fungus. Carbohydr. Polym. 2014, 106, 270–275. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Q.Y.; Xu, H.M.; Wu, M.Y.; Cheng, X.E.; Cheng, Q.H.; Liu, Z.J. Optimization of extraction process and bioactivity of protoilludane sesquiterpene aryl ester from Floccularia luteovirens. J. Zhejiang Univ. Agric. Life Sci. 2023, 1–12. (In Chinese) [Google Scholar]
- Dang, J.; Chen, C.; Ma, J.; Dawa, Y.; Wang, Q.; Tao, Y.; Wang, Q.; Ji, T. Preparative isolation of highly polar free radical inhibitor from Floccularia luteovirens using hydrophilic interaction chromatography directed by on-line HPLC-DPPH assay. J. Chromatogr. B 2020, 1142, 122043. [Google Scholar] [CrossRef]
- Ma, L. Study on Chemical Constituents and Bioactivity of Armillaria luteo-virens. Master’s Thesis, Tianjin University, Tianjin, China, 2015. (In Chinese). [Google Scholar]
- Liu, Y. The Antitumor Effect and Immunity Function of Polysaccharide from Floccularia luteovirens on Mice. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2014. (In Chinese). [Google Scholar]
- Li, S.; Gao, J.; Hou, L.; Gao, Y.; Sun, J.; Zhang, N.; Fan, B.; Wang, F. The Small Molecule Fractions of Floccularia luteovirens Induce Apoptosis of NSCLC Cells through Activating Caspase-3 Activity. Int. J. Mol. Sci. 2021, 22, 10609. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Q.H.; Ng, T.B.; Liu, H.Z.; Li, J.Q.; Chen, G.; Sheng, H.Y.; Xie, Z.L.; Wang, H.X. Isolation and characterization of a novel lectin from the mushroom Armillaria luteovirens. Biochem. Biophys. Res. Commun. 2006, 345, 1573–1578. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.M.; Chen, C.B.; Li, Y. Assessment of antioxidant and anti-inflammatory potential of the aqueous extract of Floccularia luteovirens in diabetic rats. Mycosystema 2019, 38, 1519–1526. (in Chinese). [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Wang, S.; Li, C.; Chen, C.; Wan, X.; Li, D.; Li, Y. Polysaccharides of Floccularia luteovirens Alleviate Oxidative Damage and Inflammatory Parameters of Diabetic Nephropathy in db/db Mice. Front. Biosci.-Landmrk 2023, 28, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.M.; Chen, C.B.; Li, Y. Anti-inflammatory and analgetic effects of the aqueous extract of Floccularia luteovirens on NTG-induced migraine in rats. Mycosystema 2020, 39, 917–922. (In Chinese) [Google Scholar] [CrossRef]
- Su, K.M.; Zhang, X.L.; Li, F.M.; Li, E.X.; Li, S.H. Study on Mycorrization of Pinus armandii with Tuber melanosporum. Edible Fungi China 2017, 35, 21–23. (In Chinese) [Google Scholar] [CrossRef]
- Park, K.H.; Oh, S.-Y.; Yoo, S.; Park, M.S.; Fong, J.J.; Lim, Y.W. Successional Change of the Fungal Microbiome Pine Seedling Roots Inoculated with Tricholoma matsutake. Front. Microbiol. 2020, 11, 574146. [Google Scholar] [CrossRef]
- Karwehl, S.; Stadler, M. Exploitation of Fungal Biodiversity for Discovery of Novel Antibiotics. In How to Overcome the Antibiotic Crisis; Current Topics in Microbiology and Immunology; Stadler, M., Dersch, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 398, pp. 303–338. ISBN 978-3-319-49282-7. [Google Scholar]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).