Plant Growth Promoting Bacteria and Arbuscular Mycorrhizae Improve the Growth of Persea americana var. Zutano under Salt Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of PGPR Resistant to High Osmotic Concentrations
2.2. Description of the Substrate Used for the Tests
2.3. Preparation of Avocado Seeds for the Installation of the Experiment
2.4. Selection, Propagation, and Inoculation of Arbuscular Mycorrhizae
2.5. Inoculation of the Seedlings with the Bacterial Strains
2.6. Greenhouse Environmental Conditions
2.7. Effect of Plant Growth Promoting Rhizobacteria on Salt Stress Tolerance in Persea americana var. Zutano
2.8. Effect of Inoculation with Glomeromycota fungi on Tolerance to Salt Stress in Persea americana var. Zutano
2.9. Evaluated Parameters
2.10. Statistical Analysis
3. Results
3.1. Selection of Plant Growth Promoting Rhizobacteria Tolerant to High Salinity Concentrations
3.2. Effect of Plant Growth Promoting Rhizobacteria on Salt Stress Tolerance in Persea americana var. Zutano
3.3. Effect of Plant Growth Promoting Rhizobacteria on the Accumulation of Ions Associated with Salinity in Persea americana var. Zutano
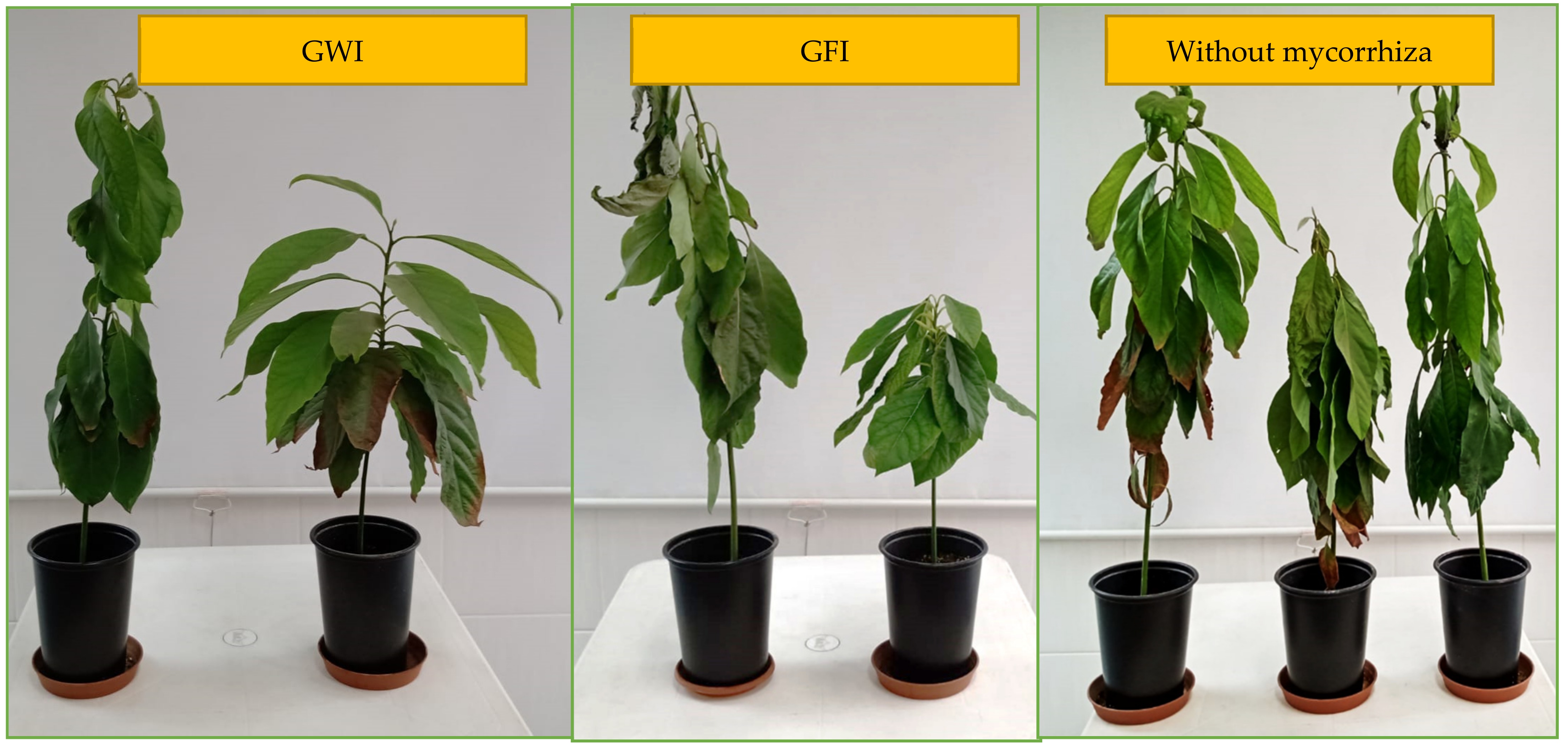
3.4. Effect of Inoculation with Glomeromycota fungi (GFI and GWI) on Tolerance to Salt Stress in Persea americana var. Zutano
4. Discussion
4.1. Effect of Plant Growth Promoting Rhizobacteria on Salt Stress Tolerance in Persea americana var. Zutano
4.2. Effect of Plant Growth Promoting Rhizobacteria on the Accumulation of Ions Associated with Salinity in Persea americana var. zutano
4.3. Effect of Inoculation with Glomeromycota fungi (GFI and GWI) on Tolerance to Salt Stress in Persea americana var. Zutano
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-Producing Plant Growth-Promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Bui, E.N. Soil Salinity: A Neglected Factor in Plant Ecology and Biogeography. J. Arid. Environ. 2013, 92, 14–25. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Safe 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil Salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Pompeiano, A.; Giannini, V.; Gaetani, M.; Vita, F.; Guglielminetti, L.; Bonari, E.; Volterrani, M. Response of warm–season grasses to n fertilization and salinity. Sci. Hortic. 2014, 177, 92–98. [Google Scholar] [CrossRef]
- Bernstein, N.; Meiri, A.; Zilberstaine, M. Root growth of avocado is more sensitive to salinity than shoot growth. J. Am. Soc. Hortic. Sci. 2004, 129, 188–192. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Melser, S.; Lu Arpaia, M. Salinity-induced changes in ion concentrations of ‘Hass’ avocado trees on three rootstocks. J. Plant Nutr. 2007, 30, 105–122. [Google Scholar] [CrossRef]
- Schaffer, B.; Gil, P.M.; Mickelbart, M.V.; Whiley, A.W. Ecophysiology. In The Avocado: Botany, Production and Uses, 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CAB International Press: Wallingford, UK, 2013. [Google Scholar]
- Crowley, D.; Smith, W.; Arpaia, M.L. Rootstock Selections for Improved Salinity Tolerance of Avocado. In Proceedings of the Avocado Brainstorming, Session 4, Salinity Management, Riverside, CA, USA, 27–28 October 1999; pp. 78–80. [Google Scholar]
- Vittery, L.R.; Colchao, M.V. Posicionamiento de la palta Hass peruana en el mercado estadounidense. Cienc. Neg. 2019, 1, 41–52. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Riego del Perú. La Situación del Mercado Internacional de la Palta: Su Análisis Desde una Perspectiva de las Exportaciones Peruanas; Ministerio de Agricultura y Riego del Perú: Lima, Perú, 2019; p. 41. Available online: http://minagri.gob.pe/portal/analisis–economico/analisis-2019?download=14480:la-situacion-del-mercado-internacional-de-la-palta&start=20 (accessed on 1 January 2022).
- Soca, R.; Rojas, J.; Willems, B.L.; Ocola, L.C.; Fernández, R.; Pérez, J.C. Identificación de las tierras degradadas por la salinidad del suelo en los cultivos de caña de azúcar mediante imágenes de satélite. Rev. Investig. Física 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Instituto Nacional de Desarrollo (INADE). Investigación Sobre el Problema de Salinidad en los Suelos en la Costa Peruana. 2010. Available online: http://apiperu.com.pe/Presentaciones/salina1.pdf (accessed on 3 January 2022).
- Zuñiga, L.D.L. Transformation of the Hyper-Arid Desert Soils in Arequipa Peru during Four Decades of Irrigated Agriculture. Ph.D. Thesis, Purdue University Graduate School, West Lafayette, IN, USA, 2020. [Google Scholar]
- Soto-Cordova, M.M.; Santa-Cruz-Arones, L. Early detection of saline soils by using satellite images in the peruvian province of Huaura. In Proceedings of the 2019 Congreso Internacional de Innovación y Tendencias en Ingenieria (CONIITI), Bogota, Colombia, 2–4 October 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Odoh, C.; Sam, K.; Zabbey, N.; Eze, C.; Nwankwegu, A.; Laku, C.; Dumpe, B. Microbial Consortium as Biofertilizers for Crops Growing under the Extreme Habitats. In Plant Microbiomes for Sustainable Agriculture; Springer: Cham, Switzerland, 2020; pp. 381–424. ISBN 978-3-030-38452-4. [Google Scholar]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (Wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum Subsp. Durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Ferreira, N.C.; Mazzuchelli, R.d.C.L.; Pacheco, A.C.; Araujo, F.F.d.; Antunes, J.E.L.; Araujo, A.S.F.d. Bacillus subtilis improves maize tolerance to salinity. Cienc. Rural 2018, 48, e20170910. [Google Scholar] [CrossRef]
- Costa-Gutierrez, S.B.; Lami, M.J.; Caram-Di Santo, M.C.; Zenoff, A.M.; Vincent, P.A.; Molina-Henares, M.A.; de Cristóbal, R.E. Plant growth promotion by Pseudomonas putida KT2440 under saline stress: Role of eptA. Appl. Microbiol. Biotechnol. 2020, 104, 4577–4592. [Google Scholar] [CrossRef]
- Chu, T.N.; Tran, B.T.H.; Van Bui, L.; Hoang, M.T.T. Plant Growth-Promoting Rhizobacterium Pseudomonas PS01 Induces Salt Tolerance in Arabidopsis thaliana. BMC Res. Notes 2019, 12, 11. [Google Scholar] [CrossRef]
- Miransari, M. Chapter 15—Arbuscular Mycorrhizal Fungi and Soil Salinity. In Mycorrhizal Mediation of Soil; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–277. ISBN 978-0-12-804312-7. [Google Scholar]
- Scharnagl, K.; Sanchez, V.; von Wettberg, E. The impact of salinity on mycorrhizal colonization of a rare legume, Galactia smallii, in South Florida Pine Rocklands. BMC Res. Notes 2018, 11, 2. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of Two Arbuscular Mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Cardona, W.A.; Joan Sebastián Gutiérrez, D.; Oscar Iván Monsalve, C.; Carmen Rosa Bonilla, C. Efecto de la salinidad sobre el crecimiento vegetativo de plantas de mora de Castilla (Rubus glaucus Benth.) micorrizadas y sin micorrizar. Rev. Colomb. Cienc. Hortícolas 2017, 11, 253–266. [Google Scholar] [CrossRef]
- Da Silva, K.A.V. Avaliação do Desenvolvimento da Salicornia ramosissima Submetida à Irrigação com Água Salina e à Presença de Micorriza Arbuscular. Master’s Dissertation, Universidade Católica de Pernambuco, Boa Vista, Brazil, 2019. [Google Scholar]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Altuntas, O.; Kutsal, I. Use of Some Bacteria and Mycorrhizae as Biofertilizers in Vegetable Growing and Beneficial Effects in Salinity and Drought Stress Conditions. In Physical Methods for Stimulation of Plant and Mushroom Development; El-Esawi, M.A., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-748-1. [Google Scholar]
- Moreira, H.; Pereira, S.I.A.; Vega, A.; Castro, P.M.L.; Marques, A.P.G.C. Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J. Environ. Manag. 2020, 257, 109982. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Hidri, R.; Mahmoud, O.M.-B.; Farhat, N.; Cordero, I.; Pueyo, J.J.; Debez, A.; Barea, J.-M.; Abdelly, C.; Azcon, R. Arbuscular Mycorrhizal fungus and rhizobacteria affect the physiology and performance of Sulla coronaria plants subjected to salt stress by mitigation of ionic imbalance. J. Plant Nutr. Soil Sci. 2019, 182, 451–462. [Google Scholar] [CrossRef]
- Lara, C.; Esquivel, L.; Negrete, J. Bacterias nativas solubilizadoras de fosfatos para incrementar los cultivos en el departamento de Córdova, Colombia. Rev. Biotecnol. Agropecu. Agroind. 2011, 9, 114–120. [Google Scholar]
- Patel, P.; Shaikh, S.; Sayyed, R. Modified chrome azurol s method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Castañeda, W.; Toro, M.; Solorzano, A.; Zúñiga-Dávila, D. Production and Nutritional Quality of Tomatoes (Solanum lycopersicum var. Cerasiforme) are Improved in the Presence of Biochar and Inoculation with Arbuscular Mycorrhizae. Am. J. Plant Sci. 2020, 11, 426–436. [Google Scholar] [CrossRef]
- Hepper, C.M.; Sen, R.; Maskall, C.S. Identification of vesicular-arbuscular mycorrhizal fungi in roots of leek (Allium porrum L.) and maize (Zea mays L.) on the basis of enzyme mobility during polyacrylamide gel electrophoresis. New Phytol. 1986, 102, 529–539. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Marques, G.; Tampakaki, A.; Alsina, I. Working with Microbial Symbioses of Legumes: Handbook of Protocols; Proyecto de Investigación FP7 nº 613781; Eurolegume: Vila Real, Protugal, 2014. [Google Scholar]
- Rao, N.K.; Laxman, R.H.; Shivashankara, K.S. Physiological and morphological responses of horticultural crops to abiotic stresses. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 3–17. [Google Scholar]
- Kourgialas, N.N.; Dokou, Z. Water management and salinity adaptation approaches of avocado trees: A review for hot-summer mediterranean climate. Agric. Water Manag. 2021, 252, 106923. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Saleem, M.; Arshad, M.; Hussain, S.; Bhatti, A.S. Perspective of Plant Growth Promoting Rhizobacteria (PGPR) containing ACC Deaminase in stress agriculture. J. Ind Microbiol. Biotechnol. 2007, 34, 635–648. [Google Scholar] [CrossRef]
- Ghosh, P.K.; De, T.K.; Maiti, T.K. Role of ACC Deaminase as a Stress Ameliorating Enzyme of Plant Growth-Promoting Rhizobacteria Useful in Stress Agriculture: A Review—University of Calcutta. Available online: https://research.caluniv.ac.in/publication/role-of-acc-deaminase-as-a-stress-ameliorating-enzyme-of-plant (accessed on 21 December 2022).
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-Acetic Acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Fazal, A.; Bano, A. Role of Plant Growth-Promoting Rhizobacteria (PGPR), biochar, and chemical fertilizer under salinity stress. Commun. Soil Sci. Plant Anal. 2016, 47, 1985–1993. [Google Scholar] [CrossRef]
- Singh, S.; Singh, U.B.; Trivedi, M.; Sahu, P.K.; Paul, S.; Paul, D.; Saxena, A.K. Seed biopriming with salt-tolerant endophytic Pseudomonas geniculata-modulated biochemical responses provide ecological fitness in maize (Zea mays L.) Grown in Saline Sodic Soil. Int. J. Environ. Res. Public Health 2020, 17, 253. [Google Scholar] [CrossRef]
- Barra, P.J.; Inostroza, N.G.; Mora, M.L.; Crowley, D.E.; Jorquera, M.A. Bacterial consortia inoculation mitigates the water shortage and salt stress in an avocado (Persea americana Mill.) Nursery. Appl. Soil Ecol. 2017, 111, 39–47. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.; Cruz, C. Current advances in plant growth promoting bacteria alleviating salt stress for sustainable agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Viera, W. Native mycorrhizae for improving seedling growth in avocado nursery (Persea americana Mill.). Indian J. Sci. Technol. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Shu, B.; Liu, L.; Jue, D.; Wang, Y.; Wei, Y.; Shi, S. Effects of avocado (Persea americana Mill.) scion on arbuscular mycorrhizal and root hair development in rootstock. Arch. Agron. Soil Sci. 2017, 63, 1951–1962. [Google Scholar] [CrossRef]
- Carreón Abud, Y.; Aguirre Paleo, S.; Gavito, M.E.; Mendoza Solís, D.J.; Juárez Chávez, R.; Martínez Trujillo, M.; Trejo Aguilar, D. Inoculación micorrízico arbuscular en portainjertos de plantas de aguacate cv “Hass” en viveros de Michoacán, México. Rev. Mex. Cienc. Agrícolas 2014, 5, 847–857. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na(+) root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Faust, F.; Schubert, S. Protein synthesis is the most sensitive process when potassium is substituted by sodium in the nutrition of sugar beet (Beta vulgaris). Plant Physiol. Biochem. 2016, 107, 237–247. [Google Scholar] [CrossRef]
- Tofighi, C.; Khavari-Nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Physiological and molecular responses of wheat plants to mycorrhizal and Epibrassinolide interactions under salinity. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1075–1080. [Google Scholar] [CrossRef]





| Características | Strain | ||||||
|---|---|---|---|---|---|---|---|
| P1 | P3 | P6 | P10 | P11 | BAC F | BAC M | |
| -Identity (BLAST NCBI) | Pseudomonas putida | Pseudomonas putida | Pseudomonas putida | Pseudomonas putida | Pseudomonas sp. | Bacillus subtilis | Pseudomonas plecoglossicida |
| -Phosphate solubilization capcity (%) | 200 | 100 | 0 | 100 | 183 | 0 | 266 |
| -Siderophore production | - | - | - | + | - | - | - |
| -Root biomass production(g) | 2.6 | 1.6 | 2.4 | 1.7 | 1.9 | 3.3 | 2.8 |
| -Leaves biomass production (g) | 2.7 | 2.2 | 2.8 | 1.8 | 2.4 | 4.3 | 3.8 |
| -Antagonistic activity on P. cinnamomi (%) | 40 | 48 | 47 | 42 | 40 | 45 | 28 |
| -Antagonistic activity on L. theobromae (%) | 0 | 0 | 0 | 0 | 0 | 33 | 0 |
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Fresh Aerial Biomass (g) | Dry Aerial Biomass (g) | Fresh Root Biomass (g) | Dry Root Biomass (g) | Total Dry Biomass (g) | Total Dry Biomass Decrease (%) * | |
|---|---|---|---|---|---|---|---|---|---|
| Without NaCl | B. subtilis | 73.9 f | 0.68 a | 57.16 d | 27.76 d | 20.51 cd | 16.33 cd | 44.09 e | 0.00 |
| 0.75 g/L NaCl | B. subtilis | 47.20 b | 0.63 a | 29.05 a | 16.34 bc | 17.16 bc | 17.22 d | 33.56 d | 23.88 |
| 1.5 g/L NaCl | B. subtilis | 58.80 d | 0.63 a | 22.34 a | 11.48 a | 15.30 ab | 5.64 a | 17.12 a | 61.17 |
| Without NaCl | P. plecoglossicida | 64.51 e | 0.65 a | 68.63 f | 26.12 d | 27.54 e | 13.99 bcd | 40.11 d | 0.00 |
| 0.75 g/L NaCl | P. plecoglossicida | 50.30 c | 0.62 a | 27.83 c | 13.75 bc | 24.17 de | 12.18 bc | 25.93 b | 35.35 |
| 1.5 g/L NaCl | P. plecoglossicida | 45.15 b | 0.51 a | 18.91 a | 9.56 a | 12.12 a | 3.74 a | 13.3 a | 66.84 |
| Without NaCl | Without bacteria | 63.20 e | 0.63 a | 63.07 e | 28.41 d | 35.76 f | 18.19 d | 46.6 d | 0.00 |
| 0.75 g/L NaCl | Without bacteria | 58.70 d | 0.60 a | 21.4 a | 17.84 c | 19.96 bcd | 12.38 bc | 30.22 bc | 35.15 |
| 1.5 g/L NaCl | Without bacteria | 42.15 a | 0.54 a | 27.06 bc | 11.90 a | 19.31 bcd | 9.82 b | 21.72 b | 53.39 |
| Salinity | Bacteria | K | Na | Cl |
|---|---|---|---|---|
| Without NaCl | B. subtilis | 1.02 a | 0.078 a | 0.24 a |
| P. plecoglossicida | 1.18 c | 0.008 a | 0.42 c | |
| Without bacteria | 1.11 b | 0.010 a | 0.37 b | |
| 0.75 g NaCl | B. subtilis | 1.70 g | 0.089 b | 3.66 e |
| P. plecoglossicida | 1.69 g | 0.010 c | 3.98 f | |
| Without bacteria | 1.86 h | 0.097 bc | 3.48 d | |
| 1.5 g NaCl | B. subtilis | 1.63 f | 0.27 e | 6.04 h |
| P. plecoglossicida | 1.59 e | 0.12 d | 4.67 g | |
| Without bacteria | 1.38 d | 0.37 f | 6.28 i |
| Salinity | Bacteria | K | Na | Cl |
|---|---|---|---|---|
| Without NaCl | B. subtilis | 1.26 h | 0.05 a | 0.85 b |
| P. plecoglossicida | 1.21 g | 0.04 a | 0.82 a | |
| Without bacteria | 1.15 f | 0.04 a | 0.95 c | |
| 0.75 g NaCl | B. subtilis | 0.66 d | 0.22 d | 0.86 b |
| P. plecoglossicida | 0.71 e | 0.15 b | 1.76 d | |
| Without bacteria | 0.44 c | 0.14 b | 3.37 h | |
| 1.5 g NaCl | B. subtilis | 0.41 b | 0.21 d | 1.93 e |
| P. plecoglossicida | 0.38 a | 0.18 c | 2.25 f | |
| Without bacteria | 0.45 c | 0.30 e | 2.52 g |
| Treatment | Plant Height (cm) | Fresh Aerial Biomass (g) | Fresh Root Biomass (g) | Dry Aerial Biomass (g) | Dry Root Biomass (g) | Total Dry Biomass (g) | Total Dry Biomass Increase (%) * |
|---|---|---|---|---|---|---|---|
| Without mycorrhiza/Without NaCl | 70.87 d | 47.33 d | 31.88 d | 26.54 e | 10.12 c | 36.66 e | - |
| Without mycorrhiza + 0.75 g/L NaCl | 64.10 cd | 24.13 b | 22.95 c | 15.15 bc | 6.12 b | 21.27 c | 0 |
| GFI + 0.75 g/L NaCl | 60.5 cd | 40.03 cd | 15.78 ab | 20.14 cd | 4.15 a | 24.29 cd | 14.19 |
| GWI + 0.75 g/L NaCl | 57.32 bc | 36.00 c | 19.92 bc | 21.62 de | 4.76 ab | 26.38 d | 24.02 |
| Without mycorrhiza + 1.5 gLl NaCl | 63.87 cd | 19.55 ab | 15.06 ab | 12.54 ab | 4.76 ab | 17.3 b | 0 |
| GWI + 1.5 g/L NaCl | 44.9 a | 13.99 a | 13.21 a | 9.23 a | 3.80 a | 13.03 a | −24.68 |
| GFI + 1.5 g/L NaCl | 48.87 ab | 13.03 a | 14.80 ab | 7.79 a | 3.24 a | 11.3 a | −34.68 |
| Treatments | Na | K | Cl | |
|---|---|---|---|---|
| Without mycorrhiza | Without NaCl | 0.08 a | 1.2 a | 0.50 a |
| 0.75 g NaCl | 0.60 b | 1.80 f | 3.73 b | |
| 1.5 g NaCl | 3.00 g | 1.67 c | 6.32 g | |
| GFI | 0.75 g NaCl | 0.62 c | 1.72 d | 3.94 c |
| 1.5 g NaCl | 0.80 d | 1.63 b | 4.00 d | |
| GWI | 0.75 g NaCl | 1.04 e | 1.86 g | 4.10 e |
| 1.5 g NaCl | 2.38 f | 1.74 e | 5.11 f | |
| Treatments | Na | K | Cl | |
|---|---|---|---|---|
| Without micorriza | Without NaCl | 0.34 a | 0.96 f | 0.62 a |
| 0.75 g NaCl | 1.38 d | 0.40 b | 1.49 c | |
| 1.5 g NaCl | 1.34 c | 0.28 a | 2.49 f | |
| GFI | 0.75 g NaCl | 1.60 e | 0.48 d | 1.65 d |
| 1.5 g NaCl | 2.00 f | 0.42 c | 2.89 g | |
| GWI | 0.75 g NaCl | 0.87 b | 0.43 c | 1.12 b |
| 1.5 g NaCl | 1.34 c | 0.50 e | 2.27 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano-Acosta, R.; Toro, M.; Zúñiga-Dávila, D. Plant Growth Promoting Bacteria and Arbuscular Mycorrhizae Improve the Growth of Persea americana var. Zutano under Salt Stress Conditions. J. Fungi 2023, 9, 233. https://doi.org/10.3390/jof9020233
Solórzano-Acosta R, Toro M, Zúñiga-Dávila D. Plant Growth Promoting Bacteria and Arbuscular Mycorrhizae Improve the Growth of Persea americana var. Zutano under Salt Stress Conditions. Journal of Fungi. 2023; 9(2):233. https://doi.org/10.3390/jof9020233
Chicago/Turabian StyleSolórzano-Acosta, Richard, Marcia Toro, and Doris Zúñiga-Dávila. 2023. "Plant Growth Promoting Bacteria and Arbuscular Mycorrhizae Improve the Growth of Persea americana var. Zutano under Salt Stress Conditions" Journal of Fungi 9, no. 2: 233. https://doi.org/10.3390/jof9020233
APA StyleSolórzano-Acosta, R., Toro, M., & Zúñiga-Dávila, D. (2023). Plant Growth Promoting Bacteria and Arbuscular Mycorrhizae Improve the Growth of Persea americana var. Zutano under Salt Stress Conditions. Journal of Fungi, 9(2), 233. https://doi.org/10.3390/jof9020233







