Palm Fungi and Their Key Role in Biodiversity Surveys: A Review
Abstract
1. Introduction
2. Historical Account of Research on Palm Fungi and Reflections on Their Importance
2.1. History of Systematic and Descriptive Taxonomy Studies on Palm Fungi
2.1.1. From Scattered to the First Systematic Reports on Palm Fungi
2.1.2. Hyde and Co-Workers and the Extensive Studies on Palm Fungi from Tropical Regions
2.1.3. The Palmicolous Hyphomycetes from Central American Countries
| Genus | Type Species | Host | Country/Region | Sequence Data 1 | Reference |
|---|---|---|---|---|---|
| Acarocybellina | A. arengae | On a dead leaf of Arenga engleri | Japan | N/A | [259] |
| Acarocybiopsis | A. cubitaensis | On a dead trunk of Roystonea regia | Cuba | N/A | [260] |
| Acuminatispora | A. palmarum | On decaying petioles and rachides of an unidentified palm in mangrove | Thailand | A | [261] |
| Agrabeeja | A. kavakapriya | On synnemata of Melanographium citri on a rachis of Korthalsia grandis | Singapore | N/A | [262] |
| Allodiatrype | A. arengae | On a dead petiole of Arenga pinnata | Thailand | A | [263] |
| Anabahusakala | A. amapensis | On decaying leaves of Syagrus sp. | Brazil (Amapá) | N/A | [264] |
| Anisospadicoides | A. macrocontinua (as Spadicoides macrocontinua) | On a rotten petiole of an unidentified palm | Peru | N/A | [64,265] |
| Apioclypea | A. livistonae | On a rachis of Livistona sp. | Papua New Guinea | N/A | [175] |
| Apogaeumannomyces | A. perplexus | On a decaying frond of an unidentified palm | Peru | N/A | [67] |
| Appendicospora | A. coryphae | On dead rachides of Corypha elata | Philippines | N/A | [125] |
| Appendispora | A. frondicola | On a dead rachis of Oncosperma horridum on forest floor | Brunei | N/A | [115] |
| Arecacicola | A. calami | On a trunk of Calamus sp. | Indonesia (Java) | N/A | [185] |
| Arecomyces | A. frondicola | On a rachis of Arenga undulatifolia | Brunei | N/A | [138] |
| Arecophila | A. gulubiicola | On a dead trunk of Gulubia costata | Papua New Guinea | N/A | [131] |
| Ashtaangam | A. Sundaram | On a rachis of an unidentified palm | Malaysia | N/A | [266] |
| Astrosphaeriellopsis | A. bakeriana | On a petiole of Borassus sp. | Thailand | A | [267] |
| Asymmetricospora | A. calamicola | On a dead stem of Calamus caryotoides | Australia (Queensland) | N/A | [141] |
| Atrosetaphiale | A. flagelliformis | On a decayed petiole of an unidentified palm | Peru | N/A | [65] |
| Aunstrupia | A. nodipes | On rotten and dead leaves and rotten petiole and branches of unidentified palms | China (Guangdong) | A | [268] |
| Bacusphaeria | B. nypae | On a petiole base of Nypa fruticans | Malaysia | A | [269] |
| Baipadisphaeria | B. spathulospora | On a trunk of Licuala longicalycata submerged in peat bog | Thailand | A | [270] |
| Basauxia | B. pulchra | On a rachis of an unidentified palm | Malaysia | N/A | [266] |
| Bhadradriella | B. hyalina | On fallen pods of Roystonea regia | India (Andhra Pradesh) | N/A | [271] |
| Brachysporiopsis | B. chinensis | On a decaying rachis of Livistona chinensis | China (Hong Kong) | N/A | [228] |
| Brobdingnagia | B. nigeriensis | On tissues of Calamus sp. | Nigeria | N/A | [212] |
| Brunneiapiospora | B. javensis | On a rachis of Calamus sp. | Indonesia (Java) | N/A | [143] |
| Bulbocatenospora | B. complanata | On fallen leaves of Bactris setulosa | Venezuela | N/A | [272] |
| Cannonia | C. australlis | On rotten branches of Butia yatay | Argentina | N/A | [204] |
| Capsulospora | C. frondicola | On a rachis of Daemonorops sp. | Brunei | N/A | [134] |
| Carinispora | C. nypae | On decaying intertidal fronds of Nypa fruticans | Brunei | N/A | [162] |
| Castanedospora | C. pachyanthicola | On the petiole of a dead leaf of Sabal palmetto | USA (Florida) | A | [273] |
| Caudatispora | C. palmicola | On a dead rachis of Phytelaphas | Ecuador | N/A | [119] |
| Cenangiumella | C. rattanicola | On a dead rattan sheath of Calamus conirostris | Brunei | N/A | [6] |
| Chitinasiproducens | C. palmae | ||||
| Circinoconiopsis | C. amazonica | On decaying leaves of Oenocarpus sp. | Brazil (Pará) | N/A | [274] |
| Cocoicola | C. cylindrospora | On petioles of Cocos nucifera | Papua New Guinea | N/A | [123] |
| Corynesporasca * | C. caryotae | On rotting leaves of Caryota urens | Sri Lanka | N/A | [275] |
| Curvatispora | C. singaporensis | On a fallen decaying frond of Livistona spinosa | Singapore | N/A | [153] |
| Cyanopulvis | C. australiensis | On a dead rattan of Calamus australis | Australia (Queensland) | N/A | [6] |
| Cylindrotorula | C. indica | On a decaying spathe of Cocos nucifera | India (Maharashtra) | A | [276] |
| Diabolocovidia | D. claustri | On leaves of Serenoa repens | USA (Florida) | A | [277] |
| Dictyopalmispora | D. palmae | On decaying leaves of Licuala longicalycata | Thailand | A | [278] |
| Discopycnothyrium | D. palmae | On the branches of an unidentified palm | Thailand | A | [279] |
| Durispora | D. elaeidicola | On dead rachides of Elaeis guineensis | Malaysia | N/A | [118] |
| Dwibahubeeja | D. indica | On leaves of C. tenuis | India (Uttar Pradesh) | N/A | [280] |
| Endosporoideus | E. pedicellatus (as E. pedicellata) | On a dead petiole of Phoenix hanceana | China (Hong Kong) | N/A | [235] |
| Fasciatispora | F. nypae | On a rotten frond of intertidal Nypa fruticans | Brunei | A | [161] |
| Fissuroma | F. maculans | On dead leaves of Arenga westerhoutii | Thailand | A | [281] |
| Flammispora | F. bioteca | On dead leaves of Licuala longicalycata submerged in peat swamp | Thailand | A | [282] |
| Fluviatispora | F. tunicata | On submerged rachides of Livistona sp. | Papua New Guinea | N/A | [174] |
| Frondicola | F. tunitricuspis | On decaying fronds of Nypa fruticans | Brunei | N/A | [162] |
| Frondisphaeria | F. palmicola | On a rachis of Eugeissona minor | Brunei | N/A | [170] |
| Frondispora | F. bicalcarata | On dead petioles of Chamaerops humilis | Italy | N/A | [111] |
| Gossypinidium | G. sporodochiale | On a dead rachis of Praestoea montana | Puerto Rico | A | [283] |
| Guestia | G. gonetropospora | On a dead rachis of Mauritia flexuosa | Ecuador | N/A | [150] |
| Haploanthostomella | H. elaeidis | On dead leaves and rachis of Elaeis guineensis | Thailand | A | [284] |
| Haplohelminthosporium | H. calami | On living leaves and petioles of Calamus sp. | Thailand | A | [285] |
| Helensiella (as Digitella) | H. rigidophora (as D. rigidophora) | On a rachis of an unidentified palm | Mexico (Veracruz) | N/A | [286,287] |
| Helminthosporiella | H. stilbacea | On a dead petiole of Cocos nucifera | Thailand | A | [285,288] |
| Hemisynnema # | H. malayasianum | On a rachis of an unidentified palm | Malaysia | N/A | [289] |
| Hyalobelemnospora | H. amazonica | On a rotten petiole of an unidentified palm | Peru | N/A | [64] |
| Kalamarospora | K. multiflagellata | On rachides of dead leaves of Sabal palmetto | USA (Florida) | N/A | [290] |
| Letendraeopsis | L. palmarum | On leaves of Euterpe oleracea | Brazil (Pará) | N/A | [291] |
| Lockerbia | L. palmicola | On dead rachides of an unidentified palm | Australia (Queensland) | N/A | [114] |
| Longicorpus | L. striatisporus (as L. striataspora) | On a decayed rachis of Nypa fruticans | Thailand | A | [9] |
| Mackenziella (as Mackenziea) | M. livistonae | On decaying rachides of Oraniopsis appendiculata | Australia (Queensland) | N/A | [15] |
| Maculatifrondes (as Maculatifrondis) | M. aequatoriensis | On leaves of an unidentified palm in rainforest | Ecuador | N/A | [208] |
| Maculatipalma | M. frondicola | On a leaf of Linospadix microcarya | Australia (Queensland) | N/A | [197] |
| Malthomyces | M. calamigena (as M. calamigenus) | On tissues of Calamus rudentum | Sri Lanka | N/A | [212] |
| Manokwaria | M. notabilis | On dead rachides of an unidentified palm in freshwater swamp | Indonesia | N/A | [109] |
| Monosporoschisma | M. elegans | On a dead material of an unidentifed palm | Chian (Hainan) | A | [268] |
| Neoastrosphaeriella | N. krabiensis | On a petiole of Metroxylon sagu | Thailand | A | [281] |
| Neobarrmaelia | N. hyphaenes | On leaves of Hyphaene sp. | South Africa | A | [292] |
| Neolinocarpon | N. globosicarpum | On decaying intertidal fronds of Nypa fruticans | Brunei | N/A | [162] |
| Neoxylaria | N. arengae | On a dead petiole of Arenga pinnata | Thailand | A | [293] |
| Nigromammilla (as Nigramammilla) | N. calami | On a sheath of dead rattan of Daemonorops margaritae | China (Hong Kong) | N/A | [179] |
| Nipicola | N. carbospora | On immersed fronds of Nypa fruticans | Brunei | N/A | [163] |
| Nusia | N. scheeleae | On a rachis of Scheelea insignis | Singapore | N/A | [294] |
| Nypaella | N. frondicola | On intertidal fronds of Nypa fruticans | Brunei | N/A | [164] |
| Ornatispora # | O. palmicola | On a dead rachis of an unidentified palm | Ecuador | N/A | [181] |
| Oxodeora | O. petrakii | On living fronds of Oreodoxa regia | Dominican Republic | N/A | [212] |
| Palmaria (as Palmomyces) | P. montanea (as P. montaneus) | On a leaf of Oraniopsis appendiculata | Australia (Queensland) | N/A | [143] |
| Palmeiromyces | P. chamaeropicola | On leaf spots of Chamaerops humilis | Portugal | A | [295] |
| Palmicola | P. archontophoenicis | On a fallen rachis of Archontophoenix alexandrae | Australia (Queensland) | N/A | [108] |
| Paracapsulospora | P. metroxyli | On a dead Metroxylon sagu | Thailand | A | [296] |
| Paradactylella | P. peruviana | On a rotten petiole of an unidentified palm | Peru | N/A | [64] |
| Paraproliferophorum | P. hyphaenes | On living leaves of Hyphaene sp. | South Africa | A | [297] |
| Pararamichloridium | P. livistonae | On leaves of Livistona australis | Australia (New South Wales) | A | [298] |
| Parateichospora | P. phoenicicola | On leaves of Phoenix reclinata | South Africa | A | [299] |
| Phaeochoropsis | P. neowashingtoniae | On leaves of Neowashingtonia filamentosa | USA (California) | N/A | [212] |
| Phaeomonilia | P. pleiomorpha | On a decaying petiole of an unidentified palm submerged in stream | Mexico (Veracruz) | N/A | [300] |
| Phruensis | P. brunneispora | On a dead trunk of Licuala longicalycata | Thailand | A | [301] |
| Polybulbophiale | P. palmicola | On the decaying petiole of Licuala sp. | Brunei | N/A | [190] |
| Porodiplodia | P. livistonae | On leaves of Livistona australis | Australia (New South Wales) | A | [302] |
| Pseudopalawania | P. siamensis | On a dead rachis of Caryota sp. | Thailand | A | [303] |
| Pulmosphaeria | P. archontophoenicis | On a dead petiole of Archontophoenix alexandrae | Australia (Queensland) | N/A | [194] |
| Quasiphoma | Q. hyphaenes | On leaves of Hyphaene sp. | South Africa | A | [292] |
| Rachidicola | R. palmae | On a rachis of Calamus sp. | China (Hong Kong) | N/A | [129] |
| Rattania | R. setulifera | On leaves of Calamus thwaitesii | India (Goa) | N/A | [304] |
| Rogergoosiella | R. roystoneicola | On a dead petiole of Roystonea regia | Cuba | N/A | [305] |
| Sabalicola | S. sabalensioides | On petioles of Sabal serrulata | USA (Florida) | N/A | [122] |
| Sawantomyces | S. indicus (as S. indica) | On a spathe of Cocos nucifera | India (Maharashtra) | N/A | [306] |
| Setophiale | S. unisetulata | On a decayed petiole of an unidentified palm | Peru | N/A | [65] |
| Sorokinella | S. appendicospora | On a dead petiole of Livistona chinensis | China (Hong Kong) | N/A | [6] |
| Stratiphoromyces | S. brunneisporus | On decaying petioles of Licuala sp. | Brunei | N/A | [189] |
| Striatiguttula | S. nypae | On a decayed rachis of Nypa fruticans | Thailand | A | [9] |
| Thailandiomyces | T. bisetulosus | On senescent trunks of Licuala longicalycata | Thailand | A | [307] |
| Tirisporella | T. beccariana | On decaying leaf bases of Nypa fruticans | Malaysia | N/A | [167] |
| Tretendophragmia | T. palmivora | On a rachis of Korthalsia sp. | Singapore | N/A | [308] |
| Tretocephala | T. decidua | On a leaf sheath and rachis of Oncosperma horridum | Singapore | N/A | [309] |
| Tribulatia | T. appendicospora | On a dead petiole of Archontophoenix alexandrae | Australia (Queensland) | N/A | [8] |
| Triseptatospora | T. calami | On dead petioles of Calamus sp. | Thailand | A | [310] |
| Unisetosphaeria | U. penguinoides | On a petiole of Eleiodoxa conferta submerged in peat swamp | Thailand | N/A | [245] |
| Uwemyces | U. elaeidis | On leaves of Elaeis oleifera | Colombia | A | [288] |
| Venustocephala | V. aequatorialis | On a decayed petiole of an unidentified palm | Ecuador | N/A | [65] |
| Venustisporium (as Venustusporium) | V. chelyoforme (as V. chelysforme) | On fallen rotten leaves of Bactris setulosa | Venezuela | N/A | [311] |
| Veramycella | V. bispora | On rachides of dead leaves of Sabal palmetto | USA (Florida) | N/A | [312] |
| Veramyces | V. manuensis | On a rotten petiole of an unidentified palm | Peru | N/A | [64] |
| Waihonghopes | W. australiensis | On a decaying rachis of Oraniopsis appendiculata | Australia (Queensland) | N/A | [15] |
2.1.4. Palm Fungi from Understudied Tropical Hotspots, Argentina, India, and Brazil
2.1.5. Palm Fungi and Reflections on the Recent Input from Molecular Era
Astrosphaeriella-like Taxa: A Polyphyletic Nature Hiding Cryptic Genera
Xylarialean and Related Sordariomycetes: The Enigmatic Anthostomella and Allied Genera
Palmicolous “Anamorphs”: A Plethora of Botryosphaeriaceae and Other Dothideomycetes
From Aquatic to Phytopathogenic Fungi: The Broad Taxonomic Spectrum of Palm Fungi
2.2. History of Biodiversity and Ecological Studies on Palm Fungi
3. What Are Palm Fungi? Global Figure and Taxonomic Structure
3.1. Global Figure of Palm Fungi
3.2. Taxonomic Structure of Palm Fungi
3.2.1. Palmicolous Sordariomycetes
3.2.2. Palmicolous Dothideomycetes
3.2.3. Palmicolous “Anamorphs”
| Class | Subclass | Order | Family | Genera |
|---|---|---|---|---|
| Dothideomycetes | Dothideomycetidae | Dothideales | Dothideaceae | Uleodothis |
| Mycosphaerellales | Extremaceae | Castanedospora | ||
| Mycosphaerellaceae | Cercospora, Distocercospora, Exosporium, Pallidocercospora, Passalora, Phaeophleospora, Pseudocercospora, Ramularia, Scolecostigmina, Uwemyces, Zasmidium | |||
| Teratosphaeriaceae | Palmeiromyces, Stenella | |||
| Incertae sedis | Pseudoepicoccum | |||
| Pleosporomycetidae | Acrospermales | Acrospermaceae | Gonatophragmium | |
| Pleosporales | Acrocalymmaceae | Acrocalymma | ||
| Aigialaceae | Fissuroma, Neoastrosphaeriella | |||
| Arthopyreniaceae | Mycomicrothelia | |||
| Astrosphaeriellaceae | Astrosphaeriella, Astrosphaeriellopsis, Pteridiospora, Pithomyces, Javaria, Triseptatospora, Xenoastrosphaeriella | |||
| Coniothyriaceae | Coniothyrium | |||
| Corynesporascaceae | Corynesporasca | |||
| Delitschiaceae | Delitschia | |||
| Dictyosporiaceae | Dictyocheirospora, Dictyopalmispora, Dictyosporium, Pseudocoleophoma, Sporidesmiella | |||
| Didymosphaeriaceae | Didymosphaeria, Montagnula, Paraconiothyrium, Paraphaeosphaeria, Pseudopithomyces | |||
| Hermatomycetaceae | Hermatomyces | |||
| Lentimurisporaceae | Bahusandhika | |||
| Leptosphaeriaceae | Chaetoplea, Leptosphaeria, Quasiphoma | |||
| Lindgomycetaceae | Lolia | |||
| Dothideomycetes (cont.) | Dothideomycetidae (cont.) | Pleosporales (cont.) | Lophiostomataceae | Lophiostoma, Vaginatispora |
| Massarinaceae | Haplohelminthosporium, Helminthosporiella, Helminthosporium, Massarina | |||
| Melanommataceae | Asymmetricospora, Byssosphaeria, Camposporium, Herpotrichia | |||
| Morosphaeriaceae | Helicascus | |||
| Neophaeosphaeriaceae | Neophaeosphaeria | |||
| Occultibambusaceae | Brunneofusispora, Neooccultibambusa | |||
| Periconiaceae | Periconia | |||
| Phaeosphaeriaceae | Amarenographium, Parastagonospora, Phaeosphaeria, Septoriella, Wojnowiciella | |||
| Pleosporaceae | Bipolaris, Curvularia, Exserohilum | |||
| Pseudoastrosphaeriellaceae | Carinispora, Pseudoastrosphaeriella | |||
| Pseudoberkleasmiaceae | Pseudoberkleasmium | |||
| Roussoellaceae | Appendispora, Neoroussoella, Roussoella | |||
| Salsugineaceae | Salsuginea | |||
| Striatiguttulaceae | Longicorpus, Striatiguttula | |||
| Trematosphaeriaceae | Falciformispora, Trematosphaeria | |||
| Teichosporaceae | Parateichospora | |||
| Tetraplosphaeriaceae | Ernakulamia, Tetraploa | |||
| Torulaceae | Cylindrotorula, Torula | |||
| Incertae sedis | Acuminatispora, Plectophomella, Repetophragma | |||
| Hysteriales | Hysteriaceae | Gloniopsis | ||
| Incertae sedis | Asterinales | Asterinaceae | Asterina, Cirsosia, Discopycnothyrium | |
| Lembosiaceae | Lembosia | |||
| Morenoinaceae | Morenoina | |||
| Botryosphaeriales | Botryosphaeriaceae | Barriopsis, Botryosphaeria, Diplodia, Lasiodiplodia, Neodeightonia | ||
| Dothideomycetes (cont.) | Incertae sedis (cont.) | Botryosphaeriales (cont.) | Phyllostictaceae | Phyllosticta |
| Jahnulales | Aliquandostipitaceae | Jahnula | ||
| Manglicolaceae | Manglicola | |||
| Kirschsteiniotheliales | Kirschsteiniotheliaceae | Kirschsteiniothelia | ||
| Incertae sedis | Taeniolella | |||
| Muyocopronales | Muyocopronaceae | Muyocopron, Pseudopalawania | ||
| Tubeufiales | Tubeufiaceae | Aquaphila, Berkleasmium, Helicoma, Helicomyces, Helicosporium, Thaxteriella | ||
| Wiesneriomycetaceae | Wiesneriomyces | |||
| - | Palawaniaceae | Palawania | ||
| - | Trichopeltinaceae | Acrogenotheca | ||
| - | - | Letendraeopsis, Xenosporium, Brooksia, Dianesea, Leptomeliola, Scolionema | ||
| Sordariomycetes | Diaporthomycetidae | Annulatascales | Annulatascaceae | Annulatascus, Submersisphaeria |
| Diaporthales | Diaporthaceae | Diaporthe | ||
| Gnomoniaceae | Maculatipalma | |||
| Melanconidaceae | Melanconis, Melanconium | |||
| Schizoparmaceae | Coniella | |||
| Incertae sedis | Durispora, Phruensis | |||
| Distoseptisporales | Distoseptisporaceae | Distoseptispora | ||
| Magnaporthales | Magnaporthaceae | Gaeumannomyces | ||
| Ophioceraceae | Ophioceras | |||
| Pseudohalonectriaceae | Pseudohalonectria | |||
| Ophiostomatales | Ophiostomataceae | Hyalobelemnospora | ||
| Phomatosporales | Phomatosporaceae | Phomatospora | ||
| Tirisporellales | Tirisporellaceae | Bacusphaeria, Thailandiomyces, Tirisporella | ||
| Sordariomycetes (cont.) | Diaporthomycetidae (cont.) | Xenospadicoidales | Xenospadicoidaceae | Koorchaloma, Spadicoides |
| Incertae sedis | Mesnieraceae | Bondiella | ||
| Trichosphaeriaceae | Unisetosphaeria | |||
| Thyridiaceae | Thyridium | |||
| - | Paraproliferophorum | |||
| Hypocreomycetidae | Glomerellales | Plectosphaerellaceae | Acremoniisimulans, Brunneomyces | |
| Hypocreales | Bionectriaceae | Acremonium, Clonostachys, Gossypinidium, Hydropisphaera, Ijuhya, Lasionectria, Nectriella, Paracylindrocarpon | ||
| Hypocreaceae | Verticimonosporium | |||
| Nectriaceae | Baipadisphaeria, Calonectria, Chaetopsina, Cosmospora, Dactylonectria, Fusarium, Ilyonectria, Nectria, Nectriopsis, Ophionectria, Pleiocarpon, Volutella | |||
| Neoacremoniaceae | Neoacremonium | |||
| Niessliaceae | Niesslia | |||
| Stachybotryaceae | Alfaria, Stachybotrys, Virgatospora | |||
| Microascales | Gondwanamycetaceae | Custingophora | ||
| Halosphaeriaceae | Aniptodera, Cirrenalia, Fluviatispora, Lignincola | |||
| Microascaceae | Wardomycopsis | |||
| Triadelphiaceae | Triadelphia | |||
| Ceratocystidaceae | Ceratocystis, Thielaviopsis | |||
| Pararamichloridiales | Pararamichloridiaceae | Pararamichloridium | ||
| Savoryellomycetidae | Conioscyphales | Conioscyphaceae | Conioscypha | |
| Pleurotheciales | Pleurotheciaceae | Monotosporella | ||
| Savoryellales | Savoryellaceae | Ascotaiwania, Canalisporium, Savoryella | ||
| Sordariomycetidae | Chaetosphaeriales | Chaetosphaeriaceae | Chaetosphaeria, Chloridium, Codinaea, Craspedodidymum, Cryptophiale, Dictyochaeta, Kionochaeta, Rattania, Sporoschisma, Thozetella | |
| Sordariomycetes (cont.) | Sordariomycetidae (cont.) | Chaetosphaeriales (cont.) | Helminthosphaeriaceae | Endophragmiella |
| Leptosporellaceae | Leptosporella | |||
| Linocarpaceae | Linocarpon, Neolinocarpon | |||
| Incertae sedis | Caudatispora | |||
| Coniochaetales | Incertae sedis | Cannonia | ||
| Meliolalles | Meliolaceae | Meliola | ||
| Phyllachorales | Phaeochoraceae | Cocoicola, Phaeochora, Phaeochoropsis, Serenomyces | ||
| Phyllachoraceae | Brobdingnagia, Camarotella, Coccodiella, Coccostromopsis, Maculatifrondes, Malthomyces, Ophiodothella, Oxodeora, Phyllachora, Sphaerodothis, Tribulatia | |||
| Sordariales | Chaetomiaceae | Trichocladium | ||
| Lasiosphaeriaceae | Cercophora, Lasiosphaeria | |||
| Incertae sedis | Lockerbia | |||
| Incertae sedis | - | Arecacicola, Curvatispora, Nigromammilla, Paracapsulospora | ||
| Xylariomycetidae | Amphisphaeriales | Amphisphaeriaceae | Amphisphaeria, Lepteutypa | |
| Apiosporaceae | Arthrinium, Dictyoarthrinium | |||
| Appendicosporaceae | Appendicospora | |||
| Beltraniaceae | Beltrania | |||
| Hyponectriaceae | Arecomyces, Frondicola, Hyponectria, Rachidicola | |||
| Iodosphaeriaceae | Iodosphaeria | |||
| Oxydothidaceae | Oxydothis | |||
| Pseudomassariaceae | Leiosphaerella, Pseudomassaria | |||
| Sporocadaceae | Bartalinia, Morinia, Neopestalotiopsis, Pestalotiopsis, Pseudopestalotiopsis, Robillarda, Seiridium | |||
| Xylariales | Barrmaeliaceae | Barrmaelia | ||
| Sordariomycetes (cont.) | Xylariomycetidae (cont.) | Xylariales (cont.) | Cainiaceae | Arecophila, Seynesia, Endocalyx |
| Clypeosphaeriaceae | Apioclypea, Brunneiapiospora, Palmaria | |||
| Diatrypaceae | Allocryptovalsa, Allodiatrype, Anthostoma, Cryptovalsa, Diatrype, Diatrypella, Eutypa, Eutypella, Frondisphaeria, Peroneutypa | |||
| Fasciatisporaceae | Fasciatispora | |||
| Graphostromataceae | Biscogniauxia | |||
| Hansfordiaceae | Hansfordia | |||
| Hypoxylaceae | Annulohypoxylon, Hypoxylon | |||
| Microdochiaceae | Idriella, Microdochium | |||
| Oxydothidaceae | Oxydothis | |||
| Robillardaceae | Robillarda | |||
| Xylariaceae | Anthostomella, Ascotricha, Astrocystis, Diabolocovidia, Kretzschmaria, Nemania, Neoxylaria, Rosellinia, Stilbohypoxylon, Xylaria | |||
| Zygosporiaceae | Zygosporium | |||
| Incertae sedis | Capsulospora, Circinotrichum, Cyanopulvis, Gyrothrix, Guestia, Haploanthostomella, Lasiobertia, Neobarrmaelia, Nipicola, Palmicola, Pemphidium, Pulmosphaeria, Sabalicola | |||
| Incertae sedis | Myelospermataceae | Myelosperma | ||
| - | Frondispora, Manokwaria | |||
| Incertae sedis | Catabotryales | Catabotryaceae | Catabotrys | |
| - | Acrodictyaceae | Acrodictys | ||
| - | - | Apogaeumannomyces, Flammispora, Mangrovispora |
3.2.4. Miscellaneous Palm Taxa
4. Palm Trees as Model Plants for the Study of Fungal Biodiversity
4.1. Palm Fungi and the Search for the “Missing Fungi”
| Palm Species 1 | Total Number of | |||||||
|---|---|---|---|---|---|---|---|---|
| Fungal Records 2 | Fungal Species 2,3 | Ascomycetes | Asexual Morphs | Coelomycetes | Hyphomycetes | Basidiomycetes | Zygomycetes | |
| Cocos nucifera | 1296 | 526 | 149 (28.33%) | 275 (52.28%) | 91 (17.30%) | 184 (34.98%) | 96 (18.25%) | 6 (1.14%) |
| Elaeis guineensis | 1256 | 427 | 100 (23.42%) | 235 (55.04%) | 50 (11.71%) | 185 (43.33%) | 80 (18.74%) | 12 (2.81%) |
| Phoenix dactylifera | 560 | 197 | 48 (24.37%) | 123 (62.44%) | 39 (19.80%) | 84 (42.64%) | 23 (11.68%) | 3 (1.52%) |
| Archontophoenix alexandrae | 355 | 178 | 87 (48.88%) | 86 (48.31%) | 11 (6.18%) | 75 (42.13%) | 5 (2.81%) | 0 |
| Areca catechu | 298 | 155 | 26 (16.77%) | 111 (71.61%) | 33 (21.29%) | 78 (50.32%) | 16 (10.32%) | 2 (1.29%) |
| Trachycarpus fortunei | 297 | 154 | 58 (37.66%) | 94 (61.04%) | 41 (26.62%) | 53 (34.42%) | 2 (1.30%) | 0 |
| Roystonea regia | 225 | 153 | 19 (12.41%) | 123 (80.39%) | 16 (10.46%) | 107 (69.93%) | 11 (7.19%) | 0 |
| Livistona chinensis | 189 | 95 | 47 (49.47%) | 35 (36.84%) | 10 (10.53%) | 25 (26.32%) | 13 (13.68%) | 0 |
| Phoenix loureiroi | 173 | 92 | 27 (29.35%) | 63 (68.48%) | 9 (9.78%) | 54 (58.70%) | 2 (2.17%) | 0 |
| Phoenix canariensis | 160 | 91 | 24 (26.37%) | 43 (47.25%) | 12 (13.19%) | 31 (34.07%) | 24 (26.37%) | 0 |
| Chamaerops humilis | 128 | 64 | 32 (50.00%) | 22 (34.38%) | 12 (18.75%) | 10 (15.63%) | 10 (15.63%) | 0 |
| Sabal palmetto | 128 | 88 | 45 (51.14%) | 28 (31.82%) | 6 (6.82%) | 22 (25.00%) | 15 (17.05%) | 0 |
| Arenga engleri | 122 | 64 | 14 (21.88%) | 50 (78.13%) | 4 (6.25%) | 46 (71.88%) | 0 | 0 |
| Licuala longicalycata | 119 | 89 | 49 (55.06%) | 40 (44.94%) | 3 (3.37%) | 37 (41.57%) | 0 | 0 |
| Rhopalostylis sapida | 113 | 88 | 36 (40.91%) | 41 (46.59%) | 0 | 41 (46.59%) | 11 (12.50%) | 0 |
| Summary figures 1,2,3 | Total number of palm genera from which associated fungi have been studied: 97 | |||||||
| Palm genera with a total number of fungal records ≥ 100: Cocos (1296 fungal records), Elaeis (1286), Phoenix (1146), Calamus (658), Archontophoenix (395), Areca (333), Rhopalostylis (318), Trachycarpus (306), Livistona (278), Sabal (274), Roystonea (270), Licuala (244), Arenga (229), Caryota (176), Chamaerops (128), Syagrus (112), Chamaedorea (108), and Borassus (105) | ||||||||
| Total number of palm species from which associated fungi have been studied: 262 | ||||||||
| Total number of palm species with a total number of fungal records ≥ 100: 15 | ||||||||
| Total number of palm species with 100 < total number of fungal records ≥ 50: 12 | ||||||||
| Total number of palm species with 50 < total number of fungal records ≥ 20: 26 | ||||||||
| Total number of palm species with a total number of fungal records < 20: 209 | ||||||||
| Total number of fungal records associated with Arecaceae hosts: 9339 | ||||||||
| Total number of fungal species recorded from Arecaceae hosts: 2932, including 1182 ascomycetes (40.31%), 332 basidiomycetes (11.32%), 1398 anamorphic fungi (47.68%), namely 984 ascomycetes (33.56%) and 413 coelomycetes (14.09%), and 20 zygomycetes (0.68%) | ||||||||
| Palm Species | Total Number of | Reference | ||||
|---|---|---|---|---|---|---|
| Fungal Records | Fungal Species | Ascomycetes | Asexual Morphs | Basidiomycetes | ||
| Eleiodoxa conferta | 462 | 112 | 43 (38%) | 67 (60%) | 2 (2%) | [248] |
| Licuala longicalycata | 358 | 147 | 79 (53%) | 65 (45%) | 3 (3%) | [249] |
| Metroxylon sagu | 82 | 45 | 21 (47%) | 24 (53%) | 0 | [669] |
| Nenga pumila | 184 | 47 | 19 (40%) | 28 (60%) | 0 | [669] |
| Genus | Species | Substratum | Reference |
|---|---|---|---|
| Astrocystis eleiodoxae | On a submerged petiole of Eleiodoxa conferta | [525] | |
| Baipadisphaeria | Baipadisphaeria spathulospora | On a submerged trunk of Licuala longicalycata | [270] |
| Chalara siamensis (as C. siamense) | On submerged dead petioles of E. conferta | [241] | |
| Craspedodidymum licualae | On a decaying trunk of L. longicalycata | [243] | |
| Cras. microsporum | On a decaying trunk of L. longicalycata | [243] | |
| Cras. siamense | On a decaying sheath of L. longicalycata | [243] | |
| Dactylaria flammulicornuta | On a terrestrial petiole of Nenga pumila | [245] | |
| D. palmae | On terrestrial sheath of N. pumila | [245] | |
| D. uliginicola | On a submerged rachis of E. conferta | [245] | |
| Dictyopalmispora | Dictyopalmispora palmae | On decaying leaves of L. longicalycata | [602] |
| Flammispora | Flammispora bioteca | On submerged decaying leaves of L. longicalycata | [282] |
| Goidanichiella fusiformis (as G. fusiforma) | On a submerged dead petiole of E. conferta | [236] | |
| Jahnula appendiculata | On a submerged trunk of L. longicalycata | [242] | |
| Knoxdaviesia undulatistipes (as Custingophora undulatistipes) | On a submerged dead petiole of E. conferta | [246] | |
| Phruensis | Phruensis brunneispora | On a dead trunk of L. longicalycata | [301] |
| Stachybotrys palmae | On a decaying rachis of L. longicalycata | [244] | |
| Submersisphaeria palmae | On submerged petioles, rachides, and trunks of E. conferta, N. pumila and L. longicalycata | [247] | |
| Thailandiomyces | Thailandiomyces bisetulosus | On submerged senescent trunk of L. longicalycata | [307] |
| Unisetosphaeria | Unisetosphaeria penguinoides | On a submerged dead petiole of E. conferta | [245] |
| Vanakripa minutiellipsoidea | On a submerged dead petiole of E. conferta | [246] |
| Genus | Species 1 | Substratum (Collection Site) | Reference |
|---|---|---|---|
| Acuminatispora | Acuminatispora palmarum | On a submerged decayed petiole (Thailand) | [261] |
| Aniptodera intermedia * | On an intertidal petiole (Malaysia) | [166] | |
| A. nypae * | On intertidal fronds (Malaysia) | [116] | |
| Anthostomella nypae * | On an intertidal petiole (Malaysia) | [166] | |
| A. nypensis * | On an intertidal petiole (Malaysia) | [166] | |
| A. nypicola * | On an intertidal petiole (Malaysia) | [166] | |
| Apioclypea nypicola * | On an intertidal rachis (Malaysia) | [143] | |
| Arecophila nypae * | On intertidal palm tissues (Malaysia) | [131] | |
| Astrocystis nypae * | On an intertidal frond (Malaysia) | [150] | |
| A. selangorensis * | On a dead intertidal rachis (Malaysia) | [150] | |
| Astrosphaeriella nipicola (as A. nipaecola) (basio. Melanopsamma nipicola) | On palm tissues (Indonesia) | [144] | |
| A. nypae * | On decaying intertidal fronds (Brunei) | [162] | |
| Bacusphaeria | Bacusphaeria nypae * | On petiole base (Malaysia) | [269] |
| Carinispora | Carinispora nypae * | On decaying intertidal fronds (Brunei) | [162] |
| Delitschia nypae * | On a decaying fruit pericarp (Thailand) | [535] | |
| Fasciatispora | Fasciatispora nypae * | On intertidal rotten fronds (Brunei) | [161] |
| Frondicola | Frondicola tunitricuspis * | On decaying fronds | [162] |
| Helicascus nypae * | On intertidal dead fronds (Brunei) | [160] | |
| Helicorhoidion nypicola * | On intertidal palm tissues (Brunei) | [166] | |
| Herpotrichia nypicola * | On an intertidal petiole (Malaysia) | [166] | |
| Leptosphaeria nypicola * | On an intertidal petiole (Malaysia) | [166] | |
| Lignincola nypae * | On an intertidal petiole (Malaysia) | [166] | |
| Linocarpon angustatum * | On an intertidal petiole base (Malaysia) | [165] | |
| L. appendiculatum * | On rotten fronds (Brunei) | [154] | |
| L. bipolare (as L. bipolaris) * | On intertidal fronds (Brunei) | [105] | |
| L. longisporum * | On intertidal fronds (Brunei) | [105] | |
| L. nipae (syn. Ophiobolus nipae) * | On dead petioles (Philippines) | [154] | |
| Longicorpus | Longicorpus striatisporus (syn. Astrosphaeriella striatispora) | On fronds (Brunei) | [9] |
| Neolinocarpon | Neolinocarpon globosicarpum * | On decaying intertidal fronds (Brunei) | [162] |
| N. nypicola * | On an intertidal petiole base (Malaysia) | [165] | |
| Nipicola | Nipicola carbospora * | On immersed fronds (Brunei) | [163] |
| N. selangorensis * | On an intertidal frond (Malaysia) | [116] | |
| Nypaella | Nypaella frondicola * | On intertidal fronds (Brunei) | [164] |
| Oxydothis nypae * | On rotten fronds (Brunei) | [156] | |
| O. nypicola * | On a decayed petiole (Brunei) | [117] | |
| Phomatospora nypae * | On dead intertidal leaves (Malaysia) | [110] | |
| P. nypicola * | On an intertidal petiole (Malaysia) | [166] | |
| Plectophomella nypae * | On intertidal fronds (Brunei) | [164] | |
| Pleurophomopsis nypae * | On intertidal fronds (Brunei) | [164] | |
| Savoryella nypae (basio. Trichocladium nypae) * | On intertidal palm tissues (Brunei) | [166,619] | |
| Striatiguttula | Striatiguttula nypae * | On a decayed rachis (Thailand) | [9] |
| Tirisporella | Tirisporella beccariana * | On decaying leaf bases (Malaysia and Phlippines) | [167] |
| Vaginatispora nypae * | On a decaying fruit pericarp (Thailand) | [535] | |
| V. palmae * | On an immersed rachis (Thailand) | [761] | |
| Vibrissea nypicola * | On an intertidal petiole (Malaysia) | [166] |
| Genus | Species | Substratum (Collection Site) | Reference |
|---|---|---|---|
| Aegerita queenslandica | On a rotten leaf (Queensland, Australia) | [63] | |
| Anthostomella clypeosa | On a dead rachis (Queensland, Australia) | [8] | |
| Apioclypea nonapiospora | On a dead rachis (Hong Kong, China) | [8] | |
| Astrosphaeriella immersa | On a dead petiole (Hong Kong, China) | [148] | |
| Barriopsis archontophoenicis | On dead woody tissues (Thailand) | [549] | |
| Botryosphaeria archontophoenicis | On a dead petiole (Hong Kong, China) | [8] | |
| Chaetopsina alexandrae | On a dead rachis (Queensland, Australia) | [8] | |
| Heteroconium queenslandicum | On a rotten leaf (Queensland, Australia) | [63] | |
| Hydropisphaera ciliata | On a dead sheath (Queensland, Australia) | [8] | |
| Iodosphaeria hongkongensis | On a dead petiole (Hong Kong, China) | [146] | |
| Lasiosphaeria alexandrae | On a submerged rachis (Queensland, Australia) | [185] | |
| L. alexandricola | On a dead sheath (Hong Kong, China) | [185] | |
| Linocarpon australiense * | On palm tissues (Queensland, Australia) | [172] | |
| L. luteocollum | On a dead rachis (Queensland, Australia) | [8] | |
| Maculatipalma * | Maculatipalma fronsicola * | On a living (Queensland, Australia) | [197] |
| Manokwaria * | Manokwaria notabilis * | On a dead rachis on rainforest floor (Queensland, Australia) | [109] |
| Melanographium palmicola (as M. palmicolum) | On a decaying rachis (Hong Kong, China) | [182] | |
| Muyocopron hongkongense | On a dead rachis (Hong Kong, China) | [8] | |
| Neolinocarpon inconspicuum (as N. inconspicuus) | On a dead rachis (Queensland, Australia) | [140] | |
| N. nonappendiculatum (as N. nonappendiculatus) | On a dead petiole (Queensland, Australia) | [140] | |
| Neoxylaria queenslandica (as Xylaria queenslandica) | On a dead rachis (Queensland, Australia) | [8] | |
| Oxydothis alexandrarum | On a rotten rachis (Queensland, Australia) | [112] | |
| O. australiensis | On a rachis in forest litter (Queensland, Australia) | [112] | |
| Palmicola | Palmicola archontophoenicis | On a basal sheath of a fallen rachis (Queensland, Australia) | [108] |
| P. bipolaris | On a dead petiole (Queensland, Australia) | [8] | |
| Phomatospora archontophoenicis | On a dead rachis (Queensland, Australia) | [8] | |
| Pseudohalonectria eubenangeensis | On a dead rachis (Queensland, Australia) | [200] | |
| Pulmosphaeria | Pulmosphaeria archontophoenicis | On a dead petiole (Queensland, Australia) | [194] |
| Selenosporella queenslandica | On a rotten leaf (Queensland, Australia) | [63] | |
| Sorokina frondicola | On dead rachis (Queensland, Australia) | [8] | |
| Sporidesmium queenslandicum | On a rotten leaf (Queensland, Australia) | [63] | |
| Triadelphia archontophoenicicola (as T. australiensis) | On a dead rachis (Queensland, Australia) | [8] | |
| Tribulatia | Tribulatia appendicospora | On a dead petiole (Queensland, Australia) | [8] |
| Trichoconis queenslandica | On a rotten leaf (Queensland, Australia) | [63] | |
| Volutella queenslandica | On a rotten leaf (Queensland, Australia) | [63] |
4.2. Palm Fungi and the Fungal Biodiversity Estimates
4.2.1. Fungal Specificity at Family, Genus, and Species Levels
| Palm Species/Genus | Fungal Species | Reference |
|---|---|---|
| Archontophoenix alexandrae | Hydropisphaera ciliata | [8] |
| Lasiosphaeria alexandrae | [185] | |
| Lockerbia palmicola * | [114] | |
| Neolinocarpon inconspicuum | [140] | |
| N. nonappendiculatum | [140] | |
| Oxydothis alexandrarum | [112] | |
| O. australiensis | [112] | |
| Palmicola archontophoenicis | [194] | |
| P. bipolaris | [8] | |
| Phomatospora archontophoenicis | [8] | |
| Pseudohalonectria eubenangeensis | [200] | |
| Pulmosphaeria archontophoenicis | [194] | |
| Calamus | Anthostomella bipileatus | [6] |
| Astrosphaeriella australiensis | [144] | |
| Cyanopulvis australiensis | [6] | |
| Neolinocarpon australiense | [140] | |
| Oxydothis calami | [117] | |
| O. luteaspora | [112] | |
| O. rubella | [112] | |
| O. uniseriata | [6] | |
| Pemphidium calamicola | [135] | |
| P. rattanicola | [6] | |
| Pseudohalonectria palmicola | [200] | |
| Roussoella calamicola | [147] | |
| Cocos nucifera | Mycosphaerella palmicola | [198] |
| Licuala | Ascotaiwania licualae | [6] |
| Capsulospora angustispora | [6] | |
| Nectriella erythroclypea | [121] | |
| Nipicola licualae | [6] | |
| Oxydothis angustispora | [6] | |
| O. cyrtospora | [6] | |
| O. extensa | [6] | |
| O. parasitica | [195] | |
| Linospadix | Oxydothis linospadicis | [195] |
| O. obducens | [117] | |
| Oraniopsis appendiculata | Monotosporella palmicola | [15] |
| M. sphaerica | [15] | |
| Palmaria montanea | [143] | |
| Sporidesmiella oraniopsidis | [230] | |
| Pinanga sp. | Phyllosticta candeloflamma | [187] |
| Arenga engleri (Hong Kong) | Arenga undulatifolia (Brunei) | Calamus sp. (Thailand) | Livistona chinensis (Hong Kong) | Oncosperma horridum (Brunei) | Phoenix hanceana (Hong Kong) | Salacca affinis (Brunei) |
|---|---|---|---|---|---|---|
| Piricauda cochinensis | Piricauda cochinensis | Tetraploa sp. | Astrosphaeriella bakeriana | Linocarpon livistonae | Diplococcium stoveri | Zygosporium minus |
| Diplococcium stoveri | Melanographium selemiodes | Morenoina palmicola | Lachnum palmae | Craspedodydimum nigroseptatum | Endocalyx cinctus | Linocarpon livistinae |
| Helminthosporium solani | Trichoderma harzianum | Circinoconis paradoxa | Appendicospora hongkongensis | Zygosporium minus | Cryptophiale udagawae | Peltistromella anomala |
| Melanographium palmicola | Zygosporium minus | Diaporthe sp. | Monodictys putredinis | Monotosporella setosa var. macrospora | Penzigomyces nodipes | Helicosporium griseum |
| Melanographium selenioides | Pleurophragmium sp. | Helminthosporium sp. | Oxydothis elaeicola | Neolinocarpon australiense | Thozetella effusa | Volutella ciliata |
| Monodictys putredinis | Helmithosporium velutimum | Linocarpon sp. | Trichoderma harzianum | Trichoderma harzianum | Pseudospiropes simplex | Oxydothis luteaspora |
| Oxydothis ragai | Volutella ciliata | Phaeosphaeria sp. | Neolinocarpon australiense | Oxydothis luteaspora | Dictyochaeta simplex | Periconiella sp. |
| Pestalotiopsis palmarum | Peltistromella anomala | Anthostomella sp. | Fasciatispora petrakii | Oxydothis licualae | Serenomyces shearii | Arecomyces bruneiensis |
| Guignardia manokwaria | Stachylidium sp. | Astrosphaeriella sp. 1 | Corynesporopsis isabelicae | Oxydothis elaeicola | Capsulospora brunneispora | Sporidesmium parvum |
| Dischoridium roseum | Anthostomella minutoides | Goidanichiella fusiformis | Dictyosporium elegans | Brachysporiella gayana | Harknessia globosa | Codinaea intermedia |
| Taxonomic Rank | Eleiodoxa conferta | Licuala longicalycata | Metroxylon sagu | Nenga pumila | Peat Swamp Palms |
|---|---|---|---|---|---|
| Genera | Astrosphaeriella | Astrosphaeriella | Nawawia | Diplococcium | Astrosphaeriella |
| Stilbohypoxylon | Oxydothis | Anthostomella | Dinemasporium | Microthyrium | |
| Cancellidium | Annulatascus | Oxydothis | Linocarpon | Stilbohypoxylon | |
| Xylomyces | Massarina | Apiospora | Arecomyces | Cancellidium | |
| Lophiostoma | Microthyrium | Cylindrocladium | Spadicoides | Diplococcium | |
| Microthyrium | Phaeoisaria | Dinemasporium | Lophodermium | Oxydothis | |
| Morenoina | Nectria | Tetraploa | Sporidesmium | Xylomyces | |
| Phaeoisaria | Phruensis | Apioclypea | Dactylaria | Lophiostoma | |
| Jahnula | Submersisphaeria | Ornatispora | Oxydothis | Phaeoisaria | |
| Annulatascus | Thozetella | Massarina | Jahnula | Annulatascus | |
| Species | Cancellidium applanatum | Microthyrium sp. | Anthostomella bipapillispora | Diplococcium stoveri | Microthyrium sp. |
| Xylomyces aquaticus | Phaeoisaria clematidis | Nawawia filiformis | Dinemasporium sp. | Cancellidium applanatum | |
| Astrosphaeriella aquatica-like | Annulatascus velatispora | Oxydothis-like | Arecomyces epigeni | Diplococcium stoveri | |
| Stilbohypoxylon elaeicola | Massarina bipolaris | Apioclypea eccentricospora | Linocarpon sp. 4 | Xylomyces aquaticus | |
| Lophiostoma frondisubmersa | Phruensis brunneispora | Apiospora sp. | Lophodermium sp. | Phaeoisaria clematidis | |
| Microthyrium sp. | Solheimia costaspora | Dinemasporium lanatum | Dactylaria palmae | Astrosphaeriella aquatica -like | |
| Morenoina palmicola | Thailandiomyces bisetulosus | Tetraploa aristata | Lophiostoma sp. | Stilbohypoxylon elaeicola | |
| Phaeoisaria clematidis | Nectria sp. 1 | Ornatispora sp. | Oxydothis sp. 8 | Jahnula appendiculata | |
| Stilbohypoxylon eleiodoxae | Helicoma sp. 1 | Massarina bipolaris | Spadicoides sp. 4 | Lophiostoma frondisubmersa | |
| Jahnula appendiculata | Astrosphaeriella malayensis | Acrogenospora sphaerocephala | Jahnula appendiculata | Morenoina palmicola |
| Thailand 1 | Brunei 2 | Philippines 3 |
|---|---|---|
| Trichocladium nypae | Linocarpon bipolare | Linocarpon appendiculatum |
| Linocarpon appendiculatum | Linocarpon appendiculatum | Microthyrium sp. |
| Lulworthia grandispora | Oxydothis nypae | Astrosphaeriella striatispora |
| Oxydothis nypae | Astrosphaeriella striatispora | Oxydothis nypicola |
| Astrosphaeriella striatispora | Trichocladium nypae | Halocyphina villosa |
| Helicorhoidion nypicola | Lignincola nypae | Didymella sp. |
| Aniptodera nypae | Neolinocarpon globosicarpum | Lignincola nypae |
| Lignincola laevis | Sporidesmium crassisporum | Helicorhoidion nypicola |
| Dictyosporium elegans | Helicorhoidion nypicola | Aniptodera intermedia |
| Anthostomella cf. rehmii | Aniptodera nypae | Massarina sp. |
4.2.2. Fungal Specificity at Organ/Tissue Level
4.3. Palm Fungi as Good Biogeographical Indicators
5. Why Study Palm Fungi? Biodiversity Estimates and Their Significance
| Benchmark | Basis | Reference and Reasoning | Plant:Fungus Ratio 1 | Estimate of Total Species Number 2 |
|---|---|---|---|---|
| I | Plant:fungus ratios in tropical palms | Hyde [762,763] based on extensive work on palm fungi in Australia | 1:28 | 67,600 |
| Fröhlich and Hyde [10] based on survey of fungi associated with six Licuala palms in Australia and Brunei Darussalam | 1:33 | 85,800 | ||
| II | Plant:fungus ratios in temperate palms | Hawksworth and Lücking [745] based on long-term investigations mainly on fungal collections from temperate regions | 1:9.8 | 25,480 |
| Present study based on the estimates presented by Taylor et al. [12] for the number of host-specific fungi in tropical and temperate palms growing inside and outside their natural geographic range | 1:8.7 | 22,620 | ||
| III | Plant:fungus ratios in palms inhabiting highly specialised habitats | Present study considering that 42 of the 142 fungal species recorded on Nypa fruticans are likely to be host-specific | 1:42 | 109,200 |
| Present study considering that palms inhabiting highly specialised habitats may have a higher percentage of host-specific fungi than typical tropical palms (25%) and be closer to the percentage of host-specificity estimated for temperate hosts (63%) | 1:55 | 143,000 | ||
| Mean I–III | 75,617 |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, W.J.; Dransfield, J. Beyond Genera Palmarum: Progress and prospects in palm systematics. Bot. J. Linn. Soc. 2016, 182, 207–233. [Google Scholar] [CrossRef]
- Tomlinson, P.B. Systematics and Ecology of the Palmae. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 85–107. [Google Scholar] [CrossRef]
- Johnson, D. Palms: Their Conservation and Sustained Utilization. Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 1996; Available online: https://portals.iucn.org/library/node/7027 (accessed on 15 September 2023).
- Johnson, D.V. Non-Wood Forest Products 10/Rev.1—Tropical Palms 2010 Revision; FAO: Rome, Italy, 2011; Available online: https://www.fao.org/3/i1971e/i1971e00.htm (accessed on 15 September 2023).
- Dransfield, J.; Uhl, N.W.; Asmussen, C.B.; Baker, W.J.; Harley, M.M.; Lewis, C.E. Genera Palmarum. The Evolution and Classification of the Palms, 2nd ed.; Royal Botanic Gardens, Kew: London, UK, 2008. [Google Scholar]
- Fröhlich, J.; Hyde, K.D. Palm microfungi. Fung. Divers. Res. Ser. 2000, 3, 1–393. [Google Scholar]
- Hyde, K.D.; Alias, S.A. Biodiversity and distribution of fungi associated with decomposing Nypa fruticans. Biodivers. Conserv. 2000, 9, 393–402. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D. Microfungi of tropical and temperate palms. Fungal Divers. Res. Ser. 2003, 12, 1–495. [Google Scholar]
- Zhang, S.-N.; Hyde, K.D.; Jones, E.B.G.; Jeewon, R.; Cheewangkoon, R.; Liu, J.-K. Striatiguttulaceae, a new pleosporalean family to accommodate Longicorpus and Striatiguttula gen. nov. from palms. Mycokeys 2019, 49, 99–129. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.; Hyde, K.D. Biodiversity of palm fungi in the tropics: Are global Fungal Divers. estimates realistic? Biodivers. Conserv. 1999, 8, 977–1004. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D.; Petrini, O. Endophytic fungi associated with palms. Mycol. Res. 2000, 104, 1202–1212. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. The biogeographical distribution of microfungi associated with three palm species from tropical and temperate habitats. J. Biogeogr. 2000, 27, 297–310. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Hyde, K.D. Fungal communities on decaying palm fronds in Australia, Brunei, and Hong Kong. Mycol. Res. 2001, 105, 1458–1471. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Hyde, K.D.; Goh, T.-K. Occurrence of fungi on tissues of Livistona chinensis. Fungal Divers. 2001, 6, 167–179. [Google Scholar]
- Yanna; Hyde, K.D. New saprobic fungi on fronds of palms from northern Queensland, Australia. Aust. Syst. Bot. 2002, 15, 755–764. [Google Scholar] [CrossRef]
- Pinnoi, A.; Phongpaichit, S.; Hyde, K.D.; Jones, E.B.G. Biodiversity of fungi on Calamus (Palmae) in Thailand. Cryptogam. Mycol. 2009, 30, 181–190. [Google Scholar]
- Hyde, K.D.; Fröhlich, J.; Taylor, J.E. Diversity of Ascomycetes on palms in the tropics. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 141–156. [Google Scholar]
- Penzig, A.J.O.; Saccardo, P.A. Diagnoses Fungorum Novorum in Insula Java Collectorum; Tipografia di Angelo Ciminago: Genova, Italy, 1897; Series Secunda; Volume 11, pp. 491–530. [Google Scholar]
- Hennings, P. Fungi blumenaviensis. II. a cl. Alfr. Möller lecti. Hedwigia 1902, 41, 1–33. [Google Scholar]
- Hennings, P. Fungi Amazonici II. a cl. Ernesto Ule collecti. Hedwigia 1904, 43, 242–273. [Google Scholar]
- Hennings, P. Fungi Philippenses I. Hedwigia 1908, 47, 250–265. [Google Scholar]
- Rehm, H. Ascomycetes Philippinensis, II. Philipp. J. Sci. Section C. Botany 1913, 8, 251–263. [Google Scholar]
- Rehm, H. Ascomycetes Philippinensis, III. Philipp. J. Sci. Section C. Botany 1913, 8, 391–405. [Google Scholar]
- Rehm, H. Ascomycetes Philippinensis, V. Leafl. Philipp. Bot. 1914, 6, 2191–2237. [Google Scholar]
- Rehm, H. Ascomycetes Philippinensis, VIII. Leafl. Philipp. Bot. 1916, 8, 2935–2961. [Google Scholar]
- Spegazzini, C. Fungi nonnulli senegalenses et canariensis. Anales Mus. Nac. Hist. Nat. Buenos Aires 1914, 26, 117–134. [Google Scholar]
- Sydow, H.; Sydow, P. Beitrag zur Kenntnis der Pilzflora der Philppinen–Inseln. Ann. Mycol. 1917, 15, 12–268. [Google Scholar]
- Ellis, M.B. Haplobasidion, Lacellinopsis and Lacellina. Mycol. Pap. 1957, 67, 1–15. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. I. Mycol. Pap. 1960, 76, 1–36. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. II. Mycol. Pap. 1961, 79, 1–23. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes. VIII. Periconiella, Trichodochium, etc. Mycol. Pap. 1967, 111, 1–46. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. XI. Mycol. Pap. 1972, 131, 1–25. [Google Scholar]
- Deighton, F.C. Three leaf–spotting hyphomycetes on palms. Trans. Br. Mycol. Soc. 1985, 85, 739–742. [Google Scholar] [CrossRef]
- Deighton, F.C. Pseudocercospora carpentariae sp. nov. Trans. Br. Mycol. Soc. 1987, 89, 402–404. [Google Scholar] [CrossRef]
- Rogers, J.D.; Callan, B.E.; Samuels, G.J. The Xylariaceae of the rainforests of North Sulawesi (Indonesia). Mycotaxon 1987, 29, 118–172. [Google Scholar]
- Reid, D.A. Fungi venezuelani: V: The Cyphellaceae of Venezuela. Kew Bull. 1961, 15, 261–275. [Google Scholar] [CrossRef]
- Dennis, R.W.G. Fungi Venezuelani: VII. Kew Bull. 1965, 19, 231–273. [Google Scholar] [CrossRef]
- Müller, E.; Dennis, R.W.G. Fungi venezuelani: VIII: Plectascales, Sphaeriales, Loculoascomycetes. Kew Bull. 1965, 19, 357–386. [Google Scholar] [CrossRef]
- Wright, J.E. Agaricostilbum, a new genus of Deuteromycetes on palm spathes from Argentina. Mycologia 1970, 62, 679–682. [Google Scholar] [CrossRef]
- Hino, I.; Katumoto, K. Notes on fungi from western Japan (1). Bull. Fac. Agric. Yamaguchi Univ. 1956, 7, 257–266. [Google Scholar]
- Katumoto, K. Notes on fungi from western Japan (5). J. Jap. Bot. 1962, 37, 295–299. [Google Scholar] [CrossRef]
- Katumoto, K. Notes on fungi from western Japan (9). J. Jap. Bot. 1966, 41, 329–334. [Google Scholar] [CrossRef]
- Goos, R.D. Some Helicosporous fungi from Hawaii. Mycologia 1980, 72, 595–610. [Google Scholar] [CrossRef]
- Holubová-Jechová, V. Craspedodidymum, new genus of phialosporous Hyphomycetes. Czech Mycol. 1972, 26, 70–73. [Google Scholar]
- Liu, X.J.; Liao, Y.Z. Records on some species of genus Prathigada and Stenella. Acta Microbiol. Sin. 1980, 20, 116–121. [Google Scholar]
- Samuels, G.J.; Rossman, A.Y. Studies in the Amphisphaeriaceae (sensu lato) 2. Leiosphaerella cocoes and two new species of Oxydothis on palms. Mycotaxon 1987, 28, 461–471. [Google Scholar]
- Hughes, S.J. New Zealand Fungi 1. Ceratosporium Schw. N. Z. J. Bot. 1964, 2, 305–309. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand fungi 5. Trichothallus and Plokamidomyces states of Trichopeltheca. N. Z. J. Bot. 1965, 3, 320–332. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand fungi 6. Sporoschisma Berk. and Br. N. Z. J. Bot. 1966, 4, 77–85. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand fungi 7. Capnocybe and Capnophialophora, new form genera of sooty moulds. N. Z. J. Bot. 1966, 4, 333–353. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand fungi 13. Trichocladium Harz. N. Z. J. Bot. 1969, 7, 153–157. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand Fungi 25. Miscellaneous species. N. Z. J. Bot. 1978, 16, 311–370. [Google Scholar] [CrossRef]
- Hughes, S.J. New Zealand Fungi 31. Capnobotrys, an anamorph of Metacapnodiaceae. N. Z. J. Bot. 1981, 19, 193–226. [Google Scholar] [CrossRef]
- McKenzie, E.H.C.; Buchanan, P.K.; Johnston, P.R. Checklist of fungi on nikau palm (Rhopalostylis sapida and R. baueri var. cheesemanii) in New Zealand. N. Z. J. Bot. 2004, 42, 335–355. [Google Scholar] [CrossRef]
- Pirozynski, K.A. Microfungi of Tanzania. I. Miscellaneous Fungi on oil palm. II. New hyphomycetes. Mycol. Pap. 1972, 129, 1–64. [Google Scholar]
- Matsushima, T. Microfungi of the Solomon Islands and Papua-New Guinea; Matsushima: Kobe, Japan, 1971. [Google Scholar]
- Matsushima, T. Icones Microfungorum a Matsushima Lectorum; Matsushima: Kobe, Japan, 1975. [Google Scholar]
- Matsushima, T. Saprophytic microfungi from Taiwan, part 1. Hyphomycetes. Matsushima Mycol. Mem. 1980, 1, 1–82. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 2. Matsushima Mycol. Mem. 1981, 2, 1–68. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 3. Matsushima Mycol. Mem. 1983, 3, 1–90. [Google Scholar] [CrossRef]
- Matsushima, T. Matsushima Mycological Memoirs 4. Matsushima Mycol. Mem. 1985, 4, 1–68. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 5. Matsushima Mycol. Mem. 1987, 5, 1–100. [Google Scholar] [CrossRef]
- Matsushima, T. Matsushima Mycological Memoirs 6. Matsushima Mycol. Mem. 1989, 6, 1–100. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 7. Matsushima Mycol. Mem. 1993, 7, 1–141. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 8. Matsushima Mycol. Mem. 1995, 8, 1–44. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 9. Matsushima Mycol. Mem. 1996, 9, 1–30. [Google Scholar]
- Matsushima, T. Matsushima Mycological Memoirs 10. Matsushima Mycol. Mem. 2003, 10, 1–214. [Google Scholar]
- Matsushima, K.; Matsushima, T. Fragmenta Mycologica I. Matsushima Mycol. Mem. 1995, 8, 45–54. [Google Scholar]
- Matsushima, K.; Matsushima, T. Fragmenta Mycologica II. Matsushima Mycol. Mem. 1996, 9, 31–40. [Google Scholar]
- Mercado-Sierra, A. El género Phragmospathula (Hyphomycetes: Fungi imperfecti) en Cuba. Acta Bot. Cub. 1980, 5, 1–6. [Google Scholar]
- Mercado-Sierra, A. Lista preliminar de hifomicetes demaciáceos de la Estación Ecológica de Sierra del Rosario y zonas adyacentes. Acta Bot. Cub. 1981, 6, 1–6. [Google Scholar]
- Mercado-Sierra, A. Taxonomía y aspectos ecológicos de algunos hifomicetes helicospóricos hallados en Cuba. Acta Bot. Cub. 1982, 11, 1–11. [Google Scholar]
- Mercado-Sierra, A. La palma real (Roystonea regia): Un sustrato idóneo para el desarrollo de hifomicetes demaciáceos. Acta Bot. Cub. 1983, 15, 1–13. [Google Scholar]
- Holubová-Jechová, V. New or interesting phialidic hyphomycetes from Cuba. Mycotaxon 1982, 15, 277–292. [Google Scholar]
- Holubová-Jechová, V.; Mercado-Sierra, A. Some new or interesting microfungi from Cuba. Mycotaxon 1982, 14, 309–315. [Google Scholar]
- Holubová-Jechová, V.; Mercado-Sierra, A. Hyphomycetes from Loma de la Coca and some localities of La Habana and Matanzas provinces, Cuba. Acta Bot. Cub. 1989, 76, 1–15. [Google Scholar]
- Mercado-Sierra, A.; Castañeda-Ruíz, R.F. Nueva especie de Triadelphia (Hyphomycetes, Deuteromycotina) de Cuba. Revista Jard. Bot. Nac. Univ. Habana 1983, 4, 65–79. [Google Scholar]
- Mercado-Sierra, A.; Castañeda-Ruíz, R.F. Nuevos hifomicetes tálicos de Cuba. Acta Bot. Cub. 1985, 32, 1–10. [Google Scholar]
- Castañeda-Ruíz, R.F.; Arnold, G.R.W. Deuteromycotina de Cuba. I. Hyphomycetes. Revista Jard. Bot. Nac. Univ. Habana 1985, 6, 47–67. [Google Scholar]
- Castañeda-Ruíz, R.F.; Arnold, G.R.W. Algunos hongos nuevos para Cuba. Revista Jard. Bot. Nac. Univ. Habana 1985, 6, 55–56. [Google Scholar]
- Mercado-Sierra, A.; Holubová-Jechová, V.; Mena-Portales, J.; Fraginals, G.G. Hongos imperfectos de Pinar del Río, Cuba: El ambiente y la taxonomía de hifomicetes demaciáceos hallados. Rep. Investig. Inst. Ecol. Sist. Acad. Ci. Cuba 1987, 2, 1–10. [Google Scholar]
- Mercado-Sierra, A.; Holubová-Jechová, V.; Mena-Portales, J. Estudios sobre la microflora de Cuba: Hifomicetes con tretoconidios. Rep. Investig. Inst. Ecol. Sist. Acad. Ci. Cuba 1989, 4, 1–8. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J. Hifomicetes de Topes de Collantes, Cuba I. Especies holoblásticas. Acta Bot. Hung. 1986, 32, 189–205. [Google Scholar]
- Mena-Portales, J.; Mercado-Sierra, A. Hifomicetes de Topes de Collantes, Cuba II. Especies enteroblásticas. Acta Bot. Hung. 1987, 33, 75–79. [Google Scholar]
- Mena-Portales, J.; Mercado-Sierra, A. Algunos hifomicetes de las provincias Ciudad de La Habana y La Habana, Cuba. Rep. Investig. Inst. Ecol. Sist. Acad. Ci. Cuba 1987, 17, 1–16. [Google Scholar]
- Mercado-Sierra, A. Hifomicetes demaciáceos de Cuba (1). Acta Bot. Cub. 1980, 1, 1–5. [Google Scholar]
- Mercado-Sierra, A. Hifomicetes demaciáceos de Cuba (2). Acta Bot. Cub. 1982, 14, 1–7. [Google Scholar]
- Holubová-Jechová, V. Studies on Hyphomycetes from Cuba I. Czech Mycol. 1983, 37, 12–18. [Google Scholar]
- Holubová-Jechová, V. Studies on hyphomycetes from Cuba V. Six new species of dematiaceous hyphomycetes from Havana Province. Czech Mycol. 1987, 41, 29–36. [Google Scholar]
- Holubová-Jechová, V. Studies on hyphomycetes from Cuba VI. New and rare species with tretic and phialidic conidiogenous cells. Czech Mycol. 1987, 41, 107–114. [Google Scholar]
- Holubová-Jechová, V. Studies on hyphomycetes from Cuba VII. Seven new taxa of dematiaceous hyphomycetes. Czech Mycol. 1988, 42, 23–30. [Google Scholar]
- Holubová-Jechová, V. Studies on hyphomycetes from Cuba VIII. A new genus Piricaudilium and some species new for the territory of Cuba. Czech Mycol. 1988, 42, 200–204. [Google Scholar]
- Holubová-Jechová, V.; Mercado-Sierra, A. Studies on Hyphomycetes from Cuba II. Hyphomycetes from the Isla de la Juventud. Czech Mycol. 1984, 38, 96–120. [Google Scholar]
- Holubová-Jechová, V.; Mercado-Sierra, A. Studies on hyphomycetes from Cuba IV. Dematiaceous hyphomycetes from the province Pinar del Río. Czech Mycol. 1986, 40, 142–164. [Google Scholar]
- Holubová-Jechová, V.; Castañeda-Ruíz, R.F. Studies on hyphomycetes from Cuba III. New and interesting dematiaceous taxa from leaf litter. Czech Mycol. 1986, 40, 74–85. [Google Scholar]
- Mena-Portales, J.; Mercado-Sierra, A. Nuevos o raros hifomicetes de Cuba III. Phragmospathulella. Un nuevo género trético. Revista Jard. Bot. Nac. Univ. Habana 1986, 7, 31–34. [Google Scholar]
- Mena-Portales, J.; Mercado-Sierra, A. Nuevos o raros hifomicetes de Cuba. IV. Un nuevo género lignícola con conidiogénesis trética. Acta Bot. Cub. 1988, 54, 1–6. [Google Scholar]
- Mercado-Sierra, A.; Castañeda-Ruíz, R.F. Nuevos o raros hifomicetes de Cuba. I. Especies de Cacumisporium, Guedea, Rhinocladium y Veronaea. Acta Bot. Cub. 1987, 50, 1–7. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J. Nuevos o raros hifomicetes de Cuba. II. Un nuevo género sobre Roystonea regia. Acta Bot. Cub. 1988, 53, 1–5. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J. Nuevos o raros hifomicetes de Cuba. V. Especies de Stachybotrys. Acta Bot. Cub. 1988, 55, 1–8. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J. Nuevos o raros hifomicetes de Cuba. VI. Neosporidesmium, nuevo género sinemático. Acta Bot. Cub. 1988, 59, 1–6. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J. Nuevos o raros hifomicetes de Cuba VII. Especies enteroblásticas. Acta Bot. Hung. 1992, 37, 63–73. [Google Scholar]
- Mercado-Sierra, A. Hifomicetes Demaciáceos de Sierra del Rosario, Cuba; Editorial Academia: La Habana, Cuba, 1984. [Google Scholar]
- Hyde, K.D.; Taylor, J.E.; Fröhlich, J. Genera of ascomycetes from palms. Fung. Divers. Res. Ser. 2000, 2, 1–247. [Google Scholar]
- Hyde, K.D. Fungi from palms. I. The genus Linocarpon, a revision. Sydowia 1992, 44, 32–54. [Google Scholar]
- Hyde, K.D. Fungi from palms. II. Kirschsteiniothelia aethiops from the date palm Phoenix dactylifera. Sydowia 1992, 45, 1–4. [Google Scholar]
- Hyde, K.D. Fungi from palms. III. The genus Pemphidium Montagne (Ascomycotina). Sydowia 1993, 45, 5–14. [Google Scholar]
- Hyde, K.D. Fungi from palms. IV. Palmicola archontophoenicis gen. et sp. nov. Sydowia 1993, 45, 15–20. [Google Scholar]
- Hyde, K.D. Fungi from palms. IX. Manokwaria notabilis gen. et sp. nov. (Ascomycetes) from Irian Jaya and Australia. Sydowia 1993, 45, 246–251. [Google Scholar]
- Hyde, K.D. Fungi from palms. V. Phomatospora nypae sp. nov. and notes on marine fungi from Nypa fruticans in Malaysia. Sydowia 1993, 45, 199–203. [Google Scholar]
- Hyde, K.D. Fungi from palms. VI. Reflections on Oxydothis and related genera. Sydowia 1993, 45, 204–225. [Google Scholar]
- Hyde, K.D. Fungi from palms. VII. The genus Oxydothis from rachides of palms in north Queensland, including five new species. Sydowia 1993, 45, 226–240. [Google Scholar]
- Hyde, K.D. Fungi from palms. VIII. The genus Myelosperma (Ascomycotina). Sydowia 1993, 45, 241–245. [Google Scholar]
- Hyde, K.D. Fungi from palms. X. Lockerbia palmicola, a new cleistothecial genus in the Sordariales. Sydowia 1993, 46, 23–28. [Google Scholar]
- Hyde, K.D. Fungi from palms. XI. Appendispora frondicola gen. et sp. nov. from Oncosperma horridum in Brunei. Sydowia 1994, 46, 29–34. [Google Scholar]
- Hyde, K.D. Fungi from palms. XII. Three new intertidal ascomycetes from palm fronds. Sydowia 1994, 46, 257–264. [Google Scholar]
- Hyde, K.D. Fungi from palms. XIII. The genus Oxydothis, a revision. Sydowia 1994, 46, 265–314. [Google Scholar]
- Hyde, K.D. Fungi from palms. XIV. Durispora elaeidicola gen. et sp. nov. Sydowia 1994, 46, 315–320. [Google Scholar]
- Fröhlich, J.; Hyde, K.D. Fungi from palms. XIX. Caudatispora palmicola gen. et sp. nov. in Ecuador. Sydowia 1995, 47, 38–43. [Google Scholar]
- Lu, B.-S.; Hyde, K.D. Fungi from palms. XLI. Fasciatispora sabalicola: Further collections from Florida, USA. Mycotaxon 1999, 71, 393–397. [Google Scholar]
- Fröhlich, J.; Lowen, R.; Hyde, K.D. Fungi from palms. XLV. Nectriella erythroclypea sp. nov. (Bionectriaceae, Hypocreales). Nova Hedwig. 2000, 70, 425–430. [Google Scholar] [CrossRef]
- Hyde, K.D. Fungi from palms. XV. Sabalicola gen. nov., and a new combination for Anthostomella sabalensioides. Nova Hedwig. 1995, 60, 595–598. [Google Scholar]
- Hyde, K.D. Fungi from palms. XVI. Cocoicola gen. nov. Nova Hedwig. 1995, 60, 599–604. [Google Scholar]
- Hyde, K.D. Fungi from palms. XVII. The genus Fasciatispora with notes on Amphisphaerella. Nova Hedwig. 1995, 61, 249–268. [Google Scholar]
- Hyde, K.D. Fungi from palms. XVIII. Appendicospora coryphae, a new name for Apiosporella coryphae. Sydowia 1995, 47, 31–37. [Google Scholar]
- Hyde, K.D. Fungi from palms. XX. The genus Guignardia. Sydowia 1995, 47, 180–198. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXI. The genus Seynesia. Sydowia 1995, 47, 199–212. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXII. A new species of Ascotaiwania. Sydowia 1995, 47, 213–216. [Google Scholar]
- Hyde, K.D.; Fröhlich, J. Fungi from palms. XXIII. Rachidicola gen. et sp. nov. Sydowia 1995, 47, 217–222. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXIV. The genus Bondiella. Mycotaxon 1996, 57, 347–352. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXIX. Arecophila gen. nov. (Amphisphaeriales, Ascomycota), with five new species and two new combinations. Nova Hedwig. 1996, 63, 81–100. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXV. Pestalosphaeria elaeidis. Mycotaxon 1996, 57, 353–357. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXVI. The genus Anthostomella, with ten new species. Nova Hedwig. 1996, 62, 273–340. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXVII. Capsulospora gen. nov., with three new species. Sydowia 1996, 48, 111–121. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXVIII. Two new species of Pemphidium from Australia and Indonesia. Sydowia 1996, 48, 122–130. [Google Scholar]
- Hyde, K.D.; Kang, J.C.; Kong, R.Y.C. Fungi from palms. XXX. Notes on Amphisphaeria species from palms and a description of Amphisphaeria umbrina. Nova Hedwig. 1996, 63, 101–108. [Google Scholar]
- Hyde, K.D.; Taylor, J.E. Fungi from palms. XXXI. The genus Nipicola (Ascomycetes, Xylariaceae) with one new species. Nova Hedwig. 1996, 63, 417–424. [Google Scholar]
- Hyde, K.D. Fungi from palms. XXXII. Arecomyces gen. nov., with seven new species. Sydowia 1996, 48, 224–240. [Google Scholar]
- Hyde, K.D.; Aptroot, A. Fungi from palms. XXXIII. The genus Massarina, with a new species. Nova Hedwig. 1997, 64, 491–504. [Google Scholar] [CrossRef]
- Hyde, K.D.; Taylor, J.E.; Fröhlich, J. Fungi from palms. XXXIV. The genus Neolinocarpon with five new species and one new combination. Fungal Divers. 1998, 1, 115–131. [Google Scholar]
- Fröhlich, J.; Hyde, K.D. Fungi from palms. XXXIX. Asymmetricospora gen. et sp. nov. (Melannomataceae). Sydowia 1998, 50, 182–186. [Google Scholar]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. Fungi from palms. XXXV. Thyridium chrysomallum associated with Archontophoenix alexandrae (Palmae) cultivated in Hong Kong. Sydowia 1997, 49, 94–100. [Google Scholar]
- Hyde, K.D.; Fröhlich, J.; Taylor, J.E. Fungi from palms. XXXVI. Reflections on unitunicate ascomycetes with apiospores. Sydowia 1998, 50, 21–80. [Google Scholar]
- Hyde, K.D.; Fröhlich, J. Fungi from palms. XXXVII. The genus Astrosphaeriella, including ten new species. Sydowia 1998, 50, 81–132. [Google Scholar]
- Fröhlich, J.; Hyde, K.D. Fungi from palms. XXXVIII. The genera Mycosphaerella and Sphaerella. Sydowia 1998, 50, 171–181. [Google Scholar]
- Taylor, J.E.; Hyde, K.D. Fungi from palms. XL. Iodosphaeria. Sydowia 1999, 51, 127–132. [Google Scholar]
- Hyde, K.D.; Aptroot, A.; Fröhlich, J.; Taylor, J.E. Fungi from palms. XLII. Didymosphaeria and similar ascomycetes from palms. Nova Hedwig. 1999, 69, 449–471. [Google Scholar] [CrossRef]
- Hyde, K.D.; Aptroot, A.; Fröhlich, J.; Taylor, J.E. Fungi from palms. XLIII. Lophiostoma and Astrosphaeriella species with slit–like ostioles. Nova Hedwig. 2000, 70, 143–160. [Google Scholar] [CrossRef]
- Aptroot, A.; Fröhlich, J.; Hyde, K.D. Fungi from palms. XLIV. Two new Massarina species with pigmented ostioles. Nova Hedwig. 2000, 70, 227–232. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Hyde, K.D. Fungi from palms. XLIX. Astrocystis, Biscogniauxia, Cyanopulvis, Hypoxylon, Nemania, Guestia, Rosellinia and Stilbohypoxylon. Fungal Divers. 2001, 7, 89–127. [Google Scholar]
- Guo, L.D.; Hyde, K.D. Fungi from palms. XLVI. Seynesia livistonae sp nov (Xylariaceae) from Hong Kong. Nova Hedwig. 2001, 72, 461–465. [Google Scholar] [CrossRef]
- Hosagoudar, V.B.; Abraham, T.K.; Biju, C.K.; Hyde, K.D. Fungi from palms. XLVII. A new species of Asterina on palms from India. Fungal Divers. 2001, 6, 69–73. [Google Scholar]
- Sarma, V.V.; Hyde, K.D. Fungi from palms. XLVIII. Curvatispora singaporensis gen. et sp nov on Livistona spinosa from Singapore. Nova Hedwig. 2001, 72, 479–485. [Google Scholar] [CrossRef]
- Hyde, K.D. The genus Linocarpon from the mangrove palm Nypa fruticans. Trans. Mycol. Soc. Jpn. 1988, 29, 339–350. [Google Scholar]
- Hyde, K.D. Studies on the tropical marine fungi of Brunei. Bot. J. Linn. Soc. 1988, 98, 135–151. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nakagiri, A. A new species of Oxydothis from the mangrove palm, Nypa fruticans. Trans. Mycol. Soc. Jpn. 1989, 30, 69–75. [Google Scholar]
- Hyde, K.D. Studies on the tropical marine fungi of Brunei. II. Notes on five interesting species. Trans. Mycol. Soc. Jpn. 1988, 29, 161–171. [Google Scholar]
- Hyde, K.D.; Jones, E.B.G. Marine mangrove fungi. Mar. Ecol. 1988, 9, 15–34. [Google Scholar] [CrossRef]
- Hyde, K.D. Intertidal mangrove fungi from north Sumatra. Can. J. Bot. 1989, 67, 3078–3082. [Google Scholar] [CrossRef]
- Hyde, K.D. Helicascus kanaloanus, Helicascus nypae sp. nov. and Salsuginea ramicola gen. et sp. nov. from intertidal mangrove wood. Bot. Mar. 1991, 34, 311–318. [Google Scholar] [CrossRef]
- Hyde, K.D. A new amphisphaeriaceous fungus from intertidal fronds of Nypa fruticans. Trans. Mycol. Soc. Jpn. 1991, 32, 265–271. [Google Scholar]
- Hyde, K.D. Fungi from decaying intertidal fronds of Nypa fruticans, including three new genera and four new species. Bot. J. Linn. Soc. 1992, 110, 95–110. [Google Scholar] [CrossRef]
- Hyde, K.D. Fungi from Nypa fruticans: Nipicola carbospora gen. et sp. nov. (Ascomycotina). Cryptogam. Bot. 1992, 2, 330–332. [Google Scholar]
- Hyde, K.D.; Sutton, B.C. Nypaella frondicola gen. et sp. nov., Plectophomella nypae sp. nov. and Pleurophomopsis nypae sp. nov. (Coelomycetes) from intertidal fronds of Nypa fruticans. Mycol. Res. 1992, 96, 210–214. [Google Scholar] [CrossRef]
- Hyde, K.D.; Alias, S.A. Linocarpon angustatum sp. nov., and Neolinocarpon nypicola sp. nov. from petioles of Nypa fruticans, and a list of fungi from aerial parts of this host. Mycoscience 1999, 40, 145–149. [Google Scholar] [CrossRef]
- Hyde, K.D.; Goh, T.-K.; Lu, B.-S.; Alias, S.A. Eleven new intertidal fungi from Nypa fruticans. Mycol. Res. 1999, 103, 1409–1422. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Hyde, K.D.; Read, S.J.; Moss, S.T.; Alias, S.A. Tirisporella gen nov, an ascomycete from the mangrove palm Nypa fruticans. Can. J. Bot. 1996, 74, 1487–1495. [Google Scholar] [CrossRef]
- Hyde, K.D.; Lee, S.Y. Ecology of mangrove fungi and their role in nutrient cycling: What gaps occur in our knowledge? Hydrobiologia 1995, 295, 107–118. [Google Scholar] [CrossRef]
- Hyde, K.D. The genus Saccardoella from intertidal mangrove wood. Mycologia 1992, 84, 803–810. [Google Scholar] [CrossRef]
- Hyde, K.D. Frondisphaeria palmicola gen. et sp. nov. from Brunei. Mycoscience 1996, 37, 169–171. [Google Scholar] [CrossRef]
- Hyde, K.D. The genus Roussoella, including two new species from palms in Cuyabeno, Ecuador. Mycol. Res. 1997, 101, 609–616. [Google Scholar] [CrossRef]
- Hyde, K.D. Additions to the genus Linocarpon (Ascomycetes: Hyponectriaceae). Bot. J. Linn. Soc. 1997, 123, 109–131. [Google Scholar] [CrossRef][Green Version]
- Hyde, K.D.; Philemon, E. Capitorostrum cocoes sp. nov., causing leaf spot of Cocos nucifera. Mycotaxon 1991, 42, 95–97. [Google Scholar]
- Hyde, K.D. Aquatic fungi on rachides of Livistona in the Western Province of Papua New Guinea. Mycol. Res. 1994, 98, 719–725. [Google Scholar] [CrossRef]
- Hyde, K.D. Fungi from rachides of Livistona in the western province of Papua New Guinea. Bot. J. Linn. Soc. 1994, 116, 315–324. [Google Scholar] [CrossRef]
- Hyde, K.D.; Eriksson, O.E.; Yue, J.Z. Roussoella, an ascomycete genus of uncertain relationships with a Cytoplea anamorph. Mycol. Res. 1996, 100, 1522–1528. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cannon, P.F.; Barr, M.E. Phaeochoraceae, a new ascomycete family from palms. Syst. Ascomycetum 1997, 15, 117–120. [Google Scholar]
- Hyde, K.D.; Wong, S.-W. Ultrastructural studies on the Myelospermaceae fam. nov., with a new species of Myelosperma. Mycol. Res. 1999, 103, 347–352. [Google Scholar] [CrossRef]
- Hyde, K.D.; Fröhlich, J. Nigramammilla calami gen. et sp nov and Arecomyces calami, A. licualae and Pseudohalonectria palmae spp. nov from palms. Cryptogam. Mycol. 2003, 24, 13–20. [Google Scholar]
- Hyde, K.D.; Fröhlich, J.; Taylor, J.E. Cocoicola livistonicola, sp. nov., and notes on Cocoicola cylindrospora from palms. Mycoscience 1997, 38, 255–258. [Google Scholar] [CrossRef]
- Hyde, K.D.; Goh, T.-K.; Taylor, J.E.; Fröhlich, J. Byssosphaeria, Chaetosphaeria, Niesslia and Ornatispora gen. nov., from palms. Mycol. Res. 1999, 103, 1423–1439. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Melanographium palmicolum sp. nov. from Hong Kong, and a key to the genus. Mycol. Res. 1997, 101, 1097–1100. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. The generic distinction between Chaetopsina and Kionochaeta, with descriptions of two new species. Mycol. Res. 1997, 101, 1517–1523. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D.; Ho, W.H.; Yanna. A revision of the genus Dictyosporium, with descriptions of three new species. Fungal Divers. 1999, 2, 65–100. [Google Scholar]
- Taylor, J.E.; Fröhlich, J.; Hyde, K.D. Lasiosphaeria and a similar new genus from palms. Mycoscience 2001, 42, 369–377. [Google Scholar] [CrossRef]
- Lu, B.-S.; Hyde, K.D.; Ho, H.W.; Tsui, K.M.; Taylor, J.E.; Wong, K.M.; Yanna; Zhou, D. Checklist of Hong Kong fungi. Fung. Divers. Res. Ser. 2000, 5, 1–207. [Google Scholar]
- Fröhlich, J.; Hyde, K.D. Guignardia candeloflamma sp. nov. causing leaf spots of Pinanga spp. Mycol. Res. 1995, 99, 110–112. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Spadicoides palmicola sp. nov. on Licuala sp. from Brunei, and a note on Spadicoides heterocolorata comb. nov. Can. J. Bot. 1998, 76, 1698–1702. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Stratiphoromyces brunneisporus gen. et sp. nov., an undescribed dematiaceous hyphomycete on Licuala palms. Mycol. Res. 1998, 102, 1149–1152. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Polybulbophiale palmicola gen. et sp. nov. (Hyphomycetes) from Brunei. Mycotaxon 1998, 69, 145–151. [Google Scholar]
- Tsui, C.K.M.; Leung, Y.M.; Hyde, K.D.; Hodgkiss, I.J. Three new Ophioceras species (Ascomycetes) from the tropics. Mycoscience 2001, 42, 321–326. [Google Scholar] [CrossRef]
- Phengsintham, P.; Hyde, K.D. Fungi of Laos I: Ascomycetes from Palms. In Proceedings of the Building Capacity in Biodiversity Information Sharing, 2003: Joint International Forum on Biodiversity Information, Building Capacity in Asia and Oceania, Tsukuba, Japan, 4–10 October 2003; National Institute for Environmental Studies: Tsukuba, Japan, 2003; pp. 174–183. [Google Scholar]
- Phengsintham, P.; Hyde, K.D. Check list of Lao fungi. In Proceedings of the Building Capacity in Biodiversity Information Sharing, 2003: Joint International Forum on Biodiversity Information, Building Capacity in Asia and Oceania, Tsukuba, Japan, 4–10 October 2003; National Institute for Environmental Studies: Tsukuba, Japan, 2003; pp. 184–190. [Google Scholar]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. Pulmosphaeria archontophoenicis gen. et sp. nov. associated with Archontophoenix alexandrae (Arecaceae) in Northern Queensland. Sydowia 1996, 48, 255–262. [Google Scholar]
- Frohlich, J.; Hyde, K.D. New Oxydothis species associated with palm leaf spots in North Queensland, Australia. Mycol. Res. 1994, 98, 213–218. [Google Scholar] [CrossRef]
- Frohlich, J.; Hyde, K.D. Astrosphaeriella fronsicola sp. nov. associated with leaf spots of Oraniopsis and other palms. Mycol. Res. 1995, 99, 453–456. [Google Scholar] [CrossRef]
- Frohlich, J.; Hyde, K.D. Maculatipalma fronsicola gen. et sp. nov. causing leaf spots on palm species in North Queensland with descriptions of related genera: Apioplagiostoma and Plagiostoma. Mycol. Res. 1995, 99, 727–734. [Google Scholar] [CrossRef]
- Hyde, K.D.; Frohlich, J. Mycosphaerella palmicola associated with leaf spots of Cocos nucifera in Australia, Irian Jaya and Papua New Guinea. Mycol. Res. 1995, 99, 704–706. [Google Scholar] [CrossRef]
- Frohlich, J.; Hyde, K.D.; Guest, D.I. Fungi associated with leaf spots of palms in North Queensland, Australia. Mycol. Res. 1997, 101, 721–732. [Google Scholar] [CrossRef]
- Hyde, K.D.; Taylor, J.E.; Fröhlich, J. Two new species of Pseudohalonectria from palms. Mycologia 1999, 91, 520–524. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Delortia palmicola and two new species from wood submerged in a freshwater stream in Australia. Mycol. Res. 1997, 101, 42–46. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. A new species of Canalisporium from Australia. Mycologia 2000, 92, 589–592. [Google Scholar] [CrossRef]
- Hyde, K.D.; Goh, T.-K. Tropical Australian Freshwater Fungi XIII. A new species of Anthostomella and its sporodochial Geniculosporium anamorph. Nova Hedwig. 1998, 67, 225–233. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D. Cannonia gen. nov., from palms in the Southern Hemisphere. Mycol. Res. 1999, 103, 1398–1402. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. Lepteutypa hexagonalis sp. nov. from Pinanga sp. in Ecuador. Mycol. Res. 1997, 101, 85–88. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. A new species of Palmicola from Ecuador. Mycol. Res. 1996, 100, 714–716. [Google Scholar] [CrossRef]
- Goh, T.-K.; Hyde, K.D. A new species of Nectria from Mauritia flexuosa (Arecaceae) in Ecuador and a key to Nectria and allied genera on palms. Mycoscience 1996, 37, 277–282. [Google Scholar] [CrossRef]
- Hyde, K.D.; Stanley, S.J.; Steinke, T.D. Fungi associated with leaf spots of palms. Maculatifrondis aequatoriensis gen. et sp. nov., with a Cyclodomus anamorph, and Myelosperma parasitica sp. nov. Mycol. Res. 1996, 100, 1509–1514. [Google Scholar] [CrossRef]
- Lu, B.-S.; Hyde, K.D.; Liew, E.C.Y. Eight new species of Anthostomella from South Africa. Mycol. Res. 2000, 104, 742–754. [Google Scholar] [CrossRef]
- Hyde, K.D.; Ho, W.-H.; Tsui, C.K.M. The genera Aniptodera, Halosarpheia, Nais and Phaeonectriella from freshwater habitats. Mycoscience 1999, 40, 165–183. [Google Scholar] [CrossRef]
- Wong, S.-W.; Hyde, K.D. Ultrastructural observations on Oxydothis alexandrarum. Fungal Divers. 1999, 2, 181–188. [Google Scholar]
- Hyde, K.D.; Cannon, P.F. Fungi causing tar spots on palms. Mycol. Pap. 1999, 175, 1–114. [Google Scholar]
- Barr, M.E.; Ohr, H.D.; Murphy, M.K. The genus Serenomyces on palms. Mycologia 1989, 81, 47–51. [Google Scholar] [CrossRef]
- Barr, M.E.; Ohr, H.D.; Ferrin, D.M.; Mundo-Ocampo, M. A new species of Serenomyces from date palm in California. Mycotaxon 1997, 61, 481–484. [Google Scholar]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. Endophytic fungi associated with the temperate palm, Trachycarpus fortunei, within and outside its natural geographic range. New Phytol. 1999, 142, 335–346. [Google Scholar] [CrossRef]
- Hyde, K.D.; Wong, S.-W. An ultrastructural study of the asci and banded ascospores of Fasciatispora petrakii. Fungal Divers. 1999, 2, 129–134. [Google Scholar]
- Wang, Y.-Z.; Hyde, K.D. Hyponectria buxi with notes on the Hyponectriaceae. Fungal Divers. 1999, 3, 159–172. [Google Scholar]
- Lu, B.-S.; Hyde, K.D.; Yuan, Z.Q. The genus Anthostomella in Australia. Fungal Divers. 1999, 3, 99–106. [Google Scholar]
- Lu, B.-S.; Hyde, K.D. A world monograph of Anthostomella. Fung. Divers. Res. Ser. 2000, 4, 1–376. [Google Scholar]
- Lu, B.-S.; Hyde, K.D. Anthostomella longa sp. nov. and note on other species on monocots from Hong Kong. Cryptogam. Mycol. 2000, 21, 207–214. [Google Scholar] [CrossRef]
- Lu, B.-S.; Hyde, K.D. Species of Anthostomella from Brunei, including A. oblongata sp. nov. Mycoscience 2000, 41, 223–226. [Google Scholar] [CrossRef]
- Aptroot, A. A monograph of Didymosphaeria. Stud. Mycol. 1995, 37, 1–160. [Google Scholar]
- Aptroot, A. Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwig. 1995, 60, 325–379. [Google Scholar]
- Yanna; Hyde, K.D.; Goh, T.-K. Staurophoma calami, a new coelomycete from Hong Kong. Sydowia 1998, 50, 139–143. [Google Scholar]
- Yanna; Hyde, K.D.; Fröhlich, J. A new species of Appendicospora from Hong Kong. Mycoscience 1997, 38, 395–397. [Google Scholar] [CrossRef]
- Yanna; Hyde, K.D.; Goh, T.-K. Koorchaloma novojournalis sp. nov., a new sporodochial fungus from Hong Kong. Fungal Divers. 1998, 1, 193–197. [Google Scholar]
- Yanna; Hyde, K.D.; Goh, T.-K. Endomelanconium phoenicicola, a new coelomycete from Phoenix hanceana in Hong Kong. Fungal Divers. 1999, 2, 199–204. [Google Scholar]
- Yanna; Ho, W.H.; McKenzie, E.H.C.; Hyde, K.D. New saprobic fungi on palm fronds, including Brachysporiopsis gen. nov. Cryptogam. Mycol. 2004, 25, 129–135. [Google Scholar]
- Yanna; Ho, W.H.; Goh, T.-K.; Hyde, K.D. A new species of Everhartia associated with leaf spots of Phoenix hanceana from Hong Kong. Bot. J. Linn. Soc. 2000, 134, 465–470. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Hyde, K.D.; McKenzie, E.H.C. Sporidesmiella oraniopsis, a new species of dematiaceous hyphomycete from North Queensland, Australia and synopsis of the genus. Fungal Divers. 2001, 8, 183–190. [Google Scholar]
- Zhuang, W.-Y.; Hyde, K.D. New species of Lachnum and Perrotia from Hong Kong, China. Mycologia 2001, 93, 606–611. [Google Scholar] [CrossRef]
- Wong, M.K.M.; Yanna; Goh, T.-K.; McKenzie, E.H.C. Two new species of Constantinella from Hong Kong. Fungal Divers. 2001, 8, 173–181. [Google Scholar]
- Yu, Z.-H.; Zhuang, W.-Y. New taxa and new records of Lachnum and Arachnopeziza (Helotiales, Hyaloscyphaceae) from tropical China. Nova Hedwig. 2002, 74, 415–428. [Google Scholar] [CrossRef]
- Zhuang, W.-Y. A new species of Lachnum on leaves of Livistona and a key to the Chinese species of the genus. Mycotaxon 2003, 86, 375–382. [Google Scholar]
- Ho, W.H.; Yanna; Hyde, K.D.; Goh, T.-K. Endosporoideus gen. nov., a mitosporic fungus on Phoenix hanceana. Mycologia 2005, 97, 238–245. [Google Scholar] [CrossRef]
- Hyde, K.D.; Yanna; Pinnoi, A.; Jones, E.B.G. Goidanichiella fusiforma sp. nov. from palm fronds in Brunei and Thailand. Fungal Divers. 2002, 11, 119–122. [Google Scholar]
- Yanna; Ho, W.H.; Goh, T.-K.; Hyde, K.D. Craspedodidymum nigroseptatum sp. nov., a new hyphomycete on palms from Brunei Darussalam. Mycol. Res. 2000, 104, 1146–1151. [Google Scholar] [CrossRef]
- Ho, W.H.; Yanna; Hyde, K.D. Two new species of Spadicoides from Brunei and Hong Kong. Mycologia 2002, 94, 302–306. [Google Scholar] [CrossRef][Green Version]
- Haines, J.H. Studies in the Hyaloscyphaceae VI: The genus Lachnum (ascomycetes) of the Guayana Highlands. Nova Hedwig. 1992, 54, 97–112. [Google Scholar]
- Cantrell, S.A.; Haines, J.H. New red species of Lachnum from the tropics. Mycol. Res. 1997, 101, 1081–1084. [Google Scholar] [CrossRef]
- McKenzie, E.H.C.; Pinnoi, A.; Wong, M.K.M.; Hyde, K.D.; Jones, E.B.G. Two new hyaline Chalara species, and a key to species described since 1975. Fungal Divers. 2002, 11, 129–139. [Google Scholar]
- Pinruan, U.; Jones, E.B.G.; Hyde, K.D. Aquatic fungi from peat swamp palms: Jahnula appendiculata sp. nov. Sydowia 2002, 54, 242–247. [Google Scholar]
- Pinruan, U.; Lumyong, S.; McKenzie, E.H.C.; Jones, E.B.G.; Hyde, K.D. Three new species of Craspedodidymum from palm in Thailand. Mycoscience 2004, 45, 177–180. [Google Scholar] [CrossRef]
- Pinruan, U.; McKenzie, E.H.C.; Jones, E.B.G.; Hyde, K.D. Two new species of Stachybotrys, and a key to the genus. Fungal Divers. 2004, 17, 145–157. [Google Scholar]
- Pinnoi, A.; Jones, E.B.G.; McKenzie, E.H.C.; Hyde, K.D. Aquatic fungi from peat swamp palms: Unisetosphaeria penguinoides gen. et sp. nov., and three new Dactylaria species. Mycoscience 2003, 44, 377–382. [Google Scholar] [CrossRef]
- Pinnoi, A.; McKenzie, E.C.; Jones, E.B.G.; Hyde, K.D. Palm fungi from Thailand: Custingophora undulatistipes sp. nov. and Vanakripa minutiellipsoidea sp. nov. Nova Hedwig. 2003, 77, 213–219. [Google Scholar] [CrossRef]
- Pinnoi, A.; Pinruan, U.; Hyde, K.D.; McKenzie, E.H.C.; Lumyong, S. Submersisphaeria palmae sp. nov. with a key to species, and notes on Helicoubisia. Sydowia 2004, 56, 72–78. [Google Scholar]
- Pinnoi, A.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Biodiversity of fungi on the palm Eleiodoxa conferta in Sirindhorn peat swamp forest, Narathiwat, Thailand. Fungal Divers. 2006, 22, 205–218. [Google Scholar]
- Pinruan, U.; Hyde, K.D.; Lumyong, S.; McKenzie, E.H.C.; Jones, E.B.G. Occurrence of fungi on tissues of the peat swamp palm Licuala longicalycata. Fungal Divers. 2007, 25, 157–173. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. III. Mycol. Pap. 1961, 82, 1–55. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. V. Mycol. Pap. 1963, 93, 1–33. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. IV. Mycol. Pap. 1963, 87, 1–42. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. VI. Mycol. Pap. 1965, 103, 1–46. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. VII. Curvularia, Brachysporium, etc. Mycol. Pap. 1966, 106, 1–57. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes. IX. Spiropes and Pleurophragmium. Mycol. Pap. 1968, 114, 1–44. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. X. Mycol. Pap. 1971, 125, 1–30. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Ellis, M.B. More Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1976. [Google Scholar]
- Subramanian, C.V. A reassessment of Sporidesmium (Hyphomycetes) and some related taxa. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1992, 58, 179–190. [Google Scholar]
- Mena-Portales, J.; Hernández-Gutiérrez, A.; Mercado-Sierra, A. Acarocybiopsis, a new genus of synnematous hyphomycetes from Cuba. Mycol. Res. 1999, 103, 1032–1034. [Google Scholar] [CrossRef]
- Zhang, S.-N.; Hyde, K.D.; Jones, E.B.G.; Cheewangkoon, R.; Liu, J.-K. Acuminatispora palmarum gen. et sp. nov. from mangrove habitats. Mycol. Prog. 2018, 17, 1173–1188. [Google Scholar] [CrossRef]
- Subramanian, C.V. Agrabeeja kavakapriya gen. et sp. nov. and additions to Hemicorynespora. Kavaka 1995, 20, 1–9. [Google Scholar]
- Konta, S.; Maharachchikumbura, S.S.N.; Senanayake, I.C.; McKenzie, E.H.C.; Stadler, M.; Boonmee, S.; Phookamsak, R.; Jayawardena, R.S.; Senwanna, C.; Hyde, K.D.; et al. A new genus Allodiatrype, five new species and a new host record of diatrypaceous fungi from palms (Arecaceae). Mycosphere 2020, 11, 239–268. [Google Scholar] [CrossRef]
- do Carmo, L.T.; Monteiro, J.S.; Gusmao, L.F.P.; Sotao, H.M.P.; Gutiérrez, A.H.; Castañeda-Ruíz, R.F. Anabahusakala, a new genus from the Brazilian Amazon rainforest. Mycotaxon 2014, 127, 11–15. [Google Scholar] [CrossRef]
- Qiao, M.; Li, D.-W.; Yu, Z.-F.; Zhang, K.; Castañeda-Ruíz, R.F. Spadicoides matsushimae sp. nov., and Anisospadicoides gen. nov. for two atypical Spadicoides species. Mycotaxon 2019, 134, 161–167. [Google Scholar] [CrossRef]
- Subramanian, C.V. Basauxia and Ashtaangam of Hyphomycetes from Southeast Asia. Kor. J. Mycol. 1992, 20, 281–284. [Google Scholar]
- Phookamsak, R.; Norphanphoun, C.; Tanaka, K.; Dai, D.-Q.; Luo, Z.-L.; Liu, J.-K.; Su, H.-Y.; Bhat, D.J.; Bahkali, A.H.; Mortimer, P.E.; et al. Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov. and Astrosphaeriellopsis, gen. nov. Fungal Divers. 2015, 74, 143–197. [Google Scholar] [CrossRef]
- Wu, W.-P.; Diao, Y.-Z. Anamorphic chaetosphaeriaceous fungi from China. Fungal Divers. 2022, 116, 1–546. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.A.; Dayarathne, M.C.; Suetrong, S.; Guo, S.Y.; Alias, S.A.; Bahkali, A.H.; Nagahama, T.; Elgorban, A.M.; Abdel-Aziz, F.A.; Hodhod, M.S.; et al. New saprobic marine fungi and a new combination. Bot. Mar. 2017, 60, 469–488. [Google Scholar] [CrossRef]
- Pinruan, U.; Rungjindamai, N.; Sakayaroj, J.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Baipadisphaeria gen. nov., a freshwater ascomycete (Hypocreales, Sordariomycetes) from decaying palm leaves in Thailand. Mycosphere 2010, 1, 53–63. [Google Scholar]
- Nagaraju, D.; Kunwar, I.K.; Sureshkumar, G.; Manoharachary, C. A new synnematous hyphomycetous fungus Bhadradriella gen. nov. from India. J. Mycol. Plant Pathol. 2011, 41, 238–240. [Google Scholar]
- Castañeda-Ruíz, R.F.; Iturriaga, T.; Decock, C. Bulbocatenospora, a new hyphomycete genus from Venezuela. Mycol. Res. 2000, 104, 107–109. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Miller, A.N.; Piepenbring, M. South Florida microfungi: Castanedospora, a new genus to accommodate Sporidesmium pachyanthicola (Capnodiales, Ascomycota). Cryptogam. Mycol. 2018, 39, 109–127. [Google Scholar] [CrossRef]
- Gutiérrez, A.H. New or rare fungi from eastern Amazonia. 1. Circinoconiopsis amazonica gen. and sp. nov. Mycotaxon 2013, 123, 107–111. [Google Scholar] [CrossRef]
- Sivanesan, A. Corynesporasca caryotae gen. et sp. nov. with a Corynespora anamorph, and the family Corynesporascaceae. Mycol. Res. 1996, 100, 783–788. [Google Scholar] [CrossRef]
- Boonmee, S.; Wanasinghe, D.N.; Calabon, M.S.; Huanraluek, N.; Chandrasiri, S.K.U.; Jones, G.E.B.; Rossi, W.; Leonardi, M.; Singh, S.K.; Rana, S.; et al. Fungal Diversity notes 1387–1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021, 111, 1–335. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Chooi, Y.H.; Gilchrist, C.L.M.; Lacey, E.; Pitt, J.I.; Roets, F.; Swart, W.J.; Cano–Lira, J.F.; Valenzuela–Lopez, N.; et al. Fungal Planet description sheets: 1042–1111. Persoonia 2020, 44, 301–459. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, F.A. Two new cheirosporous asexual taxa (Dictyosporiaceae, Pleosporales, Dothideomycetes) from freshwater habitats in Egypt. Mycosphere 2016, 7, 448–457. [Google Scholar] [CrossRef]
- Hongsanan, S.; Bahkali, A.H.; Chomnunti, P.; Liu, J.-K.; Yang, J.-B.; Hyde, K.D. Discopycnothyrium palmae gen. & sp. nov. (Asterinaceae). Mycotaxon 2016, 131, 859–869. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, A.K.; Kamal. New hyphopodiate hyphomycetes from North-Eastern Uttar Pradesh, India. Mycol. Res. 1995, 99, 395–396. [Google Scholar] [CrossRef]
- Liu, J.-K.; Phookamsak, R.; Jones, E.B.G.; Zhang, Y.; Ko–Ko, T.W.; Hu, H.L.; Boonmee, S.; Doilom, M.; Chukeatirote, E.; Bahkali, A.H.; et al. Astrosphaeriella is polyphyletic, with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers. 2011, 51, 135–154. [Google Scholar] [CrossRef]
- Pinruan, U.; Sakayaroj, J.; Jones, E.B.G.; Hyde, K.D. Flammispora gen. nov., a new freshwater ascomycete from decaying palm leaves. Stud. Mycol. 2004, 50, 381–386. [Google Scholar]
- Hou, L.; Giraldo, A.; Groenewald, J.Z.; Raemae, T.; Summerbell, R.C.; Huang, G.; Cai, L.; Crous, P.W. Redisposition of acremonium-like fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef]
- Konta, S.; Hyde, K.D.; Eungwanichayapant, P.D.; Karunarathna, S.C.; Samarakoon, M.C.; Xu, J.C.; Dauner, L.A.P.; Aluthwattha, S.T.; Lumyong, S.; Tibpromma, S. Multigene phylogeny reveals Haploanthostomella elaeidis gen. et sp. nov. and familial replacement of Endocalyx (Xylariales, Sordariomycetes, Ascomycota). Life 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Konta, S.; Hyde, K.V.D.; Karunarathna, S.C.; Mapook, A.; Senwanna, C.; Dauner, L.A.P.; Nanayakkara, C.M.; Xu, J.C.; Tibpromma, S.; Lumyong, S. Multi-gene phylogeny and morphology reveal Haplohelminthosporium gen. nov. and Helminthosporiella gen. nov. associated with palms in Thailand and a checklist for Helminthosporium reported worldwide. Life 2021, 11, 454. [Google Scholar] [CrossRef]
- Castañeda-Ruíz, R.F.; Heredia, G.; Arias, R.M. Digitella rigidophora and Redbia inflata, two new microfungi from Mexico. Mycotaxon 2013, 125, 227–233. [Google Scholar] [CrossRef]
- Minter, D.W. Nomenclatural novelties: David William Minter. Index Fungorum 2015, 226, 1. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Le Roux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.V. Hemisynnema malayasianum gen. et sp. nov. Nova Hedwig. 1994, 58, 223–228. [Google Scholar]
- Delgado-Rodríguez, G. South Florida microfungi: Kalamarospora multiflagellata gen. et sp. nov. (hyphomycetes), with additional new records from USA. Mycotaxon 2010, 114, 231–246. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Samuels, G.J. Letendraeopsis palrnarum, a new genus and species of loculoascomycetes. Mycologia 1994, 86, 254–258. [Google Scholar] [CrossRef]
- Crous, W.P.; Begoude, B.A.D.; Boers, J.; Braun, U.; Declercq, B.; Dijksterhuis, J.; Elliott, T.F.; Garay-Rodriguez, G.A.; Jurjević, Ž.; Kruse, J.; et al. New and interesting fungi. 5. Fungal. Syst. Evol. 2022, 10, 19–90. [Google Scholar] [CrossRef]
- Konta, S.; Hyde, K.D.; Phookamsak, R.; Xu, J.C.; Maharachchikumbura, S.S.N.; Daranagama, D.A.; McKenzie, E.H.C.; Boonmee, S.; Tibpromma, S.; Eungwanichayapant, P.D.; et al. Polyphyletic genera in Xylariaceae (Xylariales): Neoxylaria gen. nov. and Stilbohypoxylon. Mycosphere 2020, 11, 2629–2651. [Google Scholar] [CrossRef]
- Subramanian, C.V. Nusia gen. nov. for two interesting hyphomycetes. Cryptogam. Mycol. 1993, 14, 109–116. [Google Scholar]
- Pereira, D.S.; Phillips, A.J.L. A new leaf spot disease of Chamaerops humilis caused by Palmeiromyces chamaeropicola gen. et sp. nov. Phytopathol. Mediterr. 2020, 59, 353–363. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal Diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Crous, P.W.; Boers, J.; Holdom, D.; Osieck, E.R.; Steinrucken, T.V.; Tan, Y.P.; Vitelli, J.S.; Shivas, R.G.; Barrett, M.; Boxshall, A.G.; et al. Fungal Planet description sheets: 1383–1435. Persoonia 2022, 48, 261–371. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.; Smith, D.; Summerell, B.A.; Cano-Lira, J.F.; Guarro, J.; Houbraken, J.; et al. Fungal Planet description sheets: 625–715. Persoonia 2017, 39, 270–467. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs–Kölling, G.; Yilmaz, N.; Thangavel, R.; Wingfield, M.J.; Noordeloos, M.E.; Dima, B.; Brandrud, T.E.; Jansen, G.M.; et al. Fungal Planet description sheets: 1182–1283. Persoonia 2021, 46, 313–528. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ruíz, R.F.; Abarca, G.H.; Arias, R.M.; Saikawa, M.; Minter, D.W.; Stadler, M. Anamorphic fungi from submerged plant material: Phaeomonilia pleiomorpha, P. corticola and Cacumisporium pleuroconidiophorum. Mycotaxon 2007, 100, 327–336. [Google Scholar]
- Pinruan, U.; Sakayaroj, J.; Jones, E.B.G.; Hyde, K.D. Aquatic fungi from peat swamp palms: Phruensis brunneispora gen. et sp nov and its hyphomycete anamorph. Mycologia 2004, 96, 1163–1170. [Google Scholar] [CrossRef][Green Version]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.; Gene, J.; Guarro, J.; Baseia, I.G.; Garcia, D.; Gusmao, L.F.P.; Souza-Motta, C.M.; et al. Fungal Planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef]
- Mapook, A.; Macabeo, A.P.G.; Thongbai, B.; Hyde, K.D.; Stadler, M. Polyketide-derived secondary metabolites from a Dothideomycetes fungus, Pseudopalawania siamensis gen. et sp. nov., (Muyocopronales) with antimicrobial and cytotoxic activities. Biomolecules 2020, 10, 569. [Google Scholar] [CrossRef]
- Prabhugaonkar, A.; Bhat, D.J. Rattania setulifera, an undescribed endophytic hyphomycete on rattans from Western Ghats, India. Mycotaxon 2009, 108, 217–222. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, A.; Mena-Portales, J. A new helicosporous hyphomycete collected on Roystonea regia in Cuba. Mycol. Res. 1996, 100, 1483–1484. [Google Scholar] [CrossRef]
- Dubey, R.; Moonnambeth, N.A. Sawantomyces—A new hyphomycete genus from Western Ghats, India. J. New Biol. Rep. 2013, 2, 234–237. [Google Scholar]
- Pinruan, U.; Sakayaroj, J.; Hyde, K.D.; Jones, E.B.G. Thailandiomyces bisetulosus gen. et sp nov (Diaporthales, Sordariomycetidae, Sordariomycetes) and its anamorph Craspedodidymum, is described based on nuclear SSU and LSU rDNA sequences. Fungal Divers. 2008, 29, 89–98. [Google Scholar]
- Subramanian, C.V. Tretendophragmia palmivora gen. et sp. nov. an interesting hyphomycete from Singapore. Kavaka 1991, 19, 58–66. [Google Scholar]
- Subramanian, C.V. Tretocephala decidua gen. et sp. nov., an interesting new hyphomycete. Cryptogam. Mycol. 1992, 13, 65–68. [Google Scholar]
- Konta, S.; Tibpromma, S.; Karunarathna, S.C.; Samarakoon, M.C.; Steven, L.S.; Mapook, A.; Boonmee, S.; Senwanna, C.; Balasuriya, A.; Eungwanichayapant, P.D.; et al. Morphology and multigene phylogeny reveal ten novel taxa in Ascomycota from terrestrial palm substrates (Arecaceae) in Thailand. Mycosphere 2023, 14, 107–152. [Google Scholar] [CrossRef]
- Castañeda-Ruíz, R.; Iturriaga, T. Venustusporium, a new genus of hyphomycetes from Venezuela. Mycotaxon 1999, 72, 455–459. [Google Scholar]
- Delgado-Rodríguez, G. South Florida microfungi: Veramycella bispora, a new palmicolous anamorphic genus and species, with some new records for the continental USA. Mycotaxon 2009, 107, 357–373. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.-K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.-Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Voglmayr, H.; Jaklitsch, W.M. Corynespora, Exosporium and Helminthosporium revisited—New species and generic reclassification. Stud. Mycol. 2017, 87, 43–76. [Google Scholar] [CrossRef]
- Wu, W.-P.; Zhuang, W.-Y. Sporidesmium, Endophragmiella and related genera from China. Fung. Divers. Res. Ser. 2005, 15, 1–531. [Google Scholar]
- Wang, Y.; Hyde, K.D.; McKenzie, E.H.C.; Jiang, Y.-L.; Li, D.-W.; Zhao, D.-G. Overview of Stachybotrys (Memnoniella) and current species status. Fungal Divers. 2015, 71, 17–83. [Google Scholar] [CrossRef]
- Goos, R.D. On the anamorph genus Cirrenalia. Proc. Indian Acad. Sci. Plant Sci. 1985, 94, 245–252. [Google Scholar] [CrossRef]
- Goos, R.D. A review of the anamorph genus Helicomyces. Mycologia 1985, 77, 606–618. [Google Scholar] [CrossRef]
- Goos, R.D. The anamorph genus Zalerion. Mycotaxon 1985, 23, 445–449. [Google Scholar]
- Goos, R.D. A review of the anamorph genus Helicoma. Mycologia 1986, 78, 744–761. [Google Scholar] [CrossRef]
- Goos, R.D. Fungi with a twist: The helicosporous hyphomycetes. Mycologia 1987, 79, 1–22. [Google Scholar] [CrossRef]
- Goos, R.D. On the anamorph genera Helicosporium and Drepanospora. Mycologia 1989, 81, 356–374. [Google Scholar] [CrossRef]
- Goos, R.D. Review of the anamorph genus Xenosporium. Mycologia 1990, 82, 742–752. [Google Scholar] [CrossRef]
- Goos, R.D. Fungi of Barro Colorado Island, adjacent Panama, and the Cali region of Colombia. Mycotaxon 1997, 64, 375–383. [Google Scholar]
- Goos, R.D.; Abdullah, S.K.; Fisher, P.J.; Webster, J. The anamorph genus Helicodendron. Trans. Br. Mycol. Soc. 1985, 84, 423–435. [Google Scholar] [CrossRef]
- Subramanian, C.V. Validation of names of some taxa (hyphomycetes). Kavaka 1995, 20, 57–58. [Google Scholar]
- Subramanian, C.V. Hyphomycetes from South East Asia—Novelties from Singapore and Malaysia. Kavaka 1994, 22, 52–76. [Google Scholar]
- Mercado-Sierra, A.; Gonzalez-Fraginals, G.; Mena-Portales, J.; Rodríguez-Morejón, K. Las Palmas y su relación como sustratos de hongos microscópicos (Hifomicetos) en Cuba. Bol. Soc. Micol. Madrid 1997, 22, 34–44. [Google Scholar]
- Castañeda-Ruíz, R.F.; Kendrick, B. Conidial fungi from Cuba: I. Univ. Waterloo Biol. Ser. 1990, 32, 1–53. [Google Scholar]
- Castañeda-Ruíz, R.F.; Kendrick, B. Conidial fungi from Cuba: II. Univ. Waterloo Biol. Ser. 1990, 33, 1–62. [Google Scholar]
- Castañeda-Ruíz, R.F.; Kendrick, B. Ninety-nine conidial fungi from Cuba and three from Canada. Univ. Waterloo Biol. Ser. 1991, 35, 1–132. [Google Scholar]
- Hernández-Gutiérrez, A.; Mena-Portales, J. Sporidesmium coccothrinacis Hernández & Mena, sp. nov. Bol. Soc. Micol. Madrid 1994, 19, 313–314. [Google Scholar]
- Hernández-Gutiérrez, A.; Mena-Portales, J. Nuevos registros de hifomicetos sobre Roystonea regia en Cuba. Bol. Soc. Micol. Madrid 1995, 20, 15–23. [Google Scholar]
- Hernández-Gutiérrez, A.; Mena-Portales, J. Hifomicetos asociados a Coccothrinax (Palmae) en diferentes localidades de la Provincia de Camagüey (Cuba). Bol. Soc. Micol. Madrid 1995, 20, 25–33. [Google Scholar]
- Hernández-Gutiérrez, A.; Mena-Portales, J. Dictyochaeta minutissima sp. nov. on Coccothrinax miraguama from Cuba. Mycol. Res. 1996, 100, 687–688. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Mena-Portales, J. Hifomicetes dematiáceos de tres provincias orientales de Cuba. Rev. Iberoam. Micol. 1995, 12, 101–107. [Google Scholar]
- Castañeda-Ruíz, R.F.; Guarro, J.; Mayayo, E.; Decock, C. Notes on conidial fungi. XVI. A new species of Dendryphiosphaera and some new records from Cuba. Mycotaxon 1998, 67, 9–19. [Google Scholar]
- Mercado-Sierra, A.; Holubová-Jechová, V.; Mena-Portales, J. Hifomicetes Demaciáceos de Cuba. Enteroblásticos. Monografie XXIII; Museo Regionale di Scienze Naturali: Turin, Italy, 1997. [Google Scholar]
- Mercado-Sierra, A.; Mena-Portales, J.; Figueras, M.J. Revision of the genus Phragmospathula. Mycologia 1997, 89, 304–308. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Figueiras, M.J.; Gené, J. New or rare hyphomycetes from Cuba. VIII. Species of Lylea, Phaeoisaria, Arxiella, Graphium, Periconia and Ramichloridium. Mycotaxon 1997, 63, 369–375. [Google Scholar]
- Mercado-Sierra, A.; Gené, J.; Figueras, M.J.; Rodríguez-Morejón, K.; Guarro, J. New or rare hyphomycetes from Cuba. IX. Some species from Pinar del Rio Province. Mycotaxon 1998, 67, 417–426. [Google Scholar]
- Mena-Portales, J.; Delgado-Rodríguez, G.; Mercado-Sierra, A.; Gené, J.; Guarro, J.; Iacona, V. New or interesting hyphomycetes from the Biosphere Reserve of Sierra del Rosario, Cuba. Mycologia 2001, 93, 751–757. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Mena-Portales, J. Virgariella ellipsospora sp. nov. (Hyphomycetes, Anamorphic fungi) from Cuba. Cryptogam. Mycol. 2003, 24, 153–157. [Google Scholar]
- Mena-Portales, J.; Herrera-Figueroa, S.; Mercado-Sierra, A.; Brito, H.I.; Blanco-Hernández, N.; Medina, J.L.O.; González, S.M.; Herrera, G.R.; Rodríguez-Hernández, M.; Vilaró, M.C.; et al. Estrategia Para la Conservación de la Diversidad Fúngica en Cuba. Estado de Conocimiento, Estrategia y Plan de Acción; Mena-Portales, J., Herrera-Figueroa, S., Mercado-Sierra, A., Minter, D.W., Eds.; Instituto de Ecología y Sistemática: La Habana, Cuba, 2000; Available online: https://repositorio.geotech.cu/xmlui/handle/1234/2728 (accessed on 15 September 2023).
- Delgado-Rodríguez, G.; Mena-Portales, J.; Calduch, M.; Decock, C. Hyphomycetes (Hongos Mitospóricos) del Área Protegida Mil Cumbres, Cuba Occidental. Cryptogam. Mycol. 2002, 23, 277–293. [Google Scholar]
- Delgado-Rodríguez, G.; Mena-Portales, J.; Mercado-Sierra, A. Nuevos registros de hifomicetos en Alturas de Trinidad (Cuba). Bol. Soc. Micol. Madrid 2003, 27, 49–54. [Google Scholar]
- Mercado-Sierra, A.; Delgado-Rodríguez, G.; Mena-Portales, J.; Guarro, J. Some Hyphomycetes (mitosporic fungi) from “Ciénega de Zapata” Biosphere Reserve (Cuba). Bol. Soc. Micol. Madrid 2002, 26, 183–187. [Google Scholar]
- Mena-Portales, J.; Delgado-Rodríguez, G.; Hernández-Gutiérrez, A.; González-Fraginals, G.; Mercado-Sierra, A. Hifomicetes de Sierra de Cubitas, Cuba. Acta Bot. Cub. 2017, 216, 17–30. [Google Scholar]
- Mena-Portales, J.; Cantillo-Pérez, T.; Jimenez-Gómez, I. Hifomicetes de la Reserva de la Biosfera “Ciénaga de Zapata”. Acta Bot. Cub. 2018, 217, 96–108. [Google Scholar]
- Mena-Portales, J.; González-Fraginals, G.; Mercado-Sierra, A.; Cantillo-Pérez, T. Hifomicetes del Parque Nacional “Caguanes”, Reserva de la Biosfera “Buena Vista”, Cuba. Acta Bot. Cub. 2020, 219, 1–9. [Google Scholar]
- Mena-Portales, J.; Cantillo-Pérez, T.; Jiménez-Gómez, I. Hongos microscópicos interesantes hallados sobre palmas en Cuba. Acta Bot. Cub. 2021, 220, 1–5. [Google Scholar]
- Mercado-Sierra, A.; Gene, J.; Guarro, J. Some Costa Rican Hyphomycetes. I. Nova Hedwigia 1997, 64, 455–465. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Gene, J.; Guarro, J. Some Costa Rican hyphomycetes. II. Mycotaxon 1997, 64, 7–15. [Google Scholar]
- Granados-Montero, M.; Minter, D.W.; Castañeda-Ruíz, R.F. A checklist of asexual fungi from Costa Rica. Mycotaxon 2018, 133, 365. [Google Scholar] [CrossRef]
- Stevenson, J.A. The Fungi of Puerto Rico and the American Virgin Islands; Contribution of Reed Herbarium No. XXIII; Reed Herbarium: Baltimore, MD, USA, 1975. [Google Scholar]
- Huhndorf, S.M.; Fernández, F. Neotropical ascomycetes 7: Caudatispora biapiculata sp. nov. from Puerto Rico. Sydowia 1998, 50, 200–204. [Google Scholar]
- Guatimosim, E.; Pinto, H.J.; Barreto, R.W. Passalora acrocomiae sp. nov. and Exosporium acrocomiae from the palm Acrocomia aculeata in Puerto Rico. Mycotaxon 2012, 122, 61–67. [Google Scholar] [CrossRef]
- Piepenbring, M. Checklist of fungi in Panama. Preliminary version. Puente Biológico 2006, 1, 1–190. [Google Scholar]
- Delgado-Rodríguez, G. Nicaraguan fungi: A checklist of hyphomycetes. Mycotaxon 2011, 115, 534. [Google Scholar]
- Mercado-Sierra, A.; Heredia-Abarca, G. Hyphomycetes asociados a restos vegetales en el estado de Veracruz, México. Revista Mex. Micol. 1994, 10, 33–48. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Heredia-Abarca, G.; Mena-Portales, J. New species of dematiaceous hyphomycetes from Veracruz, Mexico. Mycotaxon 1995, 55, 491–499. [Google Scholar]
- Mercado-Sierra, A.; Heredia-Abarca, G.; Mena-Portales, J. Tropical hyphomycetes of Mexico I. New species of Hemicorynespora, Piricauda and Rhinocladium. Mycotaxon 1997, 63, 155–168. [Google Scholar]
- Heredia-Abarca, G.; Mena-Portales, J.; Mercado-Sierra, A. Hyphomycetes saprobios tropicales. Nuevos registros de dematiáceos para México. Revista Mex. Micol. 1997, 13, 41–51. [Google Scholar] [CrossRef]
- Heredia-Abarca, G.; Mena-Portales, J.; Mercado-Sierra, A.; Estebanez, M.R. Tropical hyphomycetes of Mexico. II. Some species from the tropical biology station “Los Tuxtlas”, Veracruz, Mexico. Mycotaxon 1997, 64, 203–223. [Google Scholar]
- Heredia-Abarca, G.H.; Mercado-Sierra, A. Tropical Hyphomycetes of Mexico. III. Some species from the Calakmul Biosphere Reserve, Campeche. Mycotaxon 1998, 68, 137–143. [Google Scholar]
- Heredia-Abarca, G.; Arias-Mota, R.M.; Estebanez, M.R. Contribución al conocimiento de los hongos Hyphomycetes de México. Acta Bot. Mex. 2000, 51, 39–51. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Guarro, J.; Heredia-Abarca, G.H. The hyphomycete genus Piricauda, with the description of a new species. Mycol. Res. 2005, 109, 723–728. [Google Scholar] [CrossRef]
- San Martín, F.E.; Lavin, P.A. Cuatro especies y una variedad del género Astrosphaeriella (Dothideales, Melanommataceae) de México. Acta Bot. Mex. 1999, 46, 19–27. [Google Scholar] [CrossRef]
- Mena-Portales, J.; Delgado-Rodríguez, G.; Heredia-Abarca, G. Nuevas combinaciones para especies de Sporidesmium S.L. (hongos mitospóricos). Bol. Soc. Micol. Madrid 2000, 25, 265–270. [Google Scholar]
- Mena-Portales, J.; Heredia-Abarca, G.; Mercado-Sierra, A.; Becerra-Hernández; Arias-Mota, R.M.; Gómez-Cornelio, S.A. Especies de Stachybotrys Corda (hongos anamorfos) de regiones tropicales y subtropicales de México. Bol. Soc. Micol. Madrid 2009, 33, 7–23. [Google Scholar]
- Heredia-Abarca, G.; Estebanez, M.R.; Arias-Mota, R.M.; Mena-Portales, J.; Mercado-Sierra, A. Adiciones al conocimiento de la diversidad de los hongos conidiales del bosque mesófilo de montaña del Estado de Veracruz. Acta Bot. Mex. 2004, 66, 1–22. [Google Scholar] [CrossRef]
- Heredia-Abarca, G.; Arias-Mota, R.M.; Mena-Portales, J.; Mercado-Sierra, A. Adiciones al conocimiento de la diversidad de los hongos conidiales del bosque mesófilo de montaña del Estado de Veracruz. II. Acta Bot. Mex. 2006, 77, 15–30. [Google Scholar] [CrossRef]
- Heredia-Abarca, G.; Castañeda-Ruíz, R.F.; Becerra-Hernández, C.I.; Arias-Mota, R.M. Contribución al conocimiento de los hongos anamorfos saprobios del Estado de Tabasco. I. Revista Mex. Micol. 2006, 23, 53–62. [Google Scholar] [CrossRef]
- Arias-Mota, R.M.; Heredia-Abarca, G.; Mena-Portales, J. Adiciones al conocimiento de la diversidad de los hongos anamorfos del bosque mesófilo de montaña del estado de Veracruz III. Acta Bot. Mex. 2010, 90, 19–42. [Google Scholar] [CrossRef]
- Arias-Mota, R.M.; Heredia-Abarca, G.; Castañeda-Ruíz, R.F. Adiciones al conocimiento de la diversidad de los hongos conidiales saprobios del bosque mesófilo de montaña del estado de Veracruz IV. Acta Bot. Mex. 2015, 113, 87–101. [Google Scholar] [CrossRef]
- Arias-Mota, R.M.; Heredia-Abarca, G.; Castañeda-Ruíz, R.F. Checklist of saprobic asexual microfungi from the tropical montane cloud forest of Veracruz, México. Mycotaxon 2018, 132, 986. [Google Scholar] [CrossRef]
- Becerra-Hernández, C.I.; Heredia-Abarca, G.; Arias-Mota, R.M. Contribución al conocimiento de los hongos anamorfos saprobios del Estado de Tabasco. II. Revista Mex. Micol. 2007, 24, 39–53. [Google Scholar] [CrossRef]
- Becerra-Hernández, C.I.; Heredia-Abarca, G.; Arias-Mota, R.M.; Castañeda-Ruíz, R.F.; Mena-Portales, J. Especies raras de hongos anamorfos saprobios en el Estado de Tabasco. Acta Bot. Mex. 2011, 96, 15–31. [Google Scholar] [CrossRef]
- Becerra-Hernández, C.I.; Heredia-Abarca, G.; Arias-Mota, R.M.; Mena-Portales, J.; Castañeda-Ruíz, R.F. Los hongos anamorfos saprobios del Estado de Tabasco. III. Rev. Mex. Micol. 2008, 28, 25–39. [Google Scholar] [CrossRef]
- Mercado-Sierra, A.; Basilico, J.C.; Iacona, V.; Luz-Zapata, M. Some interesting mitosporic fungi (hyphomycetes) from Argentina. Bol. Soc. Micol. Madrid 2000, 25, 243–250. [Google Scholar]
- Gómez-Zapata, P.A.; Salazar-Yepes, M. Camarotella colombiana sp. nov. (Phyllachoraceae) sobre Ceroxylon quindiuense (Arecaceae) en Colombia. Revista Mex. Biodivers. 2017, 88, 275–279. [Google Scholar] [CrossRef]
- Castañeda-Ruíz, R.F.; Iturriaga, T.; Heredia-Abarca, G.; Minter, D.W.; Gené, J.; Stadler, M.; Saikawa, M.; Silvera-Simón, C. Notes on Heteroconium and a new species from Venezuela. Mycotaxon 2008, 105, 175–184. [Google Scholar]
- Castañeda-Ruíz, R.F.; Iturriaga, T.; Minter, D.W.; Heredia-Abarca, G.; Stadler, M.; Saikawa, M.; Fernández, R. Two new anamorphic fungi and some microfungi recorded from ‘El Avila’, Venezuela. Mycotaxon 2009, 107, 225–237. [Google Scholar] [CrossRef]
- Castañeda-Ruíz, R.F.; Iturriaga, T.; Minter, D.W.; Saikawa, M.; Vidal, G.; Velázquez-Noa, S. Microfungi from Venezuela: A new species of Brachydesmiella, a new combination, and new records. Mycotaxon 2003, 85, 211–229. [Google Scholar]
- Castañeda-Ruíz, R.F.; Iturriaga, T.; Saikawa, M.; Cano, J.; Guarro, J. The genus Menisporopsis in Venezuela with the addition of M. anisospora anam. sp nov from a palm tree. Cryptogam. Mycol. 2001, 22, 259–263. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Gusmao, L.F.P.; Castañeda-Ruíz, R.F. Pleurothecium bicoloratum & Sporidesmiopsis pluriseptata spp. nov from Brazil. Mycotaxon 2016, 131, 145–152. [Google Scholar] [CrossRef]
- Capdet, M.; Pereira, S.; Romero, A.I. Coccostromopsis palmicola on Butia yatay from Argentina. Mycotaxon 2010, 114, 91–97. [Google Scholar] [CrossRef]
- Capdet, M.; Romero, A.I. Fungi from palms in Argentina. 1. Mycotaxon 2010, 112, 339–355. [Google Scholar] [CrossRef]
- Capdet, M.; Romero, A.I. Ascomicetes sobre palmeras nativas de la Argentina. II. Anamorfos. Bol. Soc. Argent. Bot. 2012, 47, 303–310. [Google Scholar]
- Subramanian, C.V. Fungi imperfecti from Madras—III. Beltraniella gen. nov. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1952, 36, 223–228. [Google Scholar] [CrossRef]
- Subramanian, C.V. Fungi imperfecti from Madras—II. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1952, 36, 160–169. [Google Scholar] [CrossRef]
- Subramanian, C.V. Fungi imperfecti from Madras—I. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1952, 36, 43–53. [Google Scholar] [CrossRef]
- Subramanian, C.V. Fungi imperfecti from Madras—V. Curvularia. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1953, 38, 27–39. [Google Scholar] [CrossRef]
- Subramanian, C.V. Fungi imperfecti from Madras—IV. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1953, 37, 96–105. [Google Scholar] [CrossRef]
- Subramanian, C.V. Fungi imperfecti from Madras—VI. J. Indian Bot. Soc. 1954, 33, 36–42. [Google Scholar]
- Subramanian, C.V. Fungi imperfecti from Madras—VII. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1955, 42, 283–292. [Google Scholar] [CrossRef]
- Subramanian, C.V. Hyphomycetes. An account of Indian species, except Cercosporae; Indian Council of Agricultural Research: New Delhi, India, 1971. [Google Scholar]
- Subramanian, C.V. Three new hyphomycetes. J. Indian Bot. Soc. 1954, 33, 28–35. [Google Scholar]
- Subramanian, C.V. Some species of Periconia from India. J. Indian Bot. Soc. 1955, 34, 339–361. [Google Scholar]
- Subramanian, C.V. Two new species of Petrakia. Beih. Zur Sydowia 1957, 1, 14–15. [Google Scholar]
- Subramanian, C.V.; Nair, N.G. Panchanania and Phragmospathula, two new genera of the Hyphomycetes. Antonie Leeuwenhoek 1966, 32, 381–386. [Google Scholar] [CrossRef]
- Subramanian, C.V.; Natarajan, K. Two new hyphomycetes from India. Mycologia 1975, 67, 1211–1217. [Google Scholar] [CrossRef]
- Subramanian, C.V.; Ramakrishnan, K. List of Indian Fungi 1952–1956. J. Madras Univ. Sec. B Sci. 1956, 26, 327–421. [Google Scholar]
- Subramanian, C.V.; Bhat, D.J. Developmental morphology of Ascomycetes XI. Nectria kera. Cryptogam. Mycol. 1984, 5, 135–145. [Google Scholar]
- Subramanian, C.V.; Bhat, D.J. Hyphomycetes from south India I. Some new taxa. Kavaka 1987, 15, 41–74. [Google Scholar]
- Subramanian, C.V. Hyphomycetes—II. J. Indian Bot. Soc. 1956, 35, 446–494. [Google Scholar]
- Subramanian, C.V. Hyphomycetes—I. J. Indian Bot. Soc. 1956, 35, 53–91. [Google Scholar]
- Subramanian, C.V. Hyphomycetes—IV. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1957, 46, 324–335. [Google Scholar] [CrossRef]
- Subramanian, C.V. Hyphomycetes—III. Two new genera, Dwayaloma and Sadasivania. J. Indian Bot. Soc. 1957, 36, 61–67. [Google Scholar]
- Subramanian, C.V. Hyphomycetes—VI. Two new genera, Edmundmasonia and Iyengarina. J. Indian Bot. Soc. 1958, 37, 401–407. [Google Scholar]
- Subramanian, C.V. Hyphomycetes—V. J. Indian Bot. Soc. 1958, 37, 47–64. [Google Scholar]
- Rao, D.; Rao, P.R. ‘Vrikshopama’, a new genus of Stilbaceae. Mycopathol. Mycol. Appl. 1964, 23, 287–290. [Google Scholar] [CrossRef]
- Rao, P.R.; Rao, D. Some Helicosporae from Hyderabad—II. Mycopathol. Mycol. Appl. 1964, 24, 27–34. [Google Scholar] [CrossRef]
- Rao, P.R.; Rao, D. Some species of Camposporium Harkn from India. Antonie Leeuwenhoek 1964, 30, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.R.; Rao, D. Some allied Dematiaceae-Dictyosporae from India. Mycopathol. Mycol. Appl. 1964, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.R.; Rao, D. The genus Periconia from India. Mycopathol. Mycol. Appl. 1964, 22, 285–310. [Google Scholar] [CrossRef]
- Rao, V.; Rao, D. A new Haplobasidion from Hyderabad. Curr. Sci. 1970, 39, 18–19. [Google Scholar]
- Rao, P.R.; Rao, S. A new Hansfordia Hughes from India. Curr. Sci. 1980, 49, 447. [Google Scholar]
- Rao, D.; Rao, V. Studies on Barnettella. Indian Phytopathol. 1973, 26, 233–236. [Google Scholar]
- Chaudhury, R.; Rao, P.N. Ascomycetes from Hyderabad, India I. Mycopathol. Mycol. Appl. 1964, 22, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N.; Chaudhury, R. A new species of Cochliobolus from Hyderabad—India. Mycopathol. Mycol. Appl. 1964, 23, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Some additions to fungi of India. I. Mycopathol. Mycol. Appl. 1966, 28, 45–48. [Google Scholar] [CrossRef]
- Rao, R. Two new species of Patellaria from India. Mycopathol. Mycol. Appl. 1967, 31, 29–32. [Google Scholar] [CrossRef]
- Rao, R. Some new and noteworthy fungi from India—VII. Sydowia 1971, 24, 322–325. [Google Scholar]
- Varghese, K.I.M.; Rao, V.G. Phaeoisaria Hohnel, a new generic record to Indian Hyphomycetes. Curr. Sci. 1977, 46, 156. [Google Scholar]
- Varghese, K.I.M.; Rao, V.G. Two undescribed species of dematiaceous hyphomycetes. Norw. J. Bot. 1980, 27, 55–57. [Google Scholar]
- Pande, A.; Rao, V.G. The genus Rosellinia (Sphaeriales) from peninsular India. Czech Mycol. 1995, 48, 177–182. [Google Scholar] [CrossRef]
- Bhat, D.J.; Kendrick, B. Twenty-five new conidial fungi from the Western Ghats and the Andaman Islands (India). Mycotaxon 1993, 49, 19–90. [Google Scholar]
- Bhat, D.J. Two undescribed species of conidial fungi from forests of western Ghats in southern India. J. For. Res. 1994, 17, 129–133. [Google Scholar]
- D’Souza, M.; Bhat, D.J. Two new hyphomycetes from India. In Microbes and Plants; Aruna, S., Ed.; Campus Books International: New Delhi, India, 2001; pp. 1–6. [Google Scholar]
- D’Souza, M.; Singh, S.K.; Bhat, D.J. New conidial fungi from Andaman Islands, India. Mycotaxon 2002, 82, 133–143. [Google Scholar]
- D’Souza, M.; Bhat, D.J. Diversity and abundance of endophytic fungi in four plant species of Western Ghat forest of Goa, southern India. Kavaka 2007, 35, 11–20. [Google Scholar]
- D’Souza, M.A.; Bhat, D.J. Occurrence of microfungi as litter colonizers and endophytes in varied plant species from the Western Ghats forests, Goa, India. Mycosphere 2013, 4, 567–582. [Google Scholar] [CrossRef]
- Manoharachary, C.; Sridhar, K.; Singh, R.; Adholeya, A.; Suryanarayanan, T.S.; Rawat, S.; Johri, B.N. Fungal biodiversity: Distribution, conservation and prospecting of fungi from India. Curr. Sci. 2005, 89, 58–71. [Google Scholar]
- Hosagoudar, V.B.; Mathew, S.P. A preliminary report on the mycoflora of the Andaman and Nicobar Islands, India. J. Econ. Taxon. Bot. 2000, 24, 631–640. [Google Scholar]
- Bhat, D.J. The forests of Western Ghats, an abode of novel and interesting microfungi. Kavaka 2008, 36, 1–11. [Google Scholar]
- Bhat, D.J.; Pratibha, J.; Gawas, P.; Sarita, K.Y.; Swapnaja, D. Diversity of microfungi in the forests of Western Ghats in Goa and surrounding regions. In Plant and Fungal Biodiversity and Bioprospecting; Krishnan, S., Bhat, D.J., Eds.; Broadway Book Centre: Goa, India, 2009; pp. 117–133. [Google Scholar]
- Bhat, D.J. Fascinating Microfungi (Hyphomycetes) of Western Ghats, India; Broadway Book Centre: Goa, India, 2010. [Google Scholar]
- Prabhugaonkar, A.; Bhat, D.J. New record of Megacapitula villosa and Paradictyoarthrinium diffractum from India. Mycosphere 2011, 2, 463–467. [Google Scholar]
- Hosagoudar, V.B.; Mathew, S.P.; Babu, D. Foliicolous fungi of Andaman Islands, India. J. Threat. Taxa 2014, 6, 5447–5463. [Google Scholar] [CrossRef]
- Pratibha, J.; Raghukumar, S.; Bhat, D.J. New species of Dendryphiopsis and Stauriella from Goa, India. Mycotaxon 2010, 113, 297–303. [Google Scholar] [CrossRef]
- Pratibha, S.J.; Raghukumar, S.; Bhat, D.J. Diversity of litter degrading microfungi from the forests of Western Ghats, India. In Biodiversity and Taxonomy; Kumar, A.B., Nayar, M.P., Varma, R.V., Peethambaran, C.K., Eds.; Narendra Publishing House: New Delhi, India, 2012; pp. 195–210. [Google Scholar]
- Dubey, R.; Moonnambeth, N.A. Acarocybellina arengae—A new generic and species record from India. Indian Phytopathol. 2013, 66, 326–327. [Google Scholar]
- Dubey, R.; Moonnambeth, N.A. Tharoopama livistonae sp. nov.—A new synematous hyphomycetes from India. Indian J. For. 2013, 36, 383–386. [Google Scholar]
- Dubey, R.; Moonnambeth, N.A. Some new host records for dematiaceous fungi from Western Ghats, India. Nelumbo 2013, 55, 219–221. [Google Scholar] [CrossRef]
- Dubey, R.; Moonnambeth, N.A. Solicorynespora matheranensis sp. nov.—A new species of Solicornespora from Indian subcontinent. NeBIO Int. J. Environ. Biodiversity 2014, 5, 15–18. [Google Scholar]
- Dubey, R.; Moonnambeth, N.A. Phragmospathula brachyspathula Mercado—A record of anamorphic fungi from Western Ghats, India. NeBIO Int. J. Environ. Biodiversity 2014, 5, 25–27. [Google Scholar]
- Dubey, R. Two new species of Zygosporium Mont. from Indian subcontinent. Indian J. Forest. 2014, 37, 165–168. [Google Scholar] [CrossRef]
- Dubey, R.; Neelima, A.M. Some new records of conidial fungi for India. J. New Biol. Rep. 2014, 3, 200–203. [Google Scholar]
- Niranjan, M.; Sarma, V.V. A check-list of fungi from Andaman and Nicobar Islands, India. Phytotaxa 2018, 347, 101–126. [Google Scholar] [CrossRef]
- Ram, T.; Sinha, G.P. A world key to Cryptothecia and Myriostigma (Arthoniaceae), with new species and new records from the Andaman and Nicobar Islands, India. Phytotaxa 2016, 266, 103–114. [Google Scholar] [CrossRef]
- Niranjan, M.; Sarma, V.V. Twelve new species of Ascomycetous fungi from Andaman Islands. Kavaka 2018, 50, 84–97. [Google Scholar]
- Niranjan, M.; Sarma, V.V. New Ascomycetous fungi in the family Aigialaceae from Andaman Islands, India. Curr. Res. Environ. Appl. Mycol. 2018, 8, 351–359. [Google Scholar] [CrossRef]
- Niranjan, M.; Sarma, V.V. New species and new records of Astrosphaeriellaceae from Andaman Islands, India. Kavaka 2020, 54, 38–42. [Google Scholar] [CrossRef]
- Niranjan, M.; Sarma, V.V. Diatrype: New records from Andaman Islands and a checklist from India. MycoAsia J. Mod. Mycol. 2020, 2020/02, 1–17. [Google Scholar] [CrossRef]
- Niranjan, M.; Sarma, V.V. Four novel species of Sordariomycetes from Andaman Islands, India. Kavaka 2021, 56, 105–111. [Google Scholar]
- Subramanian, C.V.; Tyagi, P.D. List of Indian Fungi 1956–1960. J. Madras Univ. Sec. B Sci. 1964, 34, 1–134. [Google Scholar]
- Jamaluddin; Goswami, M.G.; Ojha, B.M. Fungi of India 1989–2001; Scientific Publishers: Jodhpur, India, 2004. [Google Scholar]
- Pande, A. Ascomycetes of Peninsular India; Scientific Publishers: Jodhpur, India, 2008. [Google Scholar]
- Manoharachary, C.; Atri, N.S.; Devi, T.P.; Kamil, D.; Singh, S.K.; Singh, P.A. Bilgrami’s Fungi of India List and References (1988–2020); Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2022. [Google Scholar]
- Pande, A.; Waingahkar, D.; Rao, V.G. Fungal records on palms from India. J. Econ. Taxon. Bot. 2001, 25, 663–686. [Google Scholar]
- Moses, T. Palms of Brazil. Principes 1962, 6, 26–37. [Google Scholar]
- Hennen, J.F.; Ono, Y. Cerradoa palmaea: The first rust fungus on Palmae. Mycologia 1978, 70, 569–576. [Google Scholar] [CrossRef]
- Farr, M.L. A new species of Cryptophiale from Amazonas. Mycotaxon 1980, 11, 177–181. [Google Scholar]
- Farr, Μ.L. Amazonian Foliicolous Fungi III. A preliminary list of Ascomycotina, mostly Dothideales, sensu lato. Acta Amaz. 1985, 15, 29–34. [Google Scholar] [CrossRef]
- Farr, M.L. Amazonian foliicolous fungi. II. Deuteromycotina. Mycologia 1986, 78, 269–286. [Google Scholar] [CrossRef]
- Rodrigues, K.F. The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 1994, 86, 376–385. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Samuels, G.J. Preliminary study of endophytic fungi in a tropical palm. Mycol. Res. 1990, 94, 827–830. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Samuels, G.J. Idriella species endophytic in palms. Mycotaxon 1992, 43, 271–276. [Google Scholar]
- Rodrigues, K.F.; Leuchtmann, A.; Petrini, O. Endophytic species of Xylaria: Cultural and isozymic studies. Sydowia 1993, 45, 116–138. [Google Scholar]
- Rodrigues, K.F. Fungal endophytes of palms. In Endophytic Fungi in Grasses and Woody Plants. Systematics, Ecology and Evolution; Redlin, S.C., Carris, L.M., Eds.; APS Press: Saint Paul, MN, USA, 1996; pp. 121–132. [Google Scholar]
- Rodrigues, K.F.; Petrini, O. Biodiversity of endophytic fungi in tropical regions. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 57–69. [Google Scholar]
- Silva, M.S.; Minter, D.W. Fungi from Brazil recorded by Batista and co-workers. Mycol. Pap. 1995, 169, 1–585. [Google Scholar]
- Mendes, M.A.S.; da Silva, V.L.; Dianese, J.C.; Ferreira, M.A.S.V.; dos Santos, C.E.N.; Neto, E.G.; Urben, A.F.; Carlos, C. Fungos em Plantas no Brasil; Embrapa-SPI/Embrapa-Cenargen: Brasília, Brazil, 1998. [Google Scholar]
- Mendes, M.A.S.; Urben, A.F.; Dianese, J.C.; Lobo, V.L.S.; Ferreira, M.A.S.V.; Simon, M.F.; Sanchez, M.; dos Santos, L.T.P.; dos Santos, C.E.N. Fungos em Plantas no Brasil. Edição Ampliada e Revisada, 2nd ed.; Mendes, M.A.S., Urben, A.F., Dianese, J.C., Eds.; Escola Nacional de Gestão Agropecuária: Brasília, Brazil, 2019. Available online: https://enagro.agricultura.gov.br/gestao-do-conhecimento/fungos-em-plantas-no-brasil (accessed on 15 September 2023).
- da Cruz, A.C.R.; Hernández-Gutiérrez, A.; Gusmão, L.F.P. O gênero Exserticlava (Fungo Anamorfo—Hyphomycetes) no Brasil. Braz. J. Bot. 2008, 31, 357–361. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Hernández-Gutiérrez, A.; Sotão, H.M.P. Fungos anamorfos (hyphomycetes) da Floresta Nacional de Caxiuanã, Pará, Brasil: Novos registros para o Neotrópico. Acta Bot. Bras. 2010, 24, 868–870. [Google Scholar] [CrossRef]
- Monteiro, J.S.; do Carmo, L.T.; Sotão, H.M.P. A new species of Bhatia (asexual ascomycetes) and new records from Brazil. Phytotaxa 2017, 331, 263–272. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Sotão, H.M.O.; Cáceres, M.E.S.; Lücking, R.; Hernández-Gutiérrez, A. Checklist dos fungos da Floresta Nacional de Caxiuanã, Pará, Brasil. I. Fungos conidiais e liquenizados. Bol. Mus. Para. Emílio Goeldi Sér. Ciências Naturais 2018, 13, 221–245. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Sarmento, P.S.M.; Sotão, H.M.P. Saprobic conidial fungi associated with palm leaf litter in eastern Amazon, Brazil. An. Acad. Bras. Ciênc. 2019, 91, e20180545. [Google Scholar] [CrossRef]
- de Castro, C.C.; Hernández-Gutiérrez, A.; Sotão, H.M.P. Novos registros de fungos anamorfos (hifomicetos) para o Neotrópico e América do Sul. Braz. J. Bot. 2011, 34, 515–521. [Google Scholar] [CrossRef]
- de Castro, C.C.; Hernández-Gutiérrez, A.; Sotão, H.M.P. Fungos conidiais em Euterpe oleracea Mart. (açaizeiro) na Ilha do Combu, Pará-Brasil. Acta Bot. Bras. 2012, 26, 761–771. [Google Scholar] [CrossRef]
- Dianese, J.C.; Medeiros, R.B.; Santos, L.T.P. Cerradoa palmaea Hennen & Ono found in two new hosts, Syagrus commosa (Mart.) Mart. and S. flexuosa Becc. Fitopatol. Bras. 1992, 17, 198. [Google Scholar]
- Dianese, J.C.; Medeiros, R.B.; Santos, L.T.P. Biodiversity of microfungi found on native plants of the Brazilian Cerrado. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 367–417. [Google Scholar]
- Dianese, J.C.; Inácio, C.A.; Carvalho-Junior, A.A.; dos Santos, M.D.M.; Cantillo-Pérez, T.; Pinho, D.B. Exploring the overlooked diversity of plant-associated cerrado microfungi. Anu. Patol. Plantas 2022, 28, 69–101. [Google Scholar] [CrossRef]
- Medeiros, R.B.; Dianese, J.C. Passalora eitenii sp. nov. on Syagrus comosa in Brazil and a key to Passalora species. Mycotaxon 1994, 51, 509–513. [Google Scholar]
- de Souza, C.A.P.; Victória, N.S.; Bezerra, J.L.; Luz, E.D.M.N.; Inácio, C.A.; Dianese, J.C. Camarotella brasiliensis sp. nov. (Phyllachoraceae) on Syagrus schizophylla (Arecaceae) from Brazil. Mycotaxon 2008, 103, 313–317. [Google Scholar]
- Souza, E.S.C.; Pereira-Carvalho, R.C.; Sanchez, M.; Dianese, J.C. Echidnodella species (Asterinaceae, Ascomycota) on Mauritia flexuosa (Arecaceae) from the Brazilian Cerrado. Phytopathology 2013, 103, 137. [Google Scholar]
- Vitória, N.S.; Bezerra, J.L.; Gramacho, K.P.; Luz, E.D.M.N. Camaroetella torrendiella comb. nov. e C. acrocomiae: Agentes etiológicos das lixas do coqueiro. Trop. Plant Pathol. 2008, 33, 295–301. [Google Scholar] [CrossRef]
- Vitoria, N.S.; Cavalcanti, M.A.Q.; Hyde, K.D.; Bezerra, J.L. Arecomyces new to Brazil, including A. attaleae sp. nov. Cryptogam. Mycol. 2011, 32, 103–108. [Google Scholar] [CrossRef]
- Vitoria, N.S.; Cavalcanti, M.A.Q.; Luz, E.D.M.N.; Bezerra, J.L. Endocalyx melanoxanthus var. melanoxanthus (Ascomycota): New to Brazil and three new hosts. Mycotaxon 2011, 117, 109–113. [Google Scholar] [CrossRef]
- Vitoria, N.S.; Cavalcanti, M.A.Q.; Hyde, K.D.; Bezerra, J.L. Brunneiapiospora brasiliensis sp. nov. (Clypeosphaeriaceae) on palms from Brazil. Nova Hedwig. 2012, 94, 245–250. [Google Scholar] [CrossRef]
- Vitória, N.S.; Cavalcanti, M.A.Q.; Bezerra, J.L. Lasiodiplodia theobromae: A new host and a revision of plant hosts reported in Brazil. Agrotrópica 2012, 24, 63–66. [Google Scholar] [CrossRef]
- Vitoria, N.S.; Cavalcanti, M.A.Q.; dos Santos, C.D.; Pereira, J.; Bezerra, J.L. Neolinocarpon attaleae sp. nov. on Attalea funifera (Arecaceae) from Brazil. Mycotaxon 2013, 123, 141–145. [Google Scholar] [CrossRef]
- Vitória, N.S.; Cavalcanti, M.A.Q.; dos Santos, M.V.O.; Silvério, M.L.; Bezerra, J.L. Ascomycota em palmeiras: Novos registros e novos hospedeiros para o Nordeste Brasileiro. Agrotrópica 2014, 26, 35–42. [Google Scholar] [CrossRef]
- Vitória, N.S.; dos Santos, M.A.L.; Fortes, N.G.S. Comunidade fúngica de Syagrus coronata (Mart.) Becc: Ascomycota anamórficos e teleomórficos. In Ecologia e Biodiversidade do Semiárido Nordestino; de Andrade, M.J.G., Nogueira, E.M.S., dos Santos, C.A.B., Eds.; Sociedade Brasileira de Ecologia Humana: Bahia, Brazil, 2016; pp. 35–45. [Google Scholar]
- Vitória, N.S.; Cavalcanti, M.A.D.; Bezerra, J.L. Species of Astrosphaeriella and Fissuroma from palms: New records for South America and Brazil. Nova Hedwig. 2016, 102, 129–140. [Google Scholar] [CrossRef]
- Vitória, N.S.; dos Santos, M.A.L.; Bezerra, J.L. Contribuições para o conhecimento de fungos (Ascomycota) em Mauritia flexuosa L.f. e Acrocomia intumescens Drude, Brasil. Rev. Bras. Geogr. Fís. 2019, 12, 1252–1258. [Google Scholar] [CrossRef]
- Vitória, N.S.; dos Santos, M.A.L.; Bezerra, J.L. Euterpe oleracea Mart. e Elaeis guineensis Jacq. (Arecaceae): Novos hospedeiros para o registro de ocorrência de microfungos (Ascomycota) no Brasil. In Pindorama; dos Santos, F.A.R., Carneiro, C.H., Eds.; Editora da Universidade Federal do Piauí: Piauí, Brazil, 2019; pp. 119–133. [Google Scholar]
- Vitória, N.S.; Fortes, N.G.S.; dos Santos, M.A.L.; Barbosa, R.L. Mycota (Ascomycota) of Syagrus coronata (Mart.) Becc., Raso da Catarina Ecological Station, Brazil: New records. Acta Bras. 2020, 4, 110–120. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Izabel, T.S.S.; Leão-Ferreira, S.M.; Gusmão, L.F.P. Conidial fungi from the semi-arid Caatinga biome of Brazil. New species and new records of Helicosporium. Mycotaxon 2009, 110, 53–64. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Gusmão, L.F.P. Fungos conidiais na Caatinga: Espécies associadas ao folhedo. Acta Bot. Bras. 2009, 23, 999–1012. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Gusmão, L.F.P. Fungos conidiais na Caatinga: Espécies lignícolas. Acta Bot. Bras. 2009, 23, 1133–1144. [Google Scholar] [CrossRef]
- Adamcík, S.; Cai, L.; Chakraborty, D.; Chen, X.-H.; Cotter, H.V.; Dai, D.-Q.; Dai, Y.-C.; Das, K.; Deng, C.-Y.; Ghobad-Nejhad, M.; et al. Fungal Biodiversity Profiles 1–10. Cryptogam. Mycol. 2015, 36, 121–166. [Google Scholar] [CrossRef]
- de Oliveira, R.J.V.; Bezerra, J.L.; Lima, T.E.F.; da Silva, G.A.; Cavalcantil, M.A.D. Phaeosphaeria nodulispora, a new endophytic coelomycete isolated from tropical palm (Cocos nucifera) in Brazil. Nova Hedwig. 2016, 103, 185–192. [Google Scholar] [CrossRef]
- dos Santos, M.A.L.; Vitória, N.S.; Bezerra, J.L. Fungos colonizando palmeiras em áreas de Caatinga do Sertão da Bahia. Agrotrópica 2016, 28, 37–46. [Google Scholar] [CrossRef]
- dos Santos, E.C.S.; Vitória, N.S. Espécies de Ascomycota em Syagrus coronata (Mart.) Becc., Água Branca, Alagoas, Brasil. Rev. Ouricuri 2017, 7, 80–97. [Google Scholar]
- dos Santos, M.A.L.; Fortes, N.G.S.; Silva, T.E.F.; Vitória, N.S. Ascomycota (lichenized and non-lichenized) on Syagrus coronata in the Caatinga biome: New and interesting records for Brazil and South America. Mycotaxon 2019, 134, 737. [Google Scholar] [CrossRef]
- dos Santos, M.A.L.; Bezerra, J.L.; Vitória, N.S. Phaeoseptum aquaticum (Halotthiaceae): New record for American continent in a new host for science. Rodriguésia 2019, 70, e00282018. [Google Scholar] [CrossRef]
- dos Santos, M.A.L.; Vitória, N.S.; de Oliveira, R.J.V.; Bezerra, J.L. Diatrypella heveae Senwanna, Phookamsak & K.D. Hyde (Diatrypaceae, Xylariales): A new record for the Neotropics. Check List 2020, 16, 1703–1708. [Google Scholar] [CrossRef]
- Rocha, P.Q.; Vitória, N.S. New occurrences of ascomycetes for South America and the Neotropics. Agrotrópica 2020, 32, 31–36. [Google Scholar] [CrossRef]
- Fortes, N.G.S.; dos Santos, M.A.L.; Vitória, N.S. Apiosordaria nigeriensis (Ascomycota): A new record for the Americas. Rodriguésia 2020, 71, e00852018. [Google Scholar] [CrossRef]
- da Silva, M.S.R.; Vitória, N.S. Fungos endofíticos em frutos de Syagrus coronata (Mart.) Becc. Encicl. Biosf. 2023, 20, 188–203. [Google Scholar] [CrossRef]
- Vitória, N.S.; dos Santos, M.A.L.; Souza, V.M.F.; da Silva, T.B.M.; Bezerra, J.L. Sexual morph of Stachybotrys frondicola (Ascomycota): First record in Brazil. Encicl. Biosf. 2022, 19, 35–45. [Google Scholar] [CrossRef]
- Rocha, P.Q.; Barbosa, R.L.; Vitória, N.S. Ascomycetes in Syagrus coronata (Mart.) Becc. in the Raso da Catarina Ecological Station, with new distribution records. Rev. Ouricuri 2023, 13, 222–247. [Google Scholar] [CrossRef]
- McKenzie, E.H.C. A new species of Lylea (hyphomycetes) on Rhopalostylis (Arecaceae) in New Zealand. Mycotaxon 2009, 109, 39–42. [Google Scholar] [CrossRef]
- McKenzie, E.H.C. Two new dictyosporous hyphomycetes on Rhopalostylis sapida (Arecaceae) in New Zealand. Mycotaxon 2010, 111, 155–160. [Google Scholar] [CrossRef]
- Petrini, L.E. Rosellinia and related genera in New Zealand. N. Z. J. Bot. 2003, 41, 71–138. [Google Scholar] [CrossRef]
- Braun, U.; Hill, C.F.; Schubert, K. New species and new records of biotrophic micromycetes from Australia, Fiji, New Zealand and Thailand. Fungal Divers. 2006, 22, 13–35. [Google Scholar]
- Johnston, P.R.; Whitton, S.R.; Buchanan, P.K.; Park, D.; Pennycook, S.R.; Johnson, J.E.; Moncalvo, J.M. The basidiomycete genus Favolaschia in New Zealand. N. Z. J. Bot. 2006, 44, 65–87. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hawksworth, D.L.; Hyde, K.D.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Manamgoda, D.S.; Thambugala, K.M.; Udayanga, D.; Camporesi, E.; Daranagama, A.; et al. Epitypification and neotypification: Guidelines with appropriate and inappropriate examples. Fungal Divers. 2014, 69, 57–91. [Google Scholar] [CrossRef]
- Pinruan, U.; Rungjindamai, N.; Choeyklin, R.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Occurrence and diversity of basidiomycetous endophytes from the oil palm, Elaeis guineensis in Thailand. Fungal Divers. 2010, 41, 71–88. [Google Scholar] [CrossRef]
- Pinnoi, A.; Jeewon, R.; Sakayaroj, J.; Hyde, K.D.; Jones, E.B.G. Berkleasmium crunisia sp. nov. and its phylogenetic affinities to the Pleosporales based on 18S and 28S rDNA sequence analyses. Mycologia 2007, 99, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Pinnoi, A.; Phongpaichit, P.; Jeewon, R.; Tang, A.M.C.; Hyde, K.D.; Jones, E.B.G. Phylogenetic relationships of Astrocystis eleiodoxae sp. nov. (Xylariaceae). Mycosphere 2010, 1, 1–9. [Google Scholar]
- Bahl, J.; Jeewon, R.; Hyde, K.D. Phylogeny of Rosellinia capetribulensis sp nov and its allies (Xylariaceae). Mycologia 2005, 97, 1102–1110. [Google Scholar] [CrossRef]
- Hidayat, I.; Jeewon, R.; To-Anun, C.; Hyde, K.D. The genus Oxydothis: New palmicolous taxa and phylogenetic relationships within the Xylariales. Fungal Diver. 2006, 23, 159–179. [Google Scholar]
- Konta, S.; Hongsanan, S.; Tibpromma, S.; Thongbai, B.; Maharachchikumbura, S.S.N.; Bahkali, A.H.; Hyde, K.D.; Boonmee, S. An advance in the endophyte story: Oxydothidaceae fam. nov. with six new species of Oxydothis. Mycosphere 2016, 7, 1425–1446. [Google Scholar] [CrossRef]
- Hu, H.M.; Liu, L.L.; Zhang, X.; Lin, Y.; Shen, X.C.; Long, S.H.; Kang, J.C.; Wijayawardene, N.N.; Li, Q.R.; Long, Q.D. New species and records of Neomassaria, Oxydothis and Roussoella (Pezizomycotina, Ascomycota) associated with palm and bamboo from China. Mycokeys 2022, 93, 165–191. [Google Scholar] [CrossRef]
- Konta, S.; Hongsanan, S.; Liu, J.K.; Eungwanichayapant, P.D.; Jeewon, R.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Boonmee, S. Leptosporella (Leptosporellaceae fam. nov.) and Linocarpon and Neolinocarpon (Linocarpaceae fam. nov.) are accommodated in Chaetosphaeriales. Mycosphere 2017, 8, 1943–1974. [Google Scholar] [CrossRef]
- Zhang, S.N.; Hyde, K.D.; Jones, E.B.G.; Cheewangkoon, R.; Liu, J.K. Additions to Fissuroma and Neoastrosphaeriella (Aigialaceae, Pleosporales) from palms. Mycosphere 2020, 11, 269–284. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Jeewon, R.; Jones, E.B.G.; Boonmee, S.; Kaewchai, S.; Manawasinghe, I.S.; Lumyong, S.; Hyde, K.D. Novel palmicolous taxa within Pleosporales: Multigene phylogeny and taxonomic circumscription. Mycol. Prog. 2018, 17, 571–590. [Google Scholar] [CrossRef]
- Konta, S.; Hyde, K.D.; Eungwanichayapant, P.D.; Doilom, M.; Tennakoon, D.S.; Senwanna, C.; Boonmee, S. Fissuroma (Aigialaceae: Pleosporales) appears to be hyperdiverse on Arecaceae: Evidence from two new species from southern Thailand. Acta Bot. Bras. 2020, 34, 384–393. [Google Scholar] [CrossRef]
- Liu, J.K.; Phookamsak, R.; Dai, D.Q.; Tanaka, K.; Jones, E.B.G.; Xu, J.C.; Chukeatirote, E.; Hyde, K.D. Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa 2014, 181, 1–33. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; et al. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Mapook, A.; Hyde, K.D.; Hongsanan, S.; Phukhamsakda, C.; Li, J.F.; Boonmee, S. Palawaniaceae fam. nov., a new family (Dothideomycetes, Ascomycota) to accommodate Palawania species and their evolutionary time estimates. Mycosphere 2016, 7, 1732–1745. [Google Scholar] [CrossRef]
- Yu, X.D.; Zhang, S.N.; Cheewangkoon, R.; Liu, J.K. Additions to Occultibambusaceae (Pleosporales, Dothideomycetes): Unrevealing Palmicolous Fungi in China. Diversity 2021, 13, 516. [Google Scholar] [CrossRef]
- Hawksworth, D.L. ‘Misidentifications’ in fungal DNA sequence databanks. New Phytol. 2004, 161, 13–15. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Liu, X.Z.; Chamyuang, S.; Stadler, M.; Bahkali, A.H.; Hyde, K.D. Towards a natural classification of Sordariomycetes: The genera Frondisphaeria, Immersisphaeria, Lasiobertia, Pulmosphaeria and Yuea (Sordariomycetes incertae sedis). Phytotaxa 2016, 258, 153–163. [Google Scholar] [CrossRef][Green Version]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Jones, E.B.G.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Hyde, K.V.D.; Dong, Y.; Phookamsak, R.T.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Liu, N.G.; Abeywickrama, P.D.; Mapook, A.; Wei, D.P.; et al. Fungal Diversity notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020, 100, 5–277. [Google Scholar] [CrossRef]
- Hidayat, I.; Meeboon, J.; To–Anun, C. Anthostomella and Fasciatispora species (Xylariaceae) from palms including F. ujungkulonensis sp. nov. Mycotaxon 2007, 102, 347–354. [Google Scholar]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal Diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.Z.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Jeewon, R.; Liu, X.Z.; Stadler, M.; Lumyong, S.; Hyde, K.D. Taxonomic rearrangement of Anthostomella (Xylariaceae) based on a multigene phylogeny and morphology. Cryptogam. Mycol. 2016, 37, 509–538. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Miller, A.N.; Hashimoto, A.; Iida, T.; Ohkuma, M.; Okada, G. A phylogenetic assessment of Endocalyx (Cainiaceae, Xylariales) with E. grossus comb. et stat. nov. Mycol. Prog. 2022, 21, 221–242. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; Nilsson, R.H.; Bhunjun, C.S.; de Farias, A.R.G.; Sun, Y.R.; Wijesinghe, S.N.; Raza, M.; Bao, D.F.; Lu, L.; Tibpromma, S.; et al. The numbers of fungi: Contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Divers. 2022, 114, 327–386. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Pennycook, S.R.; Johnston, P.R.; Ramaley, A.; Akulov, A.; Crous, P.W. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 2008, 21, 29–55. [Google Scholar] [CrossRef]
- Konta, S.; Phillips, A.J.L.; Bahkali, A.H.; Jones, E.B.G.; Eungwanichayapant, D.P.; Hyde, K.D.; Boonmee, S. Botryosphaeriaceae from palms in Thailand—Barriopsis archontophoenicis sp. nov, from Archontophoenix alexandrae. Mycosphere 2016, 7, 921–932. [Google Scholar] [CrossRef]
- Konta, S.; Hongsanan, S.; Phillips, A.J.L.; Jones, E.B.G.; Boonmee, S.; Hyde, K.D. Botryosphaeriaceae from palms in Thailand II—Two new species of Neodeightonia, N. rattanica and N. rattanicola from Calamus (rattan palm). Mycosphere 2016, 7, 950–961. [Google Scholar] [CrossRef]
- Douanla–Meli, C.; Scharnhorst, A. Palm foliage as pathways of pathogenic Botryosphaeriaceae fungi and host of new Lasiodiplodia species from Mexico. Pathogens 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.R.; Manawasinghe, I.S.; Liao, C.F.; Hyde, K.D.; Dong, Z.Y. Neodeightonia arengae sp. nov., Botryosphaeriaceous taxa on Arenga tremula (Arecaceae) from Guangdong, China. Phytotaxa 2022, 530, 130–140. [Google Scholar] [CrossRef]
- Wu, N.; Dissanayake, A.J.; Chethana, K.W.T.; Liu, J.-K. Neodeightonia septata sp. nov. and N. subglobosa (Botryosphaeriaceae) from Northern Thailand. Phytotaxa 2022, 575, 129–139. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Groenewald, J.Z.; Xu, J.; Crous, P.W. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef]
- Liu, A.R.; Chen, S.C.; Lin, X.M.; Wu, S.Y.; Xu, T.; Cai, F.M.; Jeewon, R. Endophytic Pestalotiopsis species associated with plants of Palmae, Rhizophoraceae, Planchonellae and Podocarpaceae in Hainan, China. Afr. J. Microbiol. Res. 2010, 4, 2661–2669. [Google Scholar]
- Zhang, Y.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Hyde, K.D. A novel species of Pestalotiopsis causing leaf spots of Trachycarpus fortunei. Cryptogam. Mycol. 2012, 33, 311–318. [Google Scholar] [CrossRef]
- Geng, K.; Zhang, B.; Song, Y.; Hyde, K.D.; Kang, J.C.; Wang, Y. A new species of Pestalotiopsis from leaf spots of Licuala grandis from Hainan, China. Phytotaxa 2013, 88, 49–54. [Google Scholar] [CrossRef][Green Version]
- Xiong, Y.R.; Manawasinghe, I.S.; Maharachchikumbura, S.S.N.; Lu, L.; Dong, Z.Y.; Xiang, M.M.; Xu, B. Pestalotioid species associated with palm species from Sothern China. Curr. Res. Environ. Appl. Mycol. 2022, 12, 285–321. [Google Scholar] [CrossRef]
- Guterres, D.C.; Silva, M.A.; Martins, M.D.; Azevedo, D.M.Q.; Lisboa, D.O.; Pinho, D.B.; Furtado, G.Q. Leaf spot caused by Neopestalotiopsis species on Arecaceae in Brazil. Australas. Plant Pathol. 2023, 52, 47–62. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, Y.M.; Tian, C.M. Morphological and phylogenic evidences reveal a new Seiridium species in China. Phytotaxa 2019, 418, 287–293. [Google Scholar] [CrossRef]
- Pereira, D.S.; Phillips, A.J.L. Two new Morinia species from palms (Arecaceae) in Portugal. Mycol. Prog. 2021, 20, 83–94. [Google Scholar] [CrossRef]
- Li, J.F.; Phookamsak, R.; Jeewon, R.; Tibpromma, S.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Chukeatirote, E.; Lumyong, S.; Hyde, K.D.; McKenzie, E.H.C. Establishment of Zygosporiaceae fam. nov. (Xylariales, Sordariomycetes) based on rDNA sequence data to accommodate Zygosporium. Mycosphere 2017, 8, 1855–1868. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G. South Florida microfungi: New records of saprophytic hyphomycetes on plant debris. Fla. Sci. 2008, 71, 76–89. [Google Scholar]
- Delgado-Rodríguez, G. South Florida microfungi: A new species of Stanjehughesia (hyphomycetes) from Sabal palm. Mycotaxon 2008, 103, 229–234. [Google Scholar]
- Delgado-Rodríguez, G. South Florida microfungi: A new species of Ellisembia (hyphomycetes) with new records from the USA. Mycotaxon 2013, 123, 445–450. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G. South Florida microfungi: Linkosia longirostrata, a new hyphomycete on paurotis palm. Mycotaxon 2014, 129, 41–46. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Miller, A.N. South Florida microfungi: A new species of Taeniolella (anamorphic Sordariomycetes) isolated from cabbage palm. Nova Hedwig. 2017, 105, 1–14. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Koukol, O.; Cáceres, O.; Piepenbring, M. The phylogenetic placement of Ernakulamia cochinensis within Pleosporales (Dothideomycetes, Ascomycota). Cryptogam. Mycol. 2017, 38, 435–451. [Google Scholar] [CrossRef]
- Koukol, O.; Delgado-Rodríguez, G.; Hofmann, T.A.; Piepenbring, M. Panama, a hot spot for Hermatomyces (Hermatomycetaceae, Pleosporales) with five new species, and a critical synopsis of the genus. IMA Fungus 2018, 9, 107–141. [Google Scholar] [CrossRef] [PubMed]
- Nuankaew, S.; Suetrong, S.; Wutikhun, T.; Pinruan, U. Hermatomyces trangensis sp. nov., a new dematiaceous hyphomycete (Hermatomycetaceae, Pleosporales) on sugar palm in Thailand. Phytotaxa 2019, 391, 277–288. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, G.; Koukol, O.; Heredia-Abarca, G.; Piepenbring, M. Texas microfungi: Hermatomyces amphisporus (Pleosporales, Dothideomycetes) revisited. Czech Mycol. 2020, 72, 95–107. [Google Scholar] [CrossRef]
- Prasher, I.B.; Sushma. Hermatomyces indicus sp. nov. (Hyphomycetes) from India. Nova Hedwig. 2014, 99, 551–556. [Google Scholar] [CrossRef]
- Koukol, O.; Delgado-Rodríguez, G. Do not forget Africa—Revision of fungarium collections at Kew revealed a new species of Hermatomyces (Hermatomycetaceae, Pleosporales). Nova Hedwig. 2019, 109, 413–423. [Google Scholar] [CrossRef]
- Chen, Y.P.; Tian, W.H.; Guo, Y.B.; Madrid, H.; Maharachchikumbura, S.S.N. Synhelminthosporium gen. et sp. nov. and two new species of Helminthosporium (Massarinaceae, Pleosporales) from Sichuan Province, China. J. Fungi 2022, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Kularathnage, N.D.; Wanasinghe, D.N.; Senanayake, I.C.; Yang, Y.H.; Manawasinghe, I.S.; Phillips, A.J.L.; Hyde, K.D.; Dong, W.; Song, J.G. Microfungi associated with ornamental palms: Byssosphaeria phoenicis sp. nov. (Melanommataceae) and Pseudocoleophoma rhapidis sp. nov. (Dictyosporiaceae) from south China. Phytotaxa 2022, 568, 149–169. [Google Scholar] [CrossRef]
- Tian, X.G.; Tibpromma, S.; Karunarathna, S.C.; Dai, D.Q.; Lu, Y.Z.; Mapook, A.; Jayawardena, R.S. A new species and a new host record of Pseudoberkleasmium (Pseudoberkleasmiaceae, Dothideomycetes) from Cocos nucifera and Zea mays in northern Thailand. Phytotaxa 2022, 547, 232–242. [Google Scholar] [CrossRef]
- Kularathnage, N.D.; Senanayake, I.C.; Wanasinghe, D.N.; Doilom, M.; Stephenson, S.L.; Song, J.G.; Dong, W.; Xu, B. Plant-associated novel didymellaceous taxa in the South China Botanical Garden (Guangzhou, China). J. Fungi 2023, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Manawasinghe, I.S.; Wanasinghe, D.N.; Hongsanan, S.; Hyde, K.D.; Biao, X.; Dong, Z. Two new species and a new host record of Pleosporales (Dothideomycetes) from palm (Arecaceae) in Guangdong Province, China. N. Z. J. Bot. 2023, 1–27. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J. Two new species of Chaetopsina (Nectriaceae) from Saül (French Guiana). Ascomycete.org 2019, 11, 127–134. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J. Clonostachys spinulosispora (Hypocreales, Bionectriaceae), a new species on palm from French Guiana. Ascomycete.org 2018, 10, 127–130. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J.; Chaduli, D.; Lesage-Meessen, L.; Favel, A. Clonostachys saulensis (Bionectriaceae, Hypocreales), a new species from French Guiana. Ascomycete.org 2019, 11, 65–68. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J.; Chaduli, D.; Favel, A. Hydropisphaera palmicola (Bionectriaceae), a new species from Saül (French Guiana). Ascomycete.org 2022, 14, 81–84. [Google Scholar] [CrossRef]
- Lechat, C.; Lesage-Meessen, L.; Favel, A. A new species of Ijuhya, I. fournieri, from French Guiana. Ascomycete.org 2015, 7, 101–104. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J. Four new species of Ijuhya (Bionectriaceae) from Belgium, metropolitan France and French Guiana. Ascomycete.org 2017, 9, 11–18. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J.; Chaduli, D.; Favel, A. Lasionectria saulensis (Bionectriaceae, Hypocreales), a new species from French Guiana. Ascomycete.org 2022, 14, 85–88. [Google Scholar] [CrossRef]
- Lechat, C.; Fournier, J.; Chaduli, D.; Favel, A. Three new holomorphic species of Volutella (Nectriaceae, Hypocreales) from Saül (French Guiana). Ascomycete.org 2022, 14, 89–95. [Google Scholar] [CrossRef]
- Crane, J.L.; Miller, A.N. Studies in genera similar to Torula: Bahusaganda, Bahusandhika, Pseudotorula, and Simmonsiella gen. nov. IMA Fungus 2016, 7, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Phillips, A.J.L.; Pereira, D.S.; Dai, D.Q.; Aptroot, A.; Monteiro, J.S.; Druzhinina, I.S.; Cai, F.; Fan, X.L.; Selbmann, L.; et al. Forecasting the number of species of asexually reproducing fungi (Ascomycota and Basidiomycota). Fungal Divers. 2022, 114, 463–490. [Google Scholar] [CrossRef]
- Wulandari, N.F.; To-Anun, C.; McKenzie, E.H.C.; Hyde, K.D. Guignardia bispora and G. ellipsoidea spp. nov. and other Guignardia species from palms (Arecaceae). Mycosphere 2011, 2, 115–128. [Google Scholar]
- Lechat, C.; Fournier, J. Two new species of Lasionectria (Bionectriaceae, Hypocreales) from Guadeloupe and Martinique (French West Indies). Mycotaxon 2012, 121, 275–280. [Google Scholar] [CrossRef]
- Prasher, I.B.; Verma, R.K. Two new species of Dictyosporium from India. Phytotaxa 2015, 204, 193–202. [Google Scholar] [CrossRef]
- Xia, J.W.; Ma, Y.R.; Gao, J.M.; Zhang, X.G.; Li, Z. Two new species of Endophragmiella from southern China. Nova Hedwig. 2016, 103, 349–357. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Phillips, A.J.L.; Tibpromma, S.; Dai, D.-Q.; Selbmann, L.; Monteiro, J.S.; Aptroot, A.; Flakus, A.; Rajeshkumar, K.C.; Coleine, C.; et al. Looking for the undiscovered asexual taxa: Case studies from lesser studied life modes and habitats. Mycosphere 2021, 12, 1186–1229. [Google Scholar] [CrossRef]
- Suetrong, S.; Schoch, C.L.; Spatafora, J.W.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Sakayaroj, J.; Phongpaichit, S.; Tanaka, K.; Hirayama, K.; Jones, E.B.G. Molecular systematics of the marine Dothideomycetes. Stud. Mycol. 2009, 64, 155–173. [Google Scholar] [CrossRef]
- Suetrong, S.; Sakayaroj, J.; Phongpaichit, S.; Jones, E.B.G. Morphological and molecular characteristics of a poorly known marine ascomycete, Manglicola guatemalensis (Jahnulales: Pezizomycotina; Dothideomycetes, Incertae sedis): New lineage of marine ascomycetes. Mycologia 2010, 102, 83–92. [Google Scholar] [CrossRef]
- Suetrong, S.; Hyde, K.D.; Zhang, Y.; Bahkali, A.H.; Jones, E.B.G. Trematosphaeriaceae fam. nov. (Dothideomycetes, Ascomycota). Cryptogam. Mycol. 2011, 32, 343–358. [Google Scholar] [CrossRef]
- Suetrong, S.; Klaysuban, A.; Sakayaroj, J.; Preedanon, S.; Ruang-Areerate, P.; Phongpaichit, S.; Pang, K.L.; Jonese, E.B.G. Tirisporellaceae, a new family in the order Diaporthales (Sordariomycetes, Ascomycota). Cryptogam. Mycol. 2015, 36, 319–330. [Google Scholar] [CrossRef]
- Liu, J.K.; Jones, E.B.G.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Lignincola conchicola from palms with a key to the species of Lignincola. Mycotaxon 2011, 117, 343–349. [Google Scholar] [CrossRef]
- Abdel-Aziz, F.A. The genus Lolia from freshwater habitats in Egypt with one new species. Phytotaxa 2016, 267, 279–288. [Google Scholar] [CrossRef]
- Boonmee, S.; D’Souza, M.J.; Luo, Z.L.; Pinruan, U.; Tanaka, K.; Su, H.Y.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Taylor, J.E.; et al. Dictyosporiaceae fam. nov. Fungal Divers. 2016, 80, 457–482. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Liu, Z.Y. New species in Dictyosporium, new combinations in Dictyocheirospora and an updated backbone tree for Dictyosporiaceae. Mycokeys 2018, 36, 83–105. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Devadatha, B.; Abdel-Wahab, M.A.; Dayarathne, M.C.; Zhang, S.N.; Hyde, K.D.; Liu, J.K.; Bahkali, A.H.; Sarma, V.V.; Tibell, S.; et al. Phylogeny of new marine Dothideomycetes and Sordariomycetes from mangroves and deep-sea sediments. Bot. Mar. 2020, 63, 155–181. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.W.T.; Dai, D.-Q.; Dai, Y.-C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Fungal Diversity notes 111–252: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal Diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal Diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Wang, S.; Sun, Y.R.; Suwannarach, N.; Sysouphanthong, P.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Abeywickrama, P.D.; Abreu, V.P.; et al. Fungal Diversity notes 1512–1610: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2022, 117, 1–272. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Romberg, M.; Mel’nik, V.A.; Verkley, G.J.M.; Groenewald, J.Z. Fungal Planet description sheets: 92–106. Persoonia 2011, 27, 130–162. [Google Scholar] [CrossRef]
- Crous, P.W.; Shivas, R.G.; Wingfield, M.J.; Summerell, B.A.; Rossman, A.Y.; Alves, J.L.; Adams, G.C.; Barreto, R.W.; Bell, A.; Coutinho, M.L.; et al. Fungal Planet description sheets: 128–153. Persoonia 2012, 29, 146–201. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.K.; Summerell, B.A.; Giraldo, A.; Gené, J.; Guarro, J.; Wanasinghe, D.N.; Hyde, K.D.; Camporesi, E.; et al. Fungal Planet description sheets: 281–319. Persoonia 2014, 33, 212–292. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Guarro, J.; Hernandez-Restrepo, M.; Sutton, D.A.; Acharya, K.; Barber, P.A.; Boekhout, T.; Dimitrov, R.A.; Dueñas, M.; et al. Fungal Planet description sheets: 320–370. Persoonia 2015, 34, 167–266. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet description sheets: 469–557. Persoonia 2016, 37, 218–403. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Carnegie, A.J.; Wingfield, M.J.; Sharma, R.; Mughini, G.; Noordeloos, M.E.; Santini, A.; Shouche, Y.S.; Bezerra, J.D.P.; Dima, B.; et al. Fungal Planet description sheets: 868–950. Persoonia 2019, 42, 291–473. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsa-Ard, J.; Thangavel, R.; et al. Fungal Planet description sheets: 951–1041. Persoonia 2019, 43, 223–425. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Osieck, E.R.; Shivas, R.G.; Tan, Y.P.; Bishop-Hurley, S.L.; Esteve-Raventós, F.; Larsson, E.; Luangsa-Ard, J.J.; Pancorbo, F.; Balashov, S.; et al. Fungal Planet description sheets: 1478–1549. Persoonia 2023, 50, 158–310. [Google Scholar] [CrossRef]
- Boonmee, S.; Phookamsak, R.; Hongsanan, S.; Doilom, M.; Mapook, A.; McKenzie, E.H.C.; Bhat, D.J.; Hyde, K.D. Mycosphere notes 51–101. Revision of genera in Perisporiopsidaceae and Pseudoperisporiaceae and other Ascomycota genera incertae sedis. Mycosphere 2017, 8, 1695–1801. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chaiwan, N.; Norphanphoun, C.; Boonmee, S.; Camporesi, E.; Chethana, K.W.T.; Dayarathne, M.C.; de Silva, N.I.; Dissanayake, A.J.; Ekanayaka, A.H.; et al. Mycosphere notes 169–224. Mycosphere 2018, 9, 271–430. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Calabon, M.S.; Jones, E.B.G.; Zhang, Y.X.; Liao, C.F.; Xiong, Y.R.; Chaiwan, N.; Kularathnage, N.D.; Liu, N.G.; Tang, S.M.; et al. Mycosphere notes 345–386. Mycosphere 2022, 13, 454–557. [Google Scholar] [CrossRef]
- Pem, D.; Jeewon, R.; Bhat, D.J.; Doilom, M.; Boonmee, S.; Hongsanan, S.; Promputtha, I.; Xu, J.C.; Hyde, K.D. Mycosphere notes 275–324: A morpho-taxonomic revision and typification of obscure Dothideomycetes genera (incertae sedis). Mycosphere 2019, 10, 1115–1246. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Wanasinghe, D.N.; Papizadeh, M.; Goonasekara, I.D.; Camporesi, E.; Bhat, D.J.; McKenzie, E.H.C.; Phillips, A.J.L.; Diederich, P.; et al. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers. 2016, 77, 1–316. [Google Scholar] [CrossRef]
- Crous, W.P.; Schumacher, R.K.; Akulov, A.; Thangavel, R.; Hernández-Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfield, M.J.; Summerell, B.A.; Quaedvlieg, W.; et al. New and interesting fungi. 2. Fungal. Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef]
- Crous, W.P.; Wingfield, M.J.; Schumacher, R.K.; Akulov, A.; Bulgakov, T.S.; Carnegie, A.J.; Jurjević, Ž.; Decock, C.; Denman, S.; Lombard, L.; et al. New and interesting fungi. 3. Fungal. Syst. Evol. 2020, 6, 157–231. [Google Scholar] [CrossRef] [PubMed]
- Crous, W.P.; Hernández-Restrepo, M.; Schumacher, R.K.; Cowan, D.A.; Maggs-Kölling, G.; Marais, E.; Wingfield, M.J.; Yilmaz, N.; Adan, O.C.G.; Akulov, A.; et al. New and interesting fungi. 4. Fungal. Syst. Evol. 2021, 7, 255–343. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; García, D.; Gené, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R.K.; et al. Genera of phytopathogenic fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–124. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; de Silva, N.I.; Jeewon, R.; Bhat, D.J.; Phookamsak, R.; Doilom, M.; Boonmee, S.; Jayawardena, R.S.; Maharachchikumbura, S.S.N.; Senanayake, I.C.; et al. AJOM new records and collections of fungi: 1–100. Asian J. Mycol. 2020, 3, 22–294. [Google Scholar] [CrossRef]
- Li, W.J.; McKenzie, E.H.C.; Liu, J.K.; Bhat, D.J.; Dai, D.Q.; Camporesi, E.; Tian, Q.; Maharachchikumbura, S.S.N.; Luo, Z.L.; Shang, Q.J.; et al. Taxonomy and phylogeny of hyaline–spored coelomycetes. Fungal Divers. 2020, 100, 279–801. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Niranjan, M.; Dong, W.; Samarakoon, M.C.; Bao, D.F.; Calabon, M.S.; Chaiwan, N.; Chuankid, B.; Dayarathne, M.C.; de Silva, N.I.; et al. AJOM new records and collections of fungi: 101–150. Asian J. Mycol. 2021, 4, 113–260. [Google Scholar]
- Crous, P.W.; Carris, L.M.; Giraldo, A.; Groenewald, J.Z.; Hawksworth, D.L.; Hernández-Restrepo, M.; Jaklitsch, W.M.; Lebrun, M.H.; Schumacher, R.K.; Stielow, J.B.; et al. The Genera of Fungi—Fixing the application of the type species of generic names—G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 2015, 6, 163–198. [Google Scholar] [CrossRef] [PubMed]
- To-Anun, C.; Nguenhom, J.; Meeboon, J.; Hidayat, I. Two fungi associated with necrotic leaflets of areca palms (Areca catechu). Mycol. Prog. 2009, 8, 115–121. [Google Scholar] [CrossRef]
- Kinge, T.R.; Mih, A.M. Ganoderma ryvardense sp. nov. associated with basal stem rot (BSR) disease of oil palm in Cameroon. Mycosphere 2011, 2, 179–188. [Google Scholar]
- Mbenoun, M.; de Beer, Z.W.; Wingfield, M.J.; Wingfield, B.D.; Roux, J. Reconsidering species boundaries in the Ceratocystis paradoxa complex, including a new species from oil palm and cacao in Cameroon. Mycologia 2014, 106, 757–784. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Granata, G.; Epifani, F.; Perrone, G.; Polizzi, G.; Groenewald, J.Z.; Crous, P.W. Ilyonectria palmarum sp. nov. causing dry basal stem rot of Arecaceae. Eur. J. Plant Pathol. 2014, 138, 347–359. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Hyde, K.D. Fungal succession on fronds of Phoenix hanceana in Hong Kong. Fungal Divers. 2002, 10, 185–211. [Google Scholar]
- Southcott, K.A.; Johnson, J.A. Isolation of endophytes from two species of palm, from Bermuda. Can. J. Microbiol. 1997, 43, 789–792. [Google Scholar] [CrossRef]
- Girivasan, K.P.; Suryanarayanan, T.S. Intact leaves as substrate for fungi: Distribution of endophytes and phylloplane fungi in rattan palms. Czech Mycol. 2004, 56, 33–43. [Google Scholar] [CrossRef]
- Song, J.J.; Pongnak, W.; Soytong, K. Isolation and identification of endophytic fungi from 10 species palm trees. Int. J. Agric. Technol. 2016, 12, 349–363. [Google Scholar]
- Jiaojiao, S.; Wattanachai, P.; Kasem, S. Biological activity of endophytic fungi from palm trees against chili anthracnose caused by Colletotrichum capsica. Int. J. Agric. Technol. 2015, 11, 1927–1940. [Google Scholar]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. A method to promote sporulation in palm endophytic fungi. Fungal Divers. 1998, 1, 109–113. [Google Scholar]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol. Phylogenet. Evol. 2001, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 2006, 37, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Rungjindamai, N.; Pinruan, U.; Choeyklin, R.; Hattori, T.; Jones, E.B.G. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers. 2008, 33, 139–161. [Google Scholar]
- Mahmoud, F.M.; Krimi, Z.; Macia-Vicente, J.G.; Errahmani, M.B.; Lopez-Llorca, L.V. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Rev. Iberoam. Micol. 2017, 34, 116–120. [Google Scholar] [CrossRef]
- Saengket, M.; Hyde, K.D.; Kumar, V.; Doilom, M.; Brooks, S. Endophytic fungi from Oncosperma sp. with promising in vitro plant growth promotion and antagonistic activities. Chiang Mai J. Sci. 2021, 48, 837–852. [Google Scholar]
- Azuddin, N.F.; Mohd, M.H.; Rosely, N.F.N.; Mansor, A.; Zakaria, L. Molecular phylogeny of endophytic fungi from rattan (Calamus castaneus Griff.) spines and their antagonistic activities against plant pathogenic fungi. J. Fungi 2021, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Ben Chobba, I.; Elleuch, A.; Ayadi, I.; Khannous, L.; Namsi, A.; Cerqueira, F.; Drira, N.; Gharsallah, N.; Vallaeys, T. Fungal diversity in adult date palm (Phoenix dactylifera L.) revealed by culture-dependent and culture-independent approaches. J. Zhejiang Univ. Sci. B 2013, 14, 1084–1099. [Google Scholar] [CrossRef] [PubMed]
- Viana Diniz, F.; Doi, S.M.S.R.; Fittipaldy, M.C.P.M.; Lopes, R.F.; Margarido, S.S.O.R.; Pontes, S.M.A.; Ramos, D.P.; Araújo, A.V.; Carvalho, C.M. Isolation and identification of endophytic fungi from the amazonian palm Oenocarpus bataua Mart. S. Am. J. Basic Educ. Tech. Technol. 2021, 8, 139–153. [Google Scholar]
- Chase, A.R.; Broschat, T.K. Diseases and Disorders of Ornamental Palms; American Phytopathological Society Press: Saint Paul, MN, USA, 1991. [Google Scholar]
- Elliott, M.L. Diseases caused by “fungi”. In Compendium of Ornamental Palm Diseases and Disorders; Elliott, M.L., Broschat, T.K., Uchida, J.Y., Simone, G.W., Eds.; American Phytopathological Society Press: Saint Paul, MN, USA, 2004; pp. 8–37. [Google Scholar]
- Downer, A.J.; Uchida, J.Y.; Hodel, D.R.; Elliott, M.L. Lethal palm diseases common in the United States. HortTechnology 2009, 19, 710–716. [Google Scholar] [CrossRef]
- Mohammadi, H. Phaeoacremonium spp. and Botryosphaeriaceae spp. associated with date palm (Phoenix dactylifera L.) decline in Iran. J. Phytopathol. 2014, 162, 575–581. [Google Scholar] [CrossRef]
- Al-Hammadi, M.S.; Al-Shariqi, R.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M. Molecular identification of fungal pathogens associated with date palm root diseases in the United Arab Emirates. J. Plant Pathol. 2019, 101, 141–147. [Google Scholar] [CrossRef]
- Alwahshi, K.J.; Saeed, E.E.; Sham, A.; Alblooshi, A.A.; Alblooshi, M.M.; El-Tarabily, K.A.; AbuQamar, S.F. Molecular identification and disease management of date palm sudden decline syndrome in the United Arab Emirates. Int. J. Mol. Sci. 2019, 20, 923. [Google Scholar] [CrossRef]
- Al-Nadabi, H.; Maharachchikumbura, S.S.N.; Al-Gahaffi, Z.S.; Al-Hasani, A.S.; Velazhahan, R.; Al-Sadi, A.M. Molecular identification of fungal pathogens associated with leaf spot disease of date palms (Phoenix dactylifera). All Life 2020, 13, 587–597. [Google Scholar] [CrossRef]
- Nishad, R.; Ahmed, T.A. Survey and identification of date palm pathogens and indigenous biocontrol agents. Plant Dis. 2020, 104, 2498–2508. [Google Scholar] [CrossRef]
- Asensio, L.; López-Jiménez, J.A.; López-Llorca, L.V. Mycobiota of the date palm phylloplane: Description and interactions. Rev. Iberoam. Micol. 2007, 24, 299–304. [Google Scholar] [CrossRef]
- Kirkman, E.R.; Hilton, S.; Sethuraman, G.; Elias, D.M.O.; Taylor, A.; Clarkson, J.; Soh, A.C.; Bass, D.; Ooi, G.T.; McNamara, N.P.; et al. Diversity and ecological guild analysis of the oil palm fungal microbiome across root, rhizosphere, and soil compartments. Front. Microbiol. 2022, 13, 15. [Google Scholar] [CrossRef]
- Seephueak, P.; Preecha, C.; Seephueak, W. Diversity of macrofungi in oil palm (Elaeis guineensis jacq.) plantation in Southern Thailand. Walailak J. Sci. Technol. 2018, 15, 201–211. [Google Scholar] [CrossRef]
- Nobre, C.P.; da Costa, M.G.; Goto, B.T.; Gehring, C. Arbuscular mycorrhizal fungi associated with the babassu palm (Attalea speciosa) in the eastern periphery of Amazonia, Brazil. Acta Amaz. 2018, 48, 321–329. [Google Scholar] [CrossRef]
- Fisher, J.B.; Jayachandran, K. Beneficial role of arbuscular mycorrhizal fungi on florida native palms. Palms 2008, 52, 113–123. [Google Scholar]
- Polanco, G.; Carrillo, L.; Espadas, C.; Reyes-García, C.; Guadarrama, P.; Orellana, R. Asociación micorrízica arbuscular en Coccothrinax readii Quero. Trop. Subtrop. Agroecosystems 2013, 16, 223–233. [Google Scholar]
- Furrazola, E.; Sánchez-Rendon, J.A.; Guadarrama, P.; Pernús, M.; Torres-Arias, Y. Mycorrhizal status of Coccothrinax crinita (Arecaceae), an endangered endemic species from western Cuba. Rev. Mex. Biodivers. 2020, 91, 10. [Google Scholar] [CrossRef]
- Ambili, K.; Thomas, G.V.; Indu, P.; Gopal, M.; Gupta, A. Distribution of arbuscular mycorrhizae associated with coconut and arecanut based cropping systems. Agric. Res. 2012, 1, 338–345. [Google Scholar] [CrossRef]
- Ramos-Zapata, J.A.; Orellana, R.; Allen, E.B. Mycorrhizal dynamics and dependence of Desmoncus orthacanthos Martius (Arecaceae), a native palm of the Yucatan Peninsula, Mexico. Interciencia 2006, 31, 364–370. [Google Scholar]
- Asano, K.; Kagong, W.V.A.; Mohammad, S.M.B.; Sakazaki, K.; Abu Talip, M.S.; Sahmat, S.S.; Chan, M.K.Y.; Isoi, T.; Kano-Nakata, M.; Ehara, H. Arbuscular mycorrhizal communities in the roots of sago palm in mineral and shallow peat soils. Agriculture 2021, 11, 1161. [Google Scholar] [CrossRef]
- Bouamri, R.; Dalpé, Y.; Serrhini, M.N. Effect of seasonal variation on arbuscular mycorrhizal fungi associated with date palm. Emir. J. Food Agric. 2014, 26, 977–986. [Google Scholar] [CrossRef]
- Zougari-Elwedi, B.; Islami, W.; Msetra, A.; Sanaa, M.; Yolande, D.; Sahraoui, A.L. Monitoring the evolution of the arbuscular mycorrhizal fungi associated with date palm. J. New Sci. 2016, 31, 1822–1831. [Google Scholar]
- Pinruan, U.; Pinnoi, A.; Hyde, K.D.; Jones, E.B.G. Tropical peat swamp fungi with special reference to palms. In Freshwater Fungi and Fungal-Like Organisms; Jones, E.B.G., Hyde, K.D., Pang, K.L., Eds.; de Gruyter: Berlin, Germany, 2014; pp. 371–388. [Google Scholar] [CrossRef]
- Lateef, A.; Muid, S.; Bolhassan, M.H. Microfungi on leaves of Licuala bidentata (Arecaceae) from Sarawak, Malaysia. Makara J. Sci. 2015, 19, 161–166. [Google Scholar] [CrossRef]
- Pilantanapak, A.; Jones, E.B.G.; Eaton, R.A. Marine fungi on Nypa fruticans in Thailand. Bot. Mar. 2005, 48, 365–373. [Google Scholar] [CrossRef]
- Hyde, K.D.; Sarma, V.V. Biodiversity and ecological observations on filamentous fungi of mangrove palm Nypa fruticans Wurumb (Liliopsida, Arecales) along the Tutong River, Brunei. Indian J. Mar. Sci. 2006, 35, 297–307. [Google Scholar]
- Besitulo, A.; Moslem, M.A.; Hyde, K.D. Occurrence and distribution of fungi in a mangrove forest on Siargao Island, Philippines. Bot. Mar. 2010, 53, 535–543. [Google Scholar] [CrossRef]
- Loilong, A.; Sakayaroj, J.; Rungjindamai, N.; Choeyklin, R.; Jones, E.B.G. Biodiversity of fungi on the palm Nypa fruticans. In Marine Fungi and Fungal-Like Organisms; Jones, E.B.G., Pang, K.L., Eds.; de Gruyter: Berlin, Germany, 2012; pp. 273–290. [Google Scholar] [CrossRef]
- Sarma, V.V.; Hyde, K.D. Fungal species consortia on Nypa fruticans at Brunei. Stud. Fungi 2018, 3, 19–26. [Google Scholar] [CrossRef]
- Hyde, K.D.; Taylor, J.E. The palm fungi. In Proceedings of the Asia-Pacific Mycological Conference on Biodiversity and Biotechnology, Hua Hin, Thailand, 6–9 July 1998; pp. 34–38. [Google Scholar]
- Hyde, K.D.; Fröhlich, J. Ascomycetes associated with palms. In Ecology of Fungi; Bhat, D.J., Raghukumar, S., Eds.; Goa University: Goa, India, 2000; pp. 109–114. [Google Scholar]
- Pinnoi, A.; Pinruan, U.; Hyde, K.D.; Lumyong, S.; Jones, E.B.G. Palm fungi. In Thai Fungal Diversity; Jones, E.B.G., Tanticharoen, M., Hyde, K.D., Eds.; National Center for Genetic Engineering and Biotechnology, BIOTEC: Bangkok, Thailand, 2004; pp. 181–187. [Google Scholar]
- Schoch, C.L.; Crous, P.W.; Groenewald, J.Z.; Boehm, E.W.A.; Burgess, T.I.; de Gruyter, J.; de Hoog, G.S.; Dixon, L.J.; Grube, M.; Gueidan, C.; et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009, 64, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Hyde, K.D. Pleosporales . Fungal Divers. 2012, 53, 1–221. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Huang, S.K.; Abdel–Wahab, M.A.; Daranagama, D.A.; Dayarathne, M.; D’Souza, M.J.; Goonasekara, I.D.; et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015, 72, 199–301. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 317. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Lumbsch, H.T.; Liu, J.K.; Maharachchikumbura, S.S.N.; Ekanayaka, A.H.; Tian, Q.; Phookamsak, R. Outline of Ascomycota: 2017. Fungal Divers. 2018, 88, 167–263. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Groenewald, J.Z.; Coetzee, M.P.A.; Wingfield, M.J.; Crous, P.W. Evolution of lifestyles in Capnodiales. Stud. Mycol. 2020, 95, 381–414. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel–Wahab, M.A.; Abdel–Aziz, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Hongsanan, S.A.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, J.D.; Liu, N.G.; et al. Refined families of Dothideomycetes: Orders and families incertae sedis in Dothideomycetes. Fungal Divers. 2020, 105, 17–318. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Boonmee, S.; Lücking, R.; Bhat, D.J.; Liu, N.G.; et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1553–2107. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Hyde, K.D.; Gentekaki, E.; McKenzie, E.H.C.; Zhao, Q.; Bulgakov, T.S.; Camporesi, E. Preliminary classification of Leotiomycetes. Mycosphere 2019, 10, 310–489. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez–Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Divakar, P.K.; Rajeshkumar, K.C.; Weerahewa, D.; Delgado, G.; Wang, Y.; Fu, L. Notes for genera update—Ascomycota: 6616–6821. Mycosphere 2018, 9, 115–140. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; McKenzie, E.H.C.; Wang, Y. Notes for genera update—Ascomycota: 6822–6917. Mycosphere 2018, 9, 1222–1234. [Google Scholar] [CrossRef]
- Læssøe, T.; Spooner, B.M. Rosellinia & Astrocystis (Xylariaceae): New species and generic concepts. Kew Bull. 1993, 49, 1–70. [Google Scholar] [CrossRef]
- Li, Q.R.; Zhang, X.; Lin, Y.; Samarakoon, M.C.; Hyde, K.D.; Shen, X.C.; Liao, W.Q.; Karunarathna, A.; Long, S.H.; Kang, Y.Q.; et al. Morpho-molecular characterisation of Arecophila, with A. australis and A. clypeata sp. nov. and A. miscanthi comb. nov. Mycokeys 2022, 88, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.A.; Huhndorf, S.M. New species of Chaetosphaeria, Melanopsammella and Tainosphaeria gen. nov from the Americas. Fungal Divers. 2005, 18, 15–57. [Google Scholar]
- Elliott, M.L.; Des Jardin, E.A. Serenomyces associated with palms in southeastern USA: Isolation, culture storage and genetic variation. Mycologia 2014, 106, 698–707. [Google Scholar] [CrossRef]
- Mardones, M.; Trampe-Jaschik, T.; Oster, S.; Elliott, M.; Urbina, H.; Schmitt, I.; Piepenbring, M. Phylogeny of the order Phyllachorales (Ascomycota, Sordariomycetes): Among and within order relationships based on five molecular loci. Persoonia 2017, 39, 74–90. [Google Scholar] [CrossRef]
- Boonyuen, N.; Chuaseeharonnachai, C.; Suetrong, S.; Sri-Indrasutdhi, V.; Sivichai, S.; Jones, E.B.G.; Pang, K.L. Savoryellales (Hypocreomycetidae, Sordariomycetes): A novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella. Mycologia 2011, 103, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Dayarathne, M.C.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; Dong, W.; Devadatha, B.; Yang, J.; Ekanayaka, A.H.; De Silva, W.; Sarma, V.V.; Al-Sadi, A.M.; et al. Phylogenetic revision of Savoryellaceae and evidence for its ranking as a subclass. Front. Microbiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Zhang, S.N.; Abdel-Wahab, M.A.; Jones, E.B.G.; Hyde, K.D.; Liu, J.K. Additions to the genus Savoryella (Savoryellaceae), with the asexual morphs Savoryella nypae comb. nov. and S. sarushimana sp. nov. Phytotaxa 2019, 408, 195–207. [Google Scholar] [CrossRef]
- Pratibha, J.; Prabhugaonkar, A. Multi-gene phylogeny of Pithomyces with the sexual morph of P. flavus Berk. & Broome. Phytotaxa 2015, 218, 84–90. [Google Scholar] [CrossRef]
- Elliott, M.L.; Morales, A.D.; Des Jardin, E.A. New records of Botryosphaeriaceae genera associated with palms in Florida, USA. Sydowia 2018, 70, 169–178. [Google Scholar] [CrossRef]
- Rathnayaka, A.R.; Chethana, K.W.T.; Phillips, A.J.L.; Jones, E.B.G. Two new species of Botryosphaeriaceae (Botryosphaeriales) and new host/geographical records. Phytotaxa 2022, 564, 8–38. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, J.K.; Hyde, K.D.; Wanasinghe, D.N.; Boonmee, S.; Jayasiri, S.C.; Luo, Z.L.; Taylor, J.E.; Phillips, A.J.L.; Bhat, D.J.; et al. Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers. 2015, 74, 267–324. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Taxonomy of asexual microfungus Periconia on Phoenix in India. Mycol. Iran. 2015, 2, 65–68. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Abreu, V.P.; Bazzicalupo, A.; Chethana, K.W.T.; Clericuzio, M.; Dayarathne, M.C.; Dissanayake, A.J.; Ekanayaka, A.H.; He, M.Q.; et al. Fungal Diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017, 87, 1–235. [Google Scholar] [CrossRef]
- Reblova, M.; Kolarik, M.; Nekvindova, J.; Reblova, K.; Sklenar, F.; Miller, A.N.; Hernandez-Restrepo, M. Phylogenetic Reassessment, taxonomy, and biogeography of Codinaea and similar fungi. J. Fungi 2021, 7, 1097. [Google Scholar] [CrossRef]
- Elliott, M.L.; Des Jardin, E.A.; O’Donnell, K.; Geiser, D.M.; Harrison, N.A.; Broschat, T.K. Fusarium oxysporum f. sp. palmarum, a novel forma specialis causing a lethal disease of Syagrus romanzoffiana and Washingtonia robusta in Florida. Plant Dis. 2010, 94, 31–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.J.; Jayawardena, R.S.; Bhunjun, C.S.; Harishchandra, D.L.; Hyde, K.D. Pseudocercospora dypsidis sp. nov. (Mycosphaerellaceae) on Dypsis lutescens leaves in Thailand. Phytotaxa 2020, 474, 218–234. [Google Scholar] [CrossRef]
- Braun, U.; Crous, P.; Kamal. New species of Pseudocercospora, Pseudocercosporella, Ramularia and Stenella (cercosporoid hyphomycetes). Mycol. Prog. 2003, 2, 197–208. [Google Scholar] [CrossRef]
- Braun, U.; Crous, P.W.; Nakashima, C. Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae). IMA Fungus 2014, 5, 203–390. [Google Scholar] [CrossRef]
- Ma, L.G.; Ma, J.; Zhang, Y.D.; Zhang, X.G. Spadicoides camelliae and Diplococcium livistonae, two new hyphomycetes on dead branches from Fujian Province, China. Mycoscience 2012, 53, 25–30. [Google Scholar] [CrossRef]
- Wu, W.P.; Diao, Y.Z. The chalara-like anamorphs of Leotiomycetes. Fungal Divers. 2023, 119, 213–490. [Google Scholar] [CrossRef]
- Vinjusha, N.; Kumar, T.K.A. Revision of Ganoderma species associated with stem rot of coconut palm. Mycologia 2022, 114, 157–174. [Google Scholar] [CrossRef]
- Piepenbring, M.; Nold, F.; Trampe, T.; Kirschner, R. Revision of the genus Graphiola (Exobasidiales, Basidiomycota). Nova Hedwig. 2012, 94, 67–96. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R. Fungal biodiversity patterns. In Biodiversity of Fungi: Inventory and Monitoring Methods, 1st ed.; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 59–75. [Google Scholar]
- DeLong, D.C. Defining biodiversity. Wildl. Soc. Bull. 1996, 24, 738–749. [Google Scholar]
- Hyde, K.D.; Bussaban, B.; Paulus, B.; Crous, P.W.; Lee, S.; McKenzie, E.H.C.; Photita, W.; Lumyong, S. Diversity of saprobic microfungi. Biodivers. Conserv. 2007, 16, 7–35. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal biodiversity: What do we know? What can we predict? Biodivers. Conserv. 2007, 16, 1–5. [Google Scholar] [CrossRef]
- Schmit, J.P.; Mueller, G.M. An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 2007, 16, 99–111. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.W.; Stadler, M.; Liu, X.Z.; Xiang, M.C. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Fries, E.M. Systema orbis vegetabilis. Primas Lineas Novae Constructionis Periclitatur Elias Fries. Pars I. Plantae Homonemeae; Lundae e Typographia Academia: Lund, Sweden, 1825. [Google Scholar]
- Bisby, G.R.; Ainsworth, G.C. The numbers of fungi. Trans. Br. Mycol. Soc. 1943, 26, 16–19. [Google Scholar] [CrossRef]
- Martin, G.W. The numbers of fungi. Proc. Iowa Acad. Sci. 1951, 58, 175–178. [Google Scholar]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.M.; Vilgalys, R. Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.F. Diversity of the Phyllachoraceae with special reference to the tropics. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 255–278. [Google Scholar]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Fungal diversity and its implications for genetic resource collections. Stud. Mycol. 2004, 50, 9–17. [Google Scholar]
- Hawksworth, D.L. Global species numbers of fungi: Are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 2012, 21, 2425–2433. [Google Scholar] [CrossRef]
- Bass, D.; Richards, T.A. Three reasons to re-evaluate fungal diversity “on Earth and in the ocean”. Fungal Biol. Rev. 2011, 25, 159–164. [Google Scholar] [CrossRef]
- Blackwell, M. The fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 1–32. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjoller, R.; Koljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers—A user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Jayawardena, R.S.; Hyde, K.D. Hurdles in fungal taxonomy: Effectiveness of recent methods in discriminating taxa. Megataxa 2020, 1, 114–122. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Koljalg, U.; Abarenkov, K. Identifying the ‘unidentified’ fungi: A global-scale long–read third–generation sequencing approach. Fungal Divers. 2020, 103, 273–293. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best practices in metabarcoding of fungi: From experimental design to results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K.; Verma, R.K.; Avasthi, S.; Sushma; Bohra, Y.; Devadatha, B.; Niranjan, M.; Suwannarach, N. Current insight into traditional and modern methods in fungal diversity estimates. J. Fungi 2022, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Vetrovsky, T.; Lepinay, C.; Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 2022, 114, 539–547. [Google Scholar] [CrossRef]
- Baldrian, P.; Kohout, P.; Větrovský, T. Global fungal diversity estimated from high-throughput sequencing. In Evolution of Fungi and Fungal-Like Organisms; Pöggeler, S., James, T., Eds.; Springer Cham: Aargau, Switzerland, 2023; pp. 227–238. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Ohman, A.; Glotzer, D.; Nuhn, M.; Kirk, P.; Nilsson, R.H. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol. Rev. 2011, 25, 38–47. [Google Scholar] [CrossRef]
- Ryberg, M.; Nilsson, R.H. New light on names and naming of dark taxa. Mycokeys 2018, 30, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Ryberg, M.; Wurzbacher, C.; Tedersoo, L.; Anslan, S.; Polme, S.; Spirin, V.; Mikryukov, V.; Svantesson, S.; Hartmann, M.; et al. How, not if, is the question mycologists should be asking about DNA-based typification. Mycokeys 2023, 96, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017, 5, 17. [Google Scholar] [CrossRef]
- Lücking, R.; Hawksworth, D.L. Formal description of sequence-based voucherless Fungi: Promises and pitfalls, and how to resolve them. IMA Fungus 2018, 9, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Cheek, M.; Lughadha, E.N.; Kirk, P.; Lindon, H.; Carretero, J.; Looney, B.; Douglas, B.; Haelewaters, D.; Gaya, E.; Llewellyn, T.; et al. New scientific discoveries: Plants and fungi. Plants People Planet 2020, 2, 371–388. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rossman, A.Y. Where are all the undescribed fungi? Phytopathology 1997, 87, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D. Where are the missing fungi? Does Hong Kong have any answers? Mycol. Res. 2001, 105, 1514–1518. [Google Scholar] [CrossRef]
- Tang, A.M.C.; Shenoy, B.D.; Hyde, K.D. Fungal diversity. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Volume 72, pp. 221–249. [Google Scholar]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2017, 82, 1–105. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; McKenzie, E.H.; Phillips, A.J.; Jones, E.G.; Bhat, D.J.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Cheewangkoon, R.; Carnegie, A.J.; Burgess, T.I.; Summerell, B.A.; Edwards, J.; Taylor, P.W.J.; Groenewald, J.Z. Foliar pathogens of eucalypts. Stud. Mycol. 2019, 94, 125–298. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Hyde, K.D.; McKenzie, E.H.C.; Bhat, D.J.; Phillips, A.J.L.; Wanasinghe, D.N.; Samarakoon, M.C.; Jayawardena, R.S.; Dissanayake, A.J.; Tennakoon, D.S.; et al. Fungal Diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal Divers. 2018, 93, 1–160. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.B.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal Diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jeewon, R.; Chen, Y.J.; Bhunjun, C.S.; Calabon, M.S.; Jiang, H.B.; Lin, C.G.; Norphanphoun, C.; Sysouphanthong, P.; Pem, D.; et al. The numbers of fungi: Is the descriptive curve flattening? Fungal Divers. 2020, 103, 219–271. [Google Scholar] [CrossRef]
- Luo, Z.L.; Hyde, K.D.; Liu, J.K.; Maharachchikumbura, S.S.N.; Jeewon, R.; Bao, D.F.; Bhat, D.J.; Lin, C.G.; Li, W.L.; Yang, J.; et al. Freshwater Sordariomycetes. Fungal Divers. 2019, 99, 451–660. [Google Scholar] [CrossRef]
- Cannon, P.F.; Hawksworth, D.L. The diversity of fungi associated with vascular plants: The known, the unknown and the need to bridge the knowledge gap. In Advances in Plant Pathology; Andrews, J.H., Tommerup, I.C., Eds.; Academic Press: Cambridge, MA, USA, 1995; Volume 11, pp. 277–302. [Google Scholar]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, US National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 15 April 2022).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 15 April 2022).
- Hyde, K.D.; Tennakoon, D.S.; Jeewon, R.; Bhat, D.J.; Maharachchikumbura, S.S.N.; Rossi, W.; Leonardi, M.; Lee, H.B.; Mun, H.Y.; Houbraken, J.; et al. Fungal Diversity notes 1036–1150: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2019, 96, 1–242. [Google Scholar] [CrossRef]
- Hyde, K.D. Measuring biodiversity of microfungi in the wet tropics of North Queensland. In Proceedings of the Measuring and Monitoring Biodiversity of Tropical and Temperate Forests, Chiang Mai, Thailand, 27 August–2 September 1994; Boyle, T.J.B., Boontawee, B., Eds.; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 1995; pp. 271–286. [Google Scholar] [CrossRef][Green Version]
- Hyde, K.D. Biodiversity of microfungi in North Queensland. Aust. Syst. Bot. 1996, 9, 261–271. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Hyde, K.D. Host-specificity, host-exclusivity, and host-recurrence in saprobic fungi. Mycol. Res. 2001, 105, 1449–1457. [Google Scholar] [CrossRef]
- Shivas, R.G.; Hyde, K.D. Biodiversity of plant pathogenic fungi in the tropics. In Biodiversity of Tropical Microfungi; Hyde, K.D., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 47–56. [Google Scholar]
- Holliday, P. A Dictionary of Plant Pathology, 2nd ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Lucas, J.A. Plant Pathology and Plant Pathogens, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1998. [Google Scholar]
- Li, J.M.; Cornelissen, B.; Rep, M. Host-specificity factors in plant pathogenic fungi. Fungal Genet. Biol. 2020, 144, 11. [Google Scholar] [CrossRef]
- Hyde, K.D. Non-lichenised Australian ascomycetes. Aust. Syst. Bot. 2001, 14, 357–375. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; Ariyawansa, H.A.; Phillips, A.J.L.; Wanasinghe, D.N.; Bhat, D.J.; McKenzie, E.H.C.; Singtripop, C.; Camporesi, E.; Hyde, K.D. Additions o Sporormiaceae: Introducing two novel genera, Sparticola and Forliomyces, from Spartium. Cryptogam. Mycol. 2016, 37, 75–97. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.H.C.; Hyde, K.D.; Jeewon, R. A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Besitulo, A.; Sarma, V.V.; Hyde, K.D. Mangrove fungi from Siargao Island, Philippines. In Fungi in Marine Environments; Hyde, K.D., Ed.; Fungal Diversity Press: Hong Kong, China, 2002; pp. 267–283. [Google Scholar]
- Tomlinson, P.B. The Structural Biology of Palms; Oxford University Press: Clarendon, NY, USA, 1990. [Google Scholar]
- Pirozynski, K.A.; Weresub, L.K. A biogeographic view of the history of ascomycetes and the development of their pleomorphism. In The Whole Fungus: The Sexual and Asexual Synthesis, Alberta, Canada; Kendrick, B., Ed.; National Museum of Natural Sciences: Ottawa, ON, Canada, 1979; Volume I, pp. 93–123. [Google Scholar] [CrossRef]
- May, R.M. A fondness for fungi. Nature 1991, 352, 475–476. [Google Scholar] [CrossRef]
- Dowe, J. Extra-tropical palms: A statistical overview. Palm Enthus. 1992, 9, 4–8. [Google Scholar]
- Couvreur, T.L.P.; Forest, F.; Baker, W.J. Origin and global diversification patterns of tropical rain forests: Inferences from a complete genus-level phylogeny of palms. BMC Biol. 2011, 9, 12. [Google Scholar] [CrossRef]
- Dhillon, B.; Graham, F.; Laughinghouse, D.; Chakrabarti, S. Assessment of endophytic fungal community in palm leaves and their biocontrol potential. PhytoFront 2023. [Google Scholar] [CrossRef]
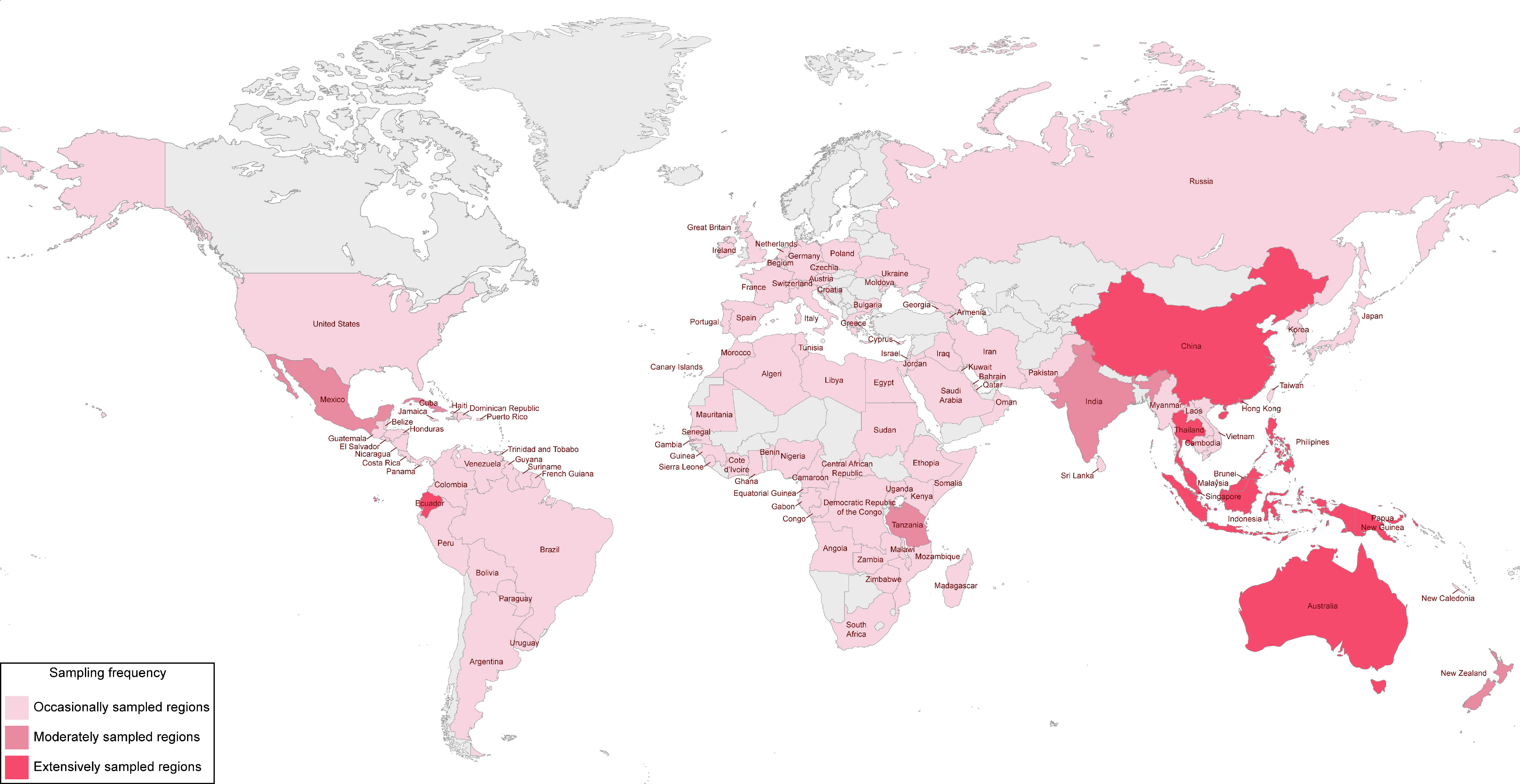
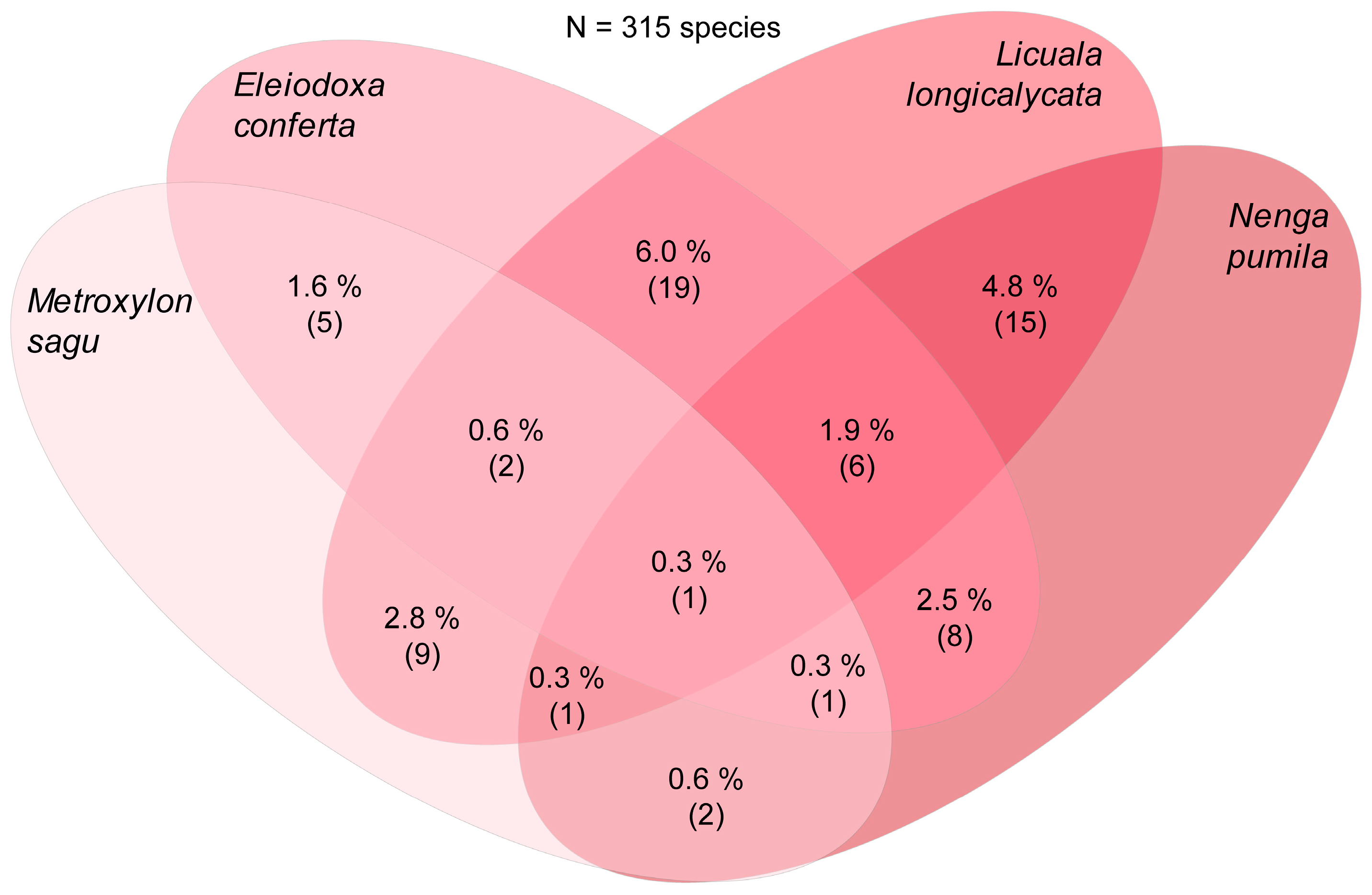
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.S.; Phillips, A.J.L. Palm Fungi and Their Key Role in Biodiversity Surveys: A Review. J. Fungi 2023, 9, 1121. https://doi.org/10.3390/jof9111121
Pereira DS, Phillips AJL. Palm Fungi and Their Key Role in Biodiversity Surveys: A Review. Journal of Fungi. 2023; 9(11):1121. https://doi.org/10.3390/jof9111121
Chicago/Turabian StylePereira, Diana S., and Alan J. L. Phillips. 2023. "Palm Fungi and Their Key Role in Biodiversity Surveys: A Review" Journal of Fungi 9, no. 11: 1121. https://doi.org/10.3390/jof9111121
APA StylePereira, D. S., & Phillips, A. J. L. (2023). Palm Fungi and Their Key Role in Biodiversity Surveys: A Review. Journal of Fungi, 9(11), 1121. https://doi.org/10.3390/jof9111121







