The Involvement of the Laccase Gene Cglac13 in Mycelial Growth, Germ Tube Development, and the Pathogenicity of Colletotrichum gloeosporioides from Mangoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Extraction of Plasmids, DNA, RNA, and Expression Levels
2.3. Primer Design
2.4. Identification of Cglac13
2.5. Construction of Plasmids and Acquisition of the Knockout Mutant and Complementary Strain
2.6. Conidia Germination, Germ Tube Elongation, and Appressorium Formation
2.7. Pathogenicity Assays
2.8. Statistical Analysis
3. Results
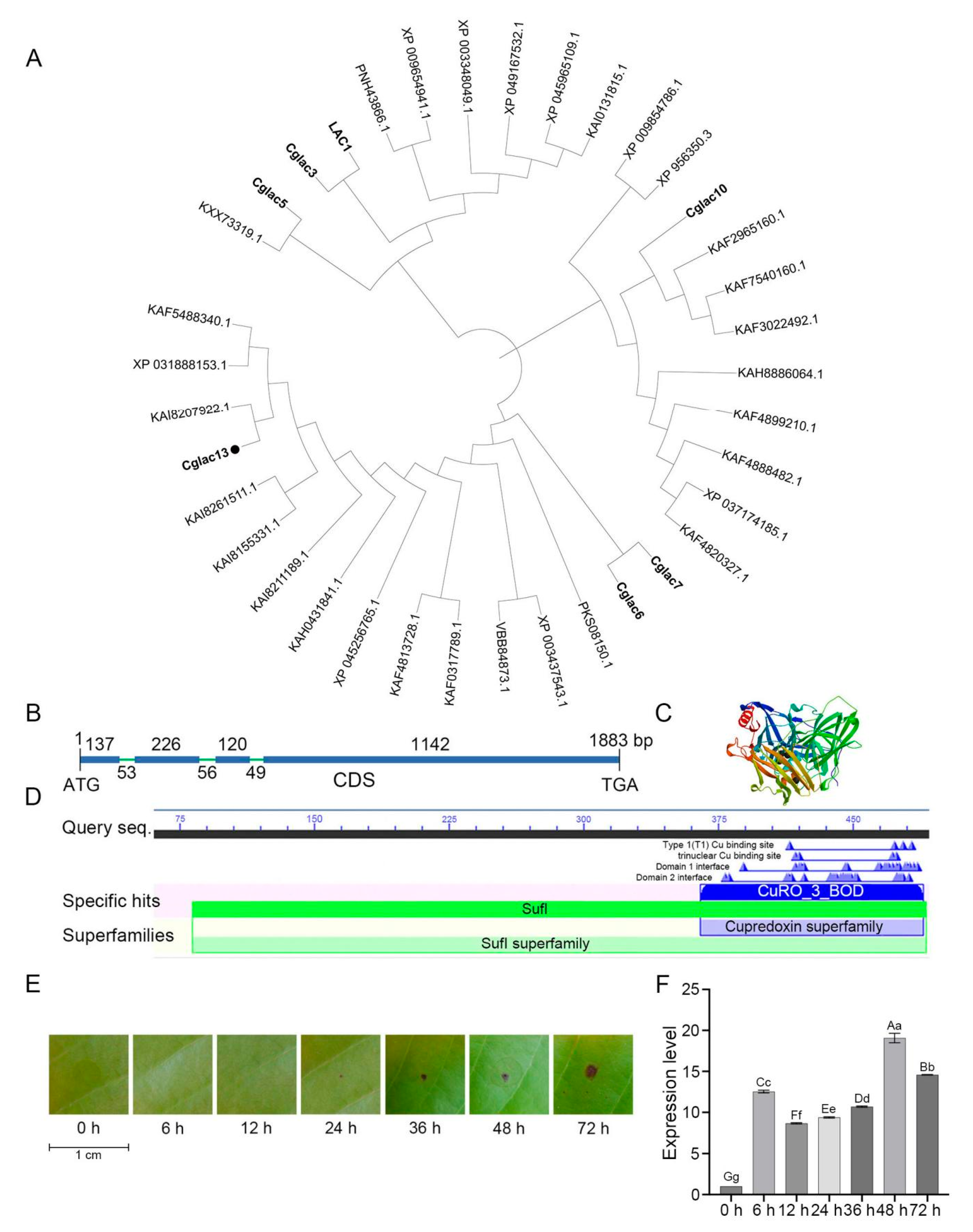
3.1. Identification and Phylogenetic Analysis of Cglac13
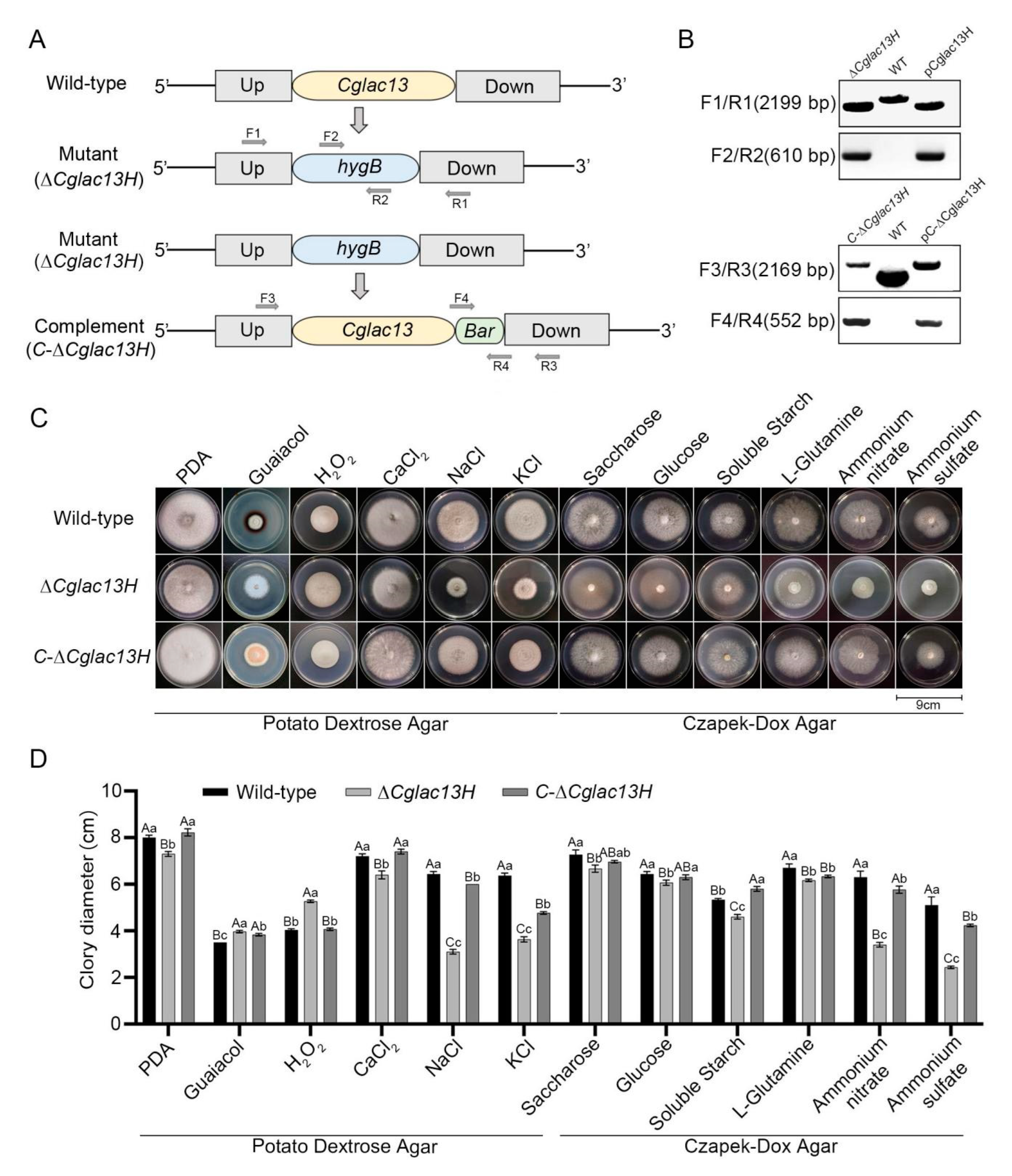
3.2. Validation of Gene Knockout Mutant and Complementary Strain
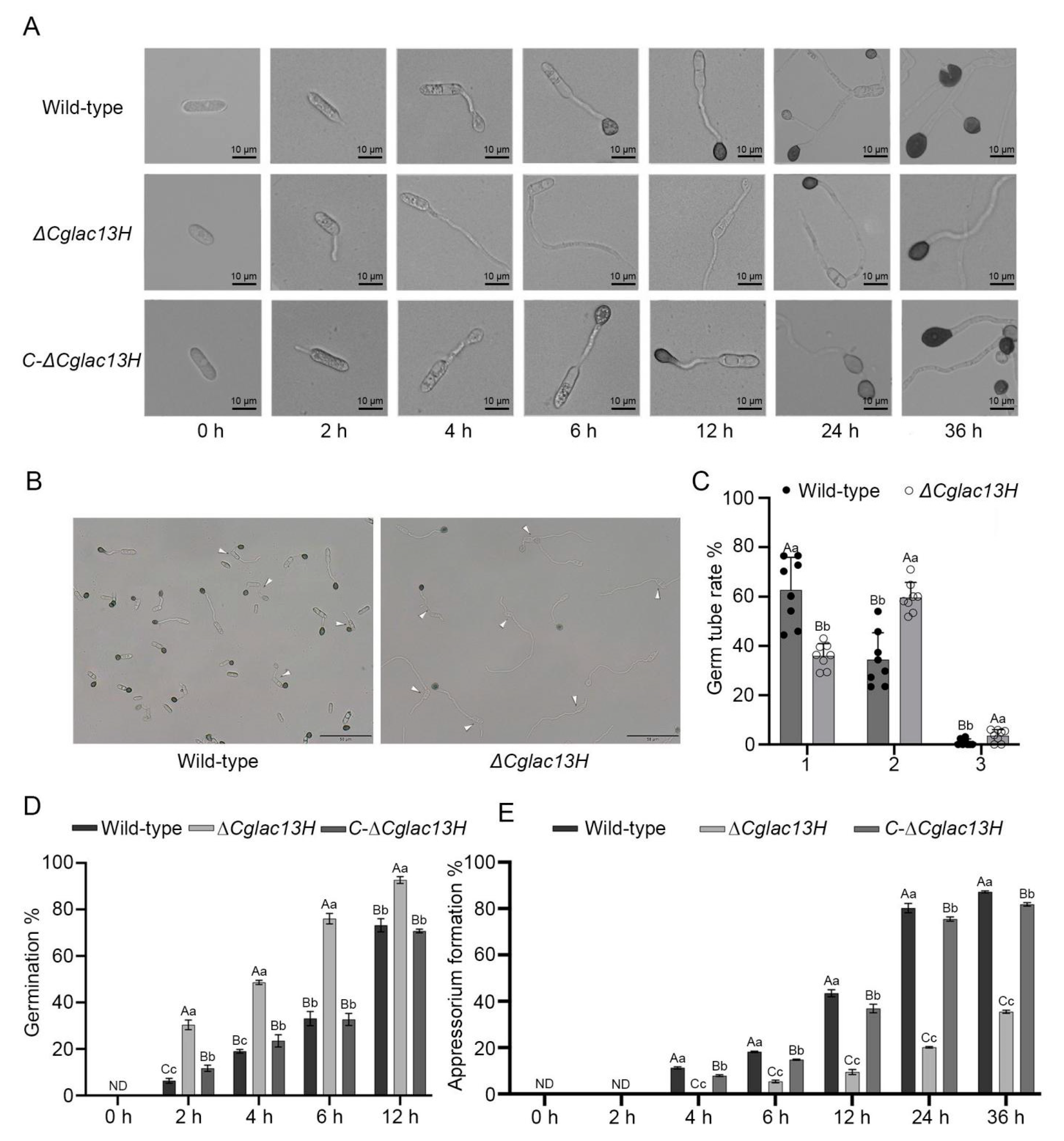
3.3. Cglac13 Involves Conidial Germination and Appressoria Formation
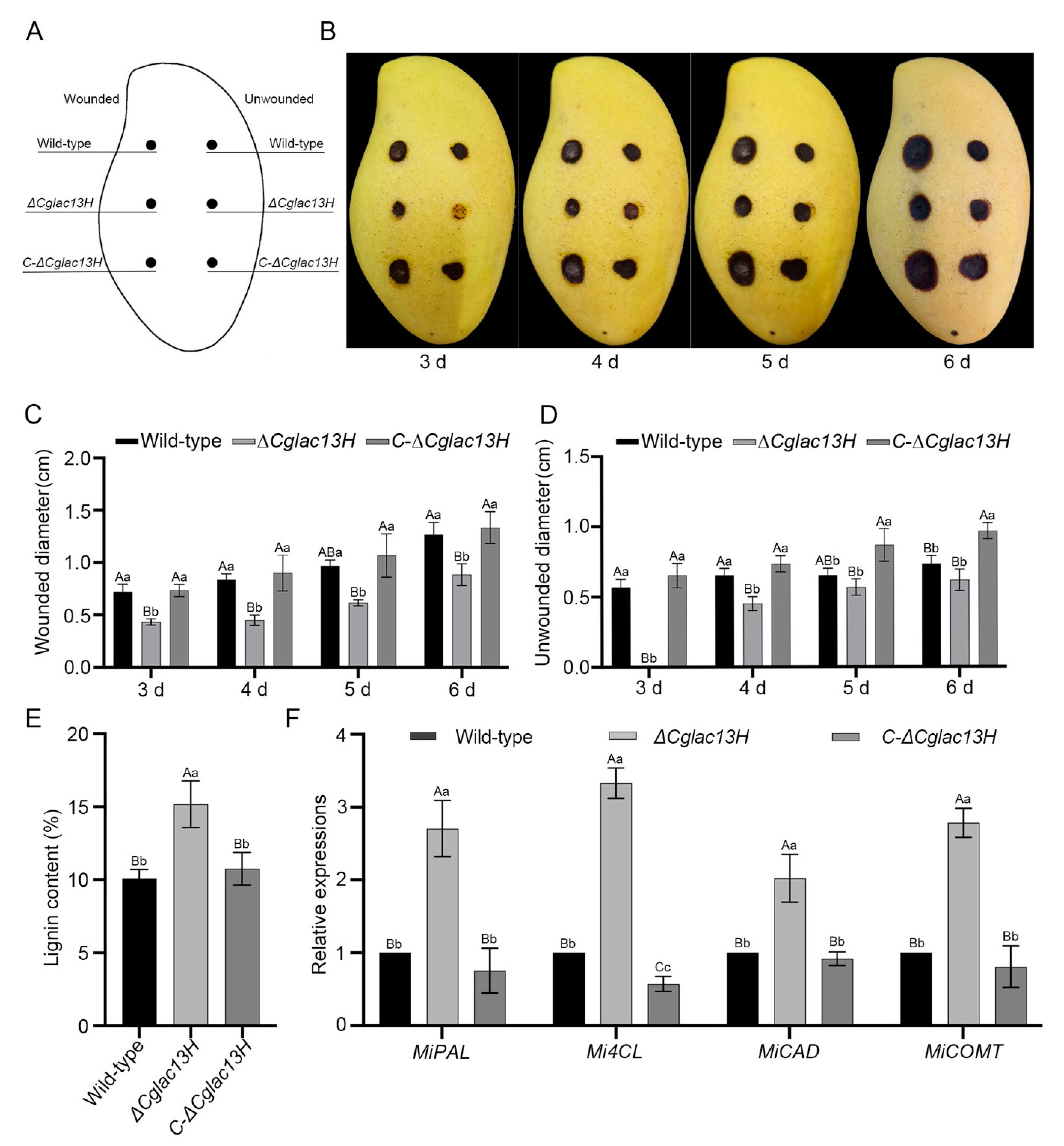
3.4. Cglac13 Is Required for Pathogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, Y.; Ali, A. Chapter 11—Colletotrichum gloeosporioides (Anthracnose). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 337–371. [Google Scholar]
- Tashiro, N.; Manabe, K.; Ide, Y. Emergence and frequency of highly benzimidazole-resistant Colletotrichum gloeosporioides, pathogen of Japanese pear anthracnose, after discontinued use of benzimidazole. J. Gen. Plant Pathol. 2012, 78, 221–226. [Google Scholar] [CrossRef]
- Azeem, M.; Zhou, Z.; Zhang, J.; Khaskheli, M.I.; Rui, J.Z.; Khaskheli, A.J.; Ali, S. Pathogenic and biological characterisation of T-DNA insertional mutants of a Colletotrichum gloeosporioides casual organism of apple anthracnose. Hortic. Sci. 2021, 48, 51–62. [Google Scholar] [CrossRef]
- Wu, J.; Hu, S.; Ye, B.; Hu, X.; Xiao, W.; Yu, H.; Zhang, C. Diversity and resistance to thiophanate-methyl of Colletotrichum spp. in strawberry nursery and the development of rapid detection ssing LAMP method. Agronomy 2022, 12, 2815. [Google Scholar] [CrossRef]
- Kaviyarasi, M.; Kamalakannan, A.; Rajendran, L.; Rajesh, S.; Kavino, M.; Swarna, L.K.R.; Shajith, B.J. Morphological and molecular characterization of Colletotrichum gloeosporioides causing mango anthracnose. Int. J. Plant Soil. Sci. 2022, 34, 837–844. [Google Scholar] [CrossRef]
- Riera, N.; Ramirez, V.D.; Barriga, M.N.; Alvarez, S.J.; Herrera, K.; Ruales, C.; Leon, R.A. First report of banana anthracnose caused by Colletotrichum gloeosporioides in Ecuador. Plant Dis. 2019, 103, 763. [Google Scholar] [CrossRef]
- Vieira, W.A.d.S.; Veloso, J.S.; Silva, A.C.d.; Nunes, A.d.S.; Doyle, V.P.; Castlebury, L.A.; Câmara, M.P.S. Elucidating the Colletotrichum spp. diversity responsible for papaya anthracnose in Brazil. Fungal Biol. 2022, 126, 623–630. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Q.; Feng, Q.; Zhang, B.; He, C.; Luo, H.; An, B. Heat shock transcription factor CgHSF1 is required for melanin biosynthesis, appressorium formation, and pathogenicity in Colletotrichum gloeosporioides. J. Fungi 2022, 8, 175. [Google Scholar] [CrossRef]
- Sangpueak, R.; Phansak, P.; Thumanu, K.; Siriwong, S.; Wongkaew, S.; Buensanteai, N. Effect of salicylic acid formulations on induced plant defense against cassava anthracnose disease. Plant Pathol. J. 2021, 37, 356–364. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, I.M.; Sharma, M.; Sharma, K.; Sharma, A. Effectiveness of fungal, bacterial and yeast antagonists for management of mango anthracnose (Colletotrichum gloeosporioides). Egypt J. Biol. Pest Control 2021, 31, 135. [Google Scholar] [CrossRef]
- Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2016, 31, 155–168. [Google Scholar] [CrossRef]
- Ren, D.; Wang, T.; Zhou, G.; Ren, W.; Duan, X.; Gao, L.; Chen, J.; Xu, L.; Zhu, P. Ethylene promotes expression of the appressorium and pathogenicity related genes via GPCR and MAPK dependent manners in Colletotrichum gloeosporioides. J. Fungi 2022, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Perez, J.J.; Hernandez-Montiel, L.G.; Vero, S.; Noa-Carrazana, J.C.; Quiñones-Aguilar, E.E.; Rincón-Enríquez, G. Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. Food Sci. Technol. 2019, 56, 4992–4999. [Google Scholar] [CrossRef] [PubMed]
- Arauz, L.F. Mango anthracnose: Economic impact and current options for integrated managaement. Plant Dis. 2000, 84, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lan, J.; Liu, L. The process of infection of mango by Colletotrichum gloeosporum and the histopathological study of its host. Chin. J. Trop. Crops 2022, 43, 361–368. (In Chinese) [Google Scholar]
- Lan, J. Study on the Taxonomy of Colletotrichum Corda et al. in China and Biological Peculiarity of Colletotrichum gloeosporioides (Penz.) Sacc. in Mango. Ph.D. Dissertation, Northwest A&F University, Xianyang, China, 2012. (In Chinese). [Google Scholar]
- Mcdowell, J.M. Genomic and transcriptomic insights into lifestyle transitions of ahemi-biotrophic fungal pathogen. New Phytol. 2013, 197, 1032–1034. [Google Scholar] [CrossRef]
- Philipp, J.; Mischo, C.E.; Gubesh, G.; Christian, S.; Becker, S.L.; Bernhard, H.; Karin, J.; Markus, B. Candida albicans adhesion to central venous catheters: Impact of blood plasma-driven germ tube formation and pathogen-derived adhesins. Virulence 2020, 11, 1453–1465. [Google Scholar]
- Zhang, J.; Zheng, X. Identification of the pathogen of Dendrobium candidum black spot and cytological study on the infection process. J. Plant Pathol. 2004, 1, 92–94. (In Chinese) [Google Scholar]
- Cao, Q.; Chi, D.; Yu, J.; Ran, Y. Scanning electron microscope and transmission electron microscope observation of the body wall of Beauveria bassiana infecting the larvae of Dendrolimus poplar. J. Beijing For. Univ. 2015, 37, 96–101. (In Chinese) [Google Scholar]
- Nakamura, K.; GO, N. Function and molecular evolution of multicopper blue proteins. Cell Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef]
- Kües, U.; Rühl, M. Multiple multi-copper oxidase gene families in basidiomycete- what for. Curr. Genom. 2011, 12, 72–94. [Google Scholar] [CrossRef]
- Liu, N.; Qu, Q.; Li, L.; Pang, Q.; Liu, J.; Zhang, Y.; Cao, Z.; Dong, J. Identification and expression pattern of laccase like copper oxidase from Fusarium graminearum. J. Plant Pathol. 2019, 49, 763–772. [Google Scholar]
- Chung, H.J.; Kwon, B.R.; Kim, J.M.; Park, S.M.; Park, J.K.; Cha, B.J.; Yang, M.S.; Kim, D.H. A tannic acid-inducible and hypoviral-regulated laccase3 contributes to the virulence of the chestnut blight fungus Cryphonectria parasitica. Mol. Plant Microbe Interact. 2008, 21, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz, T.S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington, S.P.; Leggieri, P.A.; Brown, J.L.; Swift, C.L.; Lipzen, A.; Na, H.; et al. Lignin deconstruction by anaerobic fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Ithal, N.; Recknor, J.; Nettleton, D.; Maier, T.; Baum, T.J.; Mitchum, M.G. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol. Plant Microbe Interact. 2007, 20, 510–525. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Liu, N.; Jia, H.; Shen, S.; Cao, Z.; Dong, J. Fungal laccase: Various biological functions and complex natural substrate. J. Agric. Biotechnol. 2020, 28, 333–341. (In Chinese) [Google Scholar]
- Choi, G.H.; Larson, T.G.; Nuss, D.L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol. Plant Microbe Interact. 1992, 5, 119–128. [Google Scholar] [CrossRef]
- Valeru, S.P.; Rompikuntal, P.K.; Ishikawa, T.; Vaitkevicius, K.; Sjöling, A.; Dolganov, N.; Zhu, J.; Schoolnik, G.; Wai, S.N. Role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect. Immun. 2009, 77, 935–942. [Google Scholar] [CrossRef]
- Lin, S.Y.; Okuda, S.; Ikeda, K.; Okuno, T.; Takano, Y. LAC2 encoding a secreted laccase is involved in appressorial melanization and conidial pigmentation in Colletotrichum orbiculare. Mol. Plant Microbe Interact. 2012, 25, 1552–1561. [Google Scholar] [CrossRef]
- Zhan, X. Function Analysis of Laccase Genes (StLAC) in Melanin Biosynthesis Pathway of Setosphaeria turcica; Agricultural University of Hebei: Baoding, China, 2011. (In Chinese) [Google Scholar]
- Ma, S.; Liu, N.; Jia, H.; Dai, D.; Xu, M.; Cao, Z.; Dong, J. Analysis and Expression of Laccase Gene Stlac2 in Setosphaeria turcica. Chin. Agric. Sci. 2016, 49, 4130–4139. (In Chinese) [Google Scholar]
- Jia, H.; Meng, Q.; Li, Z.; Gong, X.; Cang, J.; Hao, Z.; Cao, Z.; Dong, J. Localization of Melanin Biosynthesis Enzyme Genes in the Genome and Expression Pattern Analysis of Setosphaeria turcica. Chin. Agric. Sci. 2015, 48, 2767–2776. (In Chinese) [Google Scholar]
- Wei, Y.; Pu, J.; Zhang, H.; Liu, Y.; Zhou, F.; Zhang, K.; Liu, X. The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol. Mol. Plant Pathol. 2017, 99, 55–64. [Google Scholar] [CrossRef]
- Mehta, N.; Patil, R.; Baghela, A. Differential physiological prerequisites and gene expression profiles of conidial anastomosis tube and germ tube formation in Colletotrichum gloeosporioides. J. Fungi 2021, 7, 509. [Google Scholar] [CrossRef]
- Li, H.; Zhong, C.; Wu, Q.; Zhang, Y.; Zhang, H.; Pu, J.; Liu, X. Sequence characteristics of three pectin lyase genes of mango anthracnose and analysis of their influence by laccase gene Lac1. Chin. J. Trop. Crops 2018, 39, 2458–2464. (In Chinese) [Google Scholar]
- Hernández, C.; Farnet, D.S.A.M.; Ziarelli, F.; Perraud, G.I.; Gutiérrez, R.B.; García, P.J.A.; Alarcón, E. Laccase induction by synthetic dyes in Pycnoporus sanguineus and their possible use for sugar cane bagasse delignification. Appl. Microbiol. Biot. 2017, 101, 1189–1201. [Google Scholar] [CrossRef]
- Goswami, P.; Chinnadayyala, S.S.R.; Chakraborty, M.; Kumar, A.K.; Kakoti, A. An overview on alcohol oxidases and their potential applications. Appl. Microbiol. Biot. 2013, 97, 4259–4275. [Google Scholar] [CrossRef]
- Yan, J.; Tong, Z.; Liu, Y.; Zhang, L.; Zhang, Y.; Xie, B. Sequence characteristics and differential expression of volvariella volvacea aromatic alcohol oxidase gene vvaao1. Biotechnol. Bull. 2018, 34, 107–114. [Google Scholar]
- Gu, S. Cloning and Functional Analysis of the STK Genes that Regulates the Growth, Development and Pathogenicity of Setosphaeria turcica; Agricultural University of Hebei: Baoding, China, 2007. (In Chinese) [Google Scholar]
- Lorang, J.M.; Tuori, R.P.; Martinez, J.P.; Sawyer, T.L.; Redman, R.S.; Rollins, J.A.; Wolpert, T.J.; Johnson, K.B.; Rodriguez, R.J.; Dickman, M.B.; et al. Green fluorescent protein is lighting up fungal biology. Appl. Environ. Microb. 2001, 67, 1987–1994. [Google Scholar] [CrossRef]
- Wei, Y. Coloning and Functional Identification of Laccase Gene (Lac1) in Pathogenicity from Colletotrichum gloeosporioides the Pathogen of Mango Anthracnose Disease; Hainan University: Haikou, China, 2014. (In Chinese) [Google Scholar]
- Luo, C.; He, X.H.; Chen, H.; Hu, Y.; Ou, U.S. Molecular cloning and expression analysis of four actin genes (MiACT) from mango. Biol. Plant. 2013, 57, 238–244. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, A.; Chen, Y.; Huang, J.; Dang, Z.; Luo, R. Cloning and Sequence Analysis of PAL Gene in Mango. J. Anhui Agric. Univ. 2015, 42, 825–830. [Google Scholar]
- Wang, P.; Luo, Y.; Huang, J.; Gao, S.; Zhu, G.; Dang, Z.; Gai, J.; Yang, M.; Zhu, M.; Zhang, H.; et al. The genome evolution and domestication of tropical fruit mango. Genome Biol. 2020, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Lu, D. Study on the Biological Function of Type II Myosin Regulating Light Chain MoRlc1 of Magnaporthe grisea; Anhui Agricultural University: Hefei, China, 2020. (In Chinese) [Google Scholar]
- Cañero, D.C.; Roncero, M.I. Functional analyses of laccase genes from Fusarium oxysporum. Phytopathology 2008, 98, 509–518. [Google Scholar] [CrossRef]
- Lǚ, Z.; Kang, X.; Xiang, Z.; He, N. Laccase Gene Shlac Is Involved in the Growth and Melanin Biosynthesis of Scleromitrula shiraiana. Phytopathology 2017, 107, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Yin, Z.; Zhang, H.; Zhang, Z. Research progress on early infection mechanism of interaction between Magnaporthe oryzae and rice. Bul. Natl. Nat. Sci. Found China 2020, 34, 411–422. (In Chinese) [Google Scholar]
- Wei, Y.; Liu, X.; Zhang, H.; Zhang, X.; Qi, Y.; Xie, Y.; Lu, Y.; Cao, S.; Pu, J. Cloning and sequence analysis of Lac1 gene of Colletotrichum gloeosporioides. J. Fruit Tree 2013, 30, 202–206. (In Chinese) [Google Scholar]
- Liu, S.; Wang, Q.; Liu, N.; Luo, H.; He, C.; An, B. The histone deacetylase HOS2 controls pathogenicity through regulation of melanin biosynthesis and appressorium formation in Colletotrichum gloeosporioides. Phytopathol. Res. 2022, 4, 21. [Google Scholar] [CrossRef]
- Li, X.; Ke, Z.; Xu, S.; Tang, W.; Liu, Z. The G-protein alpha subunit CgGa1 mediates growth, sporulation, penetration and pathogenicity in Colletotrichum gloeosporioides. Microb. Pathog. 2021, 161, 105254. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tariq, M.; Ahmed, M.; Zhou, Z.; Ali, I.; Mahmood, R.T. Carbamoyl phosphate synthase subunit CgCPS1 is necessary for virulence and to regulate stress tolerance in Colletotrichum gloeosporioides. Plant Pathol. J. 2021, 37, 232–242. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Zhang, N.; Liu, Z. Function and transcriptome analysis of an oligopeptide transporter CgOPT2 in the rubber anthracnose fungus Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2021, 115, 101661. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. CgEnd3 regulates endocytosis, appressorium formation, and virulence in the poplar anthracnose fungus Colletotrichum gloeosporioides. Int. J. Mol. Sci. 2021, 22, 4029. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.B.; Qiang, G.; Lu, D.D.; Shi, S.H.; Wang, C.S. Appressorial differentiation and its association with cAMP in the insect pathogenic fungus Metarhizium anisopliae. Mycosystema 2009, 28, 712–717. [Google Scholar]
- Wang, C.S.; Typas, M.A.; Butt, T.M. Detection and characterisation of pr1 virulent gene deficiencies in the insect pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 2002, 213, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, C.; Li, H.; Shang, W.; Hu, X. Cloning and functional analysis of VdCPMO gene from Rotaea dahlia. J. Mycol. 2018, 37, 175–182. (In Chinese) [Google Scholar]
- Ahamedemujtaba, V.; Atheena, P.V.; Bhat, A.I.; Krishnamurthy, K.S.; Srinivasan, V. Symptoms of piper yellow mottle virus in black pepper as influenced by temperature and relative humidity. Virus Dis. 2021, 32, 305–313. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′-3′) | Expected Length/bp |
|---|---|---|
| Cglac13-qPCR-F | CCACTGCCACAATCTTAT | 178 |
| Cglac13-qPCR-R | TTGCACCTTCTGCACAAC | |
| 5Cglac13-MHF | AACGAAAGCCAGCGACAA | 445 |
| 5Cglac13-MHR | CTGTACTCAGGACTCAGCCAGT | |
| 3Cglac13-MHF | ATGGAGCAGGTTGATGAGATT | 458 |
| 3Cglac13-MHR | ATGGGAAGGAGGAGTGGG | |
| hygB-F | AACTGGTTCCCGGTCGGC | 1412 |
| hygB-R | AACTGATATTGAAGGAGCATTTTTT | |
| 5Cglac13-CF | TCTAGACAGACACGCAGC | 2140 |
| 5Cglac13-CR | AAATCTTACACCCCCAAT | |
| 3Cglac13-CF | TAAGATTACGGTGATGGC | 260 |
| 3Cglac13-CR | GATAGATTCTGATGGGGA | |
| F1-Cglac13H | CGGGAGCACAACAGCAAT | 2199 (Mutant) 2554 (Wild-type) 2199 (pCglac13H) |
| R1-Cglac13H | GGAGGAGTGGGTTAGCGTAG | |
| F2-H850 | AACTCACCGCGACGTCTGTC | 610 (Mutant) 0 (Wild-type) 610 (pCglac13H) |
| R2-H852 | TTGTCCGTCAGGACATTGTT | |
| F3-Cglac13 BAR | GAGTGCCCGCTCCTGTGGTA | 2169 (Complementary strain) 1618 (Wild-type) 0 (Mutant) |
| R3-Cglac13 BAR | GATTCTGATGGGGAAATTTG | |
| F4-Bar | TCAAATCTCGGTGACG | 552 (Complementary strain) 0 (Mutant; Wild-type) |
| R4-Bar | ATGAGCCCAGAACGACGC | |
| MiActin-qPCR-F | GTTTCCCAGTATTGTGGGTAGG | 167 |
| MiActin-qPCR-R | AGATCTTTTCCATATCATCCCAGTT | |
| MiPAL-qPCR-F | GTCGCATAGGAGAACGAAGC | 204 |
| MiPAL-qPCR-R | AACTTGGTGATGGCTTCCAG | |
| Mi4CL-qPCR-F | GAATACGCTTTCTCTCTCAGAG | 188 |
| Mi4CL-qPCR-R | GAGTTGGGAGAGAGTACAAATG | |
| MiCAD-qPCR-F | CGGCAAGATTACACCTTACACAT | 170 |
| MiCAD-qPCR-R | TAACCACCCCAGTAATTTCATGC | |
| MiCOMT-qPCR-F | GGCAAAGATCCCAGATTCAA | 228 |
| MiCOMT-qPCR-R | CAAAGATGGAGCATCGTCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Xiao, C.; Tan, Q.; Dong, L.; Liu, X.; Pu, J.; Zhang, H. The Involvement of the Laccase Gene Cglac13 in Mycelial Growth, Germ Tube Development, and the Pathogenicity of Colletotrichum gloeosporioides from Mangoes. J. Fungi 2023, 9, 503. https://doi.org/10.3390/jof9050503
Zhang M, Xiao C, Tan Q, Dong L, Liu X, Pu J, Zhang H. The Involvement of the Laccase Gene Cglac13 in Mycelial Growth, Germ Tube Development, and the Pathogenicity of Colletotrichum gloeosporioides from Mangoes. Journal of Fungi. 2023; 9(5):503. https://doi.org/10.3390/jof9050503
Chicago/Turabian StyleZhang, Mengting, Chunli Xiao, Qing Tan, Lingling Dong, Xiaomei Liu, Jinji Pu, and He Zhang. 2023. "The Involvement of the Laccase Gene Cglac13 in Mycelial Growth, Germ Tube Development, and the Pathogenicity of Colletotrichum gloeosporioides from Mangoes" Journal of Fungi 9, no. 5: 503. https://doi.org/10.3390/jof9050503





