What Is New in Pulmonary Mucormycosis?
Abstract
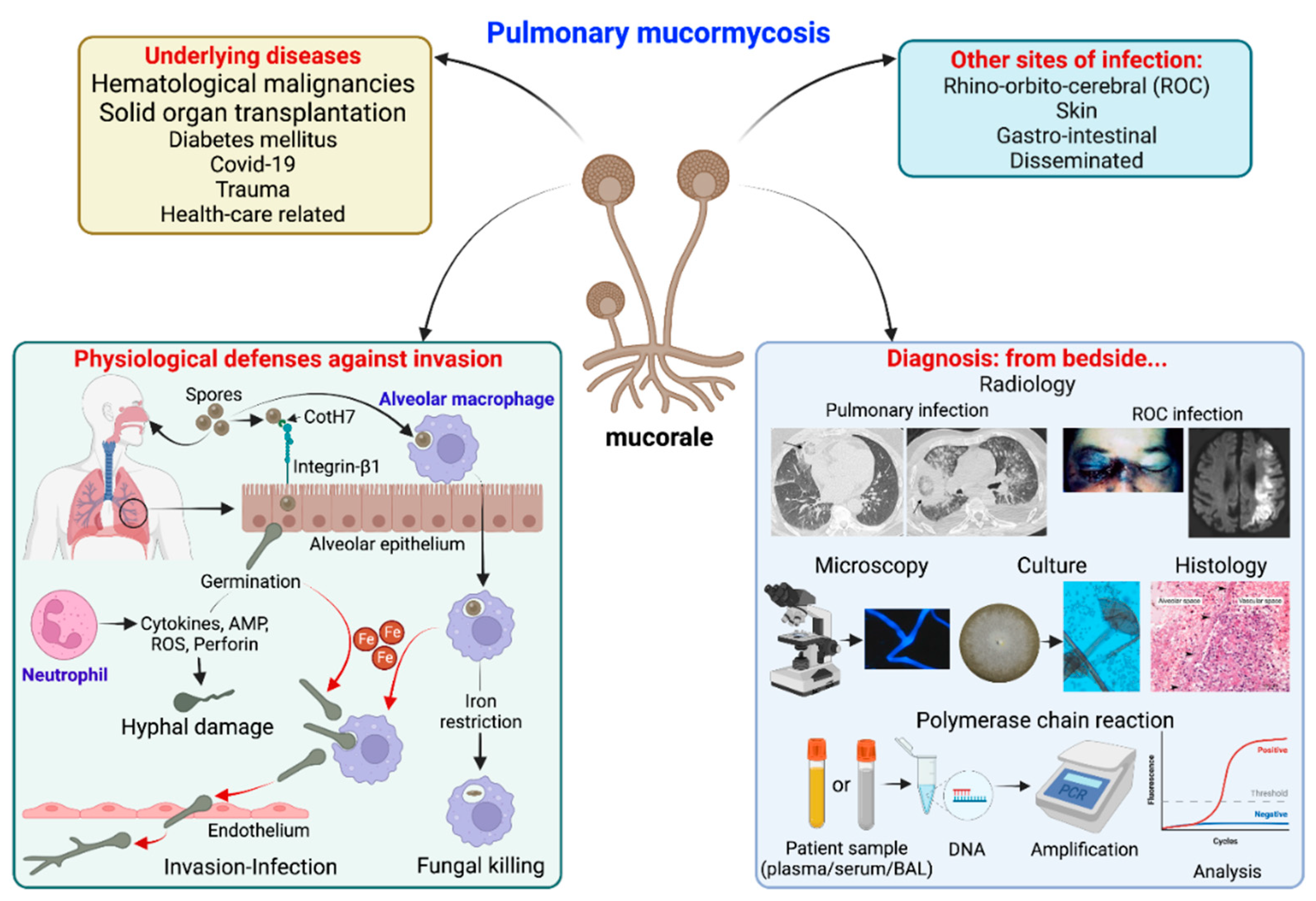
:1. Introduction
2. Risk Factors
3. Pathophysiology
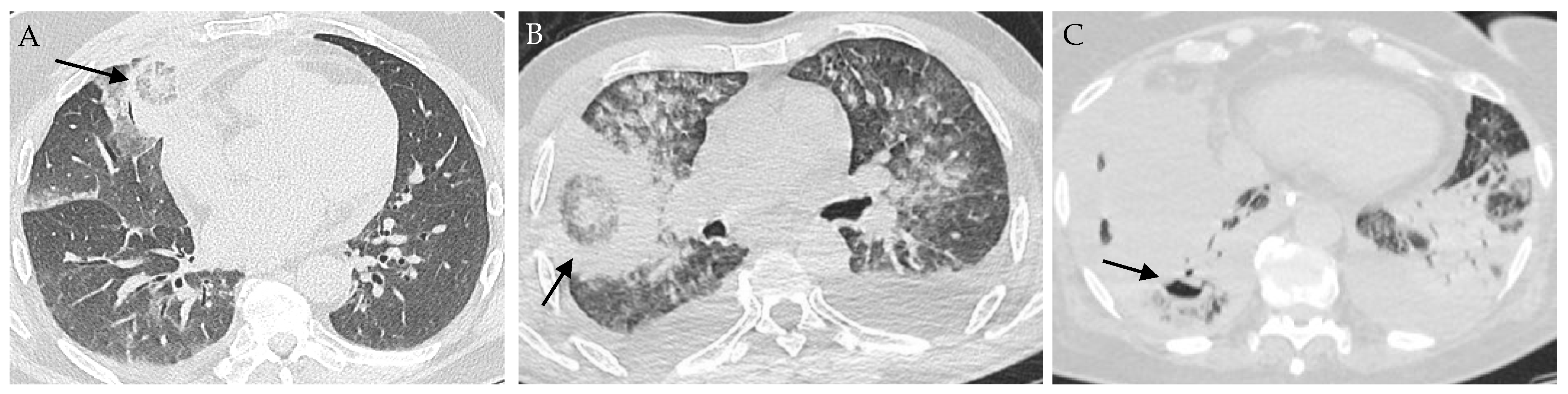
4. Clinical and Radiological Findings
5. Diagnostic Tests
6. Treatment
7. Outcome and Prognostic Factors
8. Conclusions
| Combined Therapy | Mice Model of Mucormycosis and Method of Infection | Species of Mucorales | Efficacy on Survival Compared to Monotherapy | Efficacy to Decrease Organ CFU Compared to Monotherapy | Ref. |
|---|---|---|---|---|---|
| ABLC + CAS | -DKA - via the tail vein | Rhizopus arrhizus var. delemar | yes, improve survival | not better than ABLC alone (brain and kidney CFU) | [141] |
| L-AmB + ANI or MICA | -DKA -via the tail vein | R. delemar var. delemar | yes, improve survival | not better than L-AmB alone (kidney CFU) | [142] |
| L-AmB + MICA | -DKA -via the tail vein | R. delemar var. delemar | yes, improve survival | reduce kidney CFU | [142] |
| L-AmB + POSA | -DKA and neutropenic -via the tail vein | R. arrhizus var. delemar | no benefit | not better than L-AmB alone (brain and kidney CFU) | [144] |
| L-AmB + ISA | -neutropenic -intratracheally | R. arrhizus var. delemar and M. circinelloides | yes, improve survival | reduce lung and brain CFU | [143] |
| ISA + MICA | -neutropenic -intratracheally | R. arrhizus var. delemar and M. circinelloides | no benefit | not better then monotherapy (brain and kidney CFU) | [160] |
| L-AmB + FMGX | -neutropenic -intratracheally | R. arrhizus var. delemar | yes, improve survival | reduce lung and brain CFU | [145] |
| POSA + CotH3 | -DKA -intratracheally | R. arrhizus var. delemar | yes, improve survival 100% survival | not better than monotherapy (lung and brain CFU) | [43] |
| L-AmB + CotH3 | -DKA -intratracheally | R. arrhizus var. delemar | 100% survival, trend to be better than L-AmB alone (p = 0.05) | not better than monotherapy (lung and brain CFU) | [43] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bitar, D.; Lortholary, O.; Le Strat, Y.; Nicolau, J.; Coignard, B.; Tattevin, P.; Che, D.; Dromer, F. Population-Based Analysis of Invasive Fungal Infections, France, 2001–2010. Emerg. Infect. Dis. 2014, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Bretagne, S.; Sitbon, K.; Desnos-Ollivier, M.; Garcia-Hermoso, D.; Letscher-Bru, V.; Cassaing, S.; Millon, L.; Morio, F.; Gangneux, J.-P.; Hasseine, L.; et al. Active Surveillance Program to Increase Awareness on Invasive Fungal Diseases: The French RESSIF Network (2012 to 2018). mBio 2022, 13, e0092022. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Bouchuiguir-Wafa, K.; Garbino, J. Emerging Invasive Zygomycosis in a Tertiary Care Center: Epidemiology and Associated Risk Factors. Int. J. Infect. Dis. 2010, 14 (Suppl S3), e100–e103. [Google Scholar] [CrossRef] [Green Version]
- Guinea, J.; Escribano, P.; Vena, A.; Muñoz, P.; Martínez-Jiménez, M.D.C.; Padilla, B.; Bouza, E. Increasing Incidence of Mucormycosis in a Large Spanish Hospital from 2007 to 2015: Epidemiology and Microbiological Characterization of the Isolates. PLoS ONE 2017, 12, e0179136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saegeman, V.; Maertens, J.; Meersseman, W.; Spriet, I.; Verbeken, E.; Lagrou, K. Increasing Incidence of Mucormycosis in University Hospital, Belgium. Emerg. Infect. Dis. 2010, 16, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Lynch, J.P.; Fishbein, M.C.; Clark, N.M. Mucormycosis. Semin. Respir. Crit. Care Med. 2020, 41, 99–114. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and Sites of Involvement of Invasive Fungal Infections in Patients with Haematological Malignancies: A 20-Year Autopsy Study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Prakash, H.; Chakrabarti, A. Epidemiology of Mucormycosis in India. Microorganisms 2021, 9, 523. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World. Mycopathologia 2021, 186, 739–754. [Google Scholar] [CrossRef]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; the French Mycosis Study Group. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005-2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The Epidemiology and Clinical Manifestations of Mucormycosis: A Systematic Review and Meta-Analysis of Case Reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective Antifungal Therapy (PATH) Alliance(®): Focus on Mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 Cases Accrued by the Registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007: European Study of 230 Cases of Zygomycosis. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Coste, A.; Conrad, A.; Porcher, R.; Poirée, S.; Peterlin, P.; Defrance, C.; Letscher-Bru, V.; Morio, F.; Gastinne, T.; Bougnoux, M.-E.; et al. P397 Influence of Underlying Conditions on Disease Presentation and Diagnostic Strategy during Pulmonary Mucormycosis: Anational Study of 114 Cases. Med. Mycol. 2022, 60, myac072P397. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Pulmonary Zygomycosis in Solid Organ Transplant Recipients in the Current Era. Am. J. Transplant. 2009, 9, 2166–2171. [Google Scholar] [CrossRef]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive Non-Aspergillus Mold Infections in Transplant Recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef]
- Almyroudis, N.G.; Sutton, D.A.; Linden, P.; Rinaldi, M.G.; Fung, J.; Kusne, S. Zygomycosis in Solid Organ Transplant Recipients in a Tertiary Transplant Center and Review of the Literature. Am. J. Transplant. 2006, 6, 2365–2374. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al.; RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Honavar, S.G.; Bansal, R.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.K.; Surve, A.; et al. Epidemiology, Clinical Profile, Management, and Outcome of COVID-19-Associated Rhino-Orbital-Cerebral Mucormycosis in 2826 Patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670–1692. [Google Scholar] [CrossRef] [PubMed]
- Danion, F.; Letscher-Bru, V.; Guitard, J.; Sitbon, K.; Dellière, S.; Angoulvant, A.; Desoubeaux, G.; Botterel, F.; Bellanger, A.-P.; Gargala, G.; et al. Coronavirus Disease 2019-Associated Mucormycosis in France: A Rare but Deadly Complication. Open Forum Infect. Dis. 2022, 9, ofab566. [Google Scholar] [CrossRef]
- Seidel, D.; Simon, M.; Sprute, R.; Lubnow, M.; Evert, K.; Speer, C.; Seeßle, J.; Khatamzas, E.; Merle, U.; Behrens, C.; et al. Results from a National Survey on COVID-19-Associated Mucormycosis in Germany: 13 Patients from Six Tertiary Hospitals. Mycoses 2022, 65, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Muthu, V.; Agarwal, R.; Patel, A.; Kathirvel, S.; Abraham, O.C.; Aggarwal, A.N.; Bal, A.; Bhalla, A.S.; Chhajed, P.N.; Chaudhry, D.; et al. Definition, Diagnosis, and Management of COVID-19-Associated Pulmonary Mucormycosis: Delphi Consensus Statement from the Fungal Infection Study Forum and Academy of Pulmonary Sciences, India. Lancet Infect. Dis. 2022, 22, e240–e253. [Google Scholar] [CrossRef]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A Prospective Multicenter Study on Mucormycosis in India: Epidemiology, Diagnosis, and Treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef]
- Serris, A.; Danion, F.; Lanternier, F. Disease Entities in Mucormycosis. J. Fungi 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Aboutanos, M.B.; Joshi, M.; Scalea, T.M. Isolated Pulmonary Mucormycosis in a Patient with Multiple Injuries: A Case Presentation and Review of the Literature. J. Trauma 2003, 54, 1016–1019. [Google Scholar] [CrossRef]
- Grandin, W.; Dessieux, T.; Hounfodji, P.; Viquesnel, G.; Ouchikhe, A.; Gérard, J.-L. [Pulmonary mucormycosis in a multiple-trauma patient]. Ann. Fr. Anesth. Reanim. 2006, 25, 521–524. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) Health Concerns Associated with Mold in Water-Damaged Homes after Hurricanes Katrina and Rita--New Orleans Area, Louisiana, October 2005. Morb. Mortal. Wkly Rep. 2006, 55, 41–44.
- Riggs, M.A.; Rao, C.Y.; Brown, C.M.; Van Sickle, D.; Cummings, K.J.; Dunn, K.H.; Deddens, J.A.; Ferdinands, J.; Callahan, D.; Moolenaar, R.L.; et al. Resident Cleanup Activities, Characteristics of Flood-Damaged Homes and Airborne Microbial Concentrations in New Orleans, Louisiana, October 2005. Environ. Res. 2008, 106, 401–409. [Google Scholar] [CrossRef]
- Rao, C.Y.; Kurukularatne, C.; Garcia-Diaz, J.B.; Kemmerly, S.A.; Reed, D.; Fridkin, S.K.; Morgan, J. Implications of Detecting the Mold Syncephalastrum in Clinical Specimens of New Orleans Residents after Hurricanes Katrina and Rita. J. Occup. Environ. Med. 2007, 49, 411–416. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-Associated Mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl S1), S44–S54. [Google Scholar] [CrossRef] [Green Version]
- Pongas, G.N.; Lewis, R.E.; Samonis, G.; Kontoyiannis, D.P. Voriconazole-Associated Zygomycosis: A Significant Consequence of Evolving Antifungal Prophylaxis and Immunosuppression Practices? Clin. Microbiol. Infect. 2009, 15 (Suppl S5), 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a Tertiary-Care Cancer Center in the Era of Aspergillus-Active Antifungal Therapy: A Case-Control Observational Study of 27 Recent Cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.A.; Vaughan, A.M.; Zaoutis, T.E. Trends in Zygomycosis in Children. Mycoses 2012, 55, 352–356. [Google Scholar] [CrossRef]
- Zaoutis, T.E.; Roilides, E.; Chiou, C.C.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Prasad, P.A.; et al. Zygomycosis in Children: A Systematic Review and Analysis of Reported Cases. Pediatr. Infect. Dis. J. 2007, 26, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E.; Collaborators of Zygomyco.net and/or FungiScopeTM Registries*. Invasive Mucormycosis in Children: An Epidemiologic Study in European and Non-European Countries Based on Two Registries. BMC Infect. Dis. 2016, 16, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P.; et al. GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis. mBio 2020, 11, e01087-20. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The Endothelial Cell Receptor GRP78 Is Required for Mucormycosis Pathogenesis in Diabetic Mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 Mediates Fungal Invasion of Host Cells during Mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremariam, T.; Alkhazraji, S.; Soliman, S.S.M.; Gu, Y.; Jeon, H.H.; Zhang, L.; French, S.W.; Stevens, D.A.; Edwards, J.E.; Filler, S.G.; et al. Anti-CotH3 Antibodies Protect Mice from Mucormycosis by Prevention of Invasion and Augmenting Opsonophagocytosis. Sci. Adv. 2019, 5, eaaw1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthu, V.; Dhaliwal, M.; Sharma, A.; Nair, D.; Kumar, H.M.; Rudramurthy, S.M.; Sehgal, I.S.; Choudhary, H.; Panda, N.; Chakrabarti, A.; et al. Serum Glucose-Regulated Protein 78 (GRP78) Levels in COVID-19-Associated Mucormycosis: Results of a Case-Control Study. Mycopathologia 2022, 187, 355–362. [Google Scholar] [CrossRef]
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron Restriction inside Macrophages Regulates Pulmonary Host Defense against Rhizopus Species. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [Green Version]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A Mechanism of Susceptibility to Mucormycosis in Diabetic Ketoacidosis: Transferrin and Iron Availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef]
- Brunet, K.; Arrivé, F.; Martellosio, J.-P.; Lamarche, I.; Marchand, S.; Rammaert, B. Corticosteroids Alter Alveolar Macrophage Control of Lichtheimia Corymbifera Spores in an Ex Vivo Mouse Model. Med. Mycol. 2021, 59, 694–700. [Google Scholar] [CrossRef]
- Chinn, R.Y.; Diamond, R.D. Generation of Chemotactic Factors by Rhizopus Oryzae in the Presence and Absence of Serum: Relationship to Hyphal Damage Mediated by Human Neutrophils and Effects of Hyperglycemia and Ketoacidosis. Infect. Immun. 1982, 38, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific Susceptibility to Mucormycosis in Murine Diabetes and Bronchoalveolar Macrophage Defense against Rhizopus. J. Clin. Invest. 1984, 74, 150–160. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Hu, J.; Xiao, L.; Zal, T.; Gilliet, M.; Halder, G.; Kontoyiannis, D.P. Drosophila Melanogaster as a Model Host to Dissect the Immunopathogenesis of Zygomycosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9367–9372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roilides, E.; Kontoyiannis, D.P.; Walsh, T.J. Host Defenses Against Zygomycetes. Clin. Infect. Dis. 2012, 54, S61–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamilos, G.; Lewis, R.E.; Lamaris, G.; Walsh, T.J.; Kontoyiannis, D.P. Zygomycetes Hyphae Trigger an Early, Robust Proinflammatory Response in Human Polymorphonuclear Neutrophils through Toll-Like Receptor 2 Induction but Display Relative Resistance to Oxidative Damage. Antimicrob. Agents Chemother. 2008, 52, 722–724. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, F.E.; Murcia, L.; Navarro, E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Garre, V. Mucorales Species and Macrophages. J. Fungi 2020, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lamaignere, C.; Simitsopoulou, M.; Roilides, E.; Maloukou, A.; Winn, R.M.; Walsh, T.J. Interferon-γ and Granulocyte-Macrophage Colony-Stimulating Factor Augment the Activity of Polymorphonuclear Leukocytes against Medically Important Zygomycetes. J. Infect. Dis. 2005, 191, 1180–1187. [Google Scholar] [CrossRef] [Green Version]
- Vinh, D.C.; Freeman, A.F.; Shea, Y.R.; Malech, H.L.; Abinun, M.; Weinberg, G.A.; Holland, S.M. Mucormycosis in Chronic Granulomatous Disease: Association with Iatrogenic Immunosuppression. J. Allergy Clin. Immunol. 2009, 123, 1411–1413. [Google Scholar] [CrossRef] [Green Version]
- Dotis, J.; Pana, Z.D.; Roilides, E. Non-Aspergillus Fungal Infections in Chronic Granulomatous Disease. Mycoses 2013, 56, 449–462. [Google Scholar] [CrossRef]
- Opara, N.U. A Rare Case of Pulmonary and Gastrointestinal Mucormycosis Due to Rhizopus Spp. in a Child with Chronic Granulomatous Disease. Infect. Dis. Rep. 2022, 14, 579–586. [Google Scholar] [CrossRef]
- Nadeem, A.M.; Wahla, A.S.; Al-Tarifi, A. Invasive Mediastinal Mucormycosis with Pulmonary and Cardiac Involvement in an Adult with Chronic Granulomatous Disease: Case Report and Review of the Literature. Eur. J. Case Rep. Intern. Med. 2021, 8, 002435. [Google Scholar] [CrossRef]
- Agarwal, S.; Anand, A.; Ranjan, P.; Meena, V.P.; Ray, A.; Dutta, R.; Jadon, R.S.; Vikram, N.K. Case of Mucormycosis of Mandible after Self-Extraction of Teeth Incidentally Detected to Have Chronic Granulomatous Disease: Case Report and Literature Review. Med. Mycol. Case Rep. 2020, 28, 55–59. [Google Scholar] [CrossRef]
- Winstead, M.; Ozolek, J.; Nowalk, A.; Williams, J.; Vander Lugt, M.; Lin, P. Disseminated Lichtheimia Ramosa Infection After Hematopoietic Stem Cell Transplantation in a Child With Chronic Granulomatous Disease. Pediatr. Infect. Dis. J. 2017, 36, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, A.M.; Al-Shahrani, D.A.; Al-Idrissi, E.M.; Al-Abdely, H.M. Invasive Mucormycosis in Chronic Granulomatous Disease. Saudi Med. J. 2016, 37, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, A.; Wang, X.; Li, R.; Yu, J. Cutaneous Mucormycosis Caused by Mucor Irregularis in a Patient with CARD9 Deficiency. Br. J. Dermatol. 2019, 180, 213–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Hanks, M.E.; Chandrasekaran, P.; Davis, B.C.; Hsu, A.P.; Van Wagoner, N.J.; Merlin, J.S.; Spalding, C.; La Hoz, R.M.; Holland, S.M.; et al. Gain-of-Function Signal Transducer and Activator of Transcription 1 (STAT1) Mutation–Related Primary Immunodeficiency Is Associated with Disseminated Mucormycosis. J. Allergy Clin. Immunol. 2014, 134, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, S.; Wan, Z.; Li, R.; Yu, J. In Vivo and In Vitro Impairments in T Helper Cell and Neutrophil Responses against Mucor Irregularis in Card9 Knockout Mice. Infect. Immun. 2021, 89, e00040-21. [Google Scholar] [CrossRef]
- Lee, F.Y.W.; Mossad, S.B.; Adal, K.A. Pulmonary Mucormycosis: The Last 30 Years. Arch. Intern. Med. 1999, 159, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Legouge, C.; Caillot, D.; Chretien, M.-L.; Lafon, I.; Ferrant, E.; Audia, S.; Pages, P.-B.; Roques, M.; Estivalet, L.; Martin, L.; et al. The Reversed Halo Sign: Pathognomonic Pattern of Pulmonary Mucormycosis in Leukemic Patients With Neutropenia? Clin. Infect. Dis. 2014, 58, 672–678. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of Pulmonary Zygomycosis versus Invasive Pulmonary Aspergillosis in Patients with Cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Danion, F.; Aguilar, C.; Catherinot, E.; Alanio, A.; DeWolf, S.; Lortholary, O.; Lanternier, F. Mucormycosis: New Developments into a Persistently Devastating Infection. Semin. Respir. Crit. Care Med. 2015, 36, 692–705. [Google Scholar] [CrossRef]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in Patients with Haematological Malignancies: A Retrospective Clinical Study of 37 Cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne Dell’Adulto). Br. J. Haematol. 1997, 99, 331–336. [Google Scholar] [CrossRef]
- He, R.; Hu, C.; Tang, Y.; Yang, H.; Cao, L.; Niu, R. Report of 12 Cases with Tracheobronchial Mucormycosis and a Review. Clin. Respir. J. 2018, 12, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Wahba, H.; Truong, M.T.; Lei, X.; Kontoyiannis, D.P.; Marom, E.M. Reversed Halo Sign in Invasive Pulmonary Fungal Infections. Clin. Infect. Dis. 2008, 46, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Ishiwatari, T.; Izumi, H.; Sato, F.; Aki, K.; Sasai, D.; Ando, T.; Shinozaki, M.; Natori, K.; Tochigi, N.; et al. Pathophysiological Implication of Reversed CT Halo Sign in Invasive Pulmonary Mucormycosis: A Rare Case Report. Diagn. Pathol. 2013, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Yeldandi, A.; Savas, H.; Parekh, N.D.; Lombardi, P.J.; Hart, E.M. Pulmonary Mucormycosis: Risk Factors, Radiologic Findings, and Pathologic Correlation. Radiographics 2020, 40, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, M.; Sassi, C.; Lewis, R.E.; Tolomelli, G.; Bazzocchi, A.; Cavo, M.; Vianelli, N.; Battista, G. High Resolution Computed Tomography Angiography Improves the Radiographic Diagnosis of Invasive Mold Disease in Patients with Hematological Malignancies. Clin. Infect. Dis. 2015, 60, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanzani, M.; Sassi, C.; Lewis, R.; Sartor, C.; Rasetto, G.; Cavo, M.; Battista, G. Early Low-Dose Computed Tomography with Pulmonary Angiography to Improve the Early Diagnosis of Invasive Mould Disease in Patients with Haematological Malignancies: A Pilot Study. J. Infect. 2021, 83, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; My, K.; Hj, L.; Ys, P.; So, L.; Sh, C.; Ys, K.; Jh, W.; Sh, K. Comparison of Computed Tomographic Findings in Pulmonary Mucormycosis and Invasive Pulmonary Aspergillosis. Available online: https://pubmed.ncbi.nlm.nih.gov/25882362/ (accessed on 10 November 2022).
- Lewis, R.E.; Georgiadou, S.P.; Sampsonas, F.; Chamilos, G.; Kontoyiannis, D.P. Risk Factors for Early Mortality in Haematological Malignancy Patients with Pulmonary Mucormycosis. Mycoses 2014, 57, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a Tertiary-Care Cancer Center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, R.; Luna, M.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. A Clinicopathological Study of Pulmonary Mucormycosis in Cancer Patients: Extensive Angioinvasion but Limited Inflammatory Response. J. Infect. 2009, 59, 134–138. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Paul, M.; Bernstine, H.; Vidal, L.; Ram, R.; Raanani, P.; Yeshurun, M.; Tadmor, B.; Leibovici, L.; Shpilberg, O.; et al. The Role of 18F-FDG PET/CT for the Diagnosis of Infections in Patients with Hematological Malignancies and Persistent Febrile Neutropenia. Leuk. Res. 2013, 37, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Hot, A.; Maunoury, C.; Poiree, S.; Lanternier, F.; Viard, J.P.; Loulergue, P.; Coignard, H.; Bougnoux, M.E.; Suarez, F.; Rubio, M.T.; et al. Diagnostic Contribution of Positron Emission Tomography with [18F]Fluorodeoxyglucose for Invasive Fungal Infections. Clin. Microbiol. Infect. 2011, 17, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamilos, G.; Macapinlac, H.A.; Kontoyiannis, D.P. The Use of 18F-Fluorodeoxyglucose Positron Emission Tomography for the Diagnosis and Management of Invasive Mould Infections. Med. Mycol. 2008, 46, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danion, F.; Duval, C.; Séverac, F.; Bachellier, P.; Candolfi, E.; Castelain, V.; Clere-Jehl, R.; Denis, J.; Dillenseger, L.; Epailly, E.; et al. Factors Associated with Coinfections in Invasive Aspergillosis: A Retrospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales PCR for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef]
- Lass-Florl, C.; Resch, G.; Nachbaur, D.; Mayr, A.; Gastl, G.; Auberger, J.; Bialek, R.; Freund, M.C. The Value of Computed Tomography-Guided Percutaneous Lung Biopsy for Diagnosis of Invasive Fungal Infection in Immunocompromised Patients. Clin. Infect. Dis. 2007, 45, e101–e104. [Google Scholar] [CrossRef]
- Garcia-Hermoso, D.; Alanio, A.; Lortholary, O.; Dromer, F. Agents of Systemic and Subcutaneous Mucormycosis and Entomophthoromycosis. In Manual of Clinical Microbiology; Taylor and Francis Books Limited: London, UK, 2015; pp. 2087–2108. [Google Scholar]
- Lass-Flörl, C. Zygomycosis: Conventional Laboratory Diagnosis. Clin. Microbiol. Infect. 2009, 15, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In Vitro Susceptibilities of 217 Clinical Isolates of Zygomycetes to Conventional and New Antifungal Agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef] [Green Version]
- Lamoth, F.; Kontoyiannis, D.P. Therapeutic Challenges of Non-Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob. Agents Chemother. 2019, 63, e01244-19. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.E.; Salonen, J.; Ekfors, T.O. The Use of Immunohistochemistry to Improve Sensitivity and Specificity in the Diagnosis of Systemic Mycoses in Patients with Haematological Malignancies. J. Pathol. 1997, 181, 100–105. [Google Scholar] [CrossRef]
- Dannaoui, E. Molecular Tools for Identification of Zygomycetes and the Diagnosis of Zygomycosis. Clin. Microbiol. Infect. 2009, 15, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millon, L.; Scherer, E.; Rocchi, S.; Bellanger, A.-P. Molecular Strategies to Diagnose Mucormycosis. J. Fungi 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, S.R.; Bialek, R.; Kibbler, C.C.; Cuenca-Estrella, M.; Jensen, H.E.; Kontoyiannis, D.P. Molecular Techniques for Genus and Species Determination of Fungi From Fresh and Paraffin-Embedded Formalin-Fixed Tissue in the Revised EORTC/MSGERC Definitions of Invasive Fungal Infection. Clin. Infect. Dis. 2021, 72, S109–S113. [Google Scholar] [CrossRef]
- Frater, J.L.; Hall, G.S.; Procop, G.W. Histologic Features of Zygomycosis: Emphasis on Perineural Invasion and Fungal Morphology. Arch Pathol Lab Med 2001, 125, 375–378. [Google Scholar] [CrossRef]
- Dannaoui, E. Recent Developments in the Diagnosis of Mucormycosis. J. Fungi 2022, 8, 457. [Google Scholar] [CrossRef]
- Millon, L.; Larosa, F.; Lepiller, Q.; Legrand, F.; Rocchi, S.; Daguindau, E.; Scherer, E.; Bellanger, A.-P.; Leroy, J.; Grenouillet, F. Quantitative Polymerase Chain Reaction Detection of Circulating DNA in Serum for Early Diagnosis of Mucormycosis in Immunocompromised Patients. Clin. Infect. Dis. 2013, 56, e95–e101. [Google Scholar] [CrossRef] [Green Version]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early Diagnosis and Monitoring of Mucormycosis by Detection of Circulating DNA in Serum: Retrospective Analysis of 44 Cases Collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef] [Green Version]
- Scherer, E.; Iriart, X.; Bellanger, A.P.; Dupont, D.; Guitard, J.; Gabriel, F.; Cassaing, S.; Charpentier, E.; Guenounou, S.; Cornet, M.; et al. Quantitative PCR (QPCR) Detection of Mucorales DNA in Bronchoalveolar Lavage Fluid To Diagnose Pulmonary Mucormycosis. J. Clin. Microbiol. 2018, 56, e00289-18. [Google Scholar] [CrossRef] [Green Version]
- Mercier, T.; Reynders, M.; Beuselinck, K.; Guldentops, E.; Maertens, J.; Lagrou, K. Serial Detection of Circulating Mucorales DNA in Invasive Mucormycosis: A Retrospective Multicenter Evaluation. J. Fungi 2019, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Springer, J.; Lackner, M.; Ensinger, C.; Risslegger, B.; Morton, C.O.; Nachbaur, D.; Lass-Flörl, C.; Einsele, H.; Heinz, W.J.; Loeffler, J. Clinical Evaluation of a Mucorales-Specific Real-Time PCR Assay in Tissue and Serum Samples. J. Med. Microbiol. 2016, 65, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Cornu, M.; Sendid, B.; Mery, A.; François, N.; Mikulska, M.; Letscher-Bru, V.; De Carolis, E.; Damonti, L.; Titecat, M.; Bochud, P.-Y.; et al. Evaluation of Mass Spectrometry-Based Detection of Panfungal Serum Disaccharide for Diagnosis of Invasive Fungal Infections: Results from a Collaborative Study Involving Six European Clinical Centers. J. Clin. Microbiol. 2019, 57, e01867-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnham-Marusich, A.R.; Hubbard, B.; Kvam, A.J.; Gates-Hollingsworth, M.; Green, H.R.; Soukup, E.; Limper, A.H.; Kozel, T.R. Conservation of Mannan Synthesis in Fungi of the Zygomycota and Ascomycota Reveals a Broad Diagnostic Target. mSphere 2018, 3, e00094-18. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.E.; Thornton, C.R. Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus Arrhizus (Syn. R. Oryzae), the Principal Global Agent of Mucormycosis in Humans. J. Fungi 2022, 8, 756. [Google Scholar] [CrossRef]
- Shibata, W.; Niki, M.; Sato, K.; Fujimoto, H.; Yamada, K.; Watanabe, T.; Miyazaki, Y.; Asai, K.; Obata, Y.; Tachibana, T.; et al. Detection of Rhizopus-Specific Antigen in Human and Murine Serum and Bronchoalveolar Lavage. Med. Mycol. 2020, 58, 958–964. [Google Scholar] [CrossRef]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef] [Green Version]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying Amphotericin B–Based Frontline Therapy Significantly Increases Mortality among Patients with Hematologic Malignancy Who Have Zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Lewis, R.E.; Albert, N.D.; Liao, G.; Hou, J.; Prince, R.A.; Kontoyiannis, D.P. Comparative Pharmacodynamics of Amphotericin B Lipid Complex and Liposomal Amphotericin B in a Murine Model of Pulmonary Mucormycosis. Antimicrob. Agents Chemother. 2010, 54, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Lanternier, F.; Poiree, S.; Elie, C.; Garcia-Hermoso, D.; Bakouboula, P.; Sitbon, K.; Herbrecht, R.; Wolff, M.; Ribaud, P.; Lortholary, O.; et al. Prospective Pilot Study of High-Dose (10 Mg/Kg/Day) Liposomal Amphotericin B (L-AMB) for the Initial Treatment of Mucormycosis. J. Antimicrob. Chemother. 2015, 70, 3116–3123. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter Evaluation of MIC Distributions for Epidemiologic Cutoff Value Definition to Detect Amphotericin B, Posaconazole, and Itraconazole Resistance among the Most Clinically Relevant Species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Jensen, R.H.; Meletiadis, J. In Vitro Activity of Isavuconazole and Comparators against Clinical Isolates of the Mucorales Order. Antimicrob. Agents Chemother. 2015, 59, 7735–7742. [Google Scholar] [CrossRef] [Green Version]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole Treatment for Mucormycosis: A Single-Arm Open-Label Trial and Case-Control Analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough Fungal Infections in Patients With Leukemia Receiving Isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef]
- Wurster, S.; Lewis, R.E.; Albert, N.D.; Kontoyiannis, D.P. Preexposure to Isavuconazole Increases the Virulence of Mucorales but Not Aspergillus Fumigatus in a Drosophila Melanogaster Infection Model. Antimicrob. Agents Chemother. 2019, 63, e01896-18. [Google Scholar] [CrossRef] [Green Version]
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough Mucormycosis Developing on Mucorales-Active Antifungals Portrays a Poor Prognosis in Patients with Hematologic Cancer. J. Fungi 2021, 7, 217. [Google Scholar] [CrossRef]
- van Burik, J.-A.H.; Hare, R.S.; Solomon, H.F.; Corrado, M.L.; Kontoyiannis, D.P. Posaconazole Is Effective as Salvage Therapy in Zygomycosis: A Retrospective Summary of 91 Cases. Clin. Infect. Dis. 2006, 42, e61–e65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, R.N.; Mullane, K.; van Burik, J.-A.H.; Raad, I.; Abzug, M.J.; Anstead, G.; Herbrecht, R.; Langston, A.; Marr, K.A.; Schiller, G.; et al. Posaconazole as Salvage Therapy for Zygomycosis. Antimicrob. Agents Chemother. 2006, 50, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruping, M.J.G.T.; Heinz, W.J.; Kindo, A.J.; Rickerts, V.; Lass-Florl, C.; Beisel, C.; Herbrecht, R.; Roth, Y.; Silling, G.; Ullmann, A.J.; et al. Forty-One Recent Cases of Invasive Zygomycosis from a Global Clinical Registry. J. Antimicrob. Chemother. 2010, 65, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auberger, J.; Lass-Flörl, C.; Aigner, M.; Clausen, J.; Gastl, G.; Nachbaur, D. Invasive Fungal Breakthrough Infections, Fungal Colonization and Emergence of Resistant Strains in High-Risk Patients Receiving Antifungal Prophylaxis with Posaconazole: Real-Life Data from a Single-Centre Institutional Retrospective Observational Study. J. Antimicrob. Chemother. 2012, 67, 2268–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, R.F.; López-Jiménez, J.; Cornely, O.A.; Laverdiere, M.; Helfgott, D.; Haider, S.; Chandrasekar, P.; Langston, A.; Perfect, J.; Ma, L.; et al. Phase 1b Study of New Posaconazole Tablet for Prevention of Invasive Fungal Infections in High-Risk Patients with Neutropenia. Antimicrob. Agents Chemother. 2014, 58, 5758–5765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornely, O.A.; Duarte, R.F.; Haider, S.; Chandrasekar, P.; Helfgott, D.; Jiménez, J.L.; Candoni, A.; Raad, I.; Laverdiere, M.; Langston, A.; et al. Phase 3 Pharmacokinetics and Safety Study of a Posaconazole Tablet Formulation in Patients at Risk for Invasive Fungal Disease. J. Antimicrob. Chemother. 2016, 71, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tverdek, F.P.; Heo, S.T.; Aitken, S.L.; Granwehr, B.; Kontoyiannis, D.P. Real-Life Assessment of the Safety and Effectiveness of the New Tablet and Intravenous Formulations of Posaconazole in the Prophylaxis of Invasive Fungal Infections via Analysis of 343 Courses. Antimicrob. Agents Chemother. 2017, 61, e00188-17. [Google Scholar] [CrossRef] [Green Version]
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and Exposure-Response Relationships of Isavuconazole Plasma Concentrations in the Phase 3 SECURE Trial of Patients with Invasive Mould Diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef] [Green Version]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef] [Green Version]
- Lamoth, F.; Lewis, R.E.; Kontoyiannis, D.P. Investigational Antifungal Agents for Invasive Mycoses: A Clinical Perspective. Clin. Infect. Dis. 2022, 75, 534–544. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R.; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Pulmonary Murine Mucormycosis Due to Rhizopus Arrhizus. Antimicrob. Agents Chemother. 2020, 64, e00178-20. [Google Scholar] [CrossRef] [Green Version]
- Brunet, K.; Rammaert, B. Mucormycosis Treatment: Recommendations, Latest Advances, and Perspectives. J. De Mycol. Médicale 2020, 30, 101007. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial Use of Combination Treatment Does Not Impact Survival of 106 Patients with Haematologic Malignancies and Mucormycosis: A Propensity Score Analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef] [Green Version]
- Abidi, M.Z.; Sohail, M.R.; Cummins, N.; Wilhelm, M.; Wengenack, N.; Brumble, L.; Shah, H.; Jane Hata, D.; McCullough, A.; Wendel, A.; et al. Stability in the Cumulative Incidence, Severity and Mortality of 101 Cases of Invasive Mucormycosis in High-Risk Patients from 1995 to 2011: A Comparison of Eras Immediately before and after the Availability of Voriconazole and Echinocandin-Amphotericin Combination Therapies. Mycoses 2014, 57, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.S.; Bowman, J.C.; Avanessian, V.; Brown, K.; Spellberg, B.; Edwards, J.E.; Douglas, C.M. Caspofungin Inhibits Rhizopus Oryzae 1,3-Beta-D-Glucan Synthase, Lowers Burden in Brain Measured by Quantitative PCR, and Improves Survival at a Low but Not a High Dose during Murine Disseminated Zygomycosis. Antimicrob. Agents Chemother. 2005, 49, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimko, N.N.; Khostelidi, S.N.; Volkova, A.G.; Popova, M.O.; Bogomolova, T.S.; Zuborovskaya, L.S.; Kolbin, A.S.; Medvedeva, N.V.; Zuzgin, I.S.; Simkin, S.M.; et al. Mucormycosis in Haematological Patients: Case Report and Results of Prospective Study in Saint Petersburg, Russia. Mycoses 2014, 57 (Suppl S3), 91–96. [Google Scholar] [CrossRef]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination Polyene-Caspofungin Treatment of Rhino-Orbital-Cerebral Mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Jenks, J.D.; Reed, S.L.; Seidel, D.; Koehler, P.; Cornely, O.A.; Mehta, S.R.; Hoenigl, M. Rare Mould Infections Caused by Mucorales, Lomentospora Prolificans and Fusarium, in San Diego, CA: The Role of Antifungal Combination Therapy. Int. J. Antimicrob. Agents 2018, 52, 706–712. [Google Scholar] [CrossRef]
- Pagano, L.; Cornely, O.A.; Caira, M.; Cesaro, S.; Gasbarrino, C.; Girmenia, C.; Heinz, W.J.; Herbrecht, R.; Lass-Flörl, C.; Nosari, A.; et al. Combined Antifungal Approach for the Treatment of Invasive Mucormycosis in Patients with Hematologic Diseases: A Report from the SEIFEM and FUNGISCOPE Registries. haematologica 2013, 98, e127–e130. [Google Scholar] [CrossRef]
- Spellberg, B.; Fu, Y.; Edwards, J.E.; Ibrahim, A.S. Combination Therapy with Amphotericin B Lipid Complex and Caspofungin Acetate of Disseminated Zygomycosis in Diabetic Ketoacidotic Mice. Antimicrob. Agents Chemother. 2005, 49, 830–832. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Gebremariam, T.; Fu, Y.; Edwards, J.E.; Spellberg, B. Combination Echinocandin-Polyene Treatment of Murine Mucormycosis. Antimicrob. Agents Chemother. 2008, 52, 1556–1558. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.; Gu, Y.; Singh, S.; Kitt, T.M.; Ibrahim, A.S. Combination Treatment of Liposomal Amphotericin B and Isavuconazole Is Synergistic in Treating Experimental Mucormycosis. J. Antimicrob. Chemother. 2021, 76, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebremariam, T.; Schwartz, J.A.; Edwards, J.E.; Spellberg, B. Posaconazole Mono- or Combination Therapy for Treatment of Murine Zygomycosis. Antimicrob. Agents Chemother. 2009, 53, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.; Gu, Y.; Alkhazraji, S.; Youssef, E.; Shaw, K.J.; Ibrahim, A.S. The Combination Treatment of Fosmanogepix and Liposomal Amphotericin B Is Superior to Monotherapy in Treating Experimental Invasive Mold Infections. Antimicrob. Agents Chemother. 2022, 66, e0038022. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Antimicrob. Chemother. 2012, 67, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.K.; Batra, J.S.; Michalik, D.E.; Casillas, J.; Patel, R.; Ruiz, M.E.; Hara, H.; Patel, B.; Kadapakkam, M.; Ch’Ng, J.; et al. Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor (Rhu GM-CSF) as Adjuvant Therapy for Invasive Fungal Diseases. Open Forum Infect. Dis. 2022, 9, ofac535. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T Cell Exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wurster, S.; Albert, N.D.; Bharadwaj, U.; Kasembeli, M.M.; Tarrand, J.J.; Daver, N.; Kontoyiannis, D.P. Blockade of the PD-1/PD-L1 Immune Checkpoint Pathway Improves Infection Outcomes and Enhances Fungicidal Host Defense in a Murine Model of Invasive Pulmonary Mucormycosis. Front. Immunol. 2022, 13, 838344. [Google Scholar] [CrossRef]
- Banck, J.C.; Mueller, N.; Mellinghoff, S.C.; Thelen, M.; Fraccaroli, A.; Blumenberg, V.; Koehler, P.; Kunz, W.G.; Rudelius, M.; Schrötzlmair, F.; et al. Immune Checkpoint Blockade for Aspergillosis and Mucormycosis Coinfection. Hemasphere 2021, 5, e530. [Google Scholar] [CrossRef]
- Grimaldi, D.; Pradier, O.; Hotchkiss, R.S.; Vincent, J.-L. Nivolumab plus Interferon-γ in the Treatment of Intractable Mucormycosis. Lancet Infect. Dis. 2017, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Serris, A.; Ouedrani, A.; Uhel, F.; Gazzano, M.; Bedarida, V.; Rouzaud, C.; Bougnoux, M.-E.; Raphalen, J.-H.; Poirée, S.; Lambotte, O.; et al. Case Report: Immune Checkpoint Blockade Plus Interferon-Γ Add-On Antifungal Therapy in the Treatment of Refractory Covid-Associated Pulmonary Aspergillosis and Cerebral Mucormycosis. Front. Immunol. 2022, 13, 900522. [Google Scholar] [CrossRef]
- Wurster, S.; Watowich, S.S.; Kontoyiannis, D.P. Checkpoint Inhibitors as Immunotherapy for Fungal Infections: Promises, Challenges, and Unanswered Questions. Front. Immunol. 2022, 13, 1018202. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20, 5–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skiada, A.; Lanternier, F.; Groll, A.H.; Pagano, L.; Zimmerli, S.; Herbrecht, R.; Lortholary, O.; Petrikkos, G.L.; on behalf of the third European Conference on Infections in Leukemia*. Diagnosis and Treatment of Mucormycosis in Patients with Hematological Malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013, 98, 492–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedder, M.; Spratt, J.A.; Anstadt, M.P.; Hegde, S.S.; Tedder, S.D.; Lowe, J.E. Pulmonary Mucormycosis: Results of Medical and Surgical Therapy. Ann. Thorac. Surg. 1994, 57, 1044–1050. [Google Scholar] [CrossRef]
- Multani, A.; Reveron-Thornton, R.; Garvert, D.W.; Gomez, C.A.; Montoya, J.G.; Lui, N.S. Cut It out! Thoracic Surgeon’s Approach to Pulmonary Mucormycosis and the Role of Surgical Resection in Survival. Mycoses 2019, 62, 893–907. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.; Jeon, K.; Suh, G.Y.; Shin, S.; Kim, H.K.; Kim, K.; Jeong, D.; Kim, H. Factors Affecting Surgical Resection and Treatment Outcomes in Patients with Pulmonary Mucormycosis. J. Thorac. Dis. 2019, 11, 892–900. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive Zygomycosis in India: Experience in a Tertiary Care Hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Groll, A.H.; Desai, A.; Han, D.; Howieson, C.; Kato, K.; Akhtar, S.; Kowalski, D.; Lademacher, C.; Lewis, W.; Pearlman, H.; et al. Pharmacokinetic Assessment of Drug-Drug Interactions of Isavuconazole With the Immunosuppressants Cyclosporine, Mycophenolic Acid, Prednisolone, Sirolimus, and Tacrolimus in Healthy Adults. Clin. Pharmacol. Drug Dev. 2017, 6, 76–85. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danion, F.; Coste, A.; Le Hyaric, C.; Melenotte, C.; Lamoth, F.; Calandra, T.; Garcia-Hermoso, D.; Aimanianda, V.; Lanternier, F.; Lortholary, O. What Is New in Pulmonary Mucormycosis? J. Fungi 2023, 9, 307. https://doi.org/10.3390/jof9030307
Danion F, Coste A, Le Hyaric C, Melenotte C, Lamoth F, Calandra T, Garcia-Hermoso D, Aimanianda V, Lanternier F, Lortholary O. What Is New in Pulmonary Mucormycosis? Journal of Fungi. 2023; 9(3):307. https://doi.org/10.3390/jof9030307
Chicago/Turabian StyleDanion, François, Anne Coste, Coralie Le Hyaric, Clea Melenotte, Frederic Lamoth, Thierry Calandra, Dea Garcia-Hermoso, Vishukumar Aimanianda, Fanny Lanternier, and Olivier Lortholary. 2023. "What Is New in Pulmonary Mucormycosis?" Journal of Fungi 9, no. 3: 307. https://doi.org/10.3390/jof9030307
APA StyleDanion, F., Coste, A., Le Hyaric, C., Melenotte, C., Lamoth, F., Calandra, T., Garcia-Hermoso, D., Aimanianda, V., Lanternier, F., & Lortholary, O. (2023). What Is New in Pulmonary Mucormycosis? Journal of Fungi, 9(3), 307. https://doi.org/10.3390/jof9030307









