Characterization and In Vitro Fungicide Sensitivity of Two Fusarium spp. Associated with Stem Rot of Dragon Fruit in Guizhou, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Isolation, and Conservation
2.2. Koch’s Postulates
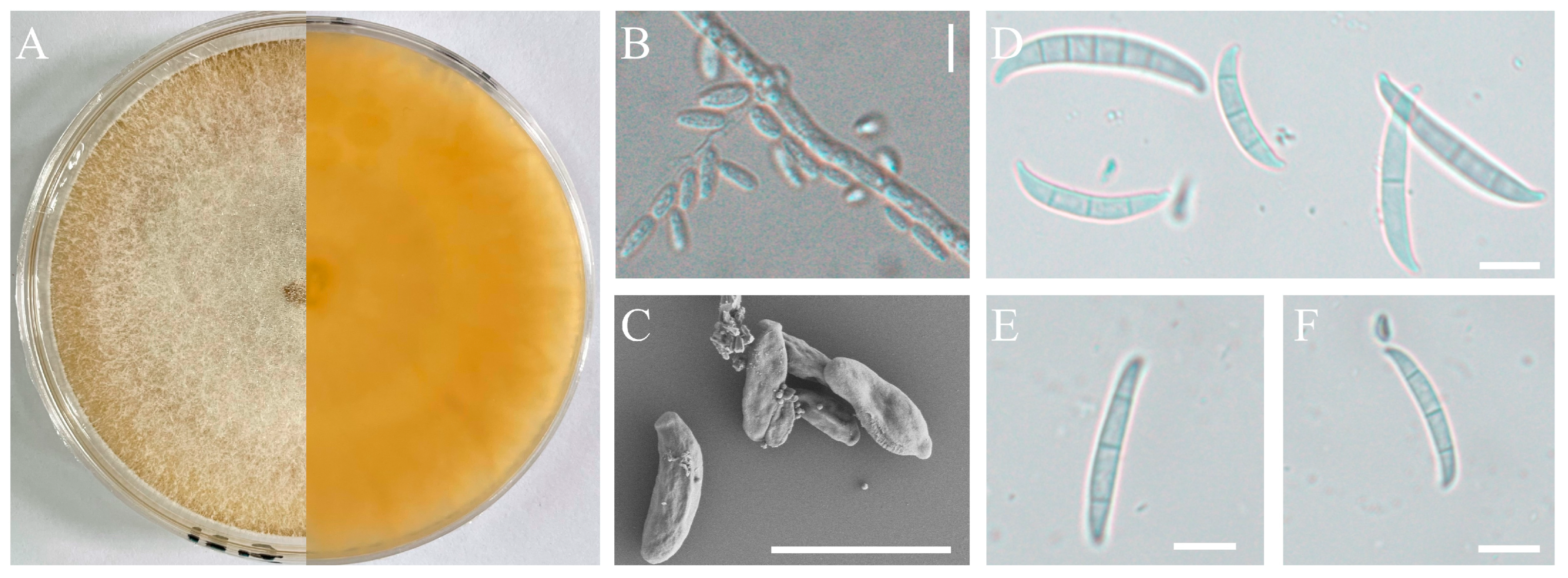
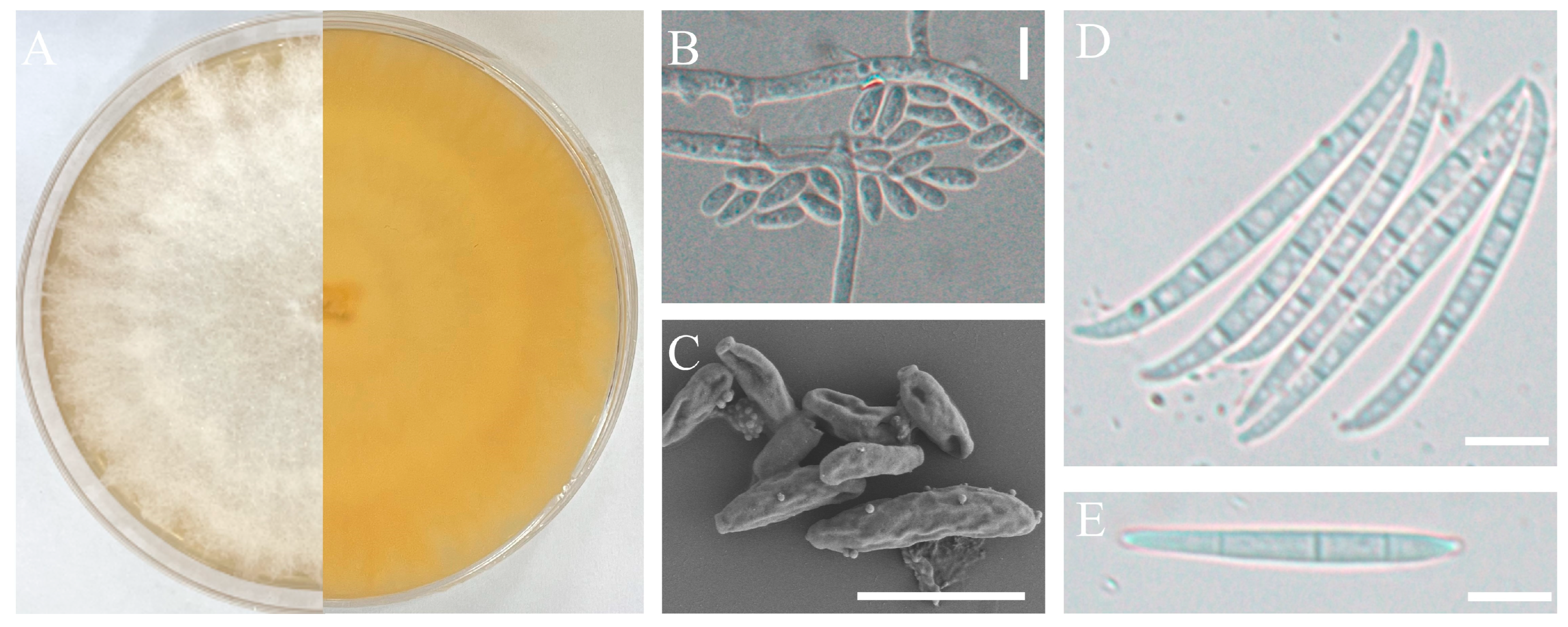
2.3. Morphological Studies
2.4. DNA Extraction, PCR Analysis, and Multi-Locus Phylogeny
2.5. Metabolic Profiling in the Biolog FF MicroPlate
2.6. Fungicide Sensitivity Testing
3. Results
3.1. Pathogenicity Test of the Isolated Strains
3.2. Isolate Identification
3.3. Fungicidal Efficacy on the Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.E.S. Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance—A review. J. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Satisha, G.C.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef] [PubMed]
- Liaotrakoon, W.; De Clercq, N.; Van Hoed, V.; Van de Walle, D.; Lewille, B.; Dewettinck, K. Impact of thermal treatment on physicochemical, antioxidative and rheological properties of white-flesh and red-flesh dragon fruit (Hylocereus spp.) purees. Food Bioprocess Technol. 2013, 6, 416–430. [Google Scholar] [CrossRef]
- Pan, L.; Fu, J.; Zhang, R.; Qin, Y.; Lu, F.; Jia, L.; Hu, Q.; Liu, C.; Huang, L.; Liang, G. Genetic diversity among germplasms of pitaya based on SSR markers. Sci. Hortic. 2017, 225, 171–176. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Jiang, L.; Yang, Q.; Luo, L.; Jiang, J.; Malichan, S.; Zhao, J.; Xie, X. Pitaya virus X coat protein acts as an RNA silencing suppressor and can be used as specific target for detection using RT-LAMP. Plant Dis. 2023, 107, 3378–3382. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Ma, L.; An, Y.; Wang, H.; Wu, W. Laboratory screening of control agents against isolated fungal pathogens causing postharvest diseases of pitaya in Guizhou, China. Front. Chem. 2022, 10, 942185. [Google Scholar] [CrossRef]
- Xu, M.; Liu, C.L.; Luo, J.; Qi, Z.; Yan, Z.; Fu, Y.; Wei, S.S.; Tang, H. Transcriptomic de novo analysis of pitaya (Hylocereus polyrhizus) canker disease caused by Neoscytalidium dimidiatum. BMC Genom. 2019, 20, 10. [Google Scholar] [CrossRef]
- Balendres, M.A.; Bengoa, J.C. Diseases of dragon fruit (Hylocereus species): Etiology and current management options. Crop Prot. 2019, 126, 104920. [Google Scholar] [CrossRef]
- Hong, C.F.; Gazis, R.; Crane, J.H.; Zhang, S. Prevalence and epidemics of Neoscytalidium stem and fruit canker on pitahaya (Hylocereus spp.) in South Florida. Plant Dis. 2020, 104, 1433–1438. [Google Scholar] [CrossRef]
- Mohd, M.H.; Salleh, B.; Zakaria, L. An overview of fungal diseases of pitaya in Malaysia. In Proceedings of the Improving Pitaya Production and Marketing Workshop, Kaohsiung, Taiwan, 13–15 September 2015; pp. 13–15. [Google Scholar]
- Zhang, R.Y.; Zhao, S.X.; Tan, Z.Q.; Zhu, C.H. First report of bacterial stem rot disease caused by Paenibacillus polymyxa on Hylocereus undulatus in China. Plant Dis. 2017, 101, 1031. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Kumar, S.; Meena, K.K.; Rane, J.; Pathak, H. Dragon fruit cultivation in India: Scope, constraints and policy issues. Tech. Bull. 2021, 27, 47. [Google Scholar]
- Rita, W.S.; Suprapta, D.N.; Sudana, I.M.; Swantara, I.M.D. First report on Fusarium solani, a pathogenic fungus causing stem rot disease on dragon fruits (Hylocereus sp.) in Bali. J. Biol. Agric. Healthc. 2013, 3, 93–99. [Google Scholar]
- Retana-Sánchez, K.; Castro-Zúñiga, O.; Blanco-Meneses, M.; Quesada-González, A. Etiology of stem rot on Hylocereus spp. cause by Enterobacter hormaechei in Costa Rica. Agron. Costarric. 2019, 43, 61–73. [Google Scholar] [CrossRef]
- Paugh, K.R.; Gordon, T.R. The Population of Fusarium oxysporum f. sp. lactucae in California and Arizona. Plant Dis. 2020, 104, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Peng, Y.; Qi, Z.; Yan, Z.; Yang, L.; He, M.D.; Li, Q.X.; Liu, C.L.; Ruan, Y.Z.; Wei, S.S.; et al. Identification of Neoscytalidium dimidiatum causing canker disease of pitaya in Hainan, China. Australas. Plant Pathol. 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Taguiam, J.D.; Evallo, E.; Bengoa, J.; Maghirang, R.; Balendres, M.A. Susceptibility of the three dragon fruit species to stem canker and growth inhibition of Neoscytalidium dimidiatum by chemicals. J. Plant Pathol. 2020, 102, 1077–1084. [Google Scholar] [CrossRef]
- Noegrohati, S.; Sulasmi, S.; Hernadi, E.; Asviastuti, S. Dissipation pattern of azoxystrobin and difenoconazole in red dragon fruit (Hylocereus polyrhizus) cultivated in Indonesian highland (West Java) and coastal area (DI Jogyakarta) and its implication for dietary risk assessment. Food Qual. Saf. 2019, 3, 99–106. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole based fungicides for fusarium head blight and deoxynivalenol control in wheat: A multivariate metaanalysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyner, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Priya, D.S.; Aiyanathan, K.E.A.; Harish, S. Efficacy of bacterial biocontrol agents and commercial fungicides against fusarium wilt of chrysanthemum. J. Pharmacogn. Phytochem. 2019, 8, 874–880. [Google Scholar]
- Baudy, P.; Konschak, M.; Sakpal, H.; Baschien, C.; Schulz, R.; Bundschuh, M.; Zubrod, J.P. The fungicide tebuconazole confounds concentrations of molecular biomarkers estimating fungal biomass. Bull. Environ. Contam. Toxicol. 2020, 105, 620–625. [Google Scholar] [CrossRef]
- Martínez-Matías, N.; Rodríguez-Medina, J.R. Fundamental concepts of azole compounds and triazole antifungals: A beginner’s review. Puerto Rico Health Sci. J. 2018, 37, 135–142. [Google Scholar]
- Amaro, A.C.E.; Baron, D.; Ono, E.O.; Rodrigues, J.D. Physiological effects of strobilurin and carboxamides on plants: An overview. Acta Physiol. Plant. 2020, 42, 4. [Google Scholar] [CrossRef]
- Wedge, D.E.; Elmer, W.H. Fusarium wilt of orchids. Icogo Bull 2008, 2, 9–10. [Google Scholar]
- Hudson, O.; Waliullah, S.; Ji, P.; Ali, M.E. Molecular characterization of laboratory mutants of Fusarium oxysporum f. sp. niveum resistant to prothioconazole, a demethylation inhibitor (DMI) fungicide. J. Fungi 2021, 7, 704. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hao, F.M.; Zhou, H.H.; Chen, J.B.; Su, H.C.; Yang, F.; Cai, Y.Y.; Li, G.L.; Zhang, M.; Zhou, F. Exploring potential mechanisms of fludioxonil resistance in Fusarium oxysporum f. sp. melonis. J. Fungi 2022, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Buyer, J.S.; Roberts, D.P.; Millner, P.; Russek-Cohen, E. Analysis of fungal communities by sole carbon source utilization profiles. J. Microbiol. Methods 2001, 45, 53–60. [Google Scholar] [CrossRef]
- Chai, X.; Yang, Z.; Fu, Q.; Pan, B.Z.; Tang, M.; Li, C.; Xu, Z.F. First report of root and basal stem rot in sacha inchi (Plukenetia volubilis) caused by Fusarium oxysporum in China. Plant Dis. 2018, 102, 242. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V. Effects of temperature and moisture on development of Fusarium graminearum perithecia in maize stalk residues. Appl. Environ. Microbiol. 2015, 82, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Trevine, V.C.; Grazziotin, F.G.; Giraudo, A.; Sallaberry-Pincheira, N.; Vianna, J.A.; Zaher, H. The systematics of Tachymenini (Serpentes, Dipsadidae): An updated classification based on molecular and morphological evidence. Zool. Scr. 2022, 51, 643–663. [Google Scholar] [CrossRef]
- Hafizi, R.; Salleh, B.; Latiffah, Z. Morphological and molecular characterization of Fusarium. solani and F. oxysporum associated with crown disease of oil palm. Braz. J. Microbiol. 2013, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Duan, C.X.; Li, S.C.; Tang, Z.L.; Luo, J.Y. First report of maize ear rot caused by Fusarium concentricum in China. Plant Dis. 2020, 104, 1539. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, W.; Xiao, M.; Xiao, T.; Zhao, Z.; Wang, H. Identification on pathogenic fungi of dragon fruit stem rot disease. Mol. Plant Breed. 2019, 17, 904–909. (In Chinese) [Google Scholar] [CrossRef]
- Caplin, I.; Unger, D.L. Molds on the Southern California deserts. Ann. Allergy 1983, 50, 260–263. [Google Scholar] [PubMed]
- Abdo, M.A.; Negm, S.E.; Hamada, M.S. Sensitivity of Fusarium oxysporum Isolates Collected from Strawberry Roots to DMI Fungicides Difenoconazole, Tebuconazole and Prochloraz. J. Plant Prot. Pathol. 2023, 14, 275–280. [Google Scholar] [CrossRef]
- Saravanakumari, K.; Thiruvudainambi, S.; Ebenezar, E.G.; Senthil, N. Efficacy of some fungicides and oil cake extracts against basal rot of onion caused by Fusarium oxysporum. Int. J. Farm Sci. 2019, 9, 93–96. [Google Scholar] [CrossRef]
- An, Y.N.; Murugesan, C.; Choi, H.; Kim, K.D.; Chun, S.C. Current Studies on Bakanae Disease in Rice: Host Range, Molecular Identification, and Disease Management. Mycobiology 2023, 51, 195–209. [Google Scholar] [CrossRef]
- An, T.J.; Kim, Y.G.; Hur, M.; Lee, J.H.; Lee, Y.J.; Cha, S.W.; Oh, S.k. Physicochemical Treatment for the Reduction of Fusarium spp. Infestedin Adlay (Coix lacryma-jobi L.) Seeds. Korean J. Med. Crop Sci. 2015, 23, 460–467. (In Korean) [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Hua, H.; Han, C.; Wu, X. Sensitivity of Rhizoctonia spp. to flutolanil and characterization of the point mutation in succinate dehydrogenase conferring fungicide resistance. Eur. J. Plant Pathol. 2019, 155, 13–23. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Falahati, R.M. Identification, virulence factors characterization, pathogenicity and aggressiveness analysis of Fusarium spp., causing wheat head blight in Iran. Eur. J. Plant Pathol. 2017, 147, 897–918. [Google Scholar] [CrossRef]


| Substrates | Isolates | Substrates | Isolates | Substrates | Isolates | |||
|---|---|---|---|---|---|---|---|---|
| H-4 | H-5 | H-4 | H-5 | H-4 | H-5 | |||
| Water | − * | − | a-Cyclodextrin | − | − | a-D-Glucose-1 -Phosphate | + | + |
| Tween 80 | +− | + | ß-Cyclodextrin | − | − | N-Acetyl-ß-D-Glucosamine | − | +− |
| Glycerol | +− | + | Glucuronamide | + | − | D-Glucuronic Acid | + | +− |
| Dextrin | − | +− | i-Erythritol | + | +− | N-Acetyl-D-Galactosamine | +− | +− |
| Glycogen | + | + | D-Fructose | − | +− | N-Acetyl-ß-D-Man nosamine | − | − |
| Adonitol | +− | +− | L-Fucose | + | +− | m-Inositol | +− | + |
| Amygdalin | +− | +− | D-Galactose | − | +− | 2-Keto-D-Gluconic Acid | + | + |
| D-Arabinose | − | +− | D-Galacturonic Acid | − | +− | a-D-Lactose | + | +− |
| L-Arabinose | − | +− | Gentiobiose | − | +− | Lactulose | − | − |
| D-Arabitol | − | +− | D-Gluconic Acid | + | +− | Maltitol | +− | − |
| Arbutin | +− | + | D-Glucosamine | − | +− | Maltose | − | +− |
| D-Cellobiose | − | + | a-D-Glucose | − | +− | Maltotriose | +− | +− |
| D-Mannitol | + | + | D-Ribose | + | +− | y-Aminobutyric Acid | + | + |
| D-Mannose | − | +− | Salicin | + | +− | Bromosuccinic Acid | + | + |
| D-Melezitose | +− | +− | Sedoheptulosan | + | +− | a-Methyl-D-Galactoside | + | − |
| D-Melibiose | + | +− | D-Sorbitol | + | +− | ß-Hydroxybutyric Acid | + | + |
| Fumaric Acid | + | + | L-Sorbose | + | +− | y- Hydroxybutyric Acid | + | + |
| L-Lactic Acid | + | + | Stachyose | + | +− | p-Hydroxy-phenylacetic Acid | + | + |
| D-Malic Acid | + | + | Sucrose | + | +− | a-Ketoglutaric Acid | + | + |
| L-Malic Acid | + | + | D-Tagatose | − | +− | D-Lactic Acid Methyl Ester | + | + |
| Quinic Acid | + | + | D-Trehalose | + | +− | ß-Methyl-D-Galactoside | + | + |
| D-Psicose | +− | + | Turanose | + | +− | a-Methyl-D-Glucoside | + | +− |
| D-Raffinose | + | + | Xylitol | + | +− | ß-Methyl-D-Glucoside | +− | +− |
| L-Rhamnose | + | + | D-Xylose | + | +− | Palatinose | − | +− |
| L-Proline | + | + | L-Alanine | +− | +− | L-Phenylalanine | + | + |
| Sebacic Acid | − | + | L-Alanyl-Glycine | + | +− | D-Saccharic Acid | + | + |
| Succinamic Acid | + | + | L-Asparagine | + | +− | L-Pyroglutamic Acid | + | + |
| Succinic Acid | + | + | L-Aspartic Acid | + | +− | Succinic Acid Mono-Methyl Ester | − | + |
| L-Serine | + | + | L-Glutamic Acid | + | +− | N-Acetyl-L-Glutamic Acid | +− | +− |
| L-Threonine | + | + | Gycyl-L-Glutamic Acid | + | +− | 2-Aminoethanol | + | + |
| Uridine | + | + | L-Ornithine | + | +− | Putrescine | + | + |
| Adenosine | + | + | L-Alaninamide | + | +− | Adenosine-5′-Monophosphate | + | − |
| Fungicides | Concentrations of Active Ingredient (μg/mL) | EC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | H-4 | H-5 | |
| Azoxystrobin 250 g/L SC | 1 | 2 | 4 | 6 | 8 | 10.65 | 2.82 |
| Difenoconazole 125 g/L·azoxystrobin 200 g/L (325 g/L SC) | 0.5 | 1 | 2 | 5 | 10 | 0.18 | 0.42 |
| Difenoconazole 150 g/L·propiconazole 150 g/L (300 g/L SC) | 0.2 | 0.5 | 1 | 2 | 4 | 0.38 | 1.06 |
| Fludioxonil 25 g/L SC | 1 | 2 | 4 | 6 | 8 | 1.15 | 0.96 |
| Difenoconazole 40% SC | 0.2 | 0.5 | 1 | 2 | 4 | 0.18 | 0.46 |
| Trifloxystrobin 25%·tebuconazole 50% (75 WG) | 0.2 | 0.5 | 1 | 2 | 4 | 0.13 | 0.14 |
| Carbendazim 50% WP | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 0.89 | 1.10 |
| Lime sulphur 45% WP | 0.05 | 0.1 | 0.5 | 1 | 2 | 5.29 | 3.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, M. Characterization and In Vitro Fungicide Sensitivity of Two Fusarium spp. Associated with Stem Rot of Dragon Fruit in Guizhou, China. J. Fungi 2023, 9, 1178. https://doi.org/10.3390/jof9121178
Zhao J, Huang M. Characterization and In Vitro Fungicide Sensitivity of Two Fusarium spp. Associated with Stem Rot of Dragon Fruit in Guizhou, China. Journal of Fungi. 2023; 9(12):1178. https://doi.org/10.3390/jof9121178
Chicago/Turabian StyleZhao, Jin, and Miao Huang. 2023. "Characterization and In Vitro Fungicide Sensitivity of Two Fusarium spp. Associated with Stem Rot of Dragon Fruit in Guizhou, China" Journal of Fungi 9, no. 12: 1178. https://doi.org/10.3390/jof9121178
APA StyleZhao, J., & Huang, M. (2023). Characterization and In Vitro Fungicide Sensitivity of Two Fusarium spp. Associated with Stem Rot of Dragon Fruit in Guizhou, China. Journal of Fungi, 9(12), 1178. https://doi.org/10.3390/jof9121178






