Ganoderma Fusions with High Yield of Ergothioneine and Comparative Analysis of Its Genomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stains and Culture Maintenance
2.2. Preparation and Regeneration of Protoplast
2.3. Protoplast Inactivation Labeling and Fusion
2.3.1. Parental Protoplast UV-Inactivation Labeling
2.3.2. Protoplast Fusion and Regeneration
2.4. Fusions Identification
2.4.1. Antagonistic Identification Test
2.4.2. Identification of Putative Fusons by ISSR-PCR
2.4.3. Determination of Ergothioneine Content of Fusions and Parents
2.5. Biological Characteristics of Fusions and Parents
2.5.1. Determination of the Mycelium Growth Rate of Fusions and Parents
2.5.2. Cultivation Characteristics of Fusions and Parents
2.5.3. Determination of Ergothioneine Content in Fusions and Parents
2.6. Re-Sequencing Analysis of Ganoderma Fusions
2.6.1. DNA Extraction and Sequencing
2.6.2. SNP and InDel Calling
3. Results
3.1. Number and Regeneration Rate of Protoplasts
3.2. Results of UV Inactivation and Labeling
3.3. Results of Ganoderma Fusion Identification
3.3.1. The Antagonism Tests
3.3.2. Results of ISSR Primer Screening
3.3.3. Amplification of Genome of Fusions by ISSR PCR
3.3.4. Ergothioneine Content in Fusions and Parents
3.4. Biological Characteristics of Fusions and Parents
3.4.1. Results of Mycelial Growth Stage
3.4.2. Results of Fruiting Body Growth Stage
3.4.3. Determination of EGT Content in Fruiting Body of Fusions and Parental Strains
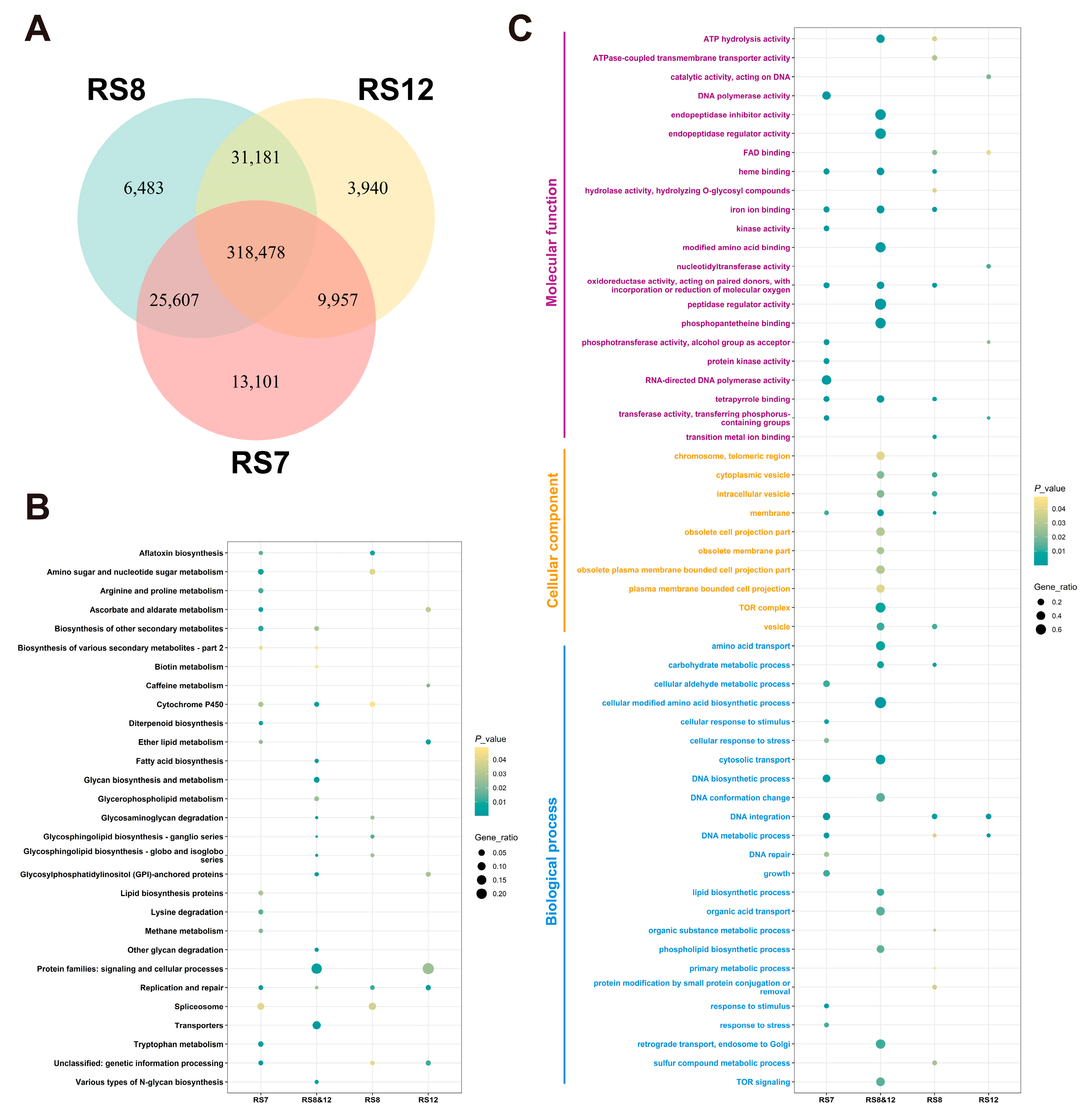
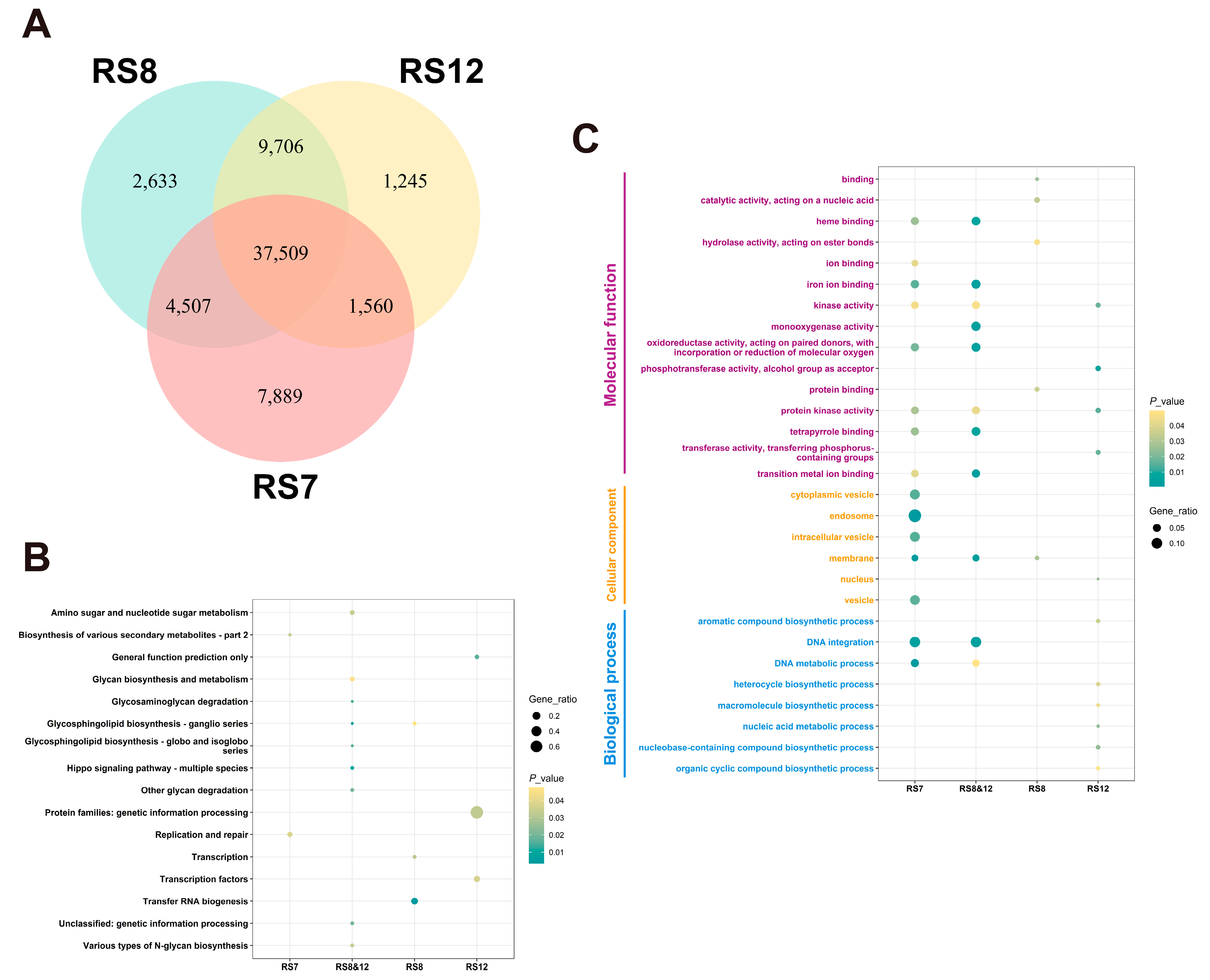
3.5. Results of Re-Sequencing of Ganoderma Fusions
3.5.1. Annotation and Functional Analysis of SNPs and InDels
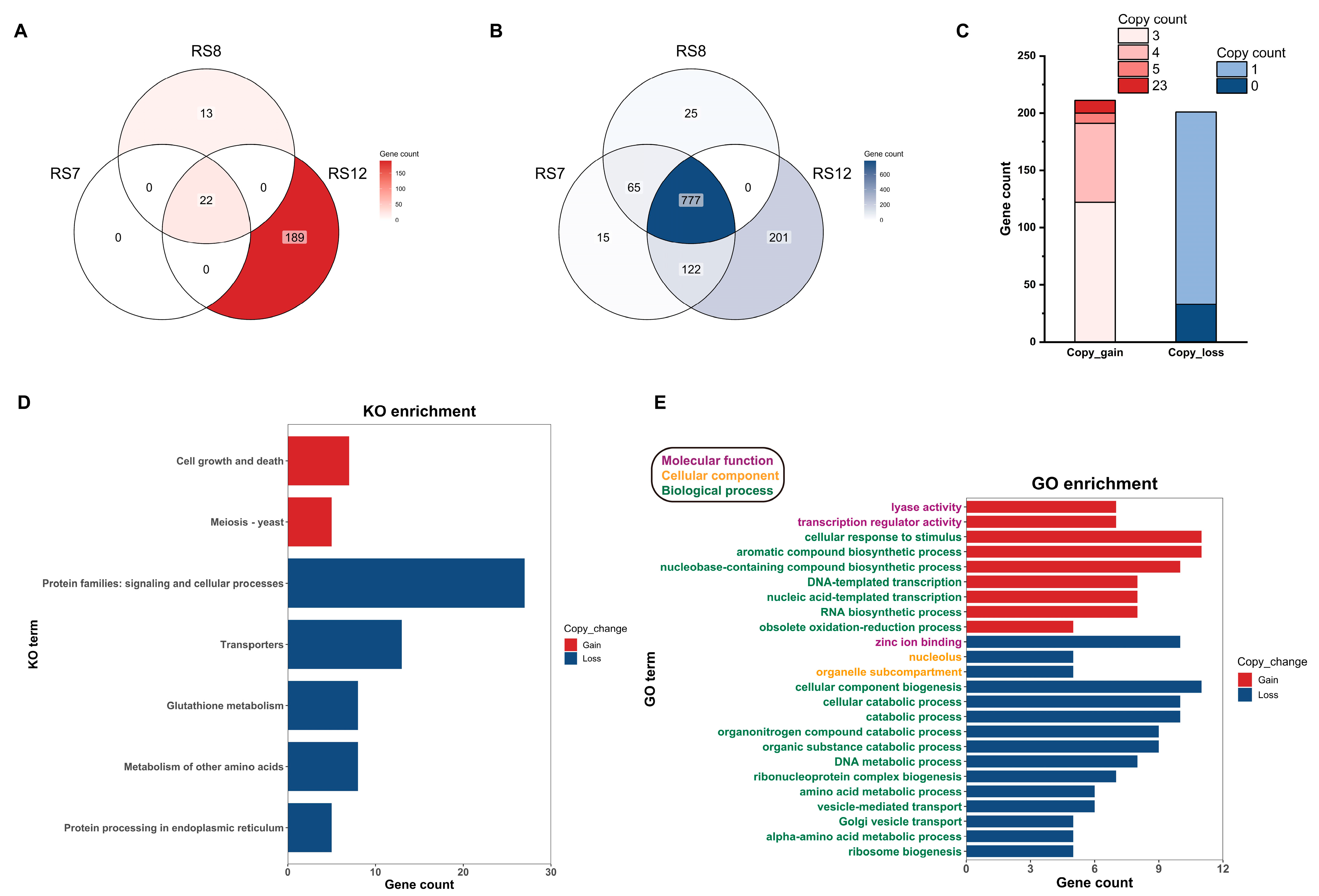
3.5.2. Annotation and Functional Analysis of Genes with Copy Number Changes
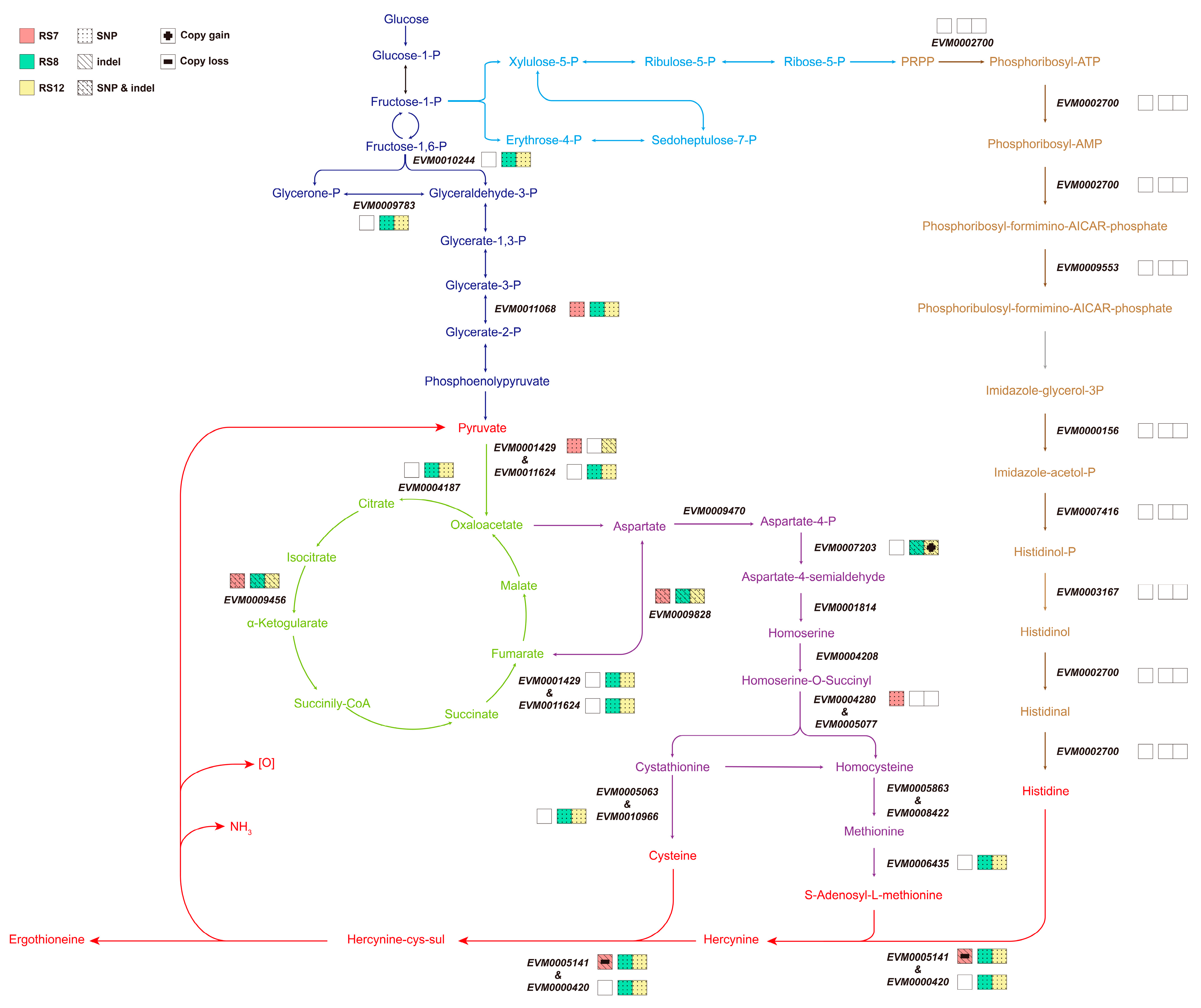
3.5.3. Effect of Gene Mutation on the EGT Synthesis Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanret, C. Sur une base nouvelle retiree du seigle ergote, l’ergothioneine. Rend. Acad. Sci. 1909, 149, 222–224. [Google Scholar]
- Cumming, B.M.; Chinta, K.C.; Reddy, V.P.; Steyn, A.J.C. Role of ergothioneine in microbial physiology and pathogenesis. Antioxid. Redox Signal. 2018, 28, 431–444. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Bioph. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Guo, L.; Lin, J. Recent advances in understanding the in vivo distribution and metabolism of ergothioneine and its roles in disease prevention. Food Sci. 2019, 40, 334–340. [Google Scholar]
- Markova, N.G.; Karaman-Jurukovska, N.; Dong, K.K.; Damaghi, N.; Smiles, K.A.; Yarosh, D.B. Skin cells and tissue are capable of using l-ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009, 46, 1168–1176. [Google Scholar] [CrossRef]
- Nakamichi, N.; Nakayama, K.; Ishimoto, T.; Masuo, Y.; Wakayama, T.; Sekiguchi, H.; Sutoh, K.; Usumi, K.; Iseki, S.; Kato, Y. Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav. 2016, 6, e00477. [Google Scholar] [CrossRef]
- Moncaster, J.A.; Walsh, D.T.; Gentlemen, S.M.; Jen, L.; Aruoma, O.I. Ergothioneine treatment protects neurons against n-methyl-d-aspartate excitotoxicity in an in vivo rat retinal model. Neurosci. Lett. 2002, 328, 55–59. [Google Scholar] [CrossRef]
- Martin, K.R. Bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J. Med. Food 2010, 13, 1340–1346. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Darlis, D.; Jalloh, M.B.; Chin, C.F.S.; Basri, N.K.M.; Besar, N.A.; Ahmad, K.; Rakib, M.R.M. Exploring the potential of bornean polypore fungi as biological control agents against pathogenic Ganoderma boninense causing basal stem rot in oil palm. Sci. Rep. 2023, 13, 10316. [Google Scholar] [CrossRef] [PubMed]
- Bharudin, I.; Ab Wahab, A.F.F.; Abd Samad, M.A.; Xin Yie, N.; Zairun, M.A.; Abu Bakar, F.D.; Abdul Murad, A.M. Review update on the life cycle, plant–microbe interaction, genomics, detection and control strategies of the oil palm pathogen Ganoderma boninense. Biology 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Boh, B.; Berovic, M.; Zhang, J.; Lin, Z.B. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Hapuarachchi, K.K. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere 2018, 9, 1025–1052. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Lin, Z. Development and innovation of ganoderma industry and products in china. In Ganoderma and Health, Biology, Chemistry and Industry; Lin, Z., Yang, B., Eds.; Springer: Singapore, 2019; pp. 187–204. [Google Scholar]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms, a rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, 75–78. [Google Scholar] [CrossRef]
- Chen, S.; Ho, K.; Hsieh, Y.; Wang, L.; Mau, J. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, H.; Lin, S.; Wu, T. Protoplast fusion technology and microbial breeding. Acta Agric. Nucleatae Sin. 2005, 19, 75–79. [Google Scholar]
- Sun, J.; Zhou, D.; Wen, P. The review of the studies on the fusion of inactive microbial protoplast. Chin. Acad. Med. Mag. Org. 2002, 2, 29–32. [Google Scholar]
- Dong, Y.; Miao, R.; Feng, R.; Wang, T.; Yan, J.; Zhao, X.; Han, X.; Gan, Y.; Lin, J.; Li, Y.; et al. Edible and medicinal fungi breeding techniques, a review, current status and future prospects. Curr. Res. Food Sci. 2022, 5, 2070–2080. [Google Scholar] [CrossRef]
- He, P.; Yu, M.; Wang, K.; Cai, Y.; Li, B.; Liu, W. Interspecific hybridization between cultivated morels Morchella importuna and Morchella sextelata by peg-induced double inactivated protoplast fusion. World J. Microbiol. Biotechnol. 2020, 36, 58. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Choi, E.C.; Kim, B.K. Studies on protoplast fusion between Lentinula edodes and Ganoderma lucidum. Arch. Pharm. Res. 1994, 17, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.W.; Luk, V.W.; Yu, S.; Lee, P.; Wai, N.; Fu, P.; Cheung, K.K.W. Artificial hybridization of Ganoderma lucidum and G. Tsugae murrill by protoplast fusion for sustainability. Int. J. Med. Mushrooms 2005, 7, 263–280. [Google Scholar] [CrossRef]
- Wu, X.P.; Liu, F.; Xie, Y.R.; Wang, Z.L.; He, Q.L.; Xie, B.G. Hybridization of Ganoderma lucidum by protoplast monokaryogenesis method. Chin. Agric. Sci. Bull. 2009, 25, 64–69. [Google Scholar]
- He, B.; You, L.; Ye, Z.; Guo, L.; Lin, J.; Wei, T.; Zheng, Q. Construction of novel cold-tolerant strains of Volvariella volvacea through protoplast fusion between Volvariella volvacea and Pleurotus eryngii. Sci. Hortic. 2018, 230, 161–168. [Google Scholar] [CrossRef]
- Chakraborty, U.; Sikdar, S.R. Production and characterization of somatic hybrids raised through protoplast fusion between edible mushroom strains Volvariella volvacea and Pleurotus florida. World J. Microb. Biot. 2008, 24, 1481–1492. [Google Scholar] [CrossRef]
- Al-Samarrai, T.H.; Schmid, J. A simple method for extraction of fungal genomic dna. Lett. Appl. Microbiol. 2000, 30, 53–56. [Google Scholar] [CrossRef]
- Dubost, N.; Ou, B.; Beelman, R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Xu, Q.; You, J.; Lin, J.; Xue, L.; Yu, Y.; Guo, L. A high-throughput detection method of ergothione in Ganoderma spp. Nat. Res. Dev. 2022, 34, 114–120. [Google Scholar]
- Ahlawat, O.P.; Gupta, P.; Dhar, B.L.; Sagar, T.G.; Rajendranath, R.; Rathnam, K. Profile of the extracellular lignocellulolytic enzymes activities as a tool to select the promising strains of Volvariella volvacea (bull. Ex fr.) Sing. Indian J. Microbiol. 2008, 48, 389–396. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows–wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Mckenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit, a mapreduce framework for analyzing next-generation dna sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, snpeff, snps in the genome of Drosophila melanogaster strain w1118, Iso-2, Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology, tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The cog database, a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Kanehisa, M. The kegg resource for deciphering the genome. Nucleic Acids Res. 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blast and psi-blast, a new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Sum, K.; Chen, H.; Li, X.; Zhao, J.; Li, H.; Li, Y. Study on the preparion and protoplast regeneration of Ganaderma lucidum. J. Fungal Res. 2015, 13, 52–54. [Google Scholar]
- Gulli, J.; Kroll, E.; Rosenzweig, F. Encapsulation enhances protoplast fusant stability. Biotechnol. Bioeng. 2020, 117, 1696–1709. [Google Scholar] [CrossRef]
- Jones, G.W.; Doyle, S.; Fitzpatrick, D.A. The evolutionary history of the genes involved in the biosynthesis of the antioxidant ergothioneine. Gene 2014, 549, 161–170. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Z.; Su, E.; Wang, L.; Sun, L.; Lei, P.; Xu, H.; Li, S. Recent strategies for the biosynthesis of ergothioneine. J. Agric. Food Chem. 2021, 69, 13682–13690. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, S.; Lin, J.; Wang, Y.; Lin, J.F.; Guo, L. The biosynthetic pathway of ergothioneine in culinary-medicinal winter mushroom, Flammulina velutipes (agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kamide, T.; Takusagawa, S.; Tanaka, N.; Ogasawara, Y.; Kawano, Y.; Ohtsu, I.; Satoh, Y.; Dairi, T. High production of ergothioneine in Escherichia coli using the sulfoxide synthase from methylobacterium strains. J. Agric. Food Chem. 2020, 68, 6390–6394. [Google Scholar] [CrossRef] [PubMed]
- Stampfli, A.R.; Goncharenko, K.V.; Meury, M.; Dubey, B.N.; Schirmer, T.; Seebeck, F.P. An alternative active site architecture for O2 activation in the ergothioneine biosynthetic egtb from Chloracidobacterium thermophilum. J. Am. Chem. Soc. 2019, 141, 5275–5285. [Google Scholar] [CrossRef]
- Park, S.; Choi, E.; Kim, B. Studies on intergeneric protoplast fusion and nuclear transfer between Ganoderma incidum and Coriolus versicolor. Arch. Pharm. Res. 1991, 14, 282–283. [Google Scholar] [CrossRef]
- Kim, C.; Choi, E.C.; Kim, B.K. Generation of nuclear hybrids overcoming the natural barrier of incompatibility, transfer of nuclei from Lentinula edodes into protoplasts of Coriolus versicolor. Arch. Pharm. Res. 2000, 23, 79–86. [Google Scholar] [CrossRef]
- Dudits, D.; Fejér, O.; Hadlaczky, G.; Koncz, C.; Lázár, G.B.; Horváth, G. Intergeneric gene transfer mediated by plant protoplast fusion. Mol. Gen. Genet. MGG 1980, 179, 283–288. [Google Scholar] [CrossRef]
- Klobutcher, L.A.; Ruddle, F.H. Phenotype stabilisation and integration of transferred material in chromosome-mediated gene transfer. Nature 1979, 280, 657–660. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Cook, P.R.; Harris, H. Correction of a genetic defect in a mammalian cell. Nat. New Biol. 1971, 230, 5–8. [Google Scholar] [CrossRef]
- Lai, W.; Wu, Y.; Yeh, T.; Hsieh, C.; Tsai, Y.; Wei, C.; Li, C.; Lu, Y.; Chang, F. The protoplast two-way fusions and fusant characteristics of Antrodia cinnamomea and Cordyceps militaris. Food Sci. Hum. Wellness 2022, 11, 1240–1251. [Google Scholar] [CrossRef]
- Peng, R.; Fu, Y.; Zou, J.; Qiu, H.; Gan, L.; Yi, H.; Yao, R.; Luo, X. Improvement of polysaccharide and triterpenoid production of Ganoderma lucidum through mutagenesis of protoplasts. Biotechnol. Biotechnol. Equip. 2016, 30, 381–387. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, E.; Ahn, J.K. Supplementation of methionine enhanced the ergothioneine accumulation in the Ganoderma neo-japonicum mycelia. Appl. Biochem. Biotech. 2009, 158, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.; Yang, Y.; Li, Y.; Mau, J.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Lo, Y.; Lin, S.; Ulziijargal, E.; Chen, S.; Chien, R.; Tzou, Y.; Mau, J. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2012, 14, 357–363. [Google Scholar] [CrossRef]


| Primer Name | Sequence (5′-3′) | Tm (°C) |
|---|---|---|
| 810 | GAGAGAGAGAGAGAGAT | 49 |
| 827 | ACACACACACACACACG | 51 |
| 844 | CTCTCTCTCTCTCTCTRC | 52 |
| 846 | CACACACACACACACART | 50 |
| 850 | GTGTGTGTGTGTGTGTYC | 52 |
| 855 | ACACACACACACACACYT | 50 |
| 856 | ACACACACACACACACYA | 50 |
| 858 | TGTGTGTGTGTGTGTGRT | 50 |
| 860 | TGTGTGTGTGTGTGTGRA | 50 |
| 864 | ATGATGATGATGATGATG | 45 |
| 865 | CCGCCGCCGCCGCCGCCG | 72 |
| 867 | GGCGGCGGCGGCGGCGGC | 72 |
| 868 | GAAGAAGAAGAAGAAGAA | 45 |
| 887 | DVDTCTCTCTCTCTCTC | 47 |
| Trial Code | Dry Weight (g) | EGT in Mycelium (mg/g DW) | Total EGT Synthesis (mg/L) |
|---|---|---|---|
| FQ16 | 0.637 ± 0.023 abc | 1.467 ± 0.015 abcd | 9.51 ± 0.38 c |
| FQ23 | 0.460 ± 0.014 a | 1.985 ± 0.020 cd | 9.13 ± 0.21 c |
| RS6 | 0.766 ± 0.093 c | 1.105 ± 0.443 ab | 8.25 ± 2.37 bc |
| RS7 | 0.561 ± 0.096 ab | 2.273 ± 0.063 c | 12.70 ± 1.85 d |
| RS8 | 0.509 ± 0.060 ab | 1.499 ± 0.299 bcd | 7.41 ± 0.26 bc |
| RS10 | 0.507 ± 0.061 ab | 1.046 ± 0.081 ab | 5.34 ± 1.06 ab |
| RS11 | 0.514 ± 0.021 ab | 1.367 ± 0.046 abc | 7.02 ± 0.18 bc |
| RS12 | 0.605 ± 0.022 abc | 1.081 ± 0.281 ab | 6.39 ± 1.56 abc |
| RS14 | 0.708 ± 0.067 bc | 0.932 ± 0.146 ab | 6.28 ± 1.28 abc |
| Strain Number | Mycelium Growth Rate (cm/d) | Full Bag Days (d) | Fresh Weight of Fruiting Body/g | Biotransformation Rate (%) | EGT in Fruiting Body (μg/g) |
|---|---|---|---|---|---|
| FQ16 | 0.71 ± 0.06 abcd | 15.5 ± 0.5 a | 38.56 ± 5.04 bcde | 16.76 ± 2.19 | 7.97 ± 0.81 ab |
| FQ23 | 0.75 ± 0.80 bcd | 14.6 ± 0.7 a | 37.53 ± 2.56 bcde | 16.32 ± 1.11 | / |
| RS6 | 0.79 ± 0.12 cd | 14.3 ± 0.9 a | 42.12 ± 1.67 cde | 18.31 ± 0.73 | 9.21 ± 0.76 abc |
| RS7 | 0.83 ± 0.07 d | 14.2 ± 0.6 a | 42.70 ± 6.60 bc | 18.57 ± 2.87 | / |
| RS8 | 0.69 ± 0.10 abc | 16.2 ± 2.4 ab | 48.16 ± 7.18 b | 20.94 ± 3.12 | / |
| RS10 | 0.74 ± 0.05 bcd | 14.7 ± 0.7 a | 39.82 ± 6.24 de | 17.31 ± 2.71 | 10.06 ± 1.03 abc |
| RS11 | 0.69 ± 0.09 abc | 15.6 ± 1.6 a | 37.27 ± 4.46 e | 16.21 ± 1.94 | 12.24 ± 1.96 cd |
| RS12 | 0.72 ± 0.09 abcd | 14.8 ± 2.4 a | 42.41 ± 3.39 f | 18.44 ± 1.47 | 7.79 ± 0.59 ab |
| RS14 | 0.63 ± 0.08 ab | 16.7 ± 1.9 ab | 36.68 ± 7.11 bcd | 15.95 ± 3.09 | 10.20 ± 1.83 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Yu, Y.; You, J.; Ye, Z.; Zhou, F.; Wang, N.; Zhong, J.; Guo, L.; Lin, J. Ganoderma Fusions with High Yield of Ergothioneine and Comparative Analysis of Its Genomics. J. Fungi 2023, 9, 1072. https://doi.org/10.3390/jof9111072
Xie J, Yu Y, You J, Ye Z, Zhou F, Wang N, Zhong J, Guo L, Lin J. Ganoderma Fusions with High Yield of Ergothioneine and Comparative Analysis of Its Genomics. Journal of Fungi. 2023; 9(11):1072. https://doi.org/10.3390/jof9111072
Chicago/Turabian StyleXie, Jiaqi, Yinghao Yu, Junjiang You, Zhiwei Ye, Fenglong Zhou, Na Wang, Jingru Zhong, Liqiong Guo, and Junfang Lin. 2023. "Ganoderma Fusions with High Yield of Ergothioneine and Comparative Analysis of Its Genomics" Journal of Fungi 9, no. 11: 1072. https://doi.org/10.3390/jof9111072





