Development of Multiple Nucleotide Polymorphism Molecular Markers for Enoki Mushroom (Flammulina filiformis) Cultivars Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. F. filiformis Strains, DNA Extraction, and Whole-Genome Sequencing
2.2. Screening and Primer Design for MNP Markers in F. filiformis
2.3. Library Construction and MNP Sequencing
2.4. Core MNP Markers and Pedigree Determination
2.5. Genetic Similarity (GS) Calculation
3. Results
3.1. Genome Resequencing, Screening of Universal MNP Markers, and MNP-Seq
3.2. MNP Markers Evaluation
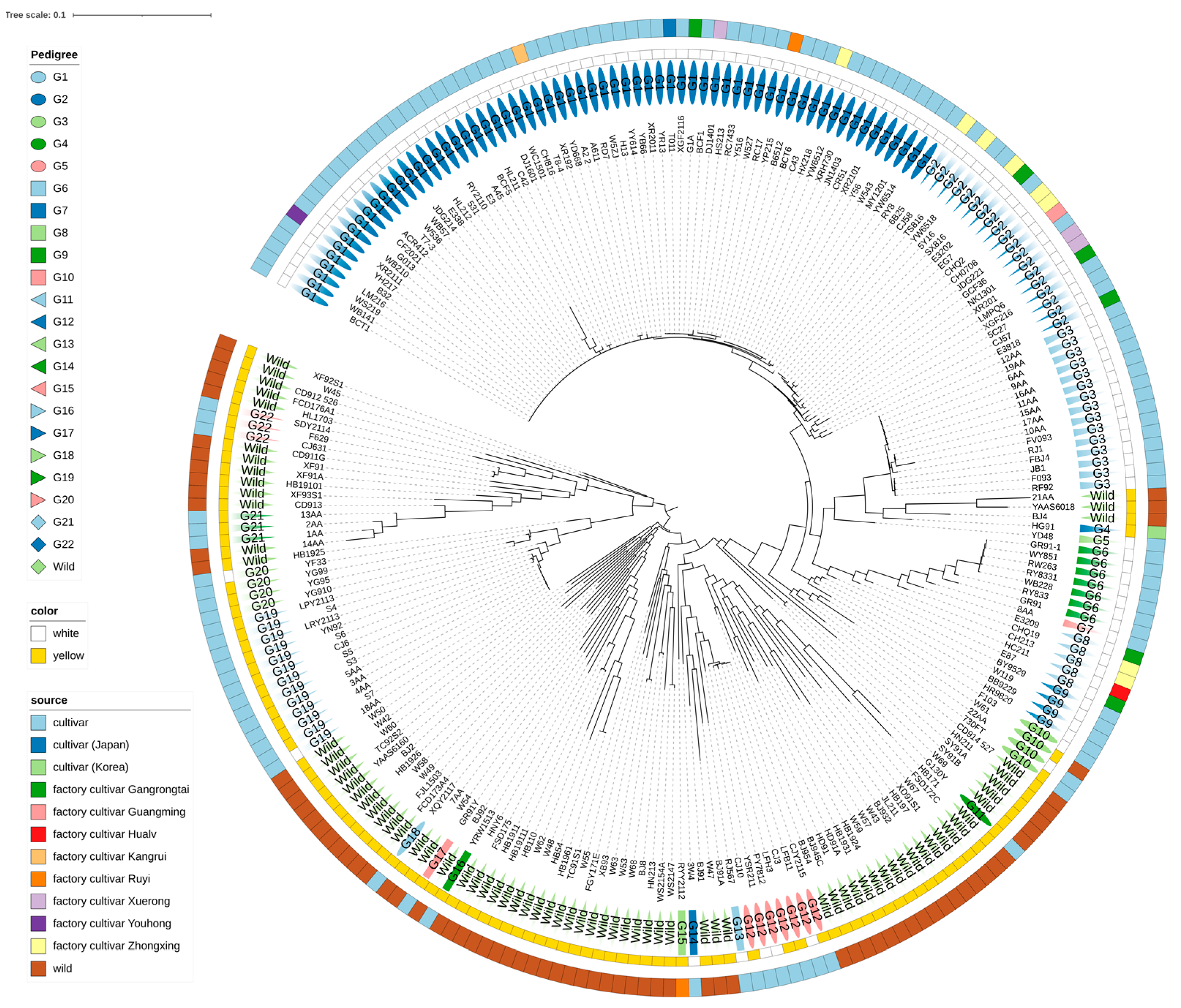
3.3. Construction of Phylogenetic Relationship Using Core MNP Sequences
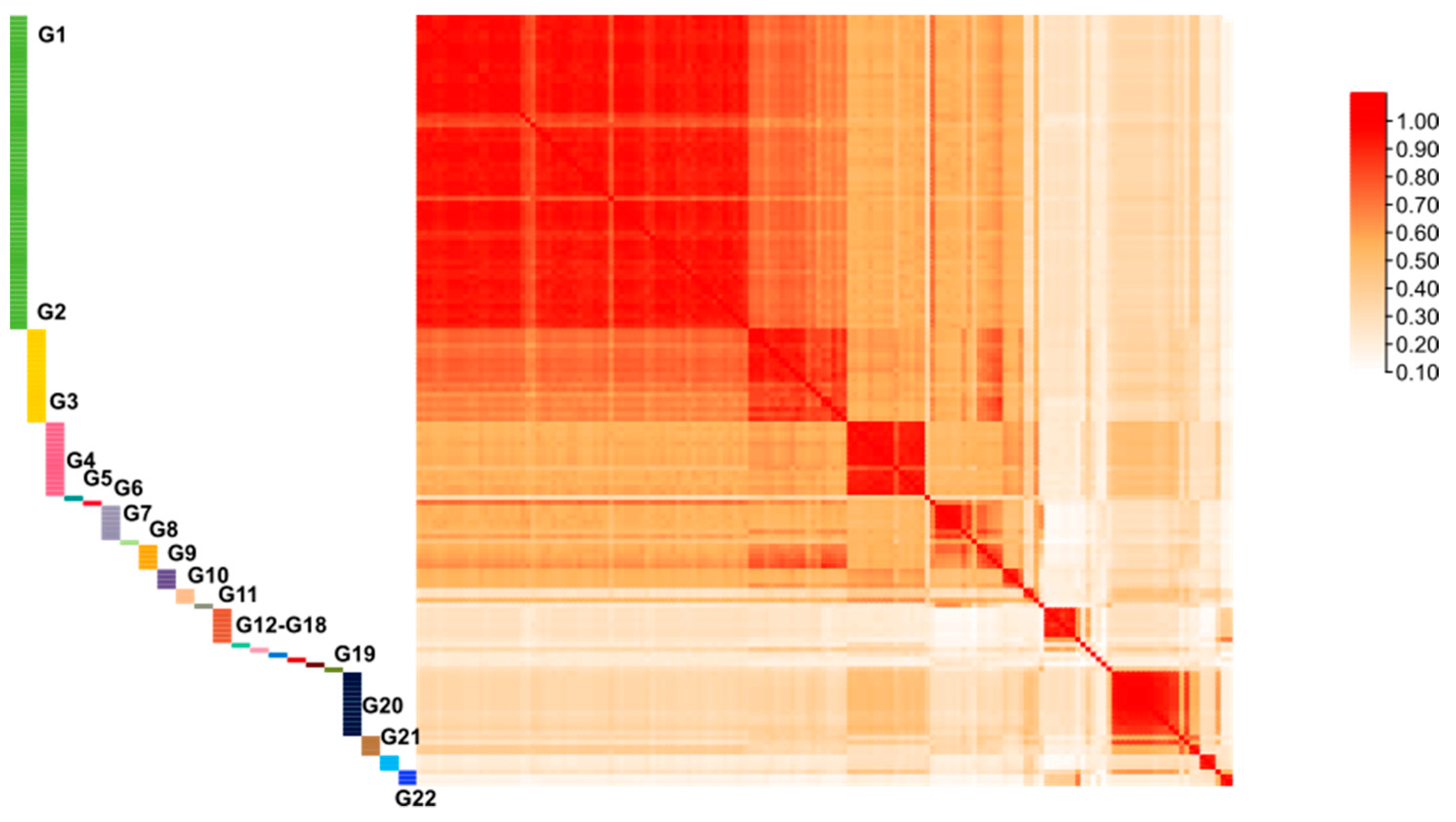
3.4. Genetic Similarity Values between Different Cultivars and Lineages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, S. Edible Mushroom Industry in China: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.I.; Yu, L.I. Quality Comparison and Analysis on White Flammulina Velutipes Grown with Bottle Lines in China. Edible Fungi China 2014, 33, 20–24. [Google Scholar]
- Flammulina Velutipes, The Culinary Medicinal Winter Mushroom|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Flammulina-velutipes-%2C-The-Culinary-Medicinal-Sharma-Kumar/61820873146f6d0281132097c4581ee2672d469c (accessed on 14 February 2023).
- Park, W.H.; Lee, H.D. Wild Fungi of Korea in Color; Kyo-Hak Publishing Co.: Seoul, Republic of Korea, 1991. [Google Scholar]
- Imazeki, R.; Otani, Y.; Hongo, T. Fungi of Japan; Yama-kei: Tokyo, Japan, 1988. [Google Scholar]
- Wang, P.M.; Liu, X.B.; Dai, Y.C.; Horak, E.; Steffen, K.; Yang, Z.L. Phylogeny and Species Delimitation of Flammulina: Taxonomic Status of Winter Mushroom in East Asia and a New European Species Identified Using an Integrated Approach. Mycol. Prog. 2018, 17, 1013–1030. [Google Scholar] [CrossRef]
- Ge, Z.-W.; Liu, X.-B.; Zhao, K.; Yang, Z.-L. Species Diversity of Flammulina in China: New Varieties and a New Record. Mycosystema 2015, 34, 589–603. [Google Scholar]
- Yy, L.; Mz, Z.; Zl, L.; Rl, Z. Evolutionary Relationship and a Novel Method of Efficient Identification of Lentinula Edodes Cultivars in China. Mycosphere 2022, 13, 56–85. [Google Scholar]
- Sonnenberg, A.S.M.; Baars, J.J.P.; Gao, W.; Visser, R.G.F. Developments in Breeding of Agaricus Bisporus Var. Bisporus: Progress Made and Technical and Legal Hurdles to Take. Appl. Microbiol. Biotechnol. 2017, 101, 1819–1829. [Google Scholar] [CrossRef]
- Palapala, V.A.; Aimi, T.; Inatomi, S.; Morinaga, T. ITS-PCR-RFLP Method for Distinguishing Commercial Cultivars of Edible Mushroom, Flammulina Velutipes. J. Food Sci. 2002, 67, 2486–2490. [Google Scholar] [CrossRef]
- Application of ISSR in Identifying <EM>Flammulina Velutipes</EM> Strains. Available online: http://www.cje.net.cn/EN/lexeme/showArticleByLexeme.do?articleID=15062 (accessed on 6 January 2023).
- Su, H.; Wang, L.; Liu, L.; Chi, X.; Zhang, Y. Use of Inter-Simple Sequence Repeat Markers to Develop Strain-Specific SCAR Markers ForFlammulina Velutipes. J. Appl. Genet. 2008, 49, 233–235. [Google Scholar] [CrossRef]
- Cocolin, L.; D’Agaro, E.; Manzano, M.; Lanari, D.; Comi, G. Rapid PCR-RFLP Method for the Identification of Marine Fish Fillets (Seabass, Seabream, Umbrine, and Dentex). J. Food Sci. 2000, 65, 1315–1317. [Google Scholar] [CrossRef]
- Genetic Diversity of Flannulina Velutipes Determined by ISSR Marker--<Edible Fungi of China>. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZSYJ200704014.htm (accessed on 6 January 2023).
- Xu, J. Fundamentals of Fungal Molecular Population Genetic Analyses. Curr. Issues Mol. Biol. 2006, 8, 75–90. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhou, J.; You, A.; Gao, L.; Li, T.; Chen, H.; Han, R.; Cui, Y.; Chen, L.; et al. Multiple Nucleotide Polymorphism DNA Markers for the Accurate Evaluation of Genetic Variations. bioRxiv 2021. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Zhou, J.; Chen, H.; Hu, Z.; Gao, L.; Chen, L.; Ren, S.; Ma, H.; Lu, L.; et al. An Accurate and Efficient Method for Large-Scale SSR Genotyping and Applications. Nucleic Acids Res. 2017, 45, e88. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Li, J.; Yang, Z.L. Genetic Diversity and Structure of Core Collection of Winter Mushroom (Flammulina Velutipes) Developed by Genomic SSR Markers. Hereditas 2018, 155, 3. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-I.; Seo, K.-I.; Jang, K.Y.; Im, J.-H.; Shin, P.-G.; Oh, Y.-L.; Oh, M.J.; Kong, W.-S. Genetic Relationships of Collected Strains Using Simple Sequence Repeat (SSR) Marker in Flammulina Velutipes. J. Mushrooms 2017, 21, 171. [Google Scholar]
- Liu, X.B.; Feng, B.; Li, J.; Yan, C.; Yang, Z.L. Genetic Diversity and Breeding History of Winter Mushroom (Flammulina Velutipes) in China Uncovered by Genomic SSR Markers. Gene 2016, 591, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Baek, J.H.; Lee, S.; Kim, C.; Rhee, H.; Kim, H.; Seo, J.-S.; Park, H.-R.; Yoon, D.-E.; Nam, J.-Y.; et al. Whole Genome and Global Gene Expression Analyses of the Model Mushroom Flammulina Velutipes Reveal a High Capacity for Lignocellulose Degradation. PLoS ONE 2014, 9, e93560. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Tang, W.; Xia, W.; Zhong, Y.; Xu, X.; Xie, B.; Tao, Y. Comprehensive Genetic Analysis of Monokaryon and Dikaryon Populations Provides Insight Into Cross-Breeding of Flammulina filiformis. Front. Microbiol. 2022, 13, 887259. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.-M.; Tang, Y.-J.; Ma, K.; Li, B.; Zeng, X.; Liu, X.-B.; Li, Y.; Yang, Z.-L.; Xu, W.-N.; et al. Genome-Wide Analysis and Prediction of Genes Involved in the Biosynthesis of Polysaccharides and Bioactive Secondary Metabolites in High-Temperature-Tolerant Wild Flammulina filiformis. BMC Genom. 2020, 21, 719. [Google Scholar] [CrossRef]
- Hosseinzadeh-Colagar, A.; Haghighatnia, M.J.; Amiri, Z.; Mohadjerani, M.; Tafrihi, M. Microsatellite (SSR) Amplification by PCR Usually Led to Polymorphic Bands: Evidence Which Shows Replication Slippage Occurs in Extend or Nascent DNA Strands. Mol. Biol. Res. Commun. 2016, 5, 167–174. [Google Scholar]
- Treangen, T.J.; Salzberg, S.L. Repetitive DNA and Next-Generation Sequencing: Computational Challenges and Solutions. Nat. Rev. Genet. 2011, 13, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor|Bioinformatics|Oxford Academic. Available online: https://academic.oup.com/bioinformatics/article/34/17/i884/5093234 (accessed on 6 January 2023).
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM 2013. Available online: https://doi.org/10.48550/arXiv.1303.3997 (accessed on 6 January 2023).
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Song, D.L.; Zeng, X.X.; Jie, L.; Chang-Wu, L.; Bao-Shan, W.U. Progress of Study on Genetic and Breeding of Flammulina Velutipes. Seed 2007, 26, 52–54. [Google Scholar]
- Al-Khayri, J.M.; Jain, S.M.; Johnson, D.V. (Eds.) Advances in Plant Breeding Strategies: Vegetable Crops: Volume 10: Leaves, Flowerheads, Green Pods, Mushrooms and Truffles; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-66968-3. [Google Scholar]

| Sample | Clean Base (bp) | Average Mapping Rate of Reads | Mean Genome Coverage | Mean Depth | Cap Color | Source | Location |
|---|---|---|---|---|---|---|---|
| W67 | 4,925,351,700 | 80.00% | 94.00% | 221.7 | yellow | wild | Beijing |
| 21AA | 6,042,946,800 | 91.90% | 88.80% | 193.3 | yellow | wild | Beijing |
| SY91B | 6,063,577,800 | 91.10% | 88.90% | 168.3 | yellow | wild | Liaoning |
| HD91A | 5,816,764,800 | 89.10% | 88.90% | 165.8 | yellow | wild | Sichuan |
| XF92S1 | 5,529,307,200 | 91.80% | 88.80% | 165.6 | yellow | wild | Xinjiang |
| XF93S1 | 4,779,288,600 | 90.90% | 88.80% | 159.5 | yellow | wild | Xinjiang |
| W63 | 5,173,407,000 | 90.00% | 88.70% | 158.5 | yellow | wild | Hebei |
| FCD173A4 | 5,787,528,300 | 91.30% | 88.20% | 157.8 | yellow | wild | Chengdu |
| W57 | 4,929,271,800 | 91.40% | 88.80% | 157.6 | yellow | wild | Hebei |
| W58 | 4,854,398,100 | 90.90% | 88.70% | 156.5 | yellow | wild | Hebei |
| YAAS6160 | 5,502,118,200 | 89.80% | 88.70% | 156.3 | yellow | wild | Yunnan |
| HN211 | 7,548,748,200 | 90.90% | 88.70% | 154.7 | yellow | wild | - |
| TC91S1 | 5,705,983,200 | 91.60% | 89.20% | 154.7 | yellow | wild | Sichuan |
| 14AA | 6,023,080,200 | 92.70% | 88.70% | 152.7 | yellow | wild | Beijing |
| BJ8 | 6,255,115,800 | 87.20% | 91.00% | 151.8 | yellow | wild | Beijing |
| WS2154A | 6,028,663,200 | 91.90% | 88.70% | 149.5 | yellow | wild | Xinjiang |
| W68 | 5,331,615,600 | 86.60% | 97.30% | 149.2 | yellow | wild | Beijing |
| W55 | 5,559,805,200 | 92.10% | 88.70% | 147.8 | yellow | wild | Shandong |
| HN213 | 9,280,081,500 | 86.00% | 88.60% | 146.1 | yellow | wild | - |
| W69 | 4,878,531,000 | 91.90% | 88.70% | 145.7 | yellow | wild | Heilongjiang |
| HB19101 | 4,991,530,200 | 91.60% | 88.60% | 145.4 | yellow | wild | Hebei |
| W62 | 5,370,762,900 | 88.90% | 88.50% | 144.7 | yellow | wild | Hebei |
| FGY171E | 5,881,290,300 | 85.10% | 88.70% | 144.5 | yellow | wild | Sichuan |
| CD913 | 4,840,125,600 | 83.30% | 88.70% | 144.3 | yellow | wild | - |
| XD91S1 | 5,379,901,800 | 83.60% | 90.40% | 144.3 | yellow | wild | Sichuan |
| W50 | 4,936,523,400 | 90.90% | 88.70% | 143.7 | yellow | wild | Liaoning |
| XF91A | 5,950,436,400 | 86.30% | 91.30% | 143.5 | yellow | wild | Xinjiang |
| XB93 | 5,463,701,400 | 89.60% | 88.70% | 141.9 | yellow | wild | Xinjiang |
| W49 | 4,947,195,300 | 90.90% | 91.20% | 141.7 | yellow | wild | Liaoning |
| W54 | 4,989,796,500 | 92.00% | 88.70% | 140.7 | yellow | wild | Shandong |
| W48 | 5,479,733,400 | 92.20% | 89.10% | 139 | yellow | wild | Sichuan |
| W61 | 5,227,953,300 | 92.60% | 87.80% | 137.2 | yellow | wild | Hebei |
| SY91A | 5,682,915,300 | 85.50% | 92.10% | 130.6 | yellow | wild | Liaoning |
| W45 | 4,996,704,900 | 89.70% | 91.00% | 126.9 | yellow | wild | Sichuan |
| 7AA | 6,039,291,000 | 87.50% | 90.20% | 126.3 | yellow | wild | Australia |
| W59 | 4,987,322,100 | 92.00% | 88.90% | 125.7 | yellow | wild | Hebei |
| HL1703 | 6,084,036,300 | 88.60% | 89.40% | 125.6 | yellow | wild | Sichuan |
| W43 | 4,937,946,000 | 82.90% | 92.80% | 125.4 | yellow | wild | Sichuan |
| W60 | 4,909,563,000 | 88.30% | 92.10% | 123.3 | yellow | wild | Hebei |
| FJL1503 | 5,474,032,200 | 86.70% | 91.20% | 122.1 | yellow | wild | Jilin |
| HB1925 | 4,921,756,800 | 89.50% | 89.80% | 122.1 | yellow | wild | Hebei |
| TC92S2 | 5,484,576,000 | 88.40% | 92.10% | 122.1 | yellow | wild | Sichuan |
| BJ2 | 5,834,235,600 | 87.60% | 90.50% | 121.4 | yellow | wild | Beijing |
| HNY6 | 6,984,377,400 | 88.90% | 92.00% | 121.2 | yellow | wild | - |
| W42 | 5,374,737,000 | 87.50% | 90.20% | 119.4 | yellow | wild | Sichuan |
| HB1924 | 5,373,926,100 | 88.40% | 91.90% | 117.6 | yellow | wild | Hebei |
| HB1926 | 5,279,833,800 | 87.90% | 90.90% | 115.2 | yellow | wild | Hebei |
| HB110 | 4,965,000,300 | 88.20% | 92.40% | 112 | yellow | wild | Hebei |
| HB1931 | 5,207,298,000 | 89.00% | 90.40% | 111.3 | yellow | wild | Hebei |
| HB19111 | 5,721,979,500 | 88.80% | 92.80% | 110.3 | yellow | wild | Hebei |
| W47 | 5,151,517,800 | 89.30% | 91.60% | 109.1 | yellow | wild | Sichuan |
| FCD176A1 | 5,711,787,300 | 86.30% | 90.90% | 108.9 | yellow | wild | Chengdu |
| FSD175 | 5,342,841,600 | 88.20% | 92.60% | 108.7 | yellow | wild | Chengdu |
| HB197 | 5,176,382,400 | 87.80% | 90.30% | 108.4 | yellow | wild | Hebei |
| CD912_526 | 6,326,020,800 | 86.30% | 89.90% | 108 | yellow | wild | Chengdu |
| BJ932 | 5,390,764,200 | 81.30% | 91.40% | 106.5 | yellow | wild | - |
| BJ91A | 5,930,747,700 | 83.80% | 91.30% | 104.6 | yellow | wild | - |
| CD914_527 | 5,281,848,600 | 64.70% | 90.90% | 80.8 | yellow | wild | Chengdu |
| FSD172C | 5,748,301,500 | 63.90% | 91.60% | 79.9 | yellow | wild | Chengdu |
| BJ945C | 4,855,080,900 | 41.70% | 91.20% | 57.4 | yellow | wild | Beijing |
| BJ91 | 5,108,668,500 | 37.10% | 91.90% | 50.1 | yellow | wild | Beijing |
| W53 | 4,953,074,400 | 28.40% | 88.30% | 49.5 | yellow | wild | Shandong |
| HB1961 | 4,988,508,600 | 32.80% | 89.30% | 43.9 | yellow | wild | Hebei |
| B6512 | 13,623,971,700 | 92.30% | 88.50% | 321.6 | white | cultivar | - |
| YY614 | 6,801,244,200 | 85.20% | 88.70% | 224.2 | white | cultivar | - |
| C43 | 4,652,067,600 | 90.20% | 88.70% | 190.4 | white | cultivar | Fujian |
| YR13 | 6,196,084,800 | 90.90% | 97.80% | 180.8 | white | cultivar | - |
| WB210 | 7,274,784,900 | 91.60% | 88.80% | 180.7 | white | cultivar | - |
| YD668 | 6,883,548,000 | 89.00% | 91.70% | 176.6 | white | cultivar | - |
| XR2111 | 6,265,922,100 | 75.60% | 91.50% | 172.4 | white | cultivar | - |
| C42 | 4,554,684,900 | 90.60% | 88.70% | 168.2 | white | cultivar | Fujian |
| XR2101 | 5,978,366,100 | 90.00% | 91.90% | 163.9 | white | cultivar | - |
| W536 | 6,755,451,000 | 85.60% | 91.50% | 163.2 | white | cultivar | - |
| RD7 | 6,676,121,400 | 93.10% | 88.50% | 161.6 | white | cultivar | Sichuan |
| BY9529 | 6,016,801,500 | 89.00% | 88.70% | 161.4 | white | cultivar | - |
| XRH730 | 6,742,733,700 | 90.00% | 88.60% | 158.6 | white | cultivar | - |
| WB228 | 5,533,026,600 | 89.60% | 88.30% | 157 | white | cultivar | - |
| XR192 | 6,175,200,600 | 90.60% | 89.20% | 157 | white | cultivar | Japan |
| W5ZJ | 6,198,809,700 | 88.50% | 91.20% | 156.9 | white | cultivar | - |
| YN92 | 5,416,198,800 | 90.70% | 88.70% | 155.9 | yellow | cultivar | - |
| GR91-1 | 6,788,983,800 | 90.30% | 88.30% | 154.4 | white | cultivar | - |
| GR91-1 | 6,788,983,800 | 90.30% | 88.30% | 154.4 | white | cultivar | - |
| G013 | 5,320,581,300 | 90.80% | 88.60% | 153.9 | white | cultivar | Japan |
| B32 | 5,648,609,700 | 91.90% | 89.00% | 153.7 | white | cultivar | Sichuan |
| W527 | 6,783,499,800 | 85.80% | 88.70% | 149.3 | white | cultivar | - |
| YRW1513 | 6,790,506,600 | 83.10% | 97.80% | 147.7 | yellow | cultivar | - |
| 9AA | 6,002,955,600 | 91.40% | 93.70% | 147.5 | white | cultivar | HongKong |
| WB141 | 5,889,080,100 | 84.10% | 88.60% | 147.3 | white | cultivar | Japan |
| WC1501 | 6,640,416,000 | 90.30% | 88.70% | 147.1 | white | cultivar | - |
| WB57 | 6,649,017,300 | 82.10% | 88.70% | 146.8 | white | cultivar | - |
| Y56 | 5,334,398,400 | 85.70% | 88.80% | 146.3 | white | cultivar | - |
| 3AA | 5,784,305,700 | 93.60% | 97.40% | 145.6 | yellow | cultivar | Hebei |
| XR2011 | 6,867,214,800 | 88.80% | 91.80% | 145.1 | white | cultivar | - |
| WS219 | 7,213,323,900 | 90.10% | 91.30% | 144.8 | white | cultivar | - |
| YSR211 | 6,521,972,100 | 91.20% | 93.70% | 144.2 | white | cultivar | - |
| RF92 | 5,552,944,800 | 90.60% | 92.30% | 143.8 | white | cultivar | Japan |
| 19AA | 5,928,289,500 | 92.50% | 96.20% | 143.3 | white | cultivar | Japan |
| 5AA | 5,330,144,700 | 92.60% | 97.80% | 142.1 | yellow | cultivar | Hunan |
| GR91 | 5,641,761,300 | 90.90% | 88.70% | 141.8 | white | cultivar | - |
| E338 | 6,093,056,400 | 88.20% | 88.40% | 141.6 | white | cultivar | Japan |
| XGF2116 | 5,892,126,000 | 91.50% | 88.70% | 141 | white | cultivar | - |
| RJ1 | 7,311,964,500 | 90.80% | 88.80% | 140.9 | white | cultivar | - |
| BCT6 | 8,254,567,800 | 90.00% | 88.60% | 140.7 | white | cultivar | - |
| 13AA | 5,966,978,100 | 91.60% | 97.20% | 139.7 | yellow | cultivar | Fujian |
| 17AA | 6,035,661,600 | 90.40% | 88.40% | 138.8 | white | cultivar | Shanghai |
| CH816 | 6,228,906,600 | 87.30% | 89.90% | 138.1 | white | cultivar | - |
| G130Y | 5,248,216,200 | 86.90% | 88.60% | 137.7 | yellow | cultivar | - |
| 18AA | 6,025,245,900 | 88.90% | 88.60% | 137.1 | white | cultivar | Japan |
| ACR412 | 6,455,527,800 | 90.40% | 88.60% | 136.5 | white | cultivar | - |
| RC17 | 6,888,097,800 | 87.70% | 88.70% | 136.5 | white | cultivar | - |
| A611 | 5,756,805,300 | 89.20% | 88.50% | 136 | white | cultivar | Japan |
| 15AA | 6,039,788,100 | 88.30% | 93.00% | 135.8 | white | cultivar | Hebei |
| BB9229 | 7,076,914,800 | 92.00% | 88.70% | 135.6 | white | cultivar | - |
| 16AA | 6,014,332,500 | 87.60% | 88.80% | 135.2 | white | cultivar | Fujian |
| 531 | 6,054,606,900 | 91.70% | 96.80% | 134.5 | white | cultivar | - |
| BCF1 | 6,116,104,800 | 81.60% | 89.00% | 134.4 | white | cultivar | - |
| YW6514 | 6,854,542,500 | 87.60% | 90.30% | 134 | white | cultivar | - |
| 10AA | 5,958,497,100 | 87.70% | 88.50% | 133 | white | cultivar | Japan |
| 2AA | 5,055,837,600 | 85.90% | 94.30% | 132.7 | yellow | cultivar | Guizhou |
| FBJ4 | 5,715,221,700 | 90.90% | 98.00% | 131.9 | white | cultivar | Shanghai |
| RC7433 | 5,878,678,500 | 91.20% | 88.70% | 131.7 | white | cultivar | - |
| A2_2 | 5,899,424,700 | 86.60% | 90.30% | 131.4 | white | cultivar | Sichuan |
| CF2021 | 6,746,666,100 | 88.40% | 92.20% | 130.8 | white | cultivar | - |
| 6AA | 5,466,440,700 | 84.20% | 88.60% | 130.3 | white | cultivar | Liaoning |
| Y516 | 6,732,100,800 | 85.60% | 88.70% | 128.5 | white | cultivar | - |
| BCF5 | 7,092,733,800 | 88.60% | 88.70% | 128.1 | white | cultivar | - |
| YB66 | 5,790,196,200 | 90.00% | 88.30% | 128 | white | cultivar | - |
| T7-3 | 6,441,932,700 | 87.00% | 90.00% | 127.9 | white | cultivar | - |
| 11AA | 6,034,369,800 | 82.80% | 88.50% | 127.1 | white | cultivar | Taiwan |
| F629 | 5,567,275,200 | 85.60% | 92.20% | 126 | yellow | cultivar | - |
| JN1403 | 6,360,789,300 | 84.70% | 89.70% | 125.8 | white | cultivar | - |
| HS213 | 6,842,381,400 | 88.50% | 93.90% | 125.5 | white | cultivar | - |
| 730FT | 6,296,794,500 | 92.00% | 97.80% | 125.2 | yellow | cultivar | Sichuan |
| JDG214 | 6,994,112,100 | 85.80% | 91.10% | 124.3 | white | cultivar | - |
| JB1 | 6,854,586,000 | 83.60% | 92.50% | 122.4 | white | cultivar | - |
| 22AA | 6,028,996,500 | 91.90% | 97.20% | 122.3 | white | cultivar | Beijing |
| 4AA | 6,006,551,100 | 92.30% | 95.40% | 121.4 | yellow | cultivar | Henan |
| CR51 | 5,990,160,600 | 79.00% | 91.00% | 121 | white | cultivar | - |
| RY8 | 7,670,523,000 | 88.20% | 92.10% | 120.3 | white | cultivar | - |
| 8AA | 5,137,149,900 | 85.50% | 91.30% | 118.7 | white | cultivar | Australia |
| LFB11 | 6,313,579,200 | 85.50% | 91.70% | 118.3 | white | cultivar | - |
| LRY2113 | 6,165,061,500 | 81.30% | 89.10% | 118.3 | yellow | cultivar | - |
| 1AA | 5,212,569,900 | 77.10% | 88.40% | 116.4 | yellow | cultivar | Yunnan |
| CJ631 | 6,686,406,300 | 85.20% | 91.80% | 116.4 | yellow | cultivar | - |
| A45 | 6,532,073,700 | 88.60% | 87.10% | 115.3 | white | cultivar | Japan |
| CJ6 | 5,651,249,100 | 80.40% | 90.50% | 114.5 | yellow | cultivar | Sichuan |
| WY851 | 6,602,663,700 | 86.40% | 91.20% | 114.3 | white | cultivar | - |
| RY2110 | 6,243,451,800 | 76.80% | 90.50% | 111.1 | white | cultivar | - |
| DJ1601 | 6,199,958,700 | 91.00% | 88.60% | 108.1 | white | cultivar | - |
| F093 | 6,686,192,400 | 88.00% | 91.60% | 108.1 | white | cultivar | - |
| 12AA | 5,564,021,100 | 76.50% | 88.20% | 107.9 | white | cultivar | Taiwan |
| RJ567 | 7,835,205,300 | 82.80% | 90.10% | 104.2 | yellow | cultivar | - |
| YW6512 | 7,055,345,700 | 71.30% | 90.40% | 102 | white | cultivar | - |
| E3 | 6,845,109,300 | 73.20% | 91.80% | 98.9 | white | cultivar | - |
| SDY2114 | 6,203,431,800 | 74.50% | 90.70% | 97.5 | yellow | cultivar | - |
| HL212 | 6,803,610,300 | 93.20% | 93.50% | 92.3 | white | cultivar | - |
| RY8331 | 6,712,070,400 | 73.50% | 91.00% | 91.4 | white | cultivar | - |
| LM216 | 6,020,644,200 | 89.70% | 88.00% | 91.2 | white | cultivar | - |
| LFH3 | 5,185,681,800 | 91.60% | 86.10% | 90.4 | yellow | cultivar | - |
| HL211 | 6,303,453,900 | 86.40% | 86.60% | 85.6 | white | cultivar | - |
| GR91Y | 6,230,154,600 | 89.90% | 91.30% | 79.6 | yellow | cultivar | - |
| H13 | 6,061,021,500 | 89.50% | 90.40% | 71.4 | white | cultivar | Japan |
| RW263 | 6,773,640,600 | 55.20% | 91.00% | 69.8 | white | cultivar | - |
| CJY2115 | 6,064,386,900 | 44.00% | 91.80% | 53.7 | yellow | cultivar | - |
| Sample | Clean Reads | Average Coverage of MNP Markers | Number of MNP Marker Detected | Cap Color | Source |
|---|---|---|---|---|---|
| XF91 | 8217560 | 20,235 | 145 | yellow | wild |
| BJ92 | 7584880 | 18,428 | 132 | yellow | wild |
| HB171 | 7272776 | 17,543 | 144 | yellow | wild |
| YAAS6018 | 12684464 | 31,165 | 133 | yellow | wild |
| BJ954 | 7633094 | 19,057 | 150 | yellow | wild |
| BJ4 | 8252300 | 17,824 | 134 | yellow | wild |
| WS2147 | 8901998 | 22,015 | 166 | yellow | wild |
| HB54 | 12034098 | 29,346 | 153 | yellow | wild |
| JL211 | 8716782 | 21,508 | 141 | yellow | wild |
| HD91 | 11488732 | 28,263 | 153 | yellow | wild |
| CD911G | 10396374 | 25,940 | 153 | yellow | wild |
| HB1911 | 7681900 | 19,159 | 122 | yellow | wild |
| SX816 | 6655314 | 16,590 | 138 | white | factory cultivar Zhongxing |
| 6B25 | 9585640 | 23,870 | 143 | white | factory cultivar Zhongxing |
| CHQ19 | 7473658 | 18,585 | 137 | white | factory cultivar Zhongxing |
| TS816 | 8376310 | 20,840 | 141 | white | factory cultivar Zhongxing |
| HX218 | 6678532 | 16,748 | 120 | white | factory cultivar Zhongxing |
| CHQ2 | 7716738 | 19,252 | 138 | white | factory cultivar Zhongxing |
| CH213 | 9069074 | 22,606 | 141 | white | factory cultivar Zhongxing |
| CH0708 | 10101620 | 25,129 | 131 | white | factory cultivar Zhongxing |
| YH217 | 9477522 | 23,555 | 151 | white | factory cultivar Youhong |
| NK1301 | 8298778 | 20,665 | 138 | white | factory cultivar Xuerong |
| DJ1401 | 8074850 | 20,094 | 135 | white | factory cultivar Xuerong |
| XR201 | 8891596 | 22,120 | 132 | white | factory cultivar Xuerong |
| RYY2112 | 8744304 | 21,595 | 143 | yellow | factory cultivar Ruyi |
| YP215 | 7452770 | 18,548 | 128 | white | factory cultivar Ruyi |
| T8-4 | 11198626 | 27,941 | 134 | white | factory cultivar Kangrui |
| HC211 | 7525108 | 18,780 | 131 | white | factory cultivar Hualv |
| JDG221 | 7407104 | 18,453 | 139 | white | factory cultivar Guangming |
| E3209 | 7651640 | 19,053 | 132 | white | factory cultivar Gangrongtai |
| LMPQ6 | 8723392 | 21,702 | 137 | white | factory cultivar Gangrongtai |
| G1A | 7270186 | 18,124 | 132 | white | factory cultivar Gangrongtai |
| E3202 | 8027632 | 20,002 | 137 | white | factory cultivar Gangrongtai |
| E3818 | 8273004 | 20,610 | 134 | white | factory cultivar Gangrongtai |
| E87 | 9291466 | 23,082 | 136 | white | factory cultivar Gangrongtai |
| XGF216 | 8798058 | 21,892 | 135 | white | factory cultivar |
| MY1201 | 8029920 | 19,940 | 141 | white | cultivar (Taiwan) |
| HG91 | 9004562 | 21,389 | 141 | yellow | cultivar (Korea) |
| T011 | 8770926 | 21,975 | 134 | white | cultivar (Japan) |
| XQY2117 | 8060156 | 20,159 | 154 | yellow | cultivar (Guangdong) |
| HR9820 | 8631350 | 20,746 | 143 | white | cultivar |
| RY833 | 9599096 | 23,888 | 145 | white | cultivar |
| 5Y16 | 7134102 | 17,761 | 134 | white | cultivar |
| YG99 | 9588270 | 23,787 | 150 | yellow | cultivar |
| CJ10 | 9040974 | 22,227 | 156 | yellow | cultivar |
| BCT1 | 7396960 | 18,446 | 139 | white | cultivar |
| PY7812 | 8825106 | 22,022 | 147 | white | cultivar |
| F103 | 7472462 | 18,523 | 163 | white | cultivar |
| FV093 | 8856082 | 22,150 | 122 | white | cultivar |
| 3W4 | 9080266 | 22,622 | 147 | white | cultivar |
| S7 | 8393386 | 20,712 | 153 | yellow | cultivar |
| YG910 | 8663284 | 21,498 | 155 | yellow | cultivar |
| YG95 | 7334222 | 18,219 | 159 | yellow | cultivar |
| GCF36 | 10356488 | 25,876 | 146 | white | cultivar |
| CJ57 | 8307228 | 20,691 | 125 | white | cultivar |
| CJ58 | 8636466 | 21,516 | 137 | white | cultivar |
| LPY2113 | 10584644 | 26,393 | 147 | yellow | cultivar |
| W543 | 8411992 | 20,871 | 135 | white | cultivar |
| YF33 | 8865860 | 21,905 | 145 | white | cultivar |
| W119 | 7222580 | 16,310 | 122 | white | cultivar |
| YW6518 | 7632486 | 18,976 | 128 | white | cultivar |
| S6 | 8391306 | 20,817 | 151 | yellow | cultivar |
| YD48 | 8491296 | 21,116 | 139 | white | cultivar |
| 5C27 | 12126742 | 30,149 | 130 | white | cultivar |
| CJ3 | 7872246 | 19,513 | 148 | yellow | cultivar |
| EG7 | 11316622 | 28,131 | 144 | white | cultivar |
| S4 | 18481160 | 45,878 | 164 | yellow | cultivar |
| S5 | 9782768 | 24,268 | 157 | yellow | cultivar |
| S3 | 10382322 | 25,833 | 150 | yellow | cultivar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, S.-H.; Jia, D.-H.; Tan, H.; Wang, B.; Zhao, R.-L. Development of Multiple Nucleotide Polymorphism Molecular Markers for Enoki Mushroom (Flammulina filiformis) Cultivars Identification. J. Fungi 2023, 9, 330. https://doi.org/10.3390/jof9030330
Liu F, Wang S-H, Jia D-H, Tan H, Wang B, Zhao R-L. Development of Multiple Nucleotide Polymorphism Molecular Markers for Enoki Mushroom (Flammulina filiformis) Cultivars Identification. Journal of Fungi. 2023; 9(3):330. https://doi.org/10.3390/jof9030330
Chicago/Turabian StyleLiu, Fei, Shi-Hui Wang, Ding-Hong Jia, Hao Tan, Bo Wang, and Rui-Lin Zhao. 2023. "Development of Multiple Nucleotide Polymorphism Molecular Markers for Enoki Mushroom (Flammulina filiformis) Cultivars Identification" Journal of Fungi 9, no. 3: 330. https://doi.org/10.3390/jof9030330
APA StyleLiu, F., Wang, S.-H., Jia, D.-H., Tan, H., Wang, B., & Zhao, R.-L. (2023). Development of Multiple Nucleotide Polymorphism Molecular Markers for Enoki Mushroom (Flammulina filiformis) Cultivars Identification. Journal of Fungi, 9(3), 330. https://doi.org/10.3390/jof9030330








