Metabolic Engineering of Pichia pastoris for the Production of Triacetic Acid Lactone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Plasmid Construction
2.3. Yeast Strain Construction
2.4. Medium and Cultivation
2.5. Analytical Methods
3. Results
3.1. Production of TAL in P. pastoris via the Introduction of 2-Pyrone Synthase
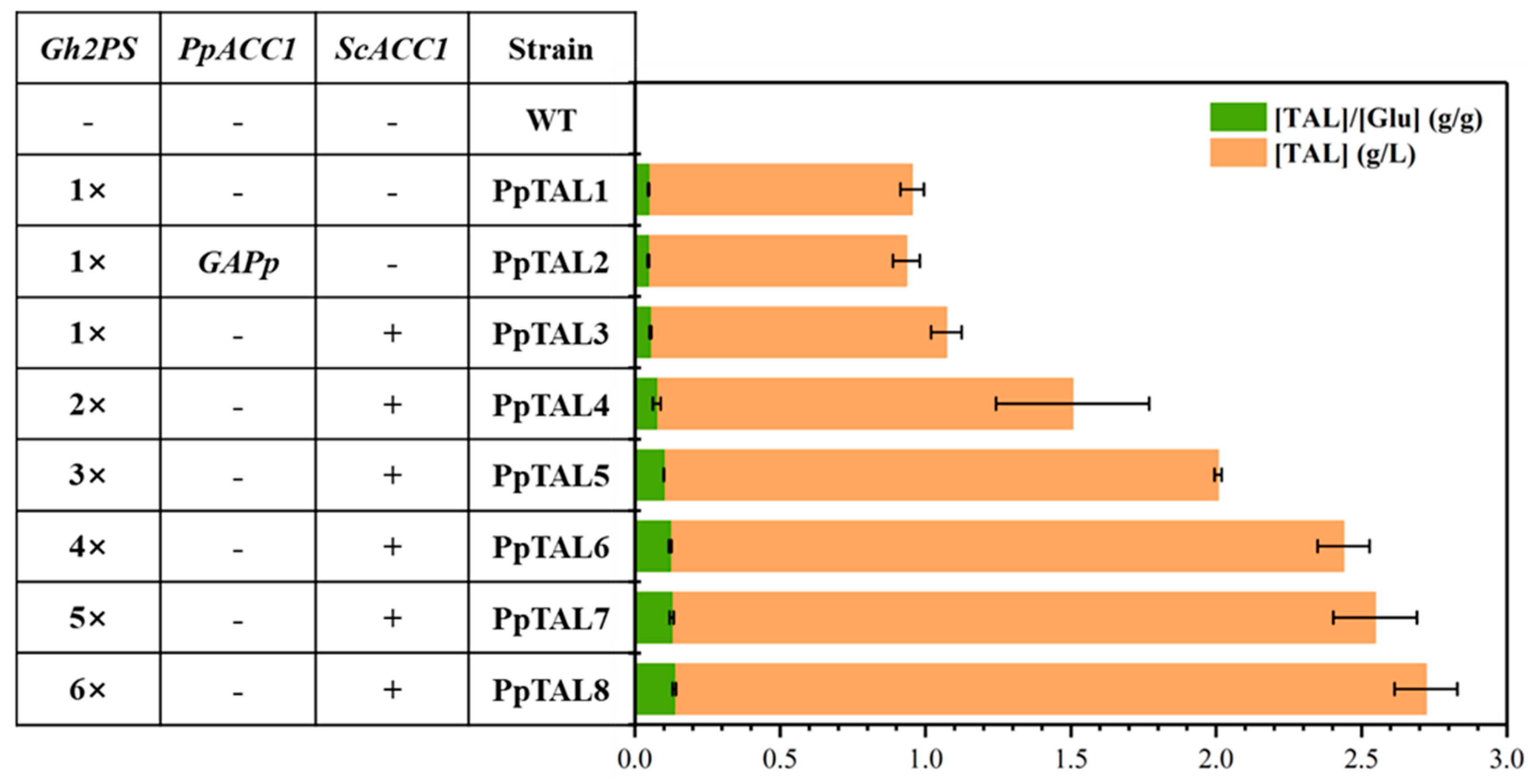
3.2. Overexpression of ScACC1* and Multi-Copy Integration of Gh2PS to Enhance TAL Production
3.3. Introduction of PK Pathway to Boost Acetyl-CoA Supply
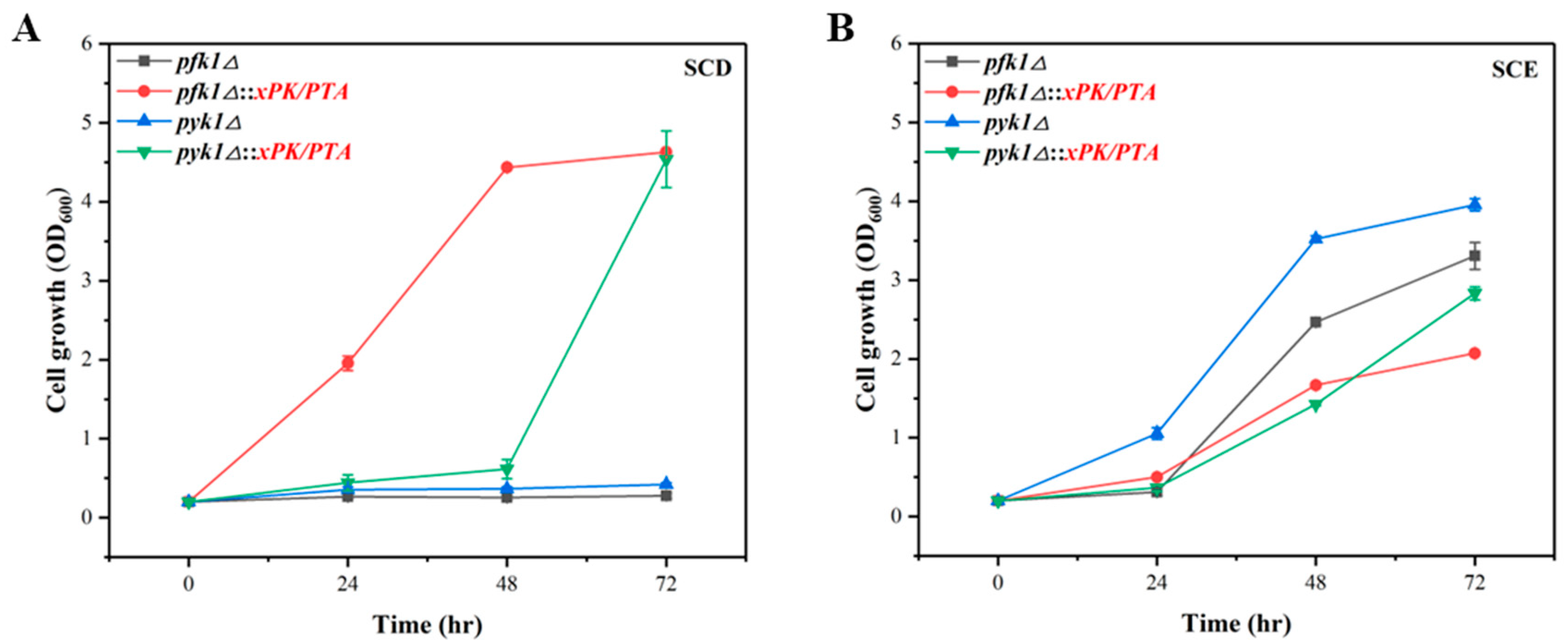
3.4. Verification of PK Pathway in P. pastoris via Growth Complementation
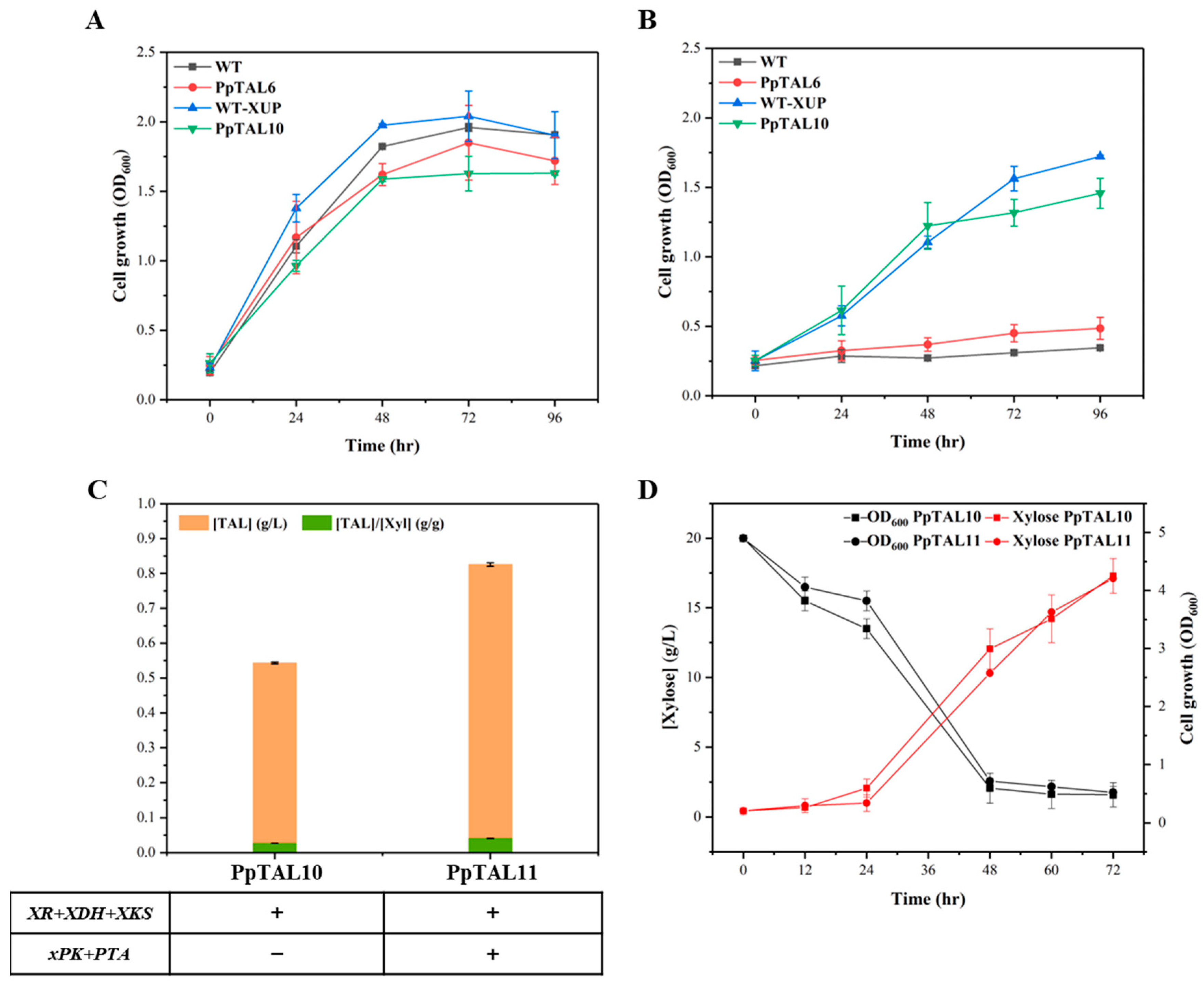
3.5. Production of TAL from Xylose
3.6. Production of TAL from Methanol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.J.; Kerkhoven, E.J.; Nielsen, J. Barriers and opportunities in bio-based production of hydrocarbons. Nat. Energy 2018, 3, 925–935. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Crumbley, A.M.; Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science 2017, 355, aag0804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Daviet, L.; Schalk, M.; Siewers, V.; Nielsen, J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab. Eng. 2013, 15, 48–54. [Google Scholar] [CrossRef]
- Nielsen, J.; Keasling, J.D. Engineering Cellular Metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef]
- Pfeifenschneider, J.; Brautaset, T.; Wendisch, V.F. Methanol as carbon substrate in the bio-economy: Metabolic engineering of aerobic methylotrophic bacteria for production of value-added chemicals. Biofuels Bioprod. Biorefining 2017, 11, 719–731. [Google Scholar] [CrossRef]
- Schwarzhans, J.-P.; Luttermann, T.; Geier, M.; Kalinowski, J.; Friehs, K. Towards systems metabolic engineering in Pichia pastoris. Biotechnol. Adv. 2017, 35, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, B.; Zhou, H.; Zhang, J. Knockout of the DAS gene increases S-adenosylmethionine production in Komagataella phaffii. Biotechnol. Biotechnol. Equip. 2020, 35, 29–36. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, L.; Lian, J. Development of synthetic biology tools to engineer Pichia pastoris as a chassis for the production of natural products. Synth. Syst. Biotechnol. 2021, 6, 110–119. [Google Scholar] [CrossRef]
- Gao, J.; Xu, J.; Zuo, Y.; Ye, C.; Jiang, L.; Feng, L.; Huang, L.; Xu, Z.; Lian, J. Synthetic biology toolkit for marker-less integration of multigene pathways into Pichia. pastoris. via CRISPR/Cas9. ACS Synth. Biol. 2022, 11, 623–633. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, Y.; Xiao, F.; Wang, Y.; Li, D.; Xu, J.; Ye, C.; Feng, L.; Jiang, L.; Liu, T.; et al. Biosynthesis of catharanthine in engineered Pichia pastoris. Nat. Synth. 2023, 2, 231–242. [Google Scholar] [CrossRef]
- Yu, J.; Landberg, J.; Shavarebi, F.; Bilanchone, V.; Okerlund, A.; Wanninayake, U.; Zhao, L.; Kraus, G.; Sandmeyer, S. Bioengineering triacetic acid lactone production in Yarrowia lipolytica for pogostone synthesis. Biotechnol. Bioeng. 2018, 115, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, J.; Da Silva, N.A. Metabolic engineering of Saccharomyces cerevisiae for the production of triacetic acid lactone. Metab. Eng. 2014, 25, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Qian, S.; Akinterinwa, O.; Frei, C.S.; Gredell, J.A.; Cirino, P.C. Screening for enhanced triacetic acid lactone production by recombinant Escherichia coli expressing a designed triacetic acid lactone reporter. J. Am. Chem. Soc. 2013, 135, 10099–10103. [Google Scholar] [CrossRef]
- Saunders, L.P.; Bowman, M.J.; Mertens, J.A.; Da Silva, N.A.; Hector, R.E. Triacetic acid lactone production in industrial Saccharomyces yeast strains. J. Ind. Microbiol. Biotechnol. 2015, 42, 711–721. [Google Scholar] [CrossRef]
- Markham, K.A.; Palmer, C.M.; Chwatko, M.; Wagner, J.M.; Murray, C.; Vazquez, S.; Swaminathan, A.; Chakravarty, I.; Lynd, N.A.; Alper, H.S. Rewiring Yarrowia lipolytica toward triacetic acid lactone for materials generation. In Proceedings of the National Academy of Sciences of the United States of America, Washington, DC, USA, 29 April 2018; Volume 115, pp. 2096–2101. [Google Scholar] [CrossRef]
- Lian, J.; Si, T.; Nair, N.U.; Zhao, H. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab. Eng. 2014, 24, 139–149. [Google Scholar] [CrossRef]
- Nielsen, J. Synthetic biology for engineering acetyl coenzyme A metabolism in yeast. mBio 2014, 5, e02153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, D.; Wang, B.; Zhao, Y.; Jia, G.; Chen, T.; Wang, Z. Advances in acetyl coenzyme A metabolic engineering with Escherichia coli. Chem. Ind. Eng. Prog. 2019, 38, 4218–4226. [Google Scholar]
- Gambacorta, F.V.; Dietrich, J.J.; Yan, Q.; Pfleger, B.F. Rewiring yeast metabolism to synthesize products beyond ethanol. Curr. Opin. Chem. Biol. 2020, 59, 182–192. [Google Scholar] [CrossRef]
- Cao, M.; Tran, V.G.; Qin, J.; Olson, A.; Mishra, S.; Schultz, J.C.; Huang, C.; Xie, D.; Zhao, H. Metabolic engineering of oleaginous yeast Rhodotorula toruloides for overproduction of triacetic acid lactone. Biotechnol. Bioeng. 2022, 119, 2529–2540. [Google Scholar] [CrossRef]
- Liu, H.; Marsafari, M.; Wang, F.; Deng, L.; Xu, P. Engineering acetyl-CoA metabolic shortcut for eco-friendly production of polyketides triacetic acid lactone in Yarrowia lipolytica. Metab. Eng. 2019, 56, 60–68. [Google Scholar] [CrossRef]
- Kamineni, A.; Consiglio, A.L.; MacEwen, K.; Chen, S.; Chifamba, G.; Shaw, A.J.; Tsakraklides, V. Increasing lipid yield in Yarrowia lipolytica through phosphoketolase and phosphotransacetylase expression in a phosphofructokinase deletion strain. Biotechnol. Biofuels 2021, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, I.W.; Lin, T.S.; Liao, J.C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 2013, 502, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Wasylenko, T.M.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 2017, 35, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, T.; Li, X.; Chen, Y.; Campbell, K.; Nielsen, J.; Chen, Y. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 2019, 10, 4976. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Siewers, V.; Nielsen, J. Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1. mBio 2014, 5, e01130-14. [Google Scholar] [CrossRef]
- Lin-Cereghino, J.; Wong, W.W.; Xiong, S.; Giang, W.; Luong, L.T.; Vu, J.; Johnson, S.D.; Lin-Cereghino, G.P. Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques 2005, 38, 44–48. [Google Scholar] [CrossRef]
- Vickery, C.R.; Cardenas, J.; Bowman, M.E.; Burkart, M.D.; Da Silva, N.A.; Noel, J.P. A coupled in vitro/in vivo approach for engineering a heterologous type III PKS to enhance polyketide biosynthesis in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2018, 115, 1394–1402. [Google Scholar] [CrossRef]
- Xu, P.; Ranganathan, S.; Fowler, Z.L.; Maranas, C.D.; Koffas, M.A.G. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab. Eng. 2011, 13, 578–587. [Google Scholar] [CrossRef]
- Zha, W.; Rubin-Pitel, S.B.; Shao, Z.; Zhao, H. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab. Eng. 2009, 11, 192–198. [Google Scholar] [CrossRef]
- Kozak, B.U.; van Rossum, H.M.; Luttik, M.A.; Akeroyd, M.; Benjamin, K.R.; Wu, L.; de Vries, S.; Daran, J.M.; Pronk, J.T.; van Maris, A.J. Engineering acetyl coenzyme A supply: Functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. mBio 2014, 5, e01696-14. [Google Scholar] [CrossRef]
- Kwak, S.; Jin, Y.S. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: A review and perspective. Microb. Cell Fact. 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Adsul, M.G.; Singhvi, M.S.; Gaikaiwari, S.A.; Gokhale, D.V. Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Gao, J.; Zhou, Y.J. Advances in engineering methylotrophic yeast for biosynthesis of valuable chemicals from methanol. Chin. Chem. Lett. 2018, 29, 681–686. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Wang, J.; Bai, P.; Shi, L.; Shen, W.; Zhou, M.; Zhou, X.; Zhang, Y.; Cai, M. Mit1 Transcription Factor Mediates Methanol Signaling and Regulates the Alcohol Oxidase 1 (AOX1) Promoter in Pichia pastoris. J. Biol. Chem. 2016, 291, 6245–6261. [Google Scholar] [CrossRef]
- van Rossum, H.M.; Kozak, B.U.; Pronk, J.T.; van Maris, A.J.A. Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing. Metab. Eng. 2016, 36, 99–115. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Yang, Y.; Wang, S.; Wang, Q.; Wang, X.; Yan, Z.; Cheng, J.; Liu, C.; Yang, X.; et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design. Nat. Commun. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Yu, W.; Zhou, Y.J. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol. Nat. Metab. 2022, 4, 932–943. [Google Scholar] [CrossRef]


| Strain | Genotype | Characteristic | Source |
|---|---|---|---|
| GS115-Cas9 | P. pastoris GS115-his4::Cas9 | Parent strain | [9] |
| PpTAL1 | GS115-Cas9 Int11::pTEF1-Gh2PS-t0547 | One copy of Gh2PS | This study |
| PpTAL2 | PpTAL1 pACC1::pGAP | ACC1 promoter replaced with pGAP based on PpTAL1 | This study |
| PpTAL3 | PpTAL1 Int39::pTEF1-ScACC1*-tAOX1 | 1 copy of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL4 | PpTAL3 Int32::pTEF1-Gh2PS-t0547 | 2 copies of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL5 | PpTAL4 Int33::pTEF1-Gh2PS-t0547 | 3 copies of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL6 | PpTAL5 Int34::pTEF1-Gh2PS-t0547 | 4 copies of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL7 | PpTAL6 Int1::pTEF1-Gh2PS-t0547 | 5 copies of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL8 | PpTAL7 Int20::pTEF1-Gh2PS-t0547 | 6 copies of Gh2PS and 1 copy of ScACC1* | This study |
| PpTAL9 | PpTAL6 Int35::pGAP-xPK-tAOX1 Int59::pTEF1-PTA-t0547 | 4 copies of Gh2PS, 1 copy of ScACC1*, and 1 copy of xPK and PTA | This study |
| PpTAL10 | PpTAL6 Int56::pGAP-XR-tAOX1-pTEF1-XDH-t0547 Int1::pTEF1-XKS-tAOX1 | 4 copies of Gh2PS, 1 copy of ScACC1*, and 1 copy of XR, XDH, and XKS | This study |
| PpTAL11 | PpTAL10 Int35::pGAP-xPK-tAOX1 Int59::pTEF1-PTA-t0547 | 4 copies of Gh2PS, 1 copy of ScACC1*, 1 copy of XR, XDH, and XKS, and 1 copy of xPK and PTA | This study |
| pfk1△ | GS115-Cas9 pfk1△ | GS115-Cas9 strain with pfk1 deletion | This study |
| pyk1△ | GS115-Cas9 pyk1△ | GS115-Cas9 strain with pyk1 deletion | This study |
| pfk1△::xPK/PTA | GS115-Cas9 pfk1△::xPK/PTA | GS115-Cas9strain with pfk1 deletion and 1 copy of xPK and PTA | This study |
| pyk1△::xPK/PTA | GS115-Cas9 pyk1△::xPK/PTA | GS115-Cas9 strain with pyk1 deletion and 1 copy of xPK and PTA | This study |
| WT-XUP | GS115-Cas9 Int56::pGAP-XR-tAOX1-pTEF1-XDH-t0547 Int1::pTEF1-XKS-tAOX1 | GS115-Cas9 strain with 1 copy of XR, XDH, and XKS | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Xu, J.; Ye, C.; Gao, J.; Huang, L.; Xu, Z.; Lian, J. Metabolic Engineering of Pichia pastoris for the Production of Triacetic Acid Lactone. J. Fungi 2023, 9, 494. https://doi.org/10.3390/jof9040494
Feng L, Xu J, Ye C, Gao J, Huang L, Xu Z, Lian J. Metabolic Engineering of Pichia pastoris for the Production of Triacetic Acid Lactone. Journal of Fungi. 2023; 9(4):494. https://doi.org/10.3390/jof9040494
Chicago/Turabian StyleFeng, Linjuan, Junhao Xu, Cuifang Ye, Jucan Gao, Lei Huang, Zhinan Xu, and Jiazhang Lian. 2023. "Metabolic Engineering of Pichia pastoris for the Production of Triacetic Acid Lactone" Journal of Fungi 9, no. 4: 494. https://doi.org/10.3390/jof9040494
APA StyleFeng, L., Xu, J., Ye, C., Gao, J., Huang, L., Xu, Z., & Lian, J. (2023). Metabolic Engineering of Pichia pastoris for the Production of Triacetic Acid Lactone. Journal of Fungi, 9(4), 494. https://doi.org/10.3390/jof9040494





