Abstract
We assessed fungal diversity in water and sediment samples obtained from five Arctic lakes in Ny-Ålesund (Svalbard Islands, High Arctic) and five Antarctic lakes on Livingston and Deception Islands (South Shetland Islands), using DNA metabarcoding. A total of 1,639,074 fungal DNA reads were detected and assigned to 5980 ASVs amplicon sequence variants (ASVs), with only 102 (1.7%) that were shared between the two Polar regions. For Arctic lakes, unknown fungal taxa dominated the sequence assemblages, suggesting the dominance of possibly undescribed fungi. The phylum Chytridiomycota was the most represented in the majority of Arctic and Antarctic samples, followed by Rozellomycota, Ascomycota, Basidiomycota, and the less frequent Monoblepharomycota, Aphelidiomycota, Mortierellomycota, Mucoromycota, and Neocallimastigomycota. At the genus level, the most abundant genera included psychrotolerant and cosmopolitan cold-adapted fungi including Alternaria, Cladosporium, Cadophora, Ulvella (Ascomycota), Leucosporidium, Vishniacozyma (Basidiomycota), and Betamyces (Chytridiomycota). The assemblages displayed high diversity and richness. The assigned diversity was composed mainly of taxa recognized as saprophytic fungi, followed by pathogenic and symbiotic fungi.
1. Introduction
The Polar regions (both Arctic and Antarctic) share the common characteristics of extremely harsh climate and offer us a unique and irreplaceable platform to discover and study extremophilic organisms [1,2]. A major feature of Arctic landscapes is the large number of lakes and ponds, which in some regions can cover up to 90% of the total surface area [3]. They contribute significantly to Arctic biodiversity, offering a diverse range of habitats for aquatic organisms, from microorganisms to animals and plants, and providing food and freshwater to migratory nesting birds, resident animals, and humans [4]. Also, the Antarctic continent and sub-Antarctic islands represent some of the most diverse and interesting lake districts on the planet [5]. Apart from subglacial lakes, here, lake ecosystems are found in the limited ice-free areas or oases, where microbial communities of viruses, archaea, bacteria, microalgae, and fungi represent the largest reservoir of biodiversity [6].
Polar regions host an extraordinary diversity of lake types, ranging from freshwater to hypersaline, from highly acidic to alkaline and from perennially ice-covered waters to concentrated brines that never freeze. Many polar lakes are ultra-oligotrophic or extremely unproductive but lakes highly enriched by animal or human activities can also be found [7].
High latitude lakes have broad global significance, acting as residences of unique species and communities and as early detectors of environmental change. Multiple stressors associated with local and global human impact such as contaminant influxes, increased exposures to ultraviolet radiation and climate change have a striking impact on these aquatic ecosystems. In fact, even small changes in physical, chemical or biological characteristics can be amplified into major shifts in limnological properties and in lake ecosystem structure and functioning [8,9,10,11]. The combination of the above-mentioned features makes polar lakes interesting and unique environments to study the taxonomy and ecology of microbial communities living under extreme conditions.
Among the different microbial groups present in polar lakes, fungi are the biggest osmotrophic specialists, producing a plethora of secretory enzymes and obtaining nutrients through extracellular digestion and endocytosis. Thanks to diverse metabolic strategies and high morphological diversity, fungi have conquered numerous ecological niches and have shared various interactions with other living organisms and inorganic surfaces. In fact, fungi are found virtually in all environments throughout the globe, including extreme environments, such as torrid and polar deserts [12,13], hypersaline salterns [14], and deep-sea [15].
The global diversity of fungi was first estimated by Hawksworth [16] to be 1.5 million species. However, the increasing development of DNA sequencing technologies which occurred in the last ten years led to expanding the estimates of fungal species numbers to 2.2–13.2 millions. Despite this high estimate diversity, to date, only around 150,000 fungal species have been described [17]. Fungi constitute a well-founded component of terrestrial ecology due to more than 100 years of research that has highlighted their role in biogeochemical cycling and promoting biodiversity [18], while aquatic ecosystems, in contrast, were long overlooked as fungal habitats. However, fungal diversity, quantitative abundance, ecological functions and, in particular, their interactions with other microorganisms remain mainly speculative, unexplored and missing from current general concepts in aquatic ecology and biogeochemistry [19].
Most previous studies of polar lake fungal communities have used traditional culture-dependent methods [1,10,20,21,22,23,24] which do not reveal the full complexity of the resident fungal diversity. Recent applications of metabarcoding approaches have focused especially on sediment of lakes of maritime Antarctica [25,26,27] and there is only one study on water samples from lakes of continental Antarctica [28]. Very few metagenomic studies have been conducted on Arctic lakes, exclusively from water samples [2,29,30].
In this paper, we used a metabarcoding approach to study the microfungal diversity both in water and sediment sampled from Arctic and Antarctic lakes, to study the fungal community composition of these two different lake matrices in both Polar regions deeper, and to better understand their ecological roles in such extreme environments.
2. Materials and Methods
2.1. Study Sites and Samples Description
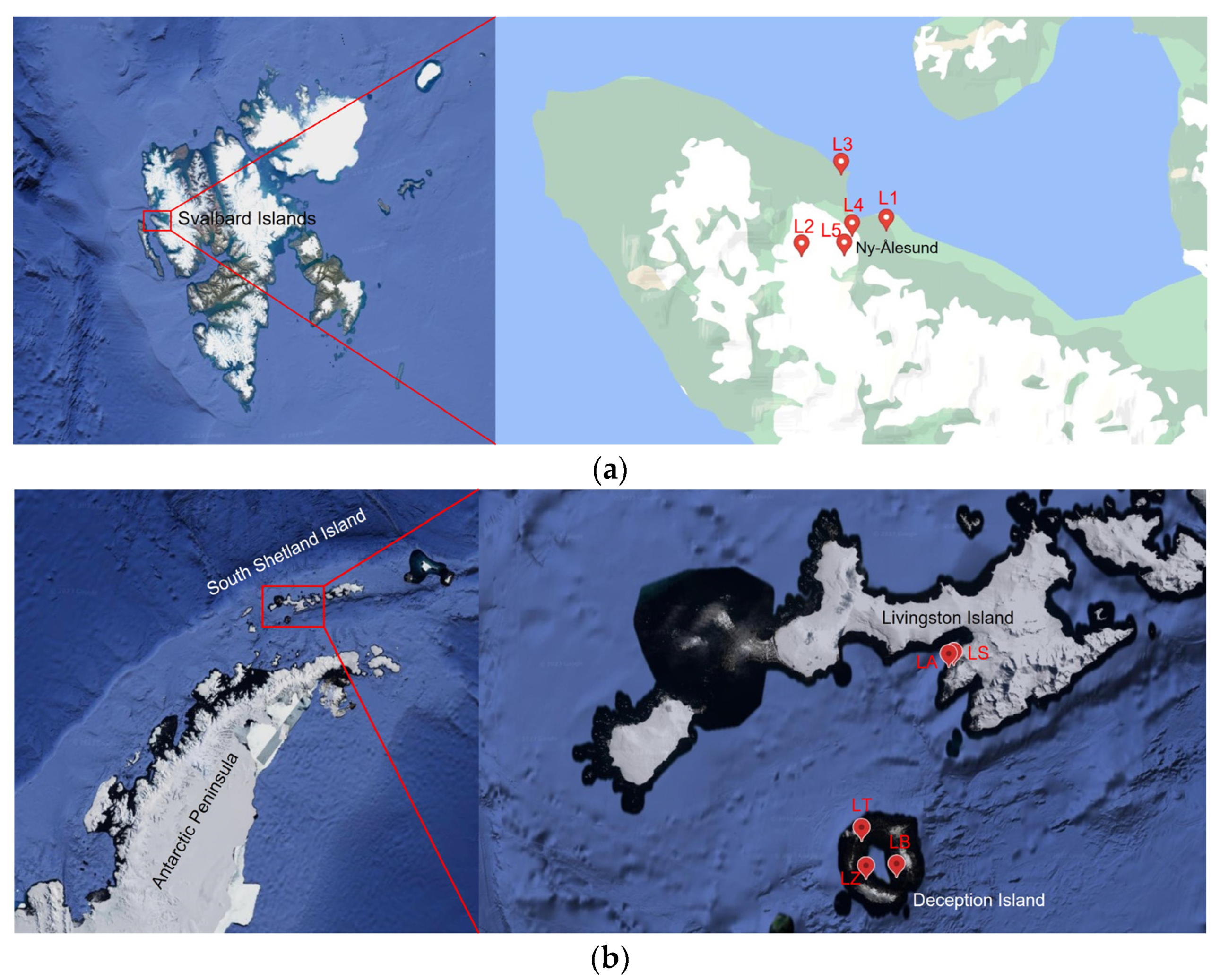
Two sampling campaigns were conducted between 5 and 18 August 2021 in the area of Ny-Ålesund (Svalbard Archipelago, High-Arctic Norway) and between 25 January and 1 February 2022 in Livingston and Deception Islands (South Shetland Islands, Antarctica), respectively. Water and sediment samples were collected from the littoral zone of five Arctic lakes which include Solvannet (L1), Glacier (L2), Knudsenheia (L3), Storvatnet (L4), Tvillingvatnet (L5), and five Antarctic lakes including Argentina (LA), Sofia (LS), Balleneros (LB), Telefon (LT), and Zapatilla (LZ). At each sampling point, the physical–chemical parameters were also measured, particularly temperature, pH, Oxygen (O2%), and conductivity (uS/cm), using manual field probes (a CyberScan PC 300 probe was used for Conductivity, pH, and temperature, while oxygen concentration was measured by a Hanna HI9143 probe). Sampling locations and physical-chemical data for each sampling point are shown in Table 1 and Figure 1.

Table 1.
Geographical and physical–chemical data for each sampling site.
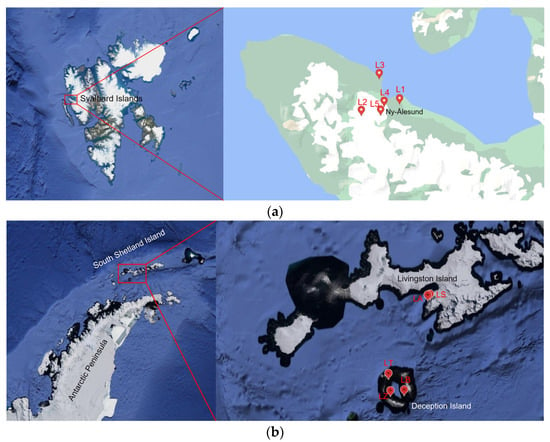
Figure 1.
Maps showing the location of the sampling sites in (a) Ny-Ålesund area (Svalbard Islands) and in (b) Livingston and Deception Islands (Antarctica). L1 = Lake Solvannet, L2 = Lake Glacier, L3 = Lake Knudsenheia, L4 = Lake Storvatnet, L5 = Lake Tvillingvatnet, LA = Lake Argentina, LS = Lake Sofia, LB = Lake Balleneros; LT = Lake Telefon, LZ = Lake Zapatilla.
Water samples (n = 10, identified with “w”) were manually collected using a presterilized 2 L-plastic bottle and immediately transported to the labs of the research station to be processed. Here, 1 L of water samples were filtered on polycarbonate membranes (diameter 47 mm; 0.22 µm pore size), in triplicate, and immediately frozen at −20 °C for transport until sample processing in the laboratory at CNR-ISP of Messina, Italy. Surface sediment samples (n = 10, identified with “s”) were collected at the interface water sediments (water depth of 30–60 cm). The first 10 cm of the surface sediment were sampled using a pre-cleaned scoop and pre-sterilized plastic containers, and immediately transported to the labs of the research station where they were directly stored at −20 °C until sample processing. Samples were than processed in the laboratory at CNR-ISPof Messina, Italy.
2.2. Total DNA Extraction, Bioinformatic Analyses and Fungal Identification
Total DNA was extracted from membranes and from 1 g of sediment using the DNeasy® PowerSoil® Pro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Before the DNA extraction, the samples were gradually thawed at 4 °C. In all DNA extraction steps, we proceeded under strict control conditions within a laminar flow hood to recover the fungal DNA and avoid contaminations. DNA concentrations and purity were quantified by a NanoDrop ND-1000 UV–Vis spectrophotometer. Extracted DNA was used as a template for generating PCR amplicons. The internal transcribed spacer 2 region (ITS2) of the nuclear ribosomal DNA was used as a DNA barcode for molecular species identification. Fungal ITS2 was amplified using the following primers: IlluAdp_ITS31_NeXTf 5′-CATCGATGAAGAACGCAG-3′ and IlluAdp_ITS4_NeXTr5′-TCCTSCGCTTAT TGATATGC-3′ [31]. Sequencing was performed using the Illumina MiSeq platforms, in paired-end form, following the standard protocols of the company EurofinsEurope Services (Germany). FastQC was used to check the quality of raw sequences [32]. Sequences were preprocessed, quality filtered, trimmed, de-noised, merged, modeled, and analyzed by R package DADA2 [33] to infer amplicon sequence variants (ASVs), i.e., biologically relevant variants rather than an arbitrarily clustered group of similar sequences. Particularly we filtered reads by length (minimum length between 150 and 140 bp), by ambiguous bases (no reads with N base were maintained in the analysis), and all sequences were trimmed at the ends after quality control (trimLeft = 17, trimRight = 15). During the analysis, filters for reducing replicate, length, and chimera errors were also applied. Fungal taxonomy annotation was performed using the ITS fungal database, UNITE—Unified system for the DNA based fungal species linked to the classification [34], formatted for DADA2, offering an updated framework for annotating fungal taxonomy (unified system for the DNA based fungal species linked to the classification, identity, similarity used cutoff 95%). Finally, a manual inspection was done, and sequences with an abundance of below 0.1% were considered together in the minor groups of retrieved fungi. All sequences have been submitted to the National Center for Biotechnology Information (NCBI) under the BioProject PRJNA1000778.
2.3. Fungal Diversity, Distribution and Predictive Functional Profiling
The numbers of reads obtained for each water and sediment sample were used to quantify taxon alpha diversity, richness and dominance, using the following indices: Fisher, Shannon, Chao1, ACE, Simpson and InvSimpson. Venn diagrams were prepared using the retrieved ASVs using InteractiVenn online tool [35] to compare the fungal assemblages present in the lake samples. Functional assignments of fungal ASVs at species and genus levels were analysed using tool FUNGuild [36]. FUNGuild v1.1 is a flat database hosted by GitHub (https://github.com/UMNFuN/FUNGuild (accessed on 20 May 2023)), accessible for use and annotation by any interested party under GNU General Public License.
2.4. Statistical Analyses
To compare the fungal community compositions across groups of samples, the Bray–Curtis similarity analysis was performed and similarity matrices were used to obtain dendrograms using R base packages. Principal component analyses (PCAs) were performed using the factoextra R package, on data from selected physical and chemical properties of sediments and waters, and the relative abundance of significant fungal groups. The environmental variables used in these analyses were as follows: oxygen (O2%), temperature (°C), water electrical conductivity (Cond uS/cm), and pH.
3. Results
3.1. Influence of Environmental Paramenters in Lake Clustering
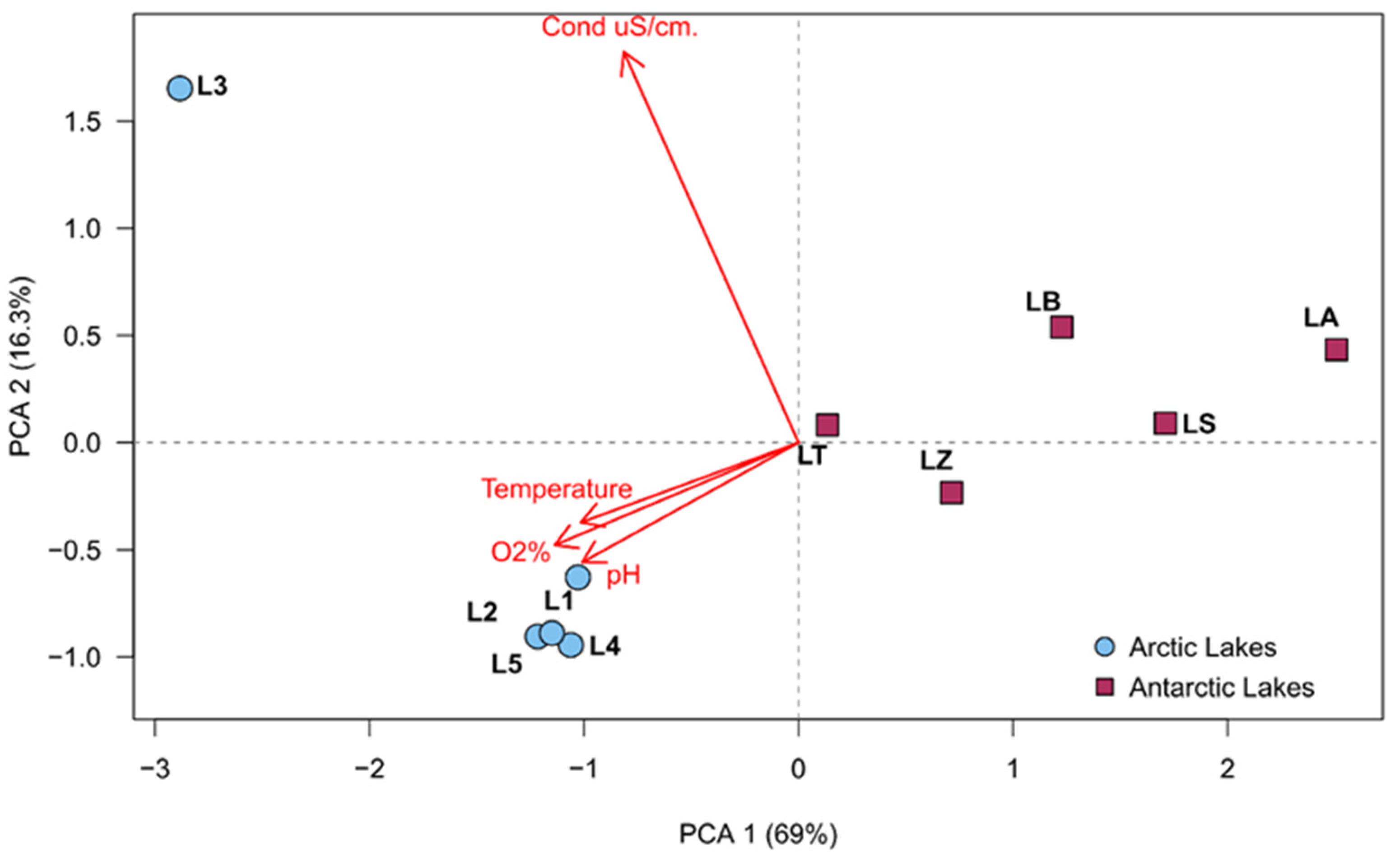
In the principal component analysis of the physico-chemical parameters, it was possible to observe that lakes were completely separated by environmental factors. In fact, the Arctic and Antarctic samples were completely distinct and generated two different groupings, with the only exception represented by L3, which was strictly related to the conductivity, due to its closeness to the sea cost and therefore it is considerably influenced by salt water. Results are shown in Figure 2.
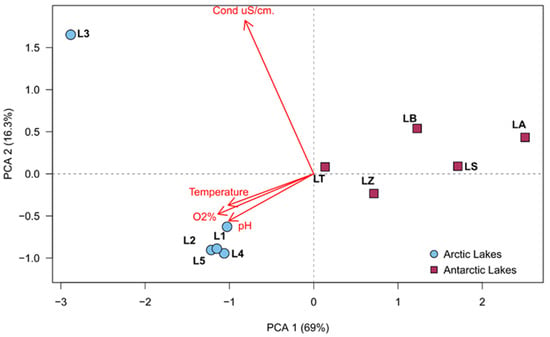
Figure 2.
Principal component analysis obtained by recorded environmental parameters, made by factoextra R package.
3.2. Fungal Taxonomy
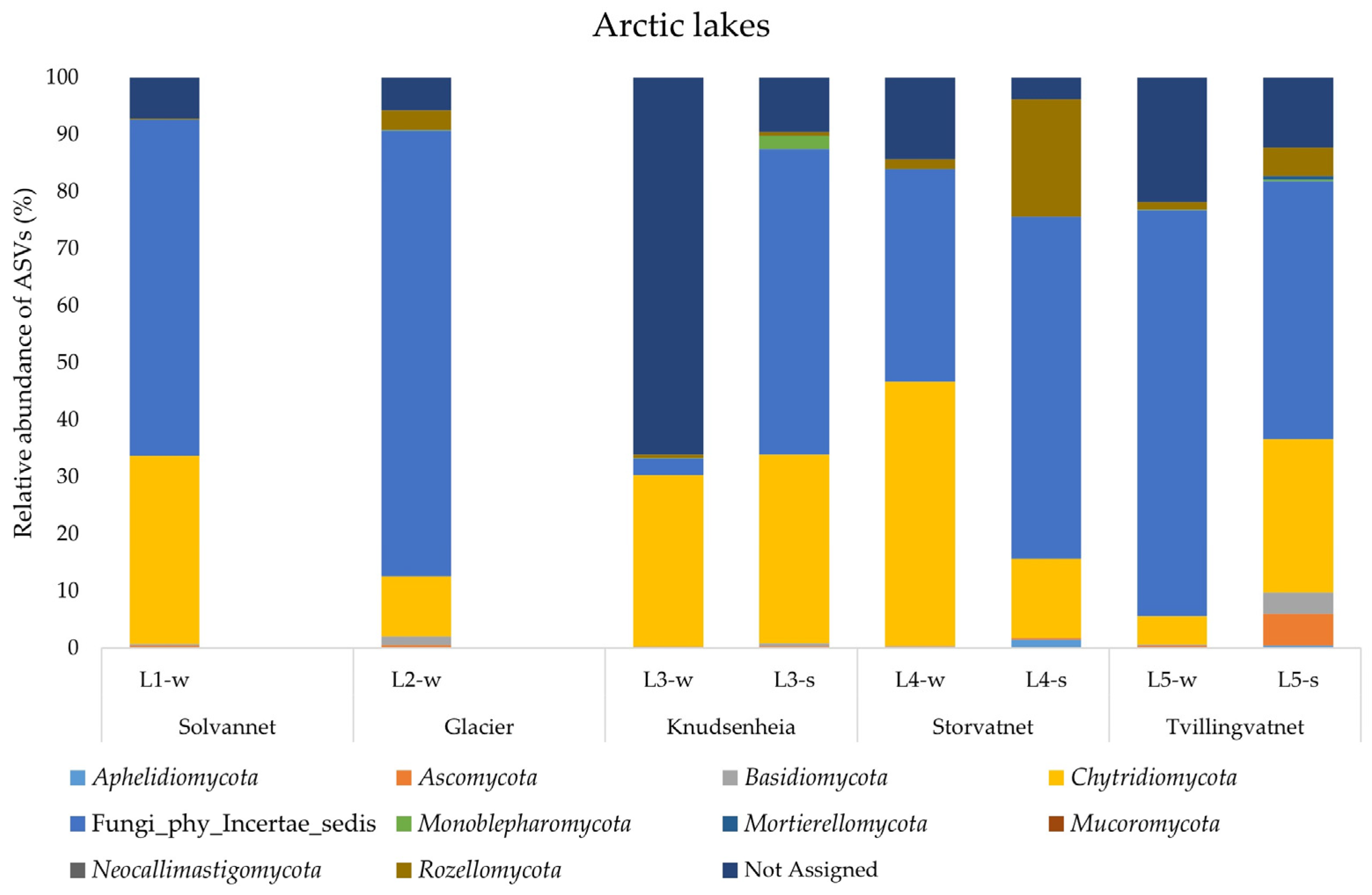
A total of 1,639,074 DNA merged reads of good quality were detected in the water and sediment samples from the ten lakes, with an average length of between 350 and 450 bp, representing 5980 ASVs. Unfortunately, two of the sequenced samples (i.e., L1-s and L2-s) did not produce good results in the first enrichment steps, with creation of a low-quality library and subsequently the impossibility to continue with the NGS sequencing. This was probably due to low the ITS DNA quantity, even though the total extracted DNA showed high concentration and good quality (Table S1). In the Arctic lakes, the analysis of phyla showed that almost in all samples an average of 50% ASVs were related to Fungi_phy_Incertae_sedis, consisting of ASVs whose taxonomical relationships and positions are unknown or not defined. The dominant phylum was represented by Chytridiomycota in all samples examined, except for L4-s, where the phylum Rozellomycota was most represented. The highest value of ASVs assigned to Chytridiomycota was observed in L4-w (46.2%) and the lowest value in L5-w (4.9%). Rozellomycota was the second most represented phylum, but with an uneven distribution: the highest abundance value was 20.5% (L4-s) and the lowest was 0.16% (L1-w). The phyla Ascomycota and Monoblepharomycota were retrieved with percentages higher than 1% only in L5-s (5.49%) and L3-s (2.41%), respectively. Finally, the phylum Basidiomycota was found with percentages higher than 1% in L2-w (1.52%) and L5-s (3.76%). The fungal community structure in water and sediment samples of the Arctic lakes is shown in Figure 3.
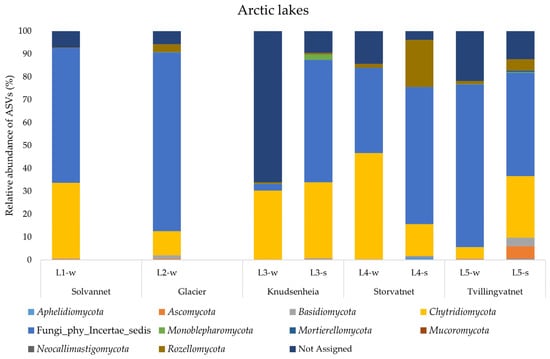
Figure 3.
Fungal community structure at the phylum level in the water and sediment samples from Arctic lakes.
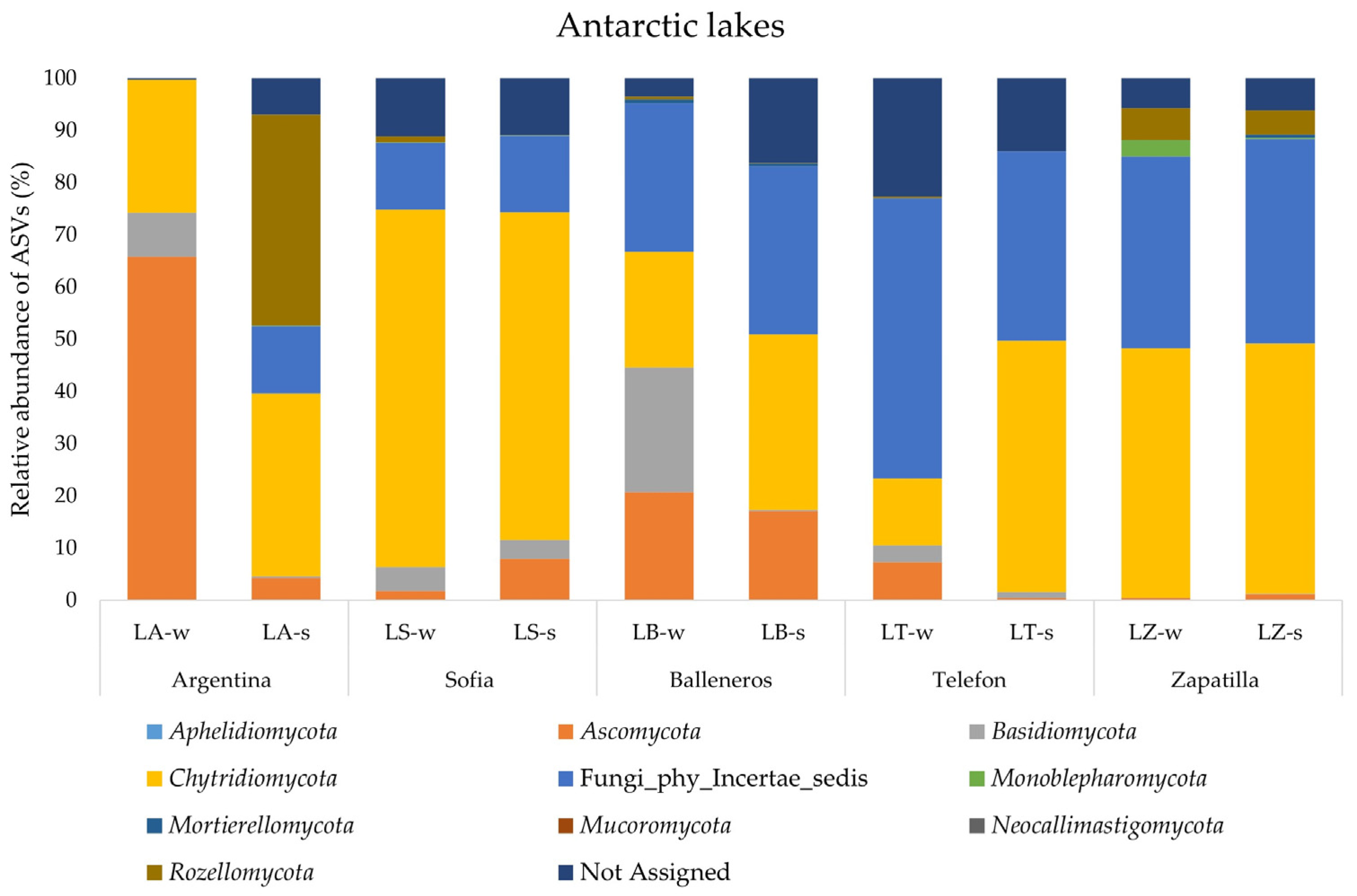
Overall, the same phyla were retrieved in Antarctic samples, but with some crucial differences. The results are shown in Figure 4. First, Fungi_phy_Incertae_sedis showed an average percentage of around 26% and were about completely absent (0.04%) in LA-w, underlining a fungal community composition related mostly to known phyla. In addition, in Antarctic samples Chytridiomycota were the most abundant phylum and ubiquitously distributed in all analyzed samples. Their highest value (68.5%) was retrieved of LS-w and the lowest value was obtained for LT-w (12.85%). The phylum Ascomycota in Antarctic lakes was retrieved with a higher value than the Arctic samples. It was found in all lakes with a value comprised between 65.8% (water of LA) and 0.45% (LZ-w). Phyla Rozellomycota and Monoblepharomycota were retrieved with percentages higher than 1% only in LA-s (40.3%) and LZ-w (3.16%), respectively. Finally, the phylum Basidiomycota was retrieved in all Antarctic samples with an average percentage of 4.5%. Their highest value was found in LB-w (23.9%) and the lowest in LZ-w (0.06%).
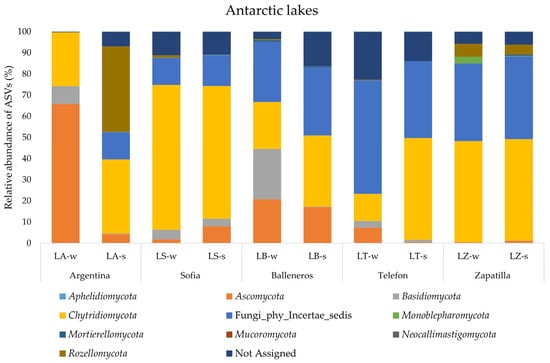
Figure 4.
Fungal community structure at the phylum level in the water and sediment samples from Antarctic lakes.
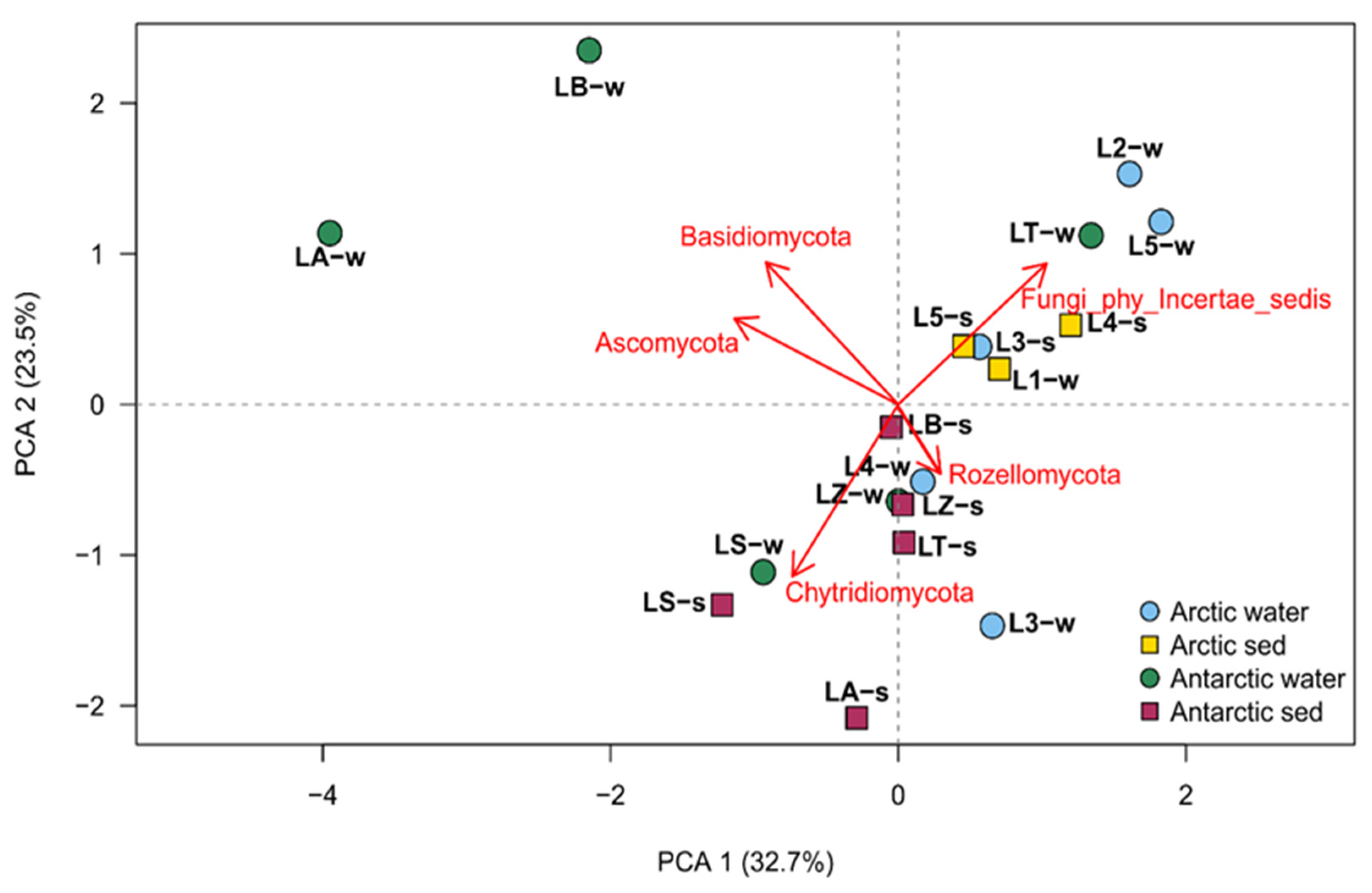
Based on all the retrieved phyla, a principal component analysis (PCA) was performed, and the results were used for selecting fungal taxa that had high variance (Ascomycota, Basidiomycota, Chytridiomycota, Rozellomycota and Fungi_phy_Incertae_sedis). With that, then was performed the construction of the PCA to identify groups of samples with similar community compositions (Figure 5).
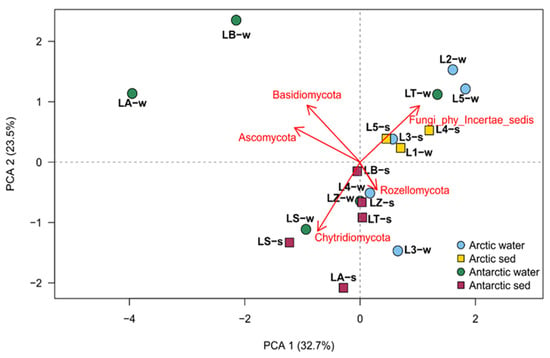
Figure 5.
Principal component analysis obtained with the retrieved phyla information parameters, made by factoextra R package.
The PCA showed a distinction between the Arctic and Antarctic samples, in fact six of the eight Arctic samples clustered together and their separation was driven by Fungi_phy_Incertae_sedis. Two exceptions in the Arctic cluster were water from both L4 and L3 which were found to be related to most Antarctic samples and related to the presence of Rozellomycota. Finally, two water samples of Antarctic region (LA and LB) were completely separated by other groups and noticeably related to the phyla Ascomycota and Basidiomycota.
The most abundant genera (percentage above 1% for at least one sample) were summarized in a heat map (Table 2). Overall, a total of 17 and 50 genera were detected in lakes from the Arctic and Antarctic regions (Table 2), respectively. In the Arctic, L5-s showed most of the genus-wide affiliated sequences. Betamyces was the most abundant genus in the Arctic lakes, and it was retrieved in all analysed samples. In particular, the highest abundance was retrieved in L5-s (16.6%). The genera Pseudeurotium and Amylocorticiellum were retrieved with more than 1% of abundance (2.1% and 2.6%, respectively) in L5-s, together with the genus Leucosporidium (1.4%) in L2-w. The genus Pseudeurotium was present in almost all samples, the genus Leucosporidium was present in half of the Arctic samples, instead Amylocorticiellum and was only found in L5-s. For Antarctic samples, LB-w showed most of the genus-wide affiliated sequences. Cladosporium represented the most abundant genus retrieved, with the highest value that was observed in LA-w (39.5%). Similarly, the genus Cadophora was mainly retrieved in LA-w with a percentage of 19.5%, followed by the genera Malassezia (8.38%) and Alternaria (5.9%), with the latter that was found exclusively in this sample. Ulvella was the most abundant genus in LB-w and LB-s (14.2% and 13.3%, respectively), followed by Vishniacozyma, which was retrieved with a percentage of 7.5% in LB-w. The genus Betamyces was found in all studied samples from Antarctic lakes, exception for LT and from LB-s, with the highest abundance in LZ-s (4.32%). In LS, Coleophoma was the most represented genus, both in sediment (6.51%) and water (1.05%). The ascomycetous genus Metschnikowia was the most abundant (3%) in LT-w. Interesting to note is that even though Arctic samples showed very few numbers of genera, Amylocorticiellum, Helicodendron, Iodophanus, Scolecolachnum (all found in L5-s), Haptocillium (L4-s), Knufia (L3-w), and Zygophlyctis (L1-w) were exclusively present in the Artic and completely absent in Antarctica.

Table 2.
Genera retrieved at a percentage above 1% in Arctic and Antarctic regions.
3.3. Fungal Diversity and Distribution
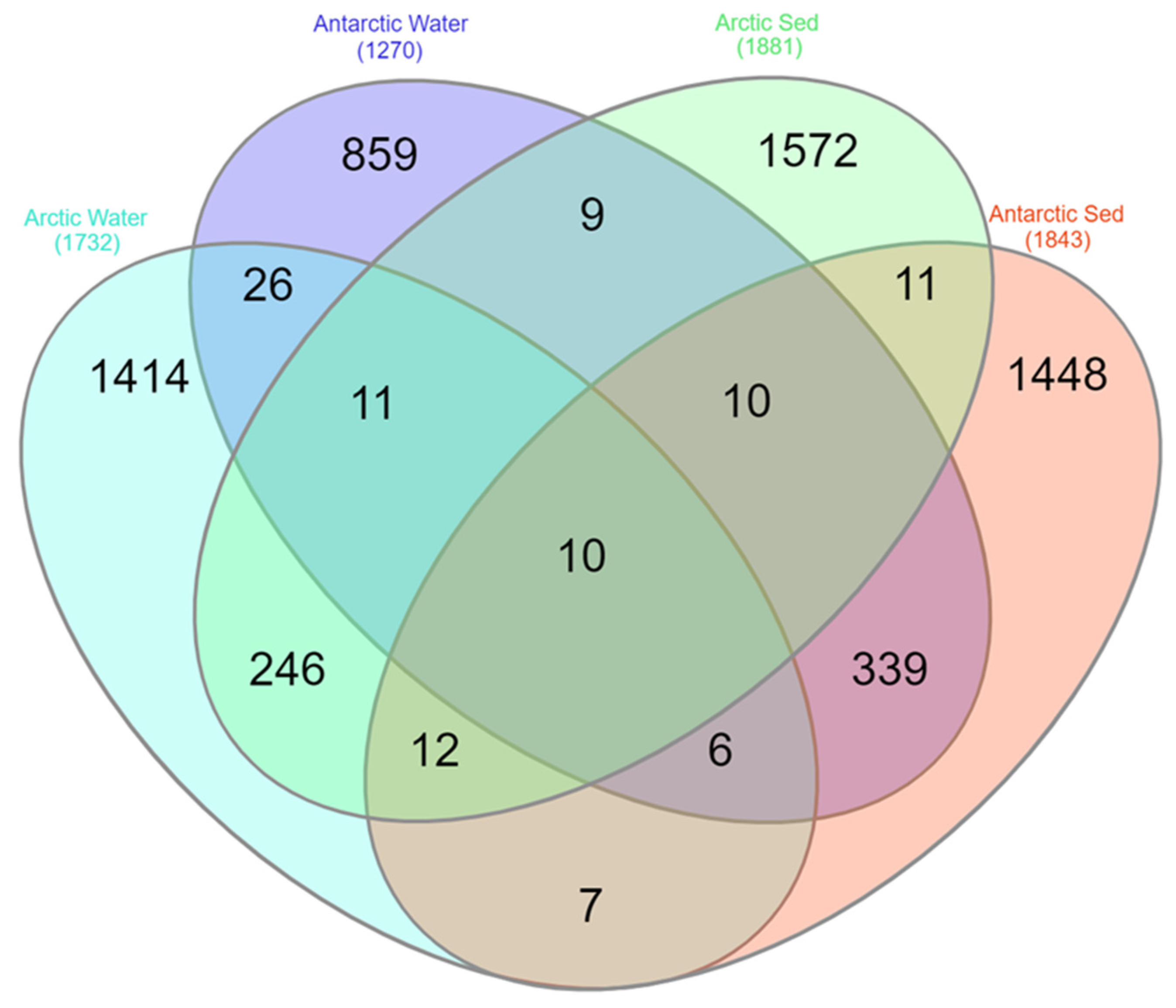
A total of 3334 ASVs were obtained from the Arctic lake samples (both water and sediment) and, of them, only 3 ASVs (0.09%) were shared between the two matrices (Table S2). Overall, lakes L4 vs. L5 and L1 vs. L3 shared the highest percentages of ASVs (7.1%and 6.9%, respectively), while L1 vs. L5 shared the lowest percentages of ASVs (2.8%). Similarly, the difference in ASV distribution between water and sediment samples was evaluated, trying to understand if lakes showed similar populations, or water and sediment have different inhabitants. Considering the two different matrices, 1732 ASVs and 1881 ASVs were obtained from Arctic water and sediment samples, respectively, with 279 ASVs (8.36%) being in common. Comparing the ASVs obtained exclusively from water samples from each Arctic lake, L1-w vs. L3-w shared the highest percentage of ASVs, followed by L2-w vs. L5-w and L4-w vs. L5-w (16.5%, 9.3% and 8.2%, respectively), while the lowest percentages of shared ASVs was observed for samples L3-w vs. L4-w (3.2%) (Table S2). Lower percentages of shared ASVs were obtained from sediment samples L3-s vs. L4-s, L3-s vs. L5-s, and L4-s vs. L5-s (1.6%, 3.4% and 3.8%, respectively) (Table S2). Antarctic lake samples (both water and sediment) gave a total of 2748 ASVs and, of them, 4 ASVs (0.15%) were shared among samples (Table S2). The highest percentages of ASVs were shared between LA vs. LS and LB vs. LT (11.6% and 10.2%, respectively), while LT vs. LZ shared the lowest percentages of ASVs (1.9%). In particular, 1270 ASVs and 1843 ASVs were obtained from Antarctic water and sediment samples, respectively, and a total of 365 ASVs (13.28%) were shared between the two matrices (Table S2). With regard to ASVs obtained exclusively from water samples from each Antarctic lake, LB-w vs. LT-w shared the highest percentage of ASVs (12.5%), while the lowest percentages of shared ASVs was observed for samples LA-w vs. LT-w (1.3%) (Table S2). For ASVs obtained exclusively from sediment samples of each Antartic lake, LA-s vs. LS-s shared the highest percentages of ASVs, followed by LB-s vs. LT-s (13.4 and 7.2%, respectively). Only 0.5% of ASVs were shared between LT-s and LZ-s (Table S2). Considering the two Polar regions, of the total 5980 ASVs retrieved, only 1.7% (102 ASVs) were shared between the Arctic and Antarctic samples (Table S2). The percentage of common ASVs between water and sediment samples were higher than that underlined between the Arctic and Antarctic samples, showing a value of 10.87% (Table S2). Finally, following the above results, the ASVs were examined separated by region (Arctic and Antarctic) and by matrix (water and sediment). The results are shown in Figure 6. Arctic vs. Antarctic water and Arctic vs. Antarctic sediment showed really low percentage of common ASVs (1.79% and 1.16%, respectively). Put differently, water vs. sediment from Arctic and water vs. sediment from Antarctic region, shared higher number of ASVs (8.36% and 13.28%, respectively).
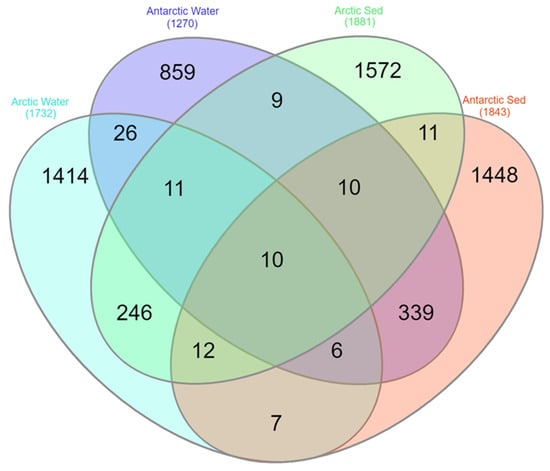
Figure 6.
Venn diagram generated using all the retrieved ASVs separated by region (Arctic and Antarctic) and matrix of origin (water and sediment). The diagram was made by InteractiVenn online tool [35].
The diversity indices were calculated for each water and sediment sample, based on final ASVs obtained after bioinformatics analyses (Table 3). Sediment showed slightly higher diversity values (mean of indices value: Chao1 512.9; ACE 513.3; Shannon 3.9; Simpson 0.9; InvSimpson 18.5; Fisher 72.5) if compared with water (mean of indices value: Chao1 354.1; ACE 354.1; Shannon 3.4; Simpson 0.8; InvSimpson 17.7; Fisher 48.2). The highest value of Shannon index was found in water sample of LS (4.82) (Livingston Island, Antarctica) and a comparable value was found also in the sediment of the same lake (4.49). Instead, the lowest Shannon diversity value of was retrieved in the water sample of L3 (Svalbard Island, High Arctic), and also sediment of the same lake showed a value lower than the average sediment Shannon index value (3.17).

Table 3.
Diversity indices calculated using the total retrieved ASVs.
3.4. Predicitive Functional Profiling of Fungal Communities
In total, a function was assigned to 103 ASVs (accounted for 1.7% of total ASVs). Genera with confidence level of “possible” were classified as “uncertained” and excluded from the functional analyses in this study. Retrieved function were assigned to saprotroph (57%), pathotroph (30.9%) and symbiotroph (12.1%) (Table S3). For the Arctic samples, the functionality was assigned to a minor number of ASVs, and pathotroph and saprotroph showed 3.03% and 6.7% in L2 and, 12.7% and 18.8% in L5, respectively. Particularly, the saprotrophic function was underlined for the ASV 24 affiliated to the taxon Betamyces (L5). Interestingly, it was also observed the assignment of symbiotroph function to a great number of sequences retrieved in LA and related to the taxon Cadophora. In general, a predictive ecological function could be assigned to a very low percentage of ASVs. This was probably due to the fact that our samples come from scarcely studied environments and they have communities composed of a great number of organisms not yet identified.
4. Discussion
Microorganisms are the dominant life forms in the Arctic and Antarctic regions. Amongst the groups of microorganisms occurring in these regions, fungi are one of the most abundant and better distributed in the various environments, playing a crucial role in the micro- and macro food webs. Particularly fungi inhabiting polar lakes play a key role in biogeochemical cycles and the mineralization of organic matter, which are essential for the balance of micro- and macronutrients in lake systems. Many fungal species display multiple stress tolerance capabilities, surviving the combination of low temperatures, high salinity, pH variation, seasonally high UV radiation and low nutrient availability experienced in different polar lakes [1,6,9]. However, despite their importance, the availability of studies of fungal diversity in polar lakes has increased only in recent years, but it still remains scant and fragmentary. To date, this work represents the first study of fungal communities in water and sediment of both Arctic and Antarctic lakes.
4.1. Fungal Diversity
The total fungal community detected through metabarcoding showed, within the analyzed lakes, comparable values of diversity for both Poles (Table 3), underlining no overall difference between the Arctic and Antarctic lakes, although in general, a slightly greater diversity for sediment than for water was observed. Comparable results were obtained by Perini et al. [30], who calculated fungal diversity in waters of a lake from Ny-Ålesund with a Shannon index H′ = 3.27, corresponding to the mean value obtained in this study (H′ = 3.3), while greater values (comprised between H′ = 3.83 and 5.24) were obtained by Zhang et al. [2]. The diversity data of fungal sequence assemblages detected in the sediment of Antarctic lakes LA, LB, LS, LT, and LZ studied here were greater than those reported in previous culture-based studies [1,10,24], and comparable with those reported in DNA metabarcode study by Ogaki et al. [25], de Souza et al. [26], Rosa et al. [27], and Gonçalves et al. [37] for other Antarctic lakes. However, results obtained by de Souza et al. [26] in sediment of Soto Lake, located in Deception Island (Antarctic Peninsula), which hosts three of the lakes examined in the present study (i.e., Balleneros, Telefon and Zapatilla), showed lower diversity indices (Fischer = 10.27). Although comparable diversity was observed between the studied lakes in the two regions (Arctic and Antarctic), a considerable difference was observed in terms of community composition. In fact, only 102 ASVs (out of 5980 ASVs; 1.7%) were shared between the Arctic and Antarctic samples. This result suggests that fungal distribution varies between the lakes in the two Polar regions and each counterpart hosts specific fungal taxa. Not only geographical distance, but also physical-chemical parameters that separate lakes of the two regions (as it is shown in Figure 2) and different sampling time could contribute to shaping the composition of the fungal community in lakes belonging to the two different regions.
4.2. Fungal Phyla
In Arctic lakes, unknown fungi dominated the sequence assemblages, with almost half of the obtained ASVs being assigned to Fungi_phy_Incertae_sedis. This assignation suggests the dominance of possibly undescribed fungi, or that these taxa provide examples of sequences not currently included in publicly accessible databases. The problem arises from the scarcity of metabarcode and metagenome studies of fungal communities in these polar ecosystems, in particular for Arctic lakes. This fact is also corroborated by the results obtained by the PCA of each fungal group for each sample (Figure 5), where almost all fungal assemblages in Arctic water and sediment correlated with the group of Fungi_phy_Incertae_sedis. The most represented identified phyla were, in order, Chytridiomycota, Rozellomycota, Ascomycota, Basidiomycota, which were commonly reported by Comeau et al. [29], Zhang et al. [2] and Perini et al. [30] in Arctic lakes. Less frequent, instead, were Monoblepharomycota, Aphelidiomycota, Mortierellomycota, Neocallimastigomycota and Mucoromycota which were never reported in Arctic lakes, but (excluding the phylum Neocallimastigomycota) were previously detected in Antarctic lakes [25,26,27,37].
In Antarctic samples, the percentage of ASVs assigned to unknown fungi was lower (average of 26.70%) than the ASVs obtained from Arctic samples, probably due to the higher number of studies of fungal communities in Antarctic lacustrine systems that increased considerably in recent years. Chytridiomycota results as the most represented phylum, followed in the order by Ascomycota, Rozellomycota, Basidiomycota, Monoblepharomycota, Mortierellomycota and Aphelidiomycota. Different studies showed that members of the phyla Chytridiomycota and Cryptomycota (Rozellomycota) dominated the fungal community composition in European freshwater lakes [38,39]. Similar results were also obtained in marine and polar freshwater environments [2,29,40] and the recent use of DNA metabarcoding approaches has revealed the presence of Chytridiomycota and Rozellomycota assigned sequences also in Antarctica, with reports from soil [41], air [42], mosses [43], permafrost [44], marine sediments [45], snow [46] and, recently, in lake sediments [25,26,27] and lake water [28]. The phyla Rozellomycota and Chytridiomycota have some physiological advantages for inhabiting aquatic ecosystems, including their mobility and capacity to parasitize numerous phytoplankton species such as diatoms, green algae, dinoflagellates and cyanobacteria [47,48]. Furthermore, the Chytridiomycota are implicated in a variety of ecological processes, such as the transfer of organic matter from phytoplankton into zooplankton via saprophytic and parasitic activity [49]. Taxa in this group are thought to mediate the transfer of organic matter from phytoplankton to zooplankton via saprophytic and parasitic activity described as the “mycoloop” [49]. The phylum Ascomycota was the most represented in water samples of LAwith a percentage of 65.87%. This dominance could be related to the origin of this lake, which derives from the ice-melting of a close glacier. In a recent DNA metabarcoding study [46], it was reported that Ascomycota represents the dominant phylum in Antarctic snow. So, by the water supply from the glacier, this phylum could enrich the fungal composition of the lake. In our study the infrequent phylum Neocallimastigomycota was found exclusively in Arctic lakes, in water of L1 and in sediment of L3, L4 and L5. This phylum was never reported in studies of polar lakes. The members of Neocallimastigomycota are anaerobic-flagellate fungi residing in the rumen and alimentary tract of larger mammalian and some reptilian, marsupial and avian herbivores, where they play an important role in the degradation of plant material [50]. The detection of this phylum in Arctic lakes analysed in this study could be due to the presence of birds or reindeers which visit these lakes.
4.3. Fungal Genera
Generally, it is very difficult to reach the genus level for fungi by metabarcoding analyses, and in our case particularly due to the scarcity of information and deposited sequences of fungi in the environments under consideration. In our study, the most represented genera found in Arctic lakes were Betamyces (Chytridiomycota), Pseuderotium (Ascomycota), Amylocorticiellum and Leucosporidium (Basidiomycota), while Cladosporium, Cadophora, Ulvella, Alternaria, Coleophoma, Metschnikowia for Ascomycota and Vishniacozyma, Malassezia for Basidiomycota were the most represented genera in Antarctic lakes, some of which have previously been reported from different environment in the Polar regions. The genus Betamyces (Chytridiomycota) was retrieved in freshwater ecosystem in Ny-Ålesund (Arctic) [2] and in sediment of Antarctic lakes, where Betamyces spp. dominated the assemblages [37]. The genus includes only one known species, Betamyces americae-meridionalis, which was isolated from pollen baits at the Paraná River (Buenos Aires, Argentina) and in soil in Costa Rica [51]. The genus Pseuderotium (Ascomycota) was previously reported in Arctic aquatic environments (streams, ponds, melting ice water, and estuaries) in the Ny-Ålesund Region using 454 pyrosequencing [2]. In a metabarcode study of a sediment core of Lake Boeckella (Antarctic peninsula) the genus Pseuderotium, with the species P. hygrophilum, represented one of the most abundant taxa [27]. The genus was isolated from different Arctic and Antarctic environments and substrata, such as lakes [1,10,30], active layer of the ice-free oases in continental Antarctica [52], sponges [53], and soil [54]. The genus Leucosporidium was described in the course of investigation of Antarctic heterobasidiomycetes [55]. It comprises 12 species, mostly phychrotolerant or psychrophilic, which occur in plant materials, soils, and marine environments of high and moderate latitudes [56,57,58]. Among Basidiomycota, Leucosporidium was the most represented genus in fungal assemblages of Arctic freshwater [29] and its isolation from Antarctic lakes was previously reported [10,20,21,26,59,60]. The basidiomycetous genus Amylocorticiellus consists of four species with a widespread distibution. The species Amylocorticiellus mollis (known in ‘building mycology’ as Leucogyrophana mollis) resulted as the dominant wood-decaying fungus in samples taken from wooden historic constructions in Svalbard [61]. Cladosporium, Cadophora and Alternaria are melanized ascomycetous fungi distributed worldwide and occupy various ecological niches. They are known to be able to resist harsh environmental conditions such as high temperatures, scarcity of water, and high UV radiation [62]. Most species of this genus are plant pathogens or endophytes [63,64], wood destroyers [61], and soil inhabitants [65]. Some species of the genus Cadophora and Cladosporium are psychrotrophs [66,67] and the presence of these genera are well documented both in Antarctica [6,54] and in the Arctic [2,30,68]. Malassezia is a lipophilous basidiomycete yeast genus typically associated with vertebrate animals, but culture-independent studies revealed their presence in diverse acquatic and terrestrial ecosystems. In particular, Malassezia is reported among the fungal genera with widest distributions across various polar niches [69], having been reported several times by Arctic and Antarctic culture-independent studies [27,30,70,71,72]. The literature about the genus Ulvella is very scarce. There is only one species, U. chlorospila synonym Pyrenula chlorospila Arnold. The genus Pyrenula is a group of crustose lichens typically growing on smooth, shaded bark. It comprises 745 named species with worldwide distribution, most represented in the tropics and Europe [73], but never reported from polar environments. Vishniacozyma is a cosmopolitan genus, and it has been reported in cold environments around the world, including subglacial ice samples from Svalbard Islands [30], and soil and wood in Antarctica [59,60,70,74]. The ascomycetous yeast genus Metschnikowia was frequently reported from polar habitats such as sea ice, invertebrates, macroalgae, marine sediment, sea water [6,75]. M. australis, which is considered an endemic Antarctic species, was isolated from biofilms sampled in Lake Kroner (Deception Island) [24]. The genus Coleophoma includes species reported as plant pathogens, saprophytes or endophytes for different plant species [76], and it was reported from sediment of Lake Wanda and from moss samples from King George Island, Antarctica [22,43].
4.4. Fungal Ecology
In our study, we deeply investigate fungal community composition additionally including the use of functional prediction. As a result, Arctic and Antarctic fungi displayed different ecological roles as saprophytes, mutualists, symbionts, and/or parasites. Saprophytic fungi dominated the assemblages detected in water and sediment of the Arctic and Antarctic lakes examined, followed by plant and animal pathogens and symbionts. Similar results were reported for sediment of lakes on Vega Island, Elephant Island, Deception Island, Jame Ross Island, and Trinity Peninsula [25,26,27,37], all in maritime Antarctica. The same functional ecological profiles were reported in metabarcoding studies of different Antarctic habitats, such as air and snow [42,77], soil [41], freshwater [78] and rock surfaces [79]. According to Schütte et al. [80], fungi present in polar environments display the capability to degrade organic matter at low temperatures, thereby releasing compounds containing carbon, nitrogen, and other elements to other organisms. The dominance of saprophytic fungi in water and sediment of the examined Arctic and Antarctic lakes might indicate, as suggest by de Souza et al. [26] the presence in these environments of a complex saprophytic fungal community that plays a vital role in the decomposition of organic matter under extreme conditions.
5. Conclusions
To date, studies on the fungal communities of lake ecosystems in Arctic and Antarctic regions are decidedly few and the majority of them have been based primarily on traditional culture-dependent approaches, thus underlining a still large gap in the understanding of these sensitive environments. This study is the first to compare fungal communities in water and sediment of Arctic and Antarctic lakes. Metabarcoding analysis revealed complex fungal communities in water and sediment of Polar lakes, which may be considered hotspots of fungal diversity, potentially including new and previously unreported species. The results obtained show clearly distinct communities between the analyzed environments, probably due to the different environmental factors and limnological differences between the analyzed lakes, however, showing some common threads. In particular, the most frequently found phyla are generally ubiquitous, even if for the first time the presence of Neocallimastigomycota is reported in Arctic samples. Furthermore, the lake water and sediment fungal assemblages were dominated by saprophytes, which may contribute to the decomposition of organic matter under extreme conditions. However, further investigation is necessary to better understand the ecological role of freshwater fungi in polar lakes, and in particular their roles in nutrient cycling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9111095/s1, Table S1: Concentration and quality of DNA extracted from water and sediment samples; Table S2: ASVs distribution in Arctic and Antarctic lakes; Table S3: Predictive functional profile of fungal community in Polar lakes.
Author Contributions
Conceptualization M.P., A.L.G. and F.D.L.; methodology A.M. and M.P.; software M.P.; investigation A.M., M.P., A.C.R., A.C. and C.R. (Carlos Rochera); resources A.L.G., C.U., C.R. (Carmen Rizzo), A.C. and M.A.; writing—original draft preparation A.M. and F.D.L.; writing—review and editing A.M., F.D.L., M.P., C.R. (Carlos Rochera) and A.L.G.; supervision F.D.L., M.P. and A.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian National Antarctic Research Program (grant n. PNRA18_00194; project MicroPolArS 2020-2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences have been submitted to the National Center for Biotechnology Information (NCBI) and are associated to the BioProject PRJNA1000778.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gonçalves, V.N.; Vaz, A.B.; Rosa, C.A.; Rosa, L.H. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol. Ecol. 2012, 82, 459–471. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of aquatic fungal communities in the Ny-Ålesund region, Svalbard (High Arctic). Microb. Ecol. 2016, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Pienitz, R.; Doran, P.T.; Lamoureux, S.F. Origin and geomorphology of lakes in the polar regions. In Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems; Vincent, W.F., Laybourn-Parry, J., Eds.; Oxford University Press: New York, NY, USA, 2008; pp. 25–41. [Google Scholar]
- Rautio, M.; Dufresne, F.; Laurion, I.; Bonilla, S.; Vincent, W.F.; Christoffersen, K.S. Shallow freshwater ecosystems of the circumpolar Arctic. Écoscience 2011, 18, 204–222. [Google Scholar] [CrossRef]
- Hodgson, D.A. Antarctic lakes. In Encyclopedia of Lakes and Reservoirs; Bengtsson, L., Herschy, R.W., Fairbridge, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 26–31. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Vieira, R.; Lírio, J.M.; Rosa, C.A.; Rosa, L.H. Diversity and ecology of fungal assemblages present in lakes of Antarctica. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 69–97. [Google Scholar] [CrossRef]
- Vincent, W.F.; Hobbie, J.E.; Laybourn-Parry, J. Introduction to the limnology of high-latitude lake and river ecosystems. In Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems; Vincent, W.F., Laybourn-Parry, J., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 1–24. [Google Scholar]
- Quayle, W.C.; Convey, P.; Peck, L.S.; Ellis-Evans, J.C.; Butler, H.G.; Peat, H.J. Ecological responses of maritime Antarctic lakes to regional climate change. In Antarctic Peninsula Climate Variability: Historical and Palaeoenvironmental Perspectives; Domack, E., Burnett, A., Leventer, A., Convey, P., Kirby, M., Bindschadler, R., Eds.; American Geophysical Union: Washington, DC, USA, 2003; pp. 159–170. [Google Scholar] [CrossRef]
- Rosa, L.H.; Zani, C.L.; Cantrell, C.L.; Duke, S.O.; van Dijck, P.; Desideri, A.; Rosa, C.A. Fungi in Antarctica: Diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Vieira, R.; Muniz, M.C.; Zani, C.L.; Alves, T.; Junior, P.A.; Murt, S.M.F.; Barbosa, E.C.; Oliveira, J.G.; Ceravolo, I.P.; et al. Diversity, ecology, and bioprospecting of culturable fungi in lakes impacted by anthropogenic activities in Maritime Antarctica. Extremophiles 2020, 24, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Rochera, C.; Picazo, A. Effect of experimentally increased nutrient availability on the structure, metabolic activities, and potential microbial functions of a maritime Antarctic microbial mat. Front. Microb. 2022, 13, 900158. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; de Hoog, G.S.; Mazzaglia, A.; Friedmann, E.I.; Onofri, S. Fungi at the edge of life: Cryptendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Gonçalves, V.N.; Cantrell, C.L.; Wedge, D.E.; Ferreira, M.C.; Soares, M.A.; Jacob, M.R.; Oliveira, F.S.; Galante, D.; Rodrigues, F.; Alves, T.M.A.; et al. Fungi associated with rocks of the Atacama Desert: Taxonomy, distribution, diversity, ecology and bioprospection for bioactive compounds. Environ. Microbiol. 2016, 18, 232–245. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Zalar, P.; de Hoog, G.S.; Plemenitaš, A. Hypersaline waters in salterns: Natural ecological niches for halophilic black yeasts. FEMS Microbiol. Ecol. 2000, 32, 235–340. [Google Scholar] [CrossRef]
- Marchetta, A.; Gerrits van den Ende, B.; Al-Hatmi, A.M.S.; Hagen, F.; Zalar, P.; Sudhadham, M.; Gunde-Cimerman, N.; Urzì, C.; de Hoog, G.S.; De Leo, F. Global molecular diversity of the halotolerant fungus Hortaea werneckii. Life 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Hyde, K.D. The numbers of fungi. Fungal Divers. 2022, 114, 1. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Evans, J.C. Fungi from maritime Antarctic freshwater environments. Br. Antarct. Surv. Bull. 1985, 68, 37–45. [Google Scholar]
- Brunati, M.; Rojas, J.L.; Sponga, F.; Ciciliato, I.; Losi, D.; Göttlich, E.; de Hoog, G.S.; Genilloud, O.E.; Marinelli, F. Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar. Genom. 2009, 2, 43–50. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Teixeira, D.R.; Vieira, R.; Lírio, J.M.; Felizardo, J.P.; Abuchacra, R.C.; Cardoso, R.P.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; et al. Diversity and bioprospecting of cultivable fungal assemblages in sediments of lakes in the Antarctic Peninsula. Fungal Biol. 2020, 124, 601–611. [Google Scholar] [CrossRef]
- Connell, L.; Segee, B.; Redman, R.; Rodriguez, R.J.; Staudige, H. Biodiversity and abundance of cultured microfungi from the permanently ice-covered Lake Fryxell, Antarctica. Life 2018, 8, 37. [Google Scholar] [CrossRef]
- de Souza, L.M.D.; Ogaki, M.B.; Texeira, E.A.A.; de Mendes, G.C.A.; Convey, P.; Rosa, C.A.; Rosa, L.H. Communities of culturable freshwater fungi present in Antarctic lakes and detection of their low-temperature-active enzymes. Braz. J. Microbiol. 2022. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Camara, P.E.A.S.; Pinto, O.H.B.; Lirio, J.M.; Coria, S.H.; Vieira, R.; Carvalho-Silva, M.; Convey, P.; Rosa, C.A.; Rosa, L.H. Diversity of fungal DNA in lake sediments on Vega Island, north-east Antarctic Peninsula assessed using DNA metabarcoding. Extremophiles 2021, 25, 257–265. [Google Scholar] [CrossRef]
- de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; de Oliveira, F.S.; Rosa, C.A.; Câmara, P.E.A.S.; Rosa, L.H. Diversity, distribution and ecology of fungal communities present in Antarctic lake sediments uncovered by DNA metabarcoding. Sci. Rep. 2022, 12, 8407. [Google Scholar] [CrossRef]
- Rosa, L.H.; Ogaki, M.B.; Lirio, J.M.; Vieira, R.; Coria, S.H.; Pinto, O.H.B.; Carvalho Silva, M.; Convey, P.; Rosa, C.A.; Camara, P.E.A.S. Fungal diversity in a sediment core from climate change impacted Boeckella Lake, Hope Bay, northeastern Antarctic Peninsula assessed using metabarcoding. Extremophiles 2022, 26, 1–10. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Wurzbacher, C.; Bourne, E.C.; Chiuchiolo, A.; Priscu, J.C.; Grossart, H.P. Early diverging lineages within Cryptomycota and Chytridiomycota dominate the fungal communities in ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Sci. Rep. 2017, 7, 15348. [Google Scholar] [CrossRef]
- Comeau, A.M.; Vincent, W.F.; Bernier, L.; Lovejoy, C. Novel chytrid lineages dominate fungal sequences in diverse marine and freshwater habitats. Sci. Rep. 2016, 6, 30120. [Google Scholar] [CrossRef]
- Perini, L.; Gostinčar, C.; Gunde-Cimerman, N. Fungal and bacterial diversity of Svalbard subglacial ice. Sci. Rep. 2019, 27, 20230. [Google Scholar] [CrossRef]
- Guglielmin, M.; Azzaro, M.; Buzzini, P.; Battistel, D.; Roman, M.; Ponti, S.; Turchetti, B.; Sannino, C.; Borruso, L.; Papale, M.; et al. Possible unique ecosystem in the endoglacial hypersaline brines in Antarctica. Sci. Rep. 2023, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Weißbecker, C.; Schnabel, B.; Heintz-Buschart, A. Dadasnakea Snakemake implementation of DADA2 to process amplicon sequencing data for microbial ecology. GigaScience 2020, 9, giaa135. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; de Oliveira, F.S.; Camara, P.E.A.S.; Rosa, L.H. Diversity and ecology of fungal assemblages present in lake sediments at Clearwater Mesa, James Ross Island, Antarctica, assessed using metabarcoding of environmental DNA. Fungal Biol. 2022, 126, 640–647. [Google Scholar] [CrossRef]
- Monchy, S.; Sanciu, G.; Jobard, M.; Rasconi, S.; Gerphagnon, M.; Chabé, M.; Cian, A.; Meloni, D.; Niquil, N.; Christaki, U.; et al. Exploring and quantifying fungal diversity in freshwater lake ecosystems using rDNA cloning/sequencing and SSU tag pyrosequencing. Environ. Microbiol. 2011, 13, 1433–1453. [Google Scholar] [CrossRef] [PubMed]
- Wurzbacher, C.; Warthmann, N.; Bourne, E.; Attermeyer, K.; Allgaier, M.; Powell, J.R.; Detering, H.; Mbedi, S.; Grossart, H.P.; Monaghan, M.T. High habitat-specificity in fungal communities in oligo-mesotrophic, temperate Lake Stechlin (North-East Germany). MycoKeys 2016, 16, 17–44. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (High Arctic). Sci. Rep. 2015, 5, 14524. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; da Silva, T.H.; Ogaki, M.B.; Pinto, O.H.B.; Stech, M.; Convey, P.; Caravalho-Silva, M.; Rosa, C.A.; Camara, P.E.A.S. DNA metabarcoding high-throughput sequencing uncovers cryptic fungal diversity in soils of protected and non-protected areas on Deception Island, Antarctica. Sci. Rep. 2020, 10, 21986. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; Pinto, O.H.B.; Convey, P.; Caravalho-Silva, M.; Rosa, C.A.; Camara, P.E.A.S. DNA metabarcoding to assess the diversity of airborne fungi present in air over Keller Peninsula, King George Island, Antarctica. Microb. Ecol. 2021, 82, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; da Costa Coelho, L.; Pinto, O.H.B.; Carvalho-Silva, M.; Convey, P.; Rosa, C.A.; Camara, P.E.A.S. Ecological succession of fungal and bacterial communities in Antarctic mosses affected by a fairy ring disease. Extremophiles 2021, 25, 471–481. [Google Scholar] [CrossRef]
- da Silva, T.H.; Camara, P.E.; Pinto, O.H.B.; Carvalho-Silva, M.; Oliveira, F.S.; Convey, P.; Rosa, C.A.; Rosa, L.H. Diversity of fungi present in permafrost in the south Shetland Islands, maritime Antarctic. Microb. Ecol. 2022, 83, 58–67. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Pinto, O.H.B.; Vieira, R.; Neto, A.A.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Camara, P.E.A.S.; Rosa, L.H. Fungi present in Antarctic deepsea sediments assessed using DNA metabarcoding. Microb. Ecol. 2021, 82, 157–164. [Google Scholar] [CrossRef]
- Rosa, L.H.; de Menezes, G.C.A.; Pinto, O.H.B.; Convey, P.; Carvalho-Silva, M.; Simoes, J.C.; Rosa, C.A.; Camara, P.E.A.S. Fungal diversity in seasonal snow of Martel Inlet, King George Island, South Shetland Islands, assessed using DNA metabarcoding. Polar Biol. 2022, 45, 627–636. [Google Scholar] [CrossRef]
- Kagami, M.; de Bruin, A.; Ibelings, B.W.; Van Donk, E. Parasitic chytrids: Their effects on phytoplankton communities and foodweb dynamics. Hydrobiologia 2007, 578, 113–129. [Google Scholar] [CrossRef]
- Ishida, S.; Nozaki, D.; Grossart, H.P.; Kagami, M. Novel basal, fungal lineages from freshwater phytoplankton and lake samples. Environ. Microbial. 2015, 7, 435–441. [Google Scholar] [CrossRef]
- Kagami, M.; Miki, T.; Takimoto, G. Mycoloop: Chytrids in aquatic food webs. Front. Microbiol. 2014, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, R.A.; Dagar, S.; Griffith, G.W.; Pratt, C.J.; Ypussef, N.H.; Elshahed, M.S. Taxonomy of the anaerobic gut fungi (Neocallimastigomycota): A review of classification criteria and description of currenttaxa. Int. J. Syst. Evol. Microbiol. 2022, 72, 005322. [Google Scholar] [CrossRef] [PubMed]
- Letcher, P.M.; Velez, C.G.; Schultz, S.; Powell, M.J. New taxa are delineated in Alphamycetaceae (Rhizophydiales, Chytridiomycota). Nova Hedwig. 2012, 94, 9–29. [Google Scholar] [CrossRef]
- Kochkina, G.A.; Ozerskaya, S.M.; Ivanushkina, N.E.; Chigineva, N.I.; Vasilenko, O.V.; Spirina, E.V.; Gilichinskii, D.A. Fungal diversity in the Antarctic active layer. Microbiology 2014, 83, 94–101. [Google Scholar] [CrossRef]
- Henríquez, M.; Vergara, K.; Norambuena, J.; Beiza, A.; Maza, F.; Ubilla, P.; Araya, I.; Chávez, R.; San-Martín, A.; Darias, J.; et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J. Microbiol. Biotechnol. 2014, 30, 65–76. [Google Scholar] [CrossRef]
- Arenz, B.E.; Blanchette, R.A. Investigations of fungal diversity in wooden structures and soils at historic sites on the Antarctic Peninsula. Can. J. Microbiol. 2009, 55, 46–56. [Google Scholar] [CrossRef]
- Fell, J.W.; Statzell, A.; Hunter, I.L.; Phaff, H.J. Leucosporidium gen. nov. the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie van Leeuwenhoek 1969, 35, 433–462. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Schäfer, A.M.; Begerow, D. Leucosporidium drummii sp. nov., a member of the Microbotryomycetes isolated from soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 728–734. [Google Scholar] [CrossRef]
- Laich, F.; Chávez, R.; Vaca, I. Leucosporidium escuderoi f. a., sp. nov., a basidiomycetous yeast associated with an Antarctic marine sponge. Antonie van Leeuwenhoek 2014, 105, 593–601. [Google Scholar] [CrossRef]
- de García, V.; Coelho, M.A.; Maia, T.M.; Rosa, L.H.; Vaz, A.M.; Rosa, C.A.; Sampaio, J.P.; Gonçalves, P.; van Broock, M.; Libkind, D. Sex in the cold: Taxonomic reorganization of psychrotolerant yeasts in the order Leucosporidiales. FEMS Yeast Res. 2015, 15, fov019. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.B.; Rosa, L.H.; Vieira, M.L.; Garcia, V.D.; Brandão, L.R.; Teixeira, L.C.; Rosa, C.A. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011, 42, 937–947. [Google Scholar] [CrossRef]
- Tsuji, M.; Fujiu, S.; Xiao, N.; Hanada, Y.; Kudoh, S.; Kondo, H.; Tsuda, S.; Hoshino, T. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctic. FEMS Microbiol. Lett. 2013, 346, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Flyen, A.-C.; Nunez, M. Wood-decaying fungi in protected buildings and structures on Svalbard. Agarica 2010, 29, 5–14. [Google Scholar]
- De Leo, F.; Marchetta, A.; Urzì, C. Black fungi on stone-built Heritage: Current knowledge and future outlook. Appl. Sci. 2022, 12, 3969. [Google Scholar] [CrossRef]
- Walsh, E.; Duan, W.; Mehdi, M.; Naphri, K.; Khiste, S.; Scalera, A.; Zhang, N. Cadophora meredithiae and C. interclivum, new species from roots of sedge and spruce in a western Canada subalpine forest. Mycologia 2018, 110, 201–214. [Google Scholar] [CrossRef]
- Zabouri, Y.; Cheriguene, A.; Chougrani, F.; Merzouk, Y.; Marchetta, A.; Urzi, C.; De Leo, F. Antifungal activity of lactic acid bacteria against phytopathogenic Alternaria alternata species and their molecular characterization. J. Food Nutr. Res. 2021, 60, 18–28. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of soil fungi. Eur. J. Soil Sci. 2007, 59, 1007. [Google Scholar] [CrossRef]
- Iliushin, V.A. First find of Cadophora antarctica Rodr. Andrade, Stchigel, Mac Cormack & Cano in the Arctic. Czech Polar Rep. 2020, 10, 147–152. [Google Scholar] [CrossRef]
- Brück, S.A.; Contato, A.G.; Gamboa-Trujillo, P.; de Oliveira, T.B.; Cereia, M.; de Moraes Polizeli, M.L.T. Prospection of psychrotrophic filamentous fungi isolated from the High Andean Paramo Region of Northern Ecuador: Enzymatic activity and molecular identification. Microorganisms 2022, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Kirtsideli, I.Y.; Vlasov, D.Y.; Barantsevich, E.P.; Krylenkov, V.A.; Sokolov, V.T. Microfungi from soil of polar island Izvestia Tsik (Kara Sea). Mikol. I Fitopatol. 2014, 48, 365–371. [Google Scholar]
- Zalar, P.; Gunde-Cimerman, N. Cold-Adapted Yeasts in Arctic Habitats in Cold-Adapted Yeasts. In Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 49–74. [Google Scholar]
- Arenz, B.E.; Held, B.W.; Jurgens, J.A.; Farrell, R.L.; Blanchette, R.A. Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil. Biol. Biochem. 2006, 38, 3057–3064. [Google Scholar] [CrossRef]
- Bridge, P.D.; Spooner, B.M. Non-lichenized Antarctic fungi: Transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012, 5, 381–394. [Google Scholar] [CrossRef]
- Connell, L.B.; Staudigel, H. Fungal diversity in a dark oligotrophic volcanic ecosystem (DOVE) on Mount Erebus, Antarctica. Biology 2013, 2, 798–809. [Google Scholar] [CrossRef]
- Aptroot, A. A world key to the species of Anthracothecium and Pyrenula. Lichenologist 2012, 44, 5–53. [Google Scholar] [CrossRef]
- Connell, L.; Redman, R.; Craig, S.; Scorzetti, G.; Iszard, M.; Rodriguez, R. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb. Ecol. 2008, 56, 448–459. [Google Scholar] [CrossRef]
- Butinar, L.; Strmole, T.; Gunde-Cimerman, N. Relative incidence of ascomycetous yeasts in Arctic coastal environments. Microb. Ecol. 2011, 61, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Groenewald, J.Z. They seldom occur alone. Fungal Biol. 2016, 120, 1392–1415. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; Pinto, O.H.B.; Šantl-Temkiv, T.; Convey, P.; Caravalho-Silva, M.; Rosa, C.A.; Camara, P.E.A.S. DNA metabarcoding high-throughput sequencing of fungal diversity in air and snow of Livingston Island, South Shetland Islands, Antarctica. Sci. Rep. 2020, 10, 21793. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.M.D.; Ogaki, M.B.; Câmara, P.E.A.S.; Pinto, O.H.B.; Convey, P.; Caravalho-Silva, M.; Rosa, C.A.; Rosa, L.H. Assessment of fungal diversity present in lakes of Maritime Antarctica using DNA metabarcoding: A temporal microcosm experiment. Extremophiles 2021, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, G.C.A.; Câmara, P.E.A.S.; Pinto, O.H.B.; Caravalho-Silva, M.; Oliveira, F.S.; Souza, C.D.; Schaefer, C.E.G.R.; Convey, P.; Rosa, C.A.; Rosa, L.H. Fungal diversity present on rocks from a polar desert in continental Antarctica assessed using DNA metabarcoding. Extremophiles 2021, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Schütte, U.M.; Henning, J.A.; Ye, Y.; Bowling, A.; Ford, J.; Genet, H.; Waldrop, M.P.; Turetsky, M.R.; White, J.R.; Bever, J.D. Effect of permafrost thaw on plant and soil fungal community in a boreal forest: Does fungal community change mediate plant productivity response? J. Ecol. 2019, 107, 1737–1752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).