Effects of Arbuscular Mycorrhizal Fungi on the Growth and Root Cell Ultrastructure of Eucalyptus grandis under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and AMF Colonization
2.2. Experiment Design
2.3. Growth Indicators Determination
2.3.1. Biomass
2.3.2. Mycorrhizal Colonization
2.3.3. Chlorophyll Fluorescence Parameters
2.3.4. Cd Concentration Analysis
2.3.5. Cd Localization in Roots of E. grandis
2.4. Statistical Analysis
3. Results
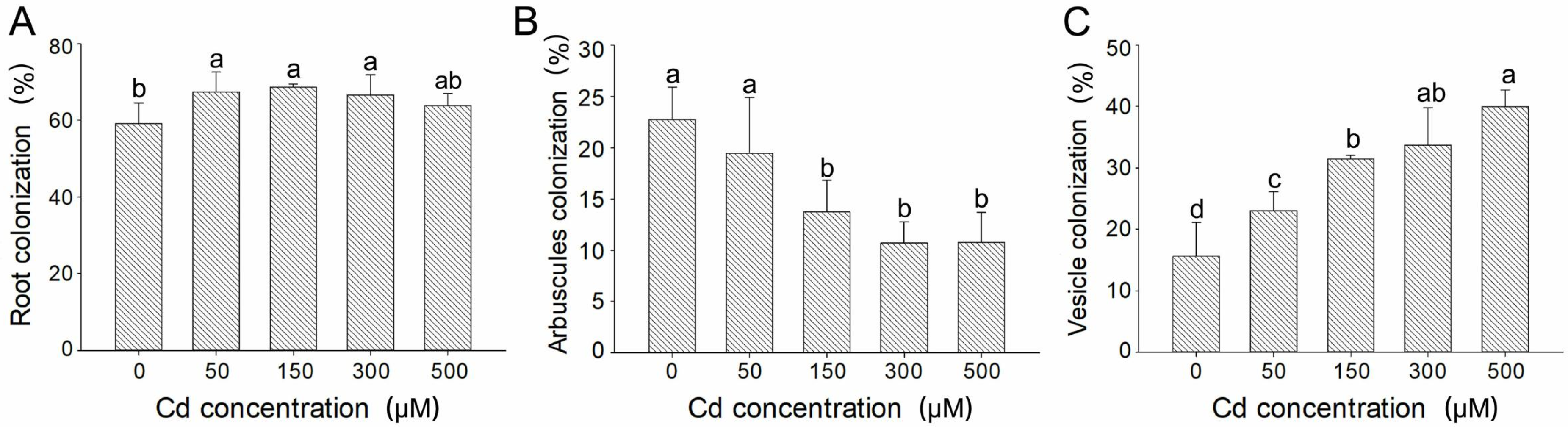
3.1. Effects of Different Cd Concentrations on AMF Colonization
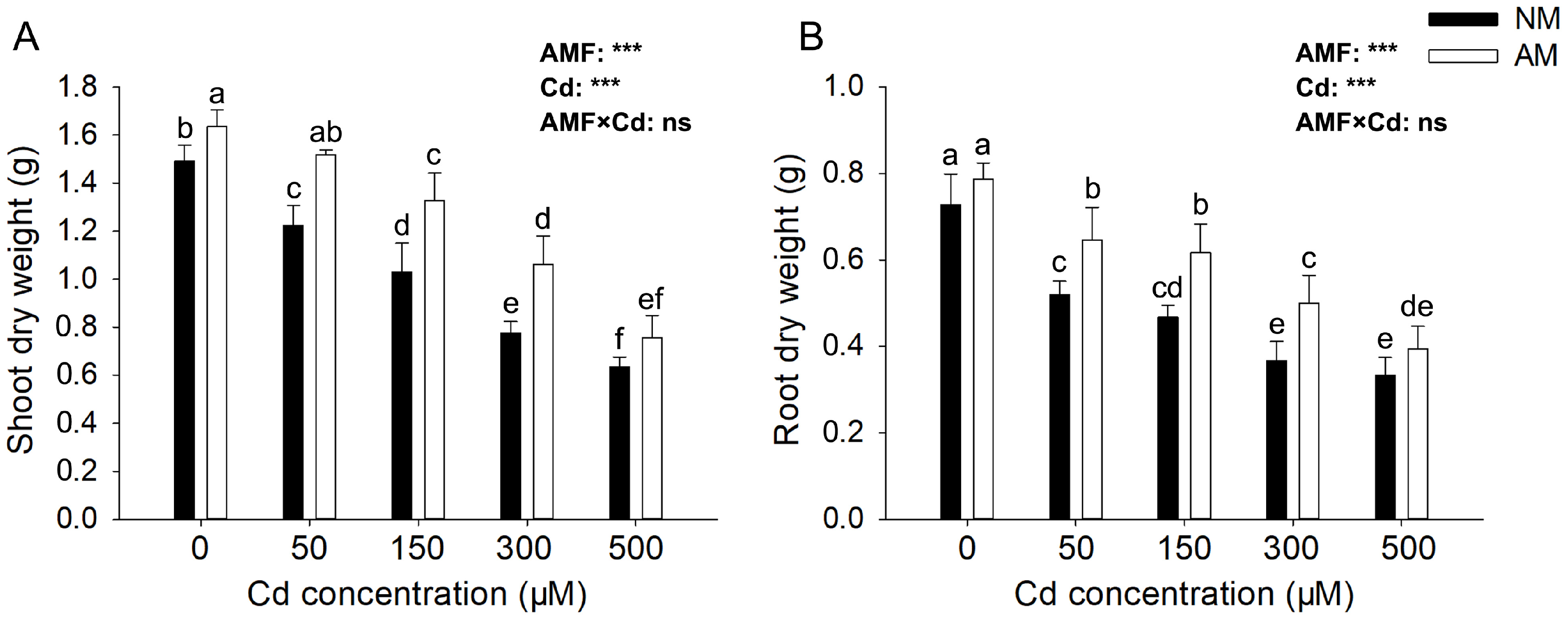
3.2. Effects of AMF on E. grandis Growth Parameters under Different Cd Concentrations
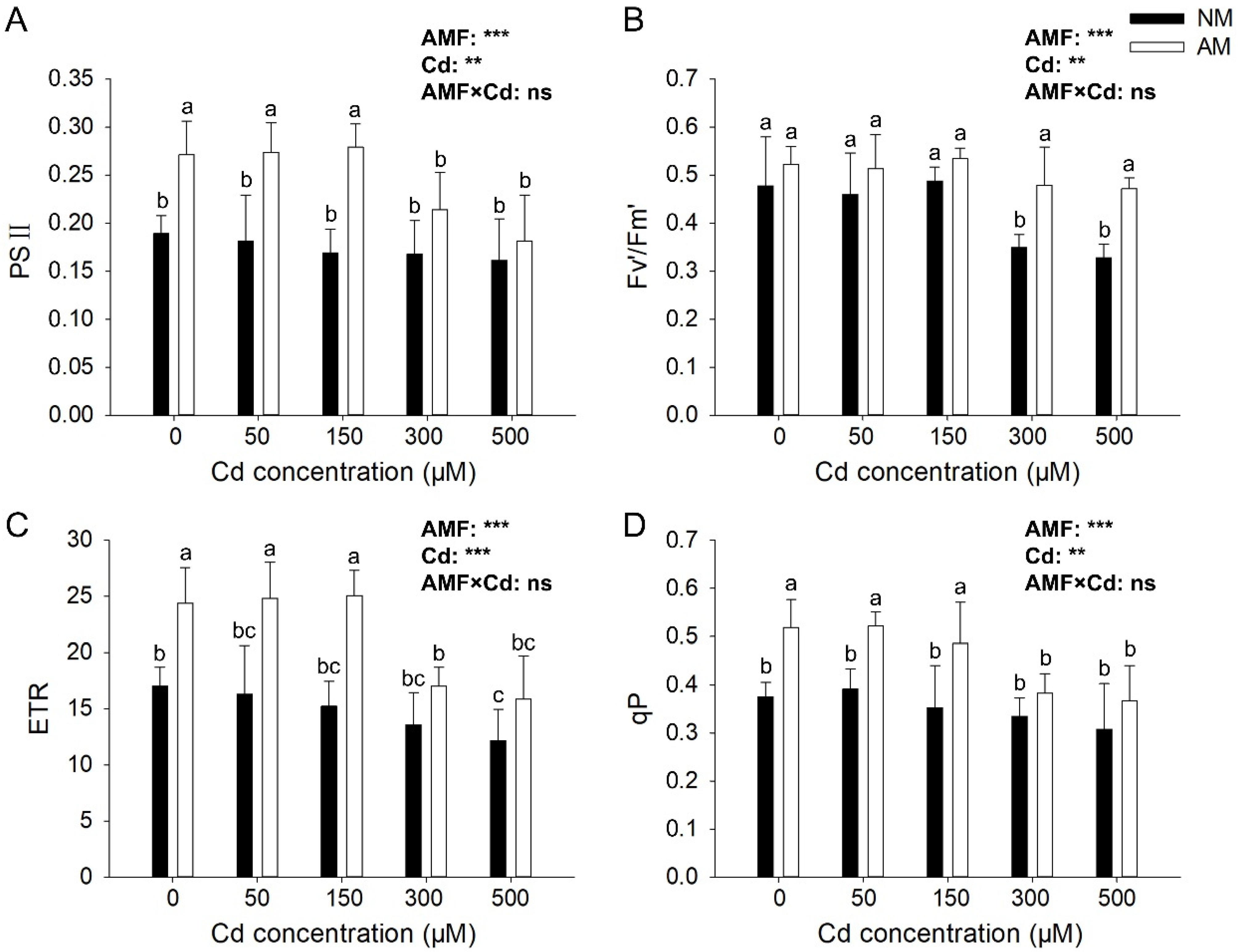
3.3. Effects of AMF on E. grandis Photosynthesis Parameters under Different Cd Concentrations
3.4. Effects of AMF on the Cd Absorption of E. grandis
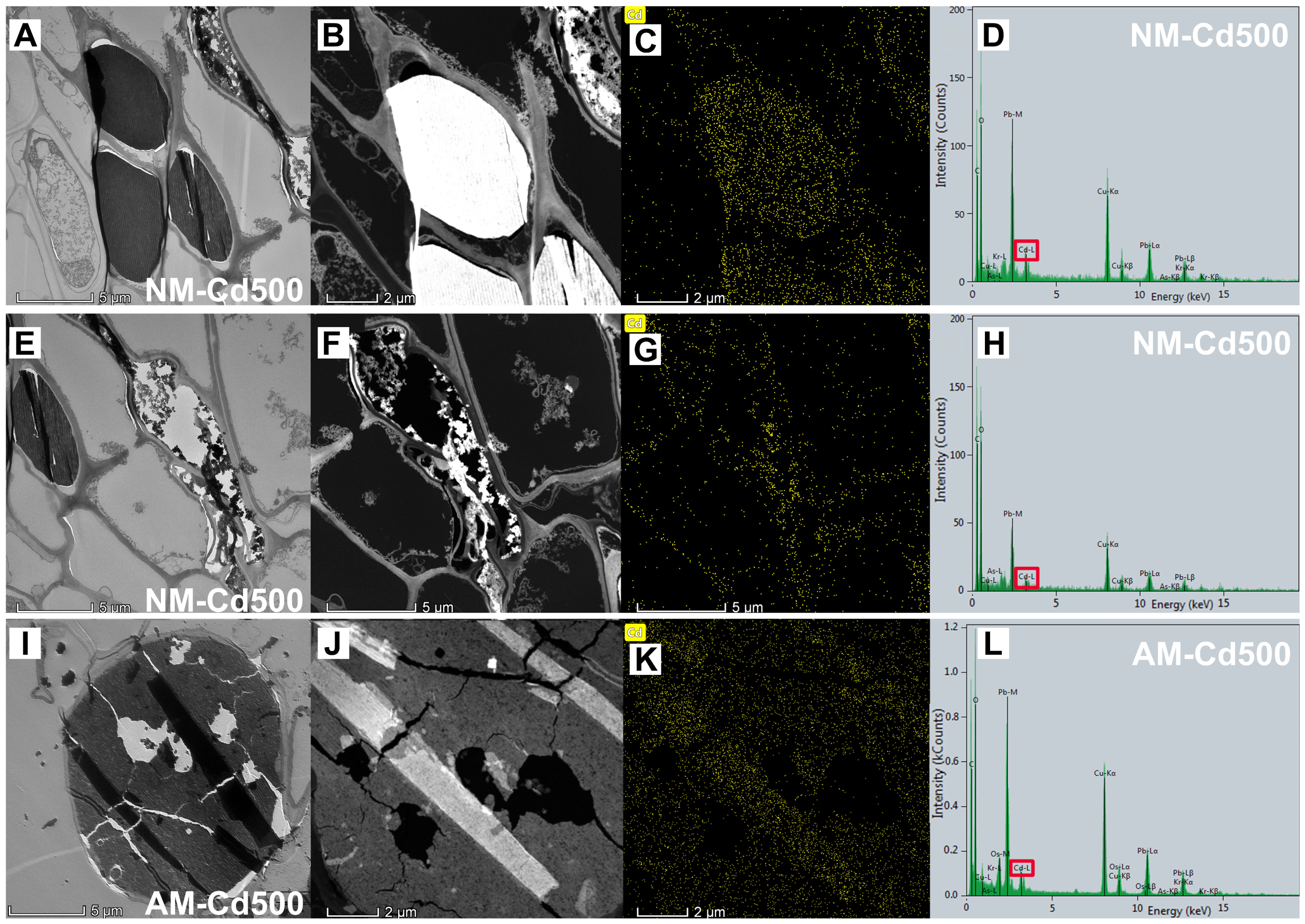
3.5. Effects of AMF and Cd Stress on E. grandis Root Cell Ultrastructure
4. Discussion
4.1. AMF Improved the Growth and Photosynthetic Efficiency of E. grandis under Cd Stress
4.2. AMF Mobilizes the Distribution and Transportation of Cd in E. grandis
4.3. Damage of Cell Ultrastructure in E. grandis Root by Cd and Positive Effect of AMF Colonization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, M.M.; Zhou, S.L.; Zhou, Y.J.; Jia, Z.Y.; Guo, T.W.; Wang, J.X. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Huang, R.; Dong, M.L.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.X.; Li, Y.W.; Hu, X.Y.; Netherway, P.; Li, Z.A. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Fernández, R.; Bertrand, A.; Casares, A.; García, R.; González, A.; Tamés, R.S. Cadmium accumulation and its effect on the in vitro growth of woody fleabane and mycorrhized white birch. Environ. Pollut. 2008, 152, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chen, J.; Shim, H.; Bai, Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ. Int. 2007, 33, 406–413. [Google Scholar] [CrossRef]
- Whitehead, D.; Beadle, C.L. Physiological regulation of productivity and water use in Eucalyptus: A review. For. Ecol. Manag. 2004, 193, 113–140. [Google Scholar] [CrossRef]
- Arriagada, C.A.; Herrera, M.A.; Ocampo, J.A. Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. J. Environ. Manag. 2007, 84, 93–99. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhao, X.Y.; Zhang, J.M.; Lu, C.; Feng, F.J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef]
- King, D.J.; Doronila, A.I.; Feenstra, C.; Baker, A.J.M.; Woodrow, I.E. Phytostabilisation of arsenical gold mine tailings using four Eucalyptus species: Growth, arsenic uptake and availability after five years. Sci. Total Environ. 2008, 40, 35–42. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, N.; Li, X.; Long, J.; Sui, X.; Wu, Y.; Li, J.; Wang, J.; Zhong, H.; Sun, G.Y. Arbuscular mycorrhizal fungi (Glomus mosseae) improve growth, photosynthesis and protects photosystem II in leaves of Lolium perenne L. in cadmium contaminated soil. Front. Plant Sci. 2018, 9, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in Populus trichocarpa. Environ. Exp. Bot. 2019, 171, 103925. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M.S. Assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal(loid) contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 318–326. [Google Scholar] [CrossRef]
- Nafady, N.A.; Elgharably, A. Mycorrhizal symbiosis and phosphorus fertilization effects on Zea mays growth and heavy metals uptake. Int. J. Phytorem. 2018, 20, 869–875. [Google Scholar] [CrossRef]
- Babadi, M.; Zalaghi, R.; Taghavi, M. A non-toxic polymer enhances sorghum-mycorrhiza symbiosis for bioremediation of Cd. Mycorrhiza 2019, 29, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Liang, J.W.; Liu, Z.Y.; Kuang, Y.X.; Han, L.N.; Chen, H.; Xie, X.A.; Hu, W.T.; Tang, M. Transcriptional regulation of metal metabolism and nutrient absorption-related genes in Eucalyptus grandis by arbuscular mycorrhizal fungi at different zinc concentrations. BMC Plant Biol. 2022, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Turnau, K.; Kottke, I.; Oberwinkler, F. Element localization in mycorrhizal roots of Pteridium aquilinum L. Kuhn collected from experimental plots treated with cadmium dust. New Phytol. 1993, 123, 313–324. [Google Scholar] [CrossRef]
- Jerusa, S.; Claudia, R.G.L.; Wesley, M.R.; Eduardo, A.; Luiz, R.G.G. Anatomy and ultrastructure alterations of Leucaena leucocephala (Lam.) inoculated with mycorrhizal fungi in response to arsenic-contaminated soil. J. Hazard. Mater. 2013, 262, 1245–1248. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhang, X.; Sun, T.Q.; Wu, Z.X.; Li, T.; Hu, T.J.; Su, D.; Lv, J.T.; Li, G.; Zhang, Z.S.; et al. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Ban, Y.H.; Yang, R.; Zhang, X.Y.; Chen, H.; Tang, M. Impact of Funneliformis mosseae on the growth, lead uptake, and localization of Sophora viciifolia. Can. J. Microbiol. 2016, 62, 361–373. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhu, S.; Ho, S.H.; Wu, J.; Kalita, P.K.; Ma, F. Unraveling the effects of arbuscular mycorrhizal fungus on uptake, translocation, and distribution of cadmium in Phragmites australis (Cav.) Trin. ex Steud. Ecotoxicol. Environ. Saf. 2018, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.D.; Keiichiro, N.; Yukari, K.; Zhang, X.; Wu, S.L.; Ryo, O. Uptake and intraradical immobilization of cadmium by arbuscular mycorrhizal fungi as revealed by a stable isotope tracer and synchrotron radiation μX-Ray fluorescence analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Hu, W.T.; Pan, L.; Chen, H.; Tang, M. VBA-AMF: A VBA program based on the magnified intersections method for quantitative recording of root colonization by arbuscular mycorrhizal fungi. Indian J. Microbiol. 2020, 60, 374–378. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable cholrophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Hoffmann, T.; Kutter, C.; Santamaria, J. Capacity of Salvinia minima Baker to tolerate and accumulate As and Pb. Eng. Life Sci. 2004, 4, 61–65. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Q.X. Distribution of forms for cadmium, lead, copper and zinc in soil land its influences by modifier. Agric. Ecosyst. Environ. 2003, 22, 541–545. [Google Scholar]
- Huang, X.; Ho, S.H.; Zhu, S.; Ma, F.; Wu, J.; Yang, J.; Wang, L. Adaptive response of arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. J. Environ. Manag. 2017, 197, 448–455. [Google Scholar] [CrossRef]
- Khan, A.R.; Waqas, M.; Ullah, I.; Khan, A.L.; Khan, M.A.; Lee, I.J.; Shin, J.H. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ. Exp. Bot. 2017, 135, 126–135. [Google Scholar] [CrossRef]
- Han, Y.; Zveushe, O.K.; Dong, F.; Ling, Q.; Chen, Y.; Sajid, S.; Zhou, L.; Dios, V.R.D. Unraveling the effects of arbuscular mycorrhizal fungi on cadmium uptake and detoxification mechanisms in perennial ryegrass (Lolium perenne). Sci. Total Environ. 2021, 798, 149222. [Google Scholar] [CrossRef]
- Fan, X.X.; Wei, C.; Feng, F.J.; Song, F.Q. Responses of photosynthesis-related parameters and chloroplast ultrastructure to atrazine in alfalfa (Medicago sativa L.) inoculated with arbuscular mycorrhizal fungi. Ecotoxicol. Environ. Saf. 2018, 166, 102–108. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Effects of AM colonization on wild tobacco plants grown in zinc-contaminated soil. Mycorrhiza 2006, 4, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Dietterich, L.; Gonneau, C.; Casper, B. Arbuscular mycorrhizal colonization has little consequence for plant heavy metal uptake in contaminated field soils. Ecol. Appl. 2017, 27, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, H.Q.; Jin, X.X.; Huang, H.C.; Zhou, L.X.; Xu, T.Y.; Tang, M. Pb transfer preference of arbuscular mycorrhizal fungus Rhizophagus irregularis in Morus alba under different light intensities. J. Fungi 2022, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Q.; Wang, L.; Ju, C.; Wang, G.; Ma, F.; Wang, Y.J.; Yang, D.G. Effects of arbuscular mycorrhizal fungi on the growth and toxic element uptake of Phragmites australis (Cav.) Trin. ex Steud under zinc/cadmium stress. Ecotoxicol. Environ. Saf. 2021, 213, 112023. [Google Scholar] [CrossRef]
- Yu, Z.H.; Zhao, X.L.; Liang, X.R.; Li, Z.R.; Wang, L.; He, Y.M.; Zhan, F.D. Arbuscular mycorrhizal fungi reduce cadmium leaching from sand columns by reducing availability and enhancing uptake by maize roots. J. Fungi 2022, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Mori, S.; Baba, K.; Kaburagi-Yada, S.; Arao, T.; Kitajima, N.; Hokura, A.; Terada, Y. Cadmium distribution in the root tissues of solanaceous plants with contrasting root-to-shoot Cd translocation efficiencies. Environ. Exp. Bot. 2011, 71, 198–206. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Xia, H. Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of bana grass and vetiver grass. Ecotoxicol. Environ. Saf. 2014, 106, 102. [Google Scholar] [CrossRef] [PubMed]
- Rask, K.A.; Johansen, J.L.; Kjoller, R.; Ekelund, F. Differences in arbuscular mycorrhizal colonisation influence cadmium uptake in plants. Environ. Exp. Bot. 2019, 162, 223–229. [Google Scholar] [CrossRef]
- Qiao, Y.H.; Crowley, D.; Wang, K.; Zhang, H.Q.; Li, H.F. Effects of biochar and arbuscular mycorrhizae on bioavailability of potentially toxic elements in an aged contaminated soil. Environ. Pollut. 2015, 206, 636–643. [Google Scholar] [CrossRef]
- Citterio, S.; Prato, N.; Fumagalli, P.; Aina, R.; Massa, N.; Santagostino, A.; Sgorbati, S.; Berta, G. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 2005, 59, 21–29. [Google Scholar] [CrossRef]
- Cui, G.; Ai, S.; Chen, K.; Wang, X. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Suski, S.; Fiedor, E.; Gregoraszczuk, E.; Kuta, E. Suspended cells of metallicolous and nonmetallicolous Viola species tolerate, accumulate and detoxify zinc and lead. Plant Physiol. Bioch. 2018, 132, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.Y.; Ali, B.; Stoffella, P.J.; Cui, X.; Yang, X.; Guo, Y. Study amino acid contents, plant growth variables and cell ultrastructural changes induced by cadmium stress between two contrasting cadmium accumulating cultivars of Brassica rapa ssp. chinensis L. (Pak Choi). Ecotoxicol. Environ. Saf. 2020, 200, 110748. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, K.; Źróbek-Sokolnik, A.; Okorski, A.; Najdzion, J. The effect of cadmium on the activity of stress-related enzymes and the ultrastructure of pea roots. Plants 2019, 8, 413. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.J.; Qiu, R.L.; Senthilkumar, P.; Jiang, D.; Chen, Z.W.; Tang, Y.T.; Liu, F.J. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ. Exp. Bot. 2009, 66, 317–325. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Li, X.; Wang, Z.; Wang, X.; Wei, H.; Chen, H.; Hu, W.; Tang, M. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Root Cell Ultrastructure of Eucalyptus grandis under Cadmium Stress. J. Fungi 2023, 9, 140. https://doi.org/10.3390/jof9020140
Kuang Y, Li X, Wang Z, Wang X, Wei H, Chen H, Hu W, Tang M. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Root Cell Ultrastructure of Eucalyptus grandis under Cadmium Stress. Journal of Fungi. 2023; 9(2):140. https://doi.org/10.3390/jof9020140
Chicago/Turabian StyleKuang, Yuxuan, Xue Li, Zhihao Wang, Xinyang Wang, Hongjian Wei, Hui Chen, Wentao Hu, and Ming Tang. 2023. "Effects of Arbuscular Mycorrhizal Fungi on the Growth and Root Cell Ultrastructure of Eucalyptus grandis under Cadmium Stress" Journal of Fungi 9, no. 2: 140. https://doi.org/10.3390/jof9020140





