Genome-Wide Characterization of Effector Protein-Encoding Genes in Sclerospora graminicola and Its Validation in Response to Pearl Millet Downy Mildew Disease Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. GeneMark-ES Suite
2.2. Identification of Signal Peptides (SP) in the N-Terminal Region
2.3. Target P Server
2.4. TMHMM v2.0
2.5. Host and Pathogen
2.6. Extraction of RNA from Scelrospora Graminicola and cDNA Synthesis
2.7. Primer Designing
2.8. PCR Amplification
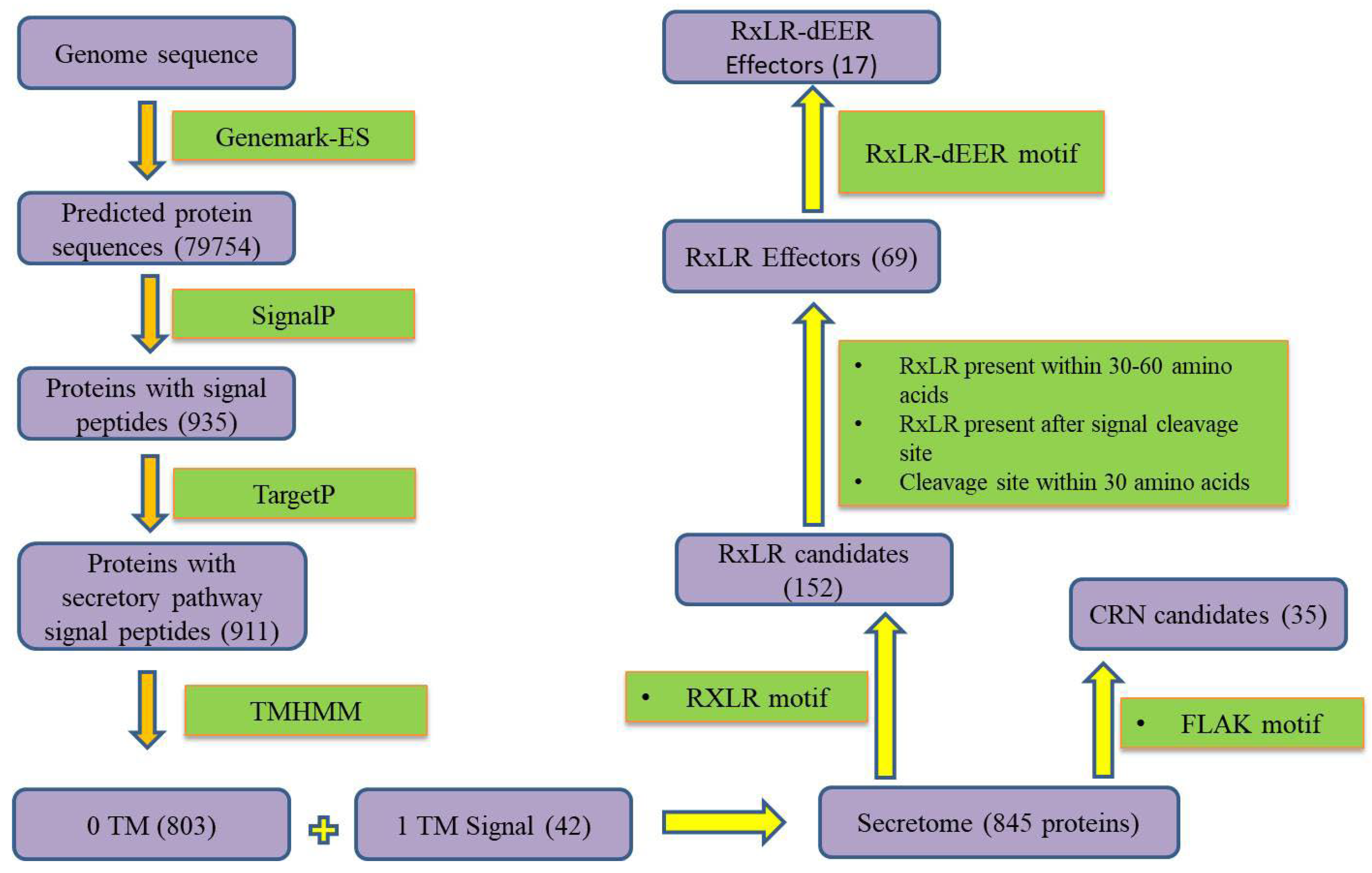
3. Results
3.1. Secretome Mapping
3.2. Annotation for Crinklerand RxLR Effectors
3.3. EffectorP Machine Learning
3.4. Similarity Search Using NCBI BLAST Tool
3.5. Confirmation of the Presence of RxLR-dEER Effectors Genes
3.6. Analysis of Overall Disorder Regions in RxLR-dEER Effector Proteins Using PONDR VL-XT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhi, N.; Gorenkin, D.; Banfield, M.J. Exploring folds, evolution and host interactions: Understanding effector structure/function in disease and immunity. New Phytol. 2020, 227, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yao, L.; Singer, S.D.; Muhammad, H.; Li, Z.; Wang, X. Constitutive heterologous overexpression of a TIR-NB-ARC-LRR gene encoding a putative disease resistance protein from wild Chinese Vitis pseudoreticulata in Arabidopsis and tobacco enhances resistance to phytopathogenic fungi and bacteria. Plant Physiol. Biochem. 2017, 112, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Newman, T.E.; Khentry, Y.; Taiwo, A.O. The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 1075–1090. [Google Scholar] [CrossRef]
- Kanja, C.; Hammond-Kosack, K.E. Proteinaceous effector discovery and characterization in filamentous plant pathogens. Mol. Plant Pathol. 2020, 21, 1353–1376. [Google Scholar] [CrossRef]
- Presti, L.L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; de Rond, L.; Bertolin, N.; Wouters, R.H.M.; Wouters, D.; Domazakis, E.; Bitew, M.K.; Win, J.; Dong, S.; et al. Divergent Evolution of PcF/SCR74 Effectors in Oomycetes Is Associated with Distinct Recognition Patterns in Solanaceous Plants. Mbio 2020, 11, e00947-20. [Google Scholar] [CrossRef]
- Outram, M.A.; Figueroa, M.; Sperschneider, J.; Williams, S.J.; Dodds, P.N. Seeing is believing: Exploiting advances in structural biology to understand and engineer plant immunity. Curr. Opin. Plant Biol. 2022, 67, 102210. [Google Scholar] [CrossRef]
- Arroyo-Velez, N.; González-Fuente, M.; Peeters, N.; Lauber, E.; Noël, L.D. From effectors to effectomes: Are functional studies of individual effectors enough to decipher plant pathogen infectious strategies? PLoS Pathog. 2020, 16, e1009059. [Google Scholar] [CrossRef] [PubMed]
- Chepsergon, J.; Motaung, T.E.; Bellieny-Rabelo, D.; Moleleki, L.N. Organize, Don’t Agonize: Strategic Success of Phytophthora Species. Microorganisms 2020, 8, 917. [Google Scholar] [CrossRef]
- Perrine-Walker, F. Phytophthorapalmivora–Cocoa Interaction. J. Fungi 2020, 6, 167. [Google Scholar] [CrossRef]
- Wang, W.; Jiao, F. Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity. Planta 2019, 250, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawra, S.; Belmonte, R.; Löbach, L.; Saraiva, M.; Willems, A.; van West, P. Secretion, delivery and function of oomycete effector proteins. Curr. Opin. Microbiol. 2012, 15, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximo, H.J.; Dalio, R.J.D.; Dias, R.O.; Litholdo, C.G.; Felizatti, H.L.; Machado, M.A. PpCRN7 and PpCRN20 of Phythophthora parasitica regulate plant cell death leading to enhancement of host susceptibility. BMC Plant Biol. 2019, 19, 544. [Google Scholar] [CrossRef] [Green Version]
- Baxter, L.; Tripathy, S.; Ishaque, N.; Boot, N.; Cabral, A.; Kemen, E.; Thines, M.; Ah-Fong, A.; Anderson, R.; Badejoko, W.; et al. Signatures of Adaptation to Obligate Biotrophy in the Hyaloperonospora arabidopsidis Genome. Science 2010, 330, 1549–1551. [Google Scholar] [CrossRef] [Green Version]
- Schornack, S.; van Damme, M.; Bozkurt, T.O.; Cano, L.M.; Smoker, M.; Thines, M.; Gaulin, E.; Kamoun, S.; Huitema, E. Ancient class of translocated oomycete effectors targets the host nucleus. Proc. Natl. Acad. Sci. USA 2010, 107, 17421–17426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecrix, Y.; Buendia, L.; Penouilh-Suzette, C.; Maréchaux, M.; Legrand, L.; Bouchez, O.; Rengel, D.; Gouzy, J.; Cottret, L.; Vear, F.; et al. Sunflower resistance to multiple downy mildew pathotypes revealed by recognition of conserved effectors of the oomycete Plasmopara halstedii. Plant J. 2018, 97, 730–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purayannur, S.; Cano, L.M.; Bowman, M.J.; Childs, K.L.; Gent, D.H.; Quesada-Ocampo, L.M. The Effector Repertoire of the Hop Downy Mildew Pathogen Pseudoperonospora humuli. Front. Genet. 2020, 11, 910. [Google Scholar] [CrossRef]
- Yang, L.-N.; Liu, H.; Duan, G.-H.; Huang, Y.-M.; Liu, S.; Fang, Z.-G.; Wu, E.-J.; Shang, L.; Zhan, J. The Phytophthora infestans AVR2 Effector Escapes R2 Recognition Through Effector Disordering. Mol. Plant-Microbe Interact. 2020, 33, 921–931. [Google Scholar] [CrossRef]
- Shen, D.; Li, Q.; Sun, P.; Zhang, M.; Dou, D. Intrinsic disorder is a common structural characteristic of RxLR effectors in oomycete pathogens. Fungal Biol. 2017, 121, 911–919. [Google Scholar] [CrossRef]
- Goss, E.M.; Press, C.M.; Grünwald, N.J. Evolution of RXLR-Class Effectors in the Oomycete Plant Pathogen Phytophthora ramorum. PLoS ONE 2013, 8, e79347. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.H.Y.; Tripathy, S.; Govers, F.; Tyler, B.M. RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc. Natl. Acad. Sci. USA 2008, 105, 4874–4879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, K.J.; Nur, M.; Gil, J.; Fletcher, K.; Lakeman, K.; Gann, D.; Gothberg, A.; Khuu, T.; Kopetzky, J.; Naqvi, S.; et al. Effector prediction and characterization in the oomycete pathogen Bremia lactucae reveal host-recognized WY domain proteins that lack the canonical RXLR motif. PLoS Pathog. 2020, 16, e1009012. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N. EffectorP 3.0: Prediction of Apoplastic and Cytoplasmic Effectors in Fungi and Oomycetes. Mol. Plant-Microbe Interact. 2022, 35, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Jogaiah, S.; Kurjogi, M.; Govind, S.R.; Huntrike, S.S.; Basappa, V.A.; Tran, L.-S.P. Isolation and evaluation of proteolytic actinomycete isolates as novel inducers of pearl millet downy mildew disease protection. Sci. Rep. 2016, 6, 30789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jogaiah, S.; Sharathchandra, R.G.; Raj, N.; Vedamurthy, A.B.; Shetty, H.S. Development of SCAR marker associated with downy mildew disease resistance in pearl millet (Pennisetum glaucum L.). Mol. Biol. Rep. 2014, 41, 7815–7824. [Google Scholar] [CrossRef]
- Thakur, R.P.; Shetty, H.S.; Khairwal, I.S. Pearl Millet Downy Mildew Research in India: Progress and Perspectives. Int. Sorghum Millets Newsl. 2006, 47, 125–130. [Google Scholar]
- Rao, V.R.; Kadwani, D.L.; Sharma, Y.K.; Sharma, R.; Thakur, R.P. Prevalence of Pearl millet Downy Mildew, Sclerospora graminicola in Gujarat and Pathogenic Characterization of Its Isolates. Indian J. Plant Prot. 2007, 35, 291–295. [Google Scholar]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Barka, E.A. Plasmopara viticola the Causal Agent of Downy Mildew of Grapevine: From Its Taxonomy to Disease Management. Front. Microbiol. 2022, 13, 889427. [Google Scholar] [CrossRef]
- Mathur, K.; Thakur, R.P.; Rao, V.P. Characterization of Colletotrichum graminicola Populations from a Sorghum Hybrid CSH 9 for Morphological and Pathogenic Variability. Indian Phytopathol. 2001, 54, 299–303. [Google Scholar]
- Tiwari, S.; Rahul, S.N.; Sehrawat, A.; Rawat, B. Circadian Redox Rhythms Play an Important Role in Plant-Pathogen Interaction. In Plant Microbiome Paradigm; Springer: Berlin/Heidelberg, Germany, 2020; pp. 147–162. [Google Scholar] [CrossRef]
- Mustafa, G.; Masood, S.; Ahmed, N.; Saboor, A.; Ahmad, S.; Hussain, S.; Bilal, M.; Ali, M.A. Seed Priming for Disease Resistance in Plants. In Priming and Pretreatment of Seeds and Seedlings; Springer: Berlin/Heidelberg, Germany, 2019; pp. 333–362. [Google Scholar]
- Feurtey, A.; Lorrain, C.; Croll, D.; Eschenbrenner, C.; Freitag, M.; Habig, M.; Haueisen, J.; Möller, M.; Schotanus, K.; Stukenbrock, E.H. Genome compartmentalization predates species divergence in the plant pathogen genus Zymoseptoria. BMC Genom. 2020, 21, 588. [Google Scholar] [CrossRef]
- Florea, L.; Hartzell, G.; Zhang, Z.; Rubin, G.M.; Miller, W. A Computer Program for Aligning a cDNA Sequence with a Genomic DNA Sequence. Genome Res. 1998, 8, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter-Hovhannisyan, V.; Lomsadze, A.; Chernoff, Y.O.; Borodovsky, M. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 2008, 18, 1979–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavy, N.; Rombauts, S.; Déhais, P.; Mathé, C.; Ramana, D.V.; Leroy, P.; Rouzé, P. Evaluation of gene prediction software using a genomic data set: Application to Arabidopsis thaliana sequences. Bioinformatics 1999, 15, 887–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, C.; Chen, C.; Zhu, L.; Liu, J.; Hui, F. Genome Sequence Resource of Albifimbria verrucaria Causing the Leaf Spot Disease of the Spinach Plant Spinacia oleracea. Plant Dis. 2022, 106, 2511–2513. [Google Scholar] [CrossRef]
- Meng, Y.; Gleason, M.L.; Zhang, R.; Sun, G. Genome Sequence Resource of the Wide-Host-Range Anthracnose Pathogen Colletotrichum siamense. Mol. Plant-Microbe Interact. 2019, 32, 931–934. [Google Scholar] [CrossRef] [Green Version]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting Subcellular Localization of Proteins Based on their N-terminal Amino Acid Sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Dahal, S.; Gour, P.; Raghuvanshi, S.; Prasad, Y.K.; Saikia, D.; Ghatani, S. Multi-stage transcriptome profiling of the neglected food-borne echinostome Artyfechino stomum sufrartyfex reveal potential diagnostic and drug targets. Acta Trop. 2022, 233, 106564. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Qi, Y.; Li, J.; Amin, R.; Yang, W.; Liu, D. Predicating the Effector Proteins Secreted by Puccinia triticina Through Transcriptomic Analysis and Multiple Prediction Approaches. Front. Microbiol. 2020, 11, 538032. [Google Scholar] [CrossRef]
- Levin, E.; Kishore, A.; Ballester, A.R.; Raphael, G.; Feigenberg, O.; Liu, Y.; Norelli, J.; Gonzalez-Candelas, L.; Wisniewski, M.; Droby, S. Identification of pathogenicity-related genes and the role of a subtilisin-related peptidase S8 (PePRT) in authophagy and virulence of Penicillium expansum on apples. Postharvest Biol. Technol. 2018, 149, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Guo, D.; Hu, W.; Niu, X. The prediction of a pathogenesis-related secretome of Puccinia helianthi through high-throughput transcriptome analysis. BMC Bioinform. 2017, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Ai, G.; Yang, K.; Ye, W.; Tian, Y.; Du, Y.; Zhu, H.; Li, T.; Xia, Q.; Shen, D.; Peng, H.; et al. Prediction and Characterization of RXLR Effectors in Pythium Species. Mol. Plant-Microbe Interact. 2020, 33, 1046–1058. [Google Scholar] [CrossRef]
- Breeze, E.; Vale, V.; McLellan, H.; Godiard, L.; Grant, M.; Frigerio, L. The Plant Endoplasmic Reticulum Is Both Receptive and Responsive to Pathogen Effectors. Plant Biol. 2020. preprint. [Google Scholar] [CrossRef]
- Divya, K.; Kishor, P.B.K.; Bhatnagar-Mathur, P.; Singam, P.; Sharma, K.K.; Vadez, V.; Reddy, P.S. Isolation and functional characterization of three abiotic stress-inducible (Apx, Dhn and Hsc70) promoters from pearl millet (Pennisetum glaucum L.). Mol. Biol. Rep. 2019, 46, 6039–6052. [Google Scholar] [CrossRef] [Green Version]
- Sudisha, J.; Kumar, S.A.; Niranjana, S.R.; Shetty, N.P.; Shetty, H.S. Cloning and Development of Pathotype-Specific SCAR Marker Associated with Sclerospora graminicola Isolates from Pearl Millet. Australas. Plant Pathol. 2009, 38, 216–221. [Google Scholar] [CrossRef]
- Evans, S.R.; West, C.; Klein-Seetharaman, J. Similarity of the non-amyloid-β component and C-terminal tail of monomeric and tetrameric alpha-synuclein with 14-3-3 sigma. Comput. Struct. Biotechnol. J. 2021, 19, 5348–5359. [Google Scholar] [CrossRef] [PubMed]
- MacCready, J.S.; Basalla, J.L.; Vecchiarelli, A.G. The McdAB Carboxysome Positioning System Is Widespread Among β-Cyanobacteria. Microbiology 2019. preprint. [Google Scholar] [CrossRef]
- Yu, L.; Liu, F.; Li, Y.; Luo, J.; Jing, R. DeepT3_4: A Hybrid Deep Neural Network Model for the Distinction between Bacterial Type III and IV Secreted Effectors. Front. Microbiol. 2021, 12, 605782. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, R.; Kiran, K.; Rajarammohan, S.; Dubey, H.; Singh, P.K.; Sharma, Y.; Deshmukh, R.; Sonah, H.; Gupta, N.; Sharma, T. Effector Biology of Biotrophic Plant Fungal Pathogens: Current Advances and Future Prospects. Microbiol. Res. 2020, 241, 126567. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; O’Hanlon, R.; Owens, R.A.; Fitzpatrick, D.A. Comparative Genomic and Proteomic Analyses of Three Widespread Phytophthora Species: Phytophthora chlamydospora, Phytophthora gonapodyides and Phytophthora pseudosyringae. Microorganisms 2020, 8, 653. [Google Scholar] [CrossRef]
- Win, J.; Morgan, W.; Bos, J.; Krasileva, K.; Cano, L.M.; Chaparro-Garcia, A.; Ammar, R.; Staskawicz, B.J.; Kamoun, S. Adaptive Evolution Has Targeted the C-Terminal Domain of the RXLR Effectors of Plant Pathogenic Oomycetes. Plant Cell 2007, 19, 2349–2369. [Google Scholar] [CrossRef] [Green Version]
- Naveed, Z.A.; Wei, X.; Chen, J.; Mubeen, H.; Ali, G.S. The PTI to ETI Continuum in Phytophthora-Plant Interactions. Front. Plant Sci. 2020, 11, 593905. [Google Scholar] [CrossRef] [PubMed]
- Chepsergon, J.; Motaung, T.E.; Moleleki, L.N. “Core” RxLR effectors in phytopathogenic oomycetes: A promising way to breeding for durable resistance in plants? Virulence 2021, 12, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.C.; Praz, C.R.; Sotiropoulos, A.G.; Menardo, F.; Kunz, L.; Schudel, S.; Oberhänsli, S.; Poretti, M.; Wehrli, A.; Bourras, S.; et al. A chromosome-scale genome assembly reveals a highly dynamic effector repertoire of wheat powdery mildew. New Phytol. 2018, 221, 2176–2189. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Li, H.; Zhou, Y.; Bao, Y.; Duan, Z.; Wang, C.; Powell, C.A.; Chen, B.; Zhang, M.; Yao, W. Predication of the Effector Proteins Secreted by Fusarium sacchari Using Genomic Analysis and Heterogenous Expression. J. Fungi 2022, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Spanu, P.D.; Abbott, J.C.; Amselem, J.; Burgis, T.A.; Soanes, D.M.; Stüber, K.; van Themaat, E.V.L.; Brown, J.K.M.; Butcher, S.A.; Gurr, S.J.; et al. Genome Expansion and Gene Loss in Powdery Mildew Fungi Reveal Tradeoffs in Extreme Parasitism. Science 2010, 330, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Kiran, K.; Rawal, H.C.; Dubey, H.; Jaswal, R.; Devanna, B.; Gupta, D.K.; Bhardwaj, S.C.; Prasad, P.; Pal, D.; Chhuneja, P.; et al. Draft Genome of the Wheat Rust Pathogen (Puccinia triticina) Unravels Genome-Wide Structural Variations during Evolution. Genome Biol. Evol. 2016, 8, 2702–2721. [Google Scholar] [CrossRef] [Green Version]
- Van de Wouw, A.P.; Idnurm, A. Biotechnological potential of engineering pathogen effector proteins for use in plant disease management. Biotechnol. Adv. 2019, 37, 107387. [Google Scholar] [CrossRef]
- Tyler, B.M.; Tripathy, S.; Zhang, X.; Dehal, P.; Jiang, R.H.Y.; Aerts, A.; Arredondo, F.D.; Baxter, L.; Bensasson, D.; Beynon, J.L.; et al. Phytophthora Genome Sequences Uncover Evolutionary Origins and Mechanisms of Pathogenesis. Science 2006, 313, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Morgan, W.; Kamoun, S. RXLR effectors of plant pathogenic oomycetes. Curr. Opin. Microbiol. 2007, 10, 332–338. [Google Scholar] [CrossRef]
- Kamoun, S. Groovy times: Filamentous pathogen effectors revealed. Curr. Opin. Plant Biol. 2007, 10, 358–365. [Google Scholar] [CrossRef]
- Cai, H.; Yu, J.; Qiao, Y.; Ma, Y.; Zheng, J.; Lin, M.; Yan, Q.; Huang, L. Effect of the Type VI Secretion System Secreted Protein Hcp on the Virulence of Aeromonas salmonicida. Microorganisms 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Dalio, R.J.D.; Paschoal, D.; Arena, G.D.; Magalhães, D.M.; Oliveira, T.S.; Merfa, M.V.; Maximo, H.J.; Machado, M.A. Hypersensitive response: From NLR pathogen recognition to cell death response. Ann. Appl. Biol. 2020, 178, 268–280. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, L.; Tarallo, M.; Gough, K.; Guo, M.; Hargreaves, C.; Loo, T.S.; McDougal, R.L.; Mesarich, C.H.; Bradshaw, R.E. Apoplastic effector candidates of a foliar forest pathogen trigger cell death in host and non-host plants. Sci. Rep. 2021, 11, 19958. [Google Scholar] [CrossRef]
- Hacquard, S.; Kracher, B.; Maekawa, T.; Vernaldi, S.; Schulze-Lefert, P.; van Themaat, E.V.L. Mosaic genome structure of the barley powdery mildew pathogen and conservation of transcriptional programs in divergent hosts. Proc. Natl. Acad. Sci. USA 2013, 110, E2219–E2228. [Google Scholar] [CrossRef] [Green Version]
- Praz, C.R.; Menardo, F.; Robinson, M.; Müller, M.C.; Wicker, T.; Bourras, S.; Keller, B. Non-parent of Origin Expression of Numerous Effector Genes Indicates a Role of Gene Regulation in Host Adaption of the Hybrid Triticale Powdery Mildew Pathogen. Front. Plant Sci. 2018, 9, 49. [Google Scholar] [CrossRef]

| Serial Number | Sequence ID | Sequence Forward   Reverse Reverse | TM 1 | AT 2 |
|---|---|---|---|---|
| 1 | 11472_g |  ATGAATAAGCGATATCTTTTG ATGAATAAGCGATATCTTTTGCCTTATAAACCAATCAATTAT  | 49 47 | 48 |
| 2 | 14151_g | ATGAAACAGATGATAAAAAGC TTAGCGTTTTGACTTTTTACC | 49 51 | 50 |
| 3 | 18087_g | ATGAACCCGAACATCATCTTC CTAGGGATTATTGACAAAGTA | 55 51 | 53 |
| 4 | 75485_g | ATGCGTCCTGTGTCCATCTTG TCACTTTGCAAAGCGTGAAAT | 59 53 | 56 |
| 5 | 7856_g | ATGAGACCGAACATTTTCTTC CTAGGGATTCTTGGCAAAGTA | 53 55 | 54 |
| 6 | 69274_g | ATGAACCCGAACATTGTATTC CTAGGGATTCTTTTTGGCAAA | 53 53 | 53 |
| 7 | 6877_g | ATGCGTCTCCGTCTGTTCCT TTACTCGCTCGCTTCACTGG | 58 58 | 58 |
| 8 | 10624_g | ATGCGCTATTCCATCCTTCTC TTAATGTTTTTTATACCAGTC | 57 47 | 52 |
| 9 | 60945_g | ATGCGGTCCGTCTCTATCCTC TTAGACTGGGTGGGCATTCTT | 61 57 | 59 |
| 10 | 8311_g | ATGCGGTCCGTCTCTATCCT TTAGACTGGGTGGGCATTCT | 58 56 | 57 |
| 11 | 35983_g | ATGATCTACGCCCCCTTAGTT TTTGAACGGGCAATGGTGTAG | 57 57 | 57 |
| 12 | 60741_g | ATGCGTTTCCATAGCCTGATT TTCCTCTGTAGCGGAAGCTCT | 55 59 | 57 |
| 13 | 38171_g | ATGGTCAACGCACTCTTGGTT TTAATTTACGCGACGTTTCTT | 57 51 | 54 |
| 14 | 46338_g | ATGATCAACAAACTCTTGGTT TTACGCGATTTTTCTTCTTTT | 51 49 | 50 |
| 15 | 74027_g | ATGATCCACAAACTCTTGGTT TTACGCGATTTTTCTTCTTTT | 53 49 | 51 |
| 16 | 10548_g | ATGAAGCTTTCTCTTCTCTTC TTACGGTGACATGAGCTTCCG | 53 59 | 56 |
| 17 | 1854_g | ATGAGAGCAACTTGTCTCCTA TCACTGACGGTACCCGTTCTT | 55 59 | 57 |
| Serial Number | Protein Number | Cytoplasmic Effector | Apoplastic Effector | Non-Effector | Prediction |
|---|---|---|---|---|---|
| 1 | 681_g | Y (0.856) | - | - | Cytoplasmic effector |
| 2 | 1492_g | - | - | Y (0.833) | Non-effector |
| 3 | 1671_g | Y (0.823) | - | - | Cytoplasmic effector |
| 4 | 3769_g | Y (0.785) | - | - | Cytoplasmic effector |
| 5 | 5109_g | Y (0.799) | - | - | Cytoplasmic effector |
| 6 | 5588_g | Y (0.89) | - | - | Cytoplasmic effector |
| 7 | 8689_g | Y (0.856) | - | - | Cytoplasmic effector |
| 8 | 8943_g | Y (0.903) | - | - | Cytoplasmic effector |
| 9 | 10070_g | Y (0.89) | - | - | Cytoplasmic effector |
| 10 | 10105_g | - | - | Y (0.735) | Non-effector |
| 11 | 12349_g | Y (0.856) | - | - | Cytoplasmic effector |
| 12 | 23122_g | Y (0.856) | - | - | Cytoplasmic effector |
| 13 | 24510_g | Y (0.887) | - | - | Cytoplasmic effector |
| 14 | 27640_g | Y (0.891) | - | - | Cytoplasmic effector |
| 15 | 27641_g | Y (0.924) | - | - | Cytoplasmic effector |
| 16 | 28426_g | Y (0.726) | - | - | Cytoplasmic effector |
| 17 | 36964_g | Y (0.575) | - | - | Cytoplasmic effector |
| 18 | 37717_g | Y (0.831) | - | - | Cytoplasmic effector |
| 19 | 38162_g | Y (0.815) | - | - | Cytoplasmic effector |
| 20 | 40025_g | Y (0.934) | - | - | Cytoplasmic effector |
| 21 | 41242_g | Y (0.934) | - | - | Cytoplasmic effector |
| 22 | 42778_g | - | - | Y (0.73) | Non-effector |
| 23 | 47345_g | Y (0.523) | - | - | Cytoplasmic effector |
| 24 | 49318_g | Y (0.785) | - | - | Cytoplasmic effector |
| 25 | 50963_g | Y (0.729) | - | - | Cytoplasmic effector |
| 26 | 53743_g | - | - | Y (0.768) | Non-effector |
| 27 | 54388_g | Y (0.835) | - | - | Cytoplasmic effector |
| 28 | 62219_g | Y (0.523) | - | - | Cytoplasmic effector |
| 29 | 62440_g | Y (0.891) | - | - | Cytoplasmic effector |
| 30 | 62442_g | Y (0.822) | - | - | Cytoplasmic effector |
| 31 | 62857_g | Y (0.89) | - | - | Cytoplasmic effector |
| 32 | 62858_g | Y (0.859) | - | - | Cytoplasmic effector |
| 33 | 68815_g | Y (0.813) | - | - | Cytoplasmic effector |
| 34 | 70580_g | Y (0.582) | - | - | Cytoplasmic effector |
| 35 | 72237_g | Y (0.824) | - | - | Cytoplasmic effector |
| Serial Number | Protein Number | Cytoplasmic Effector | Apoplastic Effector | Non-Effector | Prediction |
|---|---|---|---|---|---|
| 1 | 21379_g | Y (0.959) | - | - | Cytoplasmic effector |
| 2 | 3464_g | Y (0.809) | - | - | Cytoplasmic effector |
| 3 | 47557_g | Y (0.905) | - | - | Cytoplasmic effector |
| 4 | 12504_g | - | - | Y (0.755) | Non-effector |
| 5 | 55207_g | Y (0.791) | - | - | Cytoplasmic effector |
| 6 | 25335_g | Y (0.843) | - | - | Cytoplasmic effector |
| 7 | 11898_g | - | - | Y (0.81) | Non-effector |
| 8 | 35685_g | Y (0.903) | - | - | Cytoplasmic effector |
| 9 | 59897_g | - | - | Y (0.8) | Non-effector |
| 10 | 63043_g | - | - | Y (0.573) | Non-effector |
| 11 | 9023_g | Y (0.84) | - | - | Cytoplasmic effector |
| 12 | 18507_g | Y (0.736) | - | - | Cytoplasmic effector |
| 13 | 65651_g | Y (0.685) | - | - | Cytoplasmic effector |
| 14 | 10686_g | Y (0.906) | - | - | Cytoplasmic effector |
| 15 | 45770_g | Y (0.868) | - | - | Cytoplasmic effector |
| 16 | 26637_g | Y (0.876) | - | - | Cytoplasmic effector |
| 17 | 46575_g | Y (0.761) | - | - | Cytoplasmic effector |
| 18 | 1289_g | Y (0.905) | - | - | Cytoplasmic effector |
| 19 | 53288_g | Y (0.955) | - | - | Cytoplasmic effector |
| 20 | 10613_g | Y (0.832) | - | - | Cytoplasmic effector |
| 21 | 20048_g | Y (0.723) | - | - | Cytoplasmic effector |
| 22 | 23346_g | Y (0.832) | - | - | Cytoplasmic effector |
| 23 | 35739_g | Y (0.806) | - | - | Cytoplasmic effector |
| 24 | 44650_g | Y (0.827) | - | - | Cytoplasmic effector |
| 25 | 60111_g | Y (0.903) | - | - | Cytoplasmic effector |
| 26 | 70669_g | Y (0.86) | - | - | Cytoplasmic effector |
| 27 | 7606_g | Y (0.827) | - | - | Cytoplasmic effector |
| 28 | 9639_g | Y (0.832) | - | - | Cytoplasmic effector |
| 29 | 22687_g | Y (0.883) | - | - | Cytoplasmic effector |
| 30 | 24069_g | Y (0.921) | - | - | Cytoplasmic effector |
| 31 | 29669_g | Y (0.883) | - | - | Cytoplasmic effector |
| 32 | 35014_g | Y (0.964) | - | - | Cytoplasmic effector |
| 33 | 39202_g | Y (0.87) | - | - | Cytoplasmic effector |
| 34 | 62695_g | Y (0.964) | - | - | Cytoplasmic effector |
| 35 | 60583_g | Y (0.569) | - | - | Cytoplasmic effector |
| 36 | 68703_g | Y (0.571) | - | - | Cytoplasmic effector |
| 37 | 71584_g | Y (0.96) | - | - | Cytoplasmic effector |
| 38 | 13581_g | Y (0.808) | - | - | Cytoplasmic effector |
| 39 | 34223_g | Y (0.894) | - | - | Cytoplasmic effector |
| 40 | 37513_g | Y (0.759) | - | - | Cytoplasmic effector |
| 41 | 77025_g | Y (0.731) | - | - | Cytoplasmic effector |
| 42 | 29984_g | Y (0.746) | - | - | Cytoplasmic effector |
| 43 | 42066_g | Y (0.872) | - | - | Cytoplasmic effector |
| 44 | 42069_g | Y (0.945) | - | - | Cytoplasmic effector |
| 45 | 32234_g | Y (0.915) | - | - | Cytoplasmic effector |
| 46 | 28623_g | Y (0.927) | - | - | Cytoplasmic effector |
| 47 | 32891_g | Y (0.845) | - | - | Cytoplasmic effector |
| 48 | 1182_g | Y (0.88) | - | - | Cytoplasmic effector |
| 49 | 17918_g | Y (0.803) | - | - | Cytoplasmic effector |
| 50 | 19540_g | Y (0.64) | - | - | Cytoplasmic effector |
| 51 | 64364_g | - | - | Y (0.781) | Non-effector |
| 52 | 75770_g | Y (0.796) | - | - | Cytoplasmic effector |
| Serial Number | Sequence ID | Cytoplasmic Effector | Apoplastic Effector | Non-Effector | Prediction |
|---|---|---|---|---|---|
| 1 | 10548_g | Y (0.7) | - | - | Cytoplasmic effector |
| 2 | 14151_g | Y (0.927) | - | - | Cytoplasmic effector |
| 3 | 60741_g | Y (0.908) | - | - | Cytoplasmic effector |
| 4 | 75485_g | Y (0.946) | - | - | Cytoplasmic effector |
| 5 | 11472_g | Y (0.893) | - | - | Cytoplasmic effector |
| 6 | 6877_g | Y (0.898) | - | - | Cytoplasmic effector |
| 7 | 1854_g | - | - | Y (0.619) | Non-effector |
| 8 | 35983_g | Y (0.8) | - | - | Cytoplasmic effector |
| 9 | 38171_g | Y (0.88) | - | - | Cytoplasmic effector |
| 10 | 46338_g | Y (0.908) | - | - | Cytoplasmic effector |
| 11 | 74027_g | Y (0.908) | - | - | Cytoplasmic effector |
| 12 | 7856_g | Y (0.585) | Y (0.501) | - | Cytoplasmic/apoplastic effector |
| 13 | 18087_g | Y (0.816) | - | - | Cytoplasmic effector |
| 14 | 69274_g | Y (0.799) | - | - | Cytoplasmic effector |
| 15 | 60945_g | Y (0.921) | - | - | Cytoplasmic effector |
| 16 | 8311_g | Y (0.92) | - | - | Cytoplasmic effector |
| 17 | 10624_g | Y (0.938) | - | - | Cytoplasmic effector |
| Serial Number | Sequence ID | Gene Bank Accession Number | Size in Base Pairs |
|---|---|---|---|
| 1 | 35983_g | OM365913 | 1410 |
| 2 | 6877_g | OM135515 | 885 |
| 3 | 8311_g | OM365912 | 1254 |
| 4 | 60945_g | OM365911 | 1248 |
| 5 | 60741_g | OM365914 | 1533 |
| Band Number | Sequence ID | Gene Bank Accession Number | Overall Disorder (%) |
|---|---|---|---|
| 7 | 6877_g | OM135515 | 43.88 |
| 9 | 60945_g | OM365911 | 26.75 |
| 10 | 8311_g | OM365912 | 28.06 |
| 11 | 35983_g | OM365913 | 46.17 |
| 12 | 60741_g | OM365914 | 25.05 |
| Serial Number | Protein Name | Signal Peptide Likelihood | Cleavage Site between Position | RxLR Motif Position | DEER Motif Position |
|---|---|---|---|---|---|
| 1 | 6877_g | 0.9996 | 16 and 17 | 40 to 43 | 53 to 56 |
| 2 | 60945_g | 0.9997 | 21 and 22 | 48 to 51 | 58 to 61 |
| 3 | 8311_g | 0.9997 | 21 and 22 | 48 to 51 | 58 to 61 |
| 4 | 35983_g | 0.9998 | 19 and 20 | 43 to 46 | 55 to 58 |
| 5 | 60741_g | 0.9998 | 19 and 20 | 43 to 46 | 56 to 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadimani, S.; De Britto, S.; Udayashankar, A.C.; Geetha, N.; Nayaka, C.S.; Ali, D.; Alarifi, S.; Ito, S.-i.; Jogaiah, S. Genome-Wide Characterization of Effector Protein-Encoding Genes in Sclerospora graminicola and Its Validation in Response to Pearl Millet Downy Mildew Disease Stress. J. Fungi 2023, 9, 431. https://doi.org/10.3390/jof9040431
Hadimani S, De Britto S, Udayashankar AC, Geetha N, Nayaka CS, Ali D, Alarifi S, Ito S-i, Jogaiah S. Genome-Wide Characterization of Effector Protein-Encoding Genes in Sclerospora graminicola and Its Validation in Response to Pearl Millet Downy Mildew Disease Stress. Journal of Fungi. 2023; 9(4):431. https://doi.org/10.3390/jof9040431
Chicago/Turabian StyleHadimani, Shiva, Savitha De Britto, Arakere C. Udayashankar, Nagaraj Geetha, Chandra S. Nayaka, Daoud Ali, Saud Alarifi, Shin-ichi Ito, and Sudisha Jogaiah. 2023. "Genome-Wide Characterization of Effector Protein-Encoding Genes in Sclerospora graminicola and Its Validation in Response to Pearl Millet Downy Mildew Disease Stress" Journal of Fungi 9, no. 4: 431. https://doi.org/10.3390/jof9040431





