Mycotic Diseases in Chelonians
Abstract
:1. Introduction
2. Mycoses of Chelonians by Conventional Agents
2.1. Conventional Mycoses
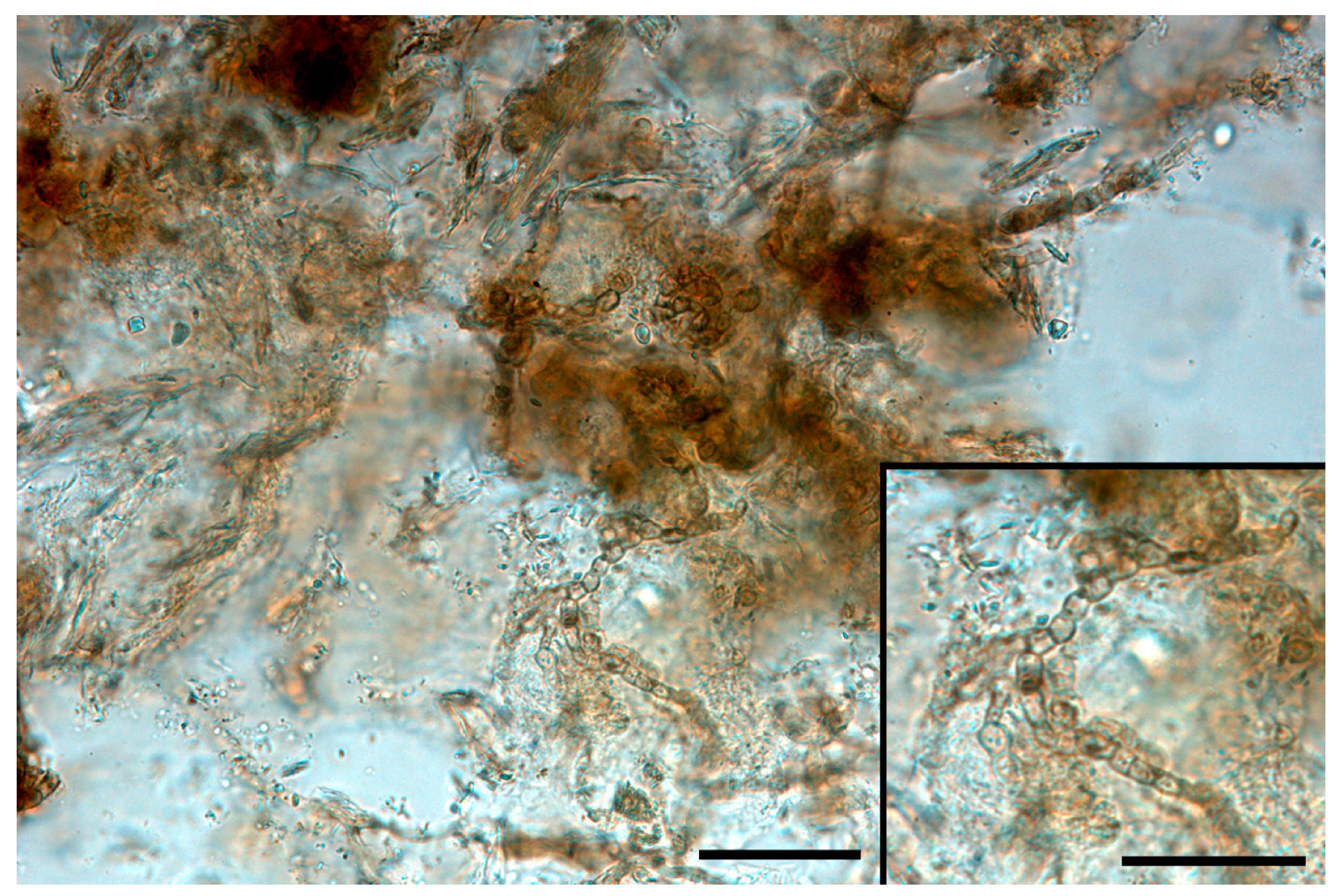
2.1.1. Dermatomycoses
Shell Mycoses
Skin Mycoses
2.1.2. Deep and Systemic Mycoses
3. Mycoses of Chelonians by Unconventional Agents
3.1. Emerging Fungal Pathogens
3.1.1. Sea Turtles’ Egg Fusariosis
3.1.2. Emydomyces testavorans Infection
3.1.3. Microsporidioses
4. Treatment of Mycoses in Chelonians
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhodin, A.G.J.; Stanford, C.B.; Dijk, P.P.V.; Eisemberg, C.; Luiselli, L.; Mittermeier, R.A.; Hudson, R.; Horne, B.D.; Goode, E.V.; Kuchling, G.; et al. Global Conservation status of turtles and tortoises (Order testudines). Chelonian Conserv. Biol. 2018, 17, 135. [Google Scholar] [CrossRef]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.J.; Paul van Dijk, P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.G.; et al. Turtles and Tortoises Are in Trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ramírez, J.M.; Abella-Pérez, E.; Phillott, A.D.; Sim, J.; van West, P.; Martín, M.P.; Marco, A.; Diéguez-Uribeondo, J. Global Distribution of Two Fungal Pathogens Threatening Endangered Sea Turtles. PLoS ONE 2014, 9, e85853. [Google Scholar] [CrossRef]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the Role of Infectious Disease in Species Extinction and Endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G. Sea Turtles: Old Viruses and New Tricks. Curr. Biol. 2004, 14, R842–R843. [Google Scholar] [CrossRef]
- Mashkour, N.; Jones, K.; Kophamel, S.; Hipolito, T.; Ahasan, S.; Walker, G.; Jakob-Hoff, R.; Whittaker, M.; Hamann, M.; Bell, I.; et al. Disease Risk Analysis in Sea Turtles: A Baseline Study to Inform Conservation Efforts. PLoS ONE 2020, 15, e0230760. [Google Scholar] [CrossRef]
- Reynolds, H.T.; Raudabaugh, D.B.; Lilje, O.; Matthew, C.; Allender, M.C.; Miller, A.M.; Gleason, F.H. Emerging mycoses and fungus-like diseases of vertebrate wildlife. In The Fungal Community: Its Organization and Role in the Ecosystem; Dighton, J., White, J.F., Eds.; CRC Taylor and Francis: Boca Raton, FL, USA, 2017; pp. 286–403. [Google Scholar]
- Austwick, P.; Keymer, I. Fungi and actinomycetes. In Diseases of the Reptilia; Cooper, J.E., Jackson, O.F., Eds.; Academic Press: New York, NY, USA, 1981; pp. 193–231. [Google Scholar]
- Kostka, V.M.; Hoffmann, L.; Balks, E.; Eskens, U.; Wimmershof, N. Review of the literature and investigations on the prevalence and consequences of yeasts in reptiles. Vet. Rec. 1997, 140, 282–287. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Cheatwood, J.L.; Maxwell, L.K. Mycotic diseases of reptiles. Semin. Avian Exot. Pet Med. 2000, 9, 94–101. [Google Scholar] [CrossRef]
- Pare, J.A. Update on fungal infections in reptiles. In Current Therapy in Reptile Medicine and Surgery; Mader, D.R., Divers, S., Eds.; Elsevier Health Sciences: Saint Louis, MO, USA, 2014; pp. 53–56. [Google Scholar]
- Hatt, J.M. Dermatological diseases in reptiles. Schweizer Archiv für Tierheilkunde 2010, 152, 123–130. [Google Scholar] [CrossRef]
- Zwart, P.; Schroder, H.D. Reptilien. In Handbuch der Zootierkrankheiten; Ippen, R., Schroder, H.D., Elze, K., Eds.; Akademia Verlag: Berlin, Germany, 1985; p. 349. [Google Scholar]
- Gabrisch, K.; Zwart, P. (Eds.) Krankheiten der Heimtiere; Schlutersche Verlagsanstalt: Berlin, Germany, 1995; p. 694. [Google Scholar]
- Hafeli, W.; Schildger, B.J. Krankheiten der Zoo- und Wildtiere; Goltenboth, E., Klos, H.G., Eds.; Blackwell Wissenschaftsverlag: Berlin, Germany, 1995; p. 530. [Google Scholar]
- Hunt, T.J. Notes on Diseases and Mortality in Testudines. Herpetologica 1957, 13, 19–23. [Google Scholar]
- Bourdeau, P.; Tronco, N. Pathologie des tortues de compagnie: Bilan de consultations à Maisons-Alfort. In Proceedings of the first International Congress of Chelonian Pathology, Gonfaron, France, 25–27 April 1992; pp. 174–188. [Google Scholar]
- Bouvard, J. Contribution a L’étude des Affections Tegumentaires des Tortues Terrestres Mediterraneennes. Observations Personnelles dans le Village des Tortues de Gonfaron (France). Ph.D. Thesis, Ecole Nationale Veterinaire de Maisons-Alfort, Maisons-Alfort, France, 22 September 1992. [Google Scholar]
- Flamant, F.; de Gentile, L.; Chermette, R.; Chabasse, D.; Bouchara., J.P. Flore fongique des lésions de la carapace des tortues terrestres de compagnie dans l’Ouest de la France. J. Mycol. Méd. 2003, 13, 67–72. [Google Scholar]
- Rose, F.L.; Koke, J.; Koehn, R.; Smith, D. Identification of the etiological agent for necrotizing scute disease in the Texas tortoise. J. Wildl. Dis. 2001, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Lungonelli, P.; Papini, R.; Mugnaini, L.; Mancianti, F. Shell Mycosis in a Group of Hermann’s Tortoises (Testudo hermanni). Vet. Rec. 2012, 170, 76. [Google Scholar] [CrossRef] [PubMed]
- Brilhante-Simoes, P.; Duque, D.; Sampaio, F.; Silva, A.; Lopes, R.; Salinas, D.; Fernandes, H.; Garces, A. First Report of a Cutaneous and Shell Mycosis in an Ouachita Map Turtle (Graptemys ouachitensis, Cagle, 1953) by Fusarium solani. Veterinarska Stanica 2023, 54, 3. [Google Scholar] [CrossRef]
- Sutton, D.A.; Marín, Y.; Thompson, E.H.; Wickes, B.L.; Fu, J.; García, D.; Swinford, A.; De Maar, T.; Guarro, J. Isolation and characterization of a new fungal genus and species, Aphanoascella galapagosensis, from carapace keratitis of a Galapagos tortoise (Chelonoidis nigra microphyes). Med. Mycol. 2013, 51, 113–120. [Google Scholar] [CrossRef]
- Stringer, E.M.; Garner, M.M.; Proudfoot, J.S.; Ramer, J.C.; Bowman, M.R.; Heng, H.G.; Bradway, D.S. Phaeohyphomycosis of the carapace in an Aldabra tortoise (Geochelone gigantea). J. Zoo Wildl. Med. 2009, 40, 160–167. [Google Scholar] [CrossRef]
- Nardoni, S. (Dipartimento di Scienze Veterinarie, Università degli Studi di Pisa, Pisa, Italy). Unpublished data. 2011. [Google Scholar]
- Luangsa-ard, J.; Houbraken, J.; Van Doorn, T.; Hong, S.-B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus: Purpureocillium, a new fungal genus for P. lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Lafortune, M.; Wellehan, J.F.X.; Terrell, S.P.; Jacobson, E.R.; Heard, D.; Kimbrough, J.W. Shell and systemic hyalohyphomycosis in fly river turtles, Carettochelys insculpta, caused by Paecilomyces lilacinus. J. Herpetol. Med. Surg. 2005, 15, 15–19. [Google Scholar] [CrossRef]
- Cafarchia, C.; Paradies, R.; Figueredo, L.A.; Iatta, R.; Desantis, S.; Di Bello, A.V.F.; Zizzo, N.; Van Diepeningen, A.D. Fusarium spp. in loggerhead sea turtles (Caretta caretta): From colonization to infection. Vet. Pathol. 2020, 57, 139–146. [Google Scholar] [CrossRef]
- Lai, O.; Tinelli, A.; Soloperto, S.; Marzano, G.; Tosches, M.; Leone, R.; Gelli, D.; Belloli, C.; Crescenzo, G. Fusarium solani hyalohyphomycosis in loggerhead sea turtles (Caretta caretta): A diagnostic and therapeutical challenge. Vet. Ital. 2020, 56, 123–132. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Calderwood, M.B.; Clubb, S.L. Mucormycosis in hatchling Florida softshell turtles. J. Am. Vet. Med. Assoc. 1980, 177, 835–837. [Google Scholar] [PubMed]
- Schildger, B.J.; Frank, H.; Gobel, T.; Weiss, R. Mycotic infections of the integument and inner organs in reptiles. Herpetopathologia 1991, 2, 81–97. [Google Scholar]
- Guého, E.; Smith, M.T.; de Hoog, G.S.; Billon-Grand, G.; Christen, R.; Batenburg-van der Vegte, W.H. Contributions to a revision of the genus Trichosporon. Antonie Van Leeuwenhoek 1992, 6, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Frye, F.L.; Dutra, F.R. Mycotic granulomata involving the forefeet of a turtle. Vet. Med. Small Anim. Clin. 1974, 69, 1554–1556. [Google Scholar] [PubMed]
- Li, X.; Zhang, C.; Fang, W.; Lin, F. White-Spot Disease of Chinese soft-shelled turtles (Trionyx sinens) caused by Paecilomyces lilacinus. J. Zhejiang Univ. Sci. B 2008, 9, 578–581. [Google Scholar] [CrossRef]
- Rebell, G. Fusarium infections in human and veterinary medicine, In Fusarium: Diseases, Biology and Taxonomy; Nelson, P.E., Tousson, T.A., Cook, R.J., Eds.; The Pennsylvania State University Press: University Park, PA, USA, 1981; pp. 210–220. [Google Scholar]
- Cabañes, F.J.; Alonso, J.M.; Castellá, G.; Alegre, F.; Domingo, M.; Pont, S. Cutaneous hyalohyphomycosis caused by Fusarium solani in a loggerhead sea turtle (Caretta caretta L.). J. Clin. Microbiol. 1997, 35, 3343–3345. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hartmann, M.; Hennequin, C.; Catteau, S.; Béatini, C.; Blanc, V. Clusters of Fusarium solani infection in juvenile captive born Caretta caretta sea turtles. J. Mycol. Med. 2017, 27, 113–118. [Google Scholar] [CrossRef]
- Williams, S.R.; Sims, M.A.; Roth-Johnson, L.; Wickes, B. Surgical Removal of an Abscess Associated with Fusarium Solani from a Kemp’s Ridley Sea Turtle (Lepidochelys kempii). J. Zoo Wildl. Med. 2012, 43, 402–406. [Google Scholar] [CrossRef]
- Castellá, G.; Cano, J.; Guarro, J.; Cabañes, F.J. DNA Fingerprinting of Fusarium solani Isolates Related to a Cutaneous Infection in a Sea Turtle. Med. Mycol. 1999, 37, 223–226. [Google Scholar] [CrossRef]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying Fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Wiederhold, N.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M. Veterinary fusarioses within the United States. J. Clin. Microbiol. 2016, 54, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Greeff-Laubscher, M.R.; Jacobs, K. Fusarium species isolated from post-hatchling loggerhead sea turtles (Caretta caretta) in South Africa. Sci. Rep. 2022, 12, 5874. [Google Scholar] [CrossRef]
- Wadhwani, K.; Srivastava, A.K. Fungi from otitis media of agricultural field workers. Mycopathologia 1984, 88, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Salvadori, M.; Poli, A.; Rocchigiani, G.; Mancianti, F. Cutaneous lesions due to Trichosporon jirovecii in a tortoise (Testudo hermanni). Med. Mycol. Case Rep. 2017, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Hamerton, A.E. Report on the deaths occurring in the Society’s garden during the year 1933. Proc. Zoo Soc. Lond. 1934, 104, 39. [Google Scholar] [CrossRef]
- Heard, D.J.; Cantor, G.H.; Jacobson, E.R.; Purich, B.; Ajello, L.; Padhye, A.A. Hyalohyphomycosis caused by Paecilomyces lilacinus in an Aldabra tortoise. J. Am. Vet. Med. Assoc. 1986, 189, 1143–1145. [Google Scholar]
- Georg, L.K.; Williamson, W.M.; Tilden, E.B.; Getty, R.E. Mycotic pulmonary disease of captive giant tortoises due to Beauveria bassiana and Paecilomyces fumoso-roseus. Sabouraudia 1962, 2, 80. [Google Scholar] [CrossRef]
- Cabo, J.F.G.; Serrano, J.E.; Asensio, M.C.B. Mycotic pulmonary disease by Beauveria bassiana in a captive tortoise. Mycoses 1995, 38, 167–169. [Google Scholar] [CrossRef]
- Schumacher, V.L.; Mangold, B.; Lenzycki, J.; Hinckley, L.; Sutton, D.A.; Frasca, S. Occurrence of fruiting structures allows determination of Purpureocillium lilacinum as an inciting agent of pleuritis and pneumonia in a loggerhead sea turtle (Caretta caretta) by histopathologic correlation to culture. Med. Mycol. Case Rep. 2014, 6, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Arpini, C.M.; Nóbrega, Y.C.; Castheloge, V.D.; Neves, D.S.; Tadokoro, C.E.; da Costa, G.L.; Oliveira, M.M.E.; de Deus Santos, M.R. Purpureocillium lilacinum infection in captive loggerhead sea turtle hatchlings. Med. Mycol. Case Rep. 2019, 23, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Posthaus, H.; Krampe, M.; Pagan, O.; Gueho, E.; Suter, C.; Bacciarini, L. Systemic paecilomycosis in a hawksbill turtle (Eretmochelys imbricata). J. Mycol. Med. 1997, 7, 223–226. [Google Scholar]
- Horgan, M.D.; Alexander, A.B.; Innis, C.; Stacy, B.A.; Gai, J.J.; Pesavento, P.A.; Highland, M.A.; Liguori, B.L.; Norton, T.M.; Wellehan, J.F.X.; et al. Pulmonary and coelomic mycoses due to Metarhizium and Beauveria species in reptiles. J. Zoo Wildl. Med. 2022, 53, 605–612. [Google Scholar] [CrossRef]
- Glazebrook, J.S.; Campbell, R.S.F. A survey of the diseases of marine turtles in northern Australia. I Farmed turtles. Dis. Aquat. Org 1990, 9, 83–95. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Gaskin, J.M.; Shields, R.P.; White, F.W. Mycotic pneumonia in mariculture-reared green sea turtles. J. Am. Vet. Med. Assoc. 1979, 175, 929–933. [Google Scholar] [PubMed]
- Manire, C.A.; Rhinehart, H.L.; Sutton, D.A.; Thompson, E.H.; Rinaldi, M.G.; Buck, J.D.; Jacobson, E. Disseminated mycotic infection caused by Colletotrichum acutatum in a Kemp’s ridley sea turtle (Lepidochelys kempi). J. Clin. Microbiol. 2002, 40, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Orós, J.; Ramírez, A.S.; Poveda, J.B.; Rodríguez, J.L.; Fernández, A. Systemic mycosis caused by Penicillium griseofulvum in a Seychelles giant tortoise (Megalochelys gigantea). Vet. Rec. 1996, 139, 295–296. [Google Scholar] [CrossRef]
- Orós, J.; Calabuig, P.; Arencibia, A.; Camacho, M.; Jensen, H. Systemic mycosis caused by Trichophyton spp. in an olive ridley sea turtle (Lepidochelys olivacea): An immunohistochemical study. N. Z. Vet. J. 2011, 59, 92–95. [Google Scholar] [CrossRef]
- Orós, J.; Delgado, C.; Fernández, L.; Jensen, H.E. Pulmonary hyalohyphomycosis caused by Fusarium spp. in a Kemp’s ridley sea turtle (Lepidochelys kempi): An immunohistochemical study. N. Z. Vet. J. 2004, 52, 150–152. [Google Scholar] [CrossRef]
- Aleksić-Kovačević, S.; Ozvegy, J.; Krstić, N.; Rusvai, M.; Jakab, C.; Stanimirović, Z.; Becskei, Z. Skin and skeletal system lesions of european pond turtles (Emys orbicularis) from natural habitats. Acta Vet. Hung. 2014, 62, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Manharth, A.; Lemberger, K.; Mylniczenko, N.; Pinkerton, M.; Pessier, A.P.; Kammeyer, P.; De Hoog, S. Disseminated phaeohyphomycosis due to an Exophiala species in a Galapagos tortoise, Geochelone nigra. J. Herpetol. Med. Surg. 2005, 15, 20–26. [Google Scholar] [CrossRef]
- Domiciano, I.G.; Domit, C.; Trigo, C.C.; de Alcântara, B.K.; Headley, S.A.; Bracarense, A.P.F.R.L. Phaeohyphomycoses in a free-ranging loggerhead turtle (Caretta caretta) from Southern Brazil. Mycopathologia 2014, 178, 123–128. [Google Scholar] [CrossRef]
- Donnelly, K.; Waltzek, T.B.; Wellehan, J.F.X.; Sutton, D.A.; Wiederhold, N.P.; Stacy, B.A. Phaeohyphomycosis resulting in obstructive tracheitis in three green sea Turtles Chelonia mydas stranded along the Florida coast. Dis. Aquat. Organ. 2015, 113, 257–262. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.J. Pulmonary candidiasis caused by Candida albicans in a Greek tortoise (Testudo graeca) and treatment with intrapulmonary amphotericin B. J. Zoo Wildl. Med. 2001, 32, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Orós, J.; Arencibia, A.; Fernández, L.; Jensen, H.E. Intestinal candidiasis in a loggerhead sea turtle (Caretta Caretta): An immunohistochemical study. Vet. J. 2004, 167, 202–207. [Google Scholar] [CrossRef]
- Juniantito, V.; Izawa, T.; Kuwamura, M.; Yonezawa, M.; Ito, S.; Yamate, J. Gastrointestinal candidiasis in an Aldabra giant tortoise (Geochelone gigantea). J. Vet. Med. Sci. 2009, 71, 1269–1272. [Google Scholar] [CrossRef]
- Zwart, P.; Buitelaar, M.N. Candida tropicalis enteric infections and their treatment in Chelonians. In Proceedings of the Annual American Association of Zoo Veterinarians, Washington DC, USA, 18–20 October 1980; pp. 58–59. [Google Scholar]
- Ruiz, J.M.; Arteaga, E.; Martinez, J.; Rubio, E.M.; Torres, J.M. Cutaneous and renal geotrichosis in a giant tortoise (Geochelone elephantopus). Sabouraudia 1980, 18, 51–59. [Google Scholar] [CrossRef]
- Iannaccone, M.; Basso, P.R.; Congiu, T.; Cavicchio, P.; Ulivi, V.; Campolo, M. Multiple Organ Dysfunction Syndrome (MODS) Induced by Candida krusei in an Aldabra giant tortoise (Aldabrachelys gigantea) and confirmed by electron microscopy analysis. Med. Mycol. Case Rep. 2018, 21, 44–48. [Google Scholar] [CrossRef]
- Wang, W.-L.; Sun, P.-L.; Kao, C.-F.; Li, W.-T.; Cheng, I.-J.; Yu, P.-H. Disseminated candidiasis and candidemia caused by Candida palmioleophila in a green sea turtle (Chelonia mydas). Animals 2021, 11, 3480. [Google Scholar] [CrossRef]
- Hoh, D.Z.; Lee, H.-H.; Wada, N.; Liu, W.-A.; Lu, M.R.; Lai, C.-K.; Ke, H.-M.; Sun, P.-F.; Tang, S.-L.; Chung, W.-H.; et al. Comparative genomic and transcriptomic analyses of trans-kingdom pathogen Fusarium solani species complex reveal degrees of compartmentalization. BMC Biol. 2022, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Phillott, A.D. Penetration of the eggshell and invasion of embryonic tissue by fungi colonizing sea turtle eggs. Herpetofauna 2004, 34, 44–47. [Google Scholar]
- Güçlü, Ö.; Bıyık, H.; Şahiner, A. Mycoflora identified from loggerhead turtle (Caretta caretta) egg shells and nest sand at Fethiye beach, Turkey. Afr. J. Microbiol. Res. 2010, 4, 408–413. [Google Scholar]
- Milena, S.C.N.; Carina, C.D.M.M.; Luciana, G.D.O. Mycobiota from the eggs, nests and stillbirths of Eretmochelys imbricata Linneus 1766 (Testudines: Cheloniidae) in Pernambuco State, Brazil. Afr. J. Microbiol. Res. 2015, 9, 1195–1199. [Google Scholar] [CrossRef]
- Sarmiento-Ramírez, J.M.; Abella, E.; Martín, M.P.; Tellería, M.T.; López-Jurado, L.F.; Marco, A.; Diéguez-Uribeondo, J. Fusarium solani is responsible for mass mortalities in nests of loggerhead sea turtle, Caretta caretta, in Boavista, Cape Verde. FEMS Microbiol. Lett. 2010, 312, 192–200. [Google Scholar] [CrossRef]
- Brofft Bailey, J.; Lamb, M.; Walker, M.; Weed, C.; Stephenson Craven, K. Detection of potential fungal pathogens Fusarium falciforme and F. keratoplasticum in unhatched loggerhead turtle eggs using a molecular approach. Endanger. Species Res. 2018, 36, 111–119. [Google Scholar] [CrossRef]
- O’Donnell, K.; Al-Hatmi, A.M.S.; Aoki, T.; Brankovics, B.; Cano-Lira, J.F.; Coleman, J.J.; de Hoog, G.S.; Di Pietro, A.; Frandsen, R.J.N.; Geiser, D.M.; et al. No to Neocosmospora: Phylogenomic and practical reasons for continued inclusion of the Fusarium solani species complex in the genus Fusarium. mSphere 2020, 5, e00810-20. [Google Scholar] [CrossRef]
- García-Martín, J.M.; Sarmiento-Ramírez, J.M.; Diéguez-Uribeondo, J. Beyond sea turtles: Fusarium keratoplasticum in eggshells of Podocnemis unifilis, a threatened Amazonian freshwater turtle. J. Fungi 2021, 7, 742. [Google Scholar] [CrossRef]
- Short, D.P.G.; O’Donnell, K.; Geiser, D.M. Clonality, recombination, and hybridization in the plumbing-inhabiting human pathogen Fusarium keratoplasticum inferred from multilocus sequence typing. BMC Evol. Biol. 2014, 14, 91. [Google Scholar] [CrossRef]
- Short, D.P.G.; O’Donnell, K.; Thrane, U.; Nielsen, K.F.; Zhang, N.; Juba, J.H.; Geiser, D.M. Phylogenetic relationships among members of the Fusarium solani species complex in human infections and the descriptions of F. keratoplasticum sp. nov. and F. petroliphilum stat. nov. Fungal Genet. Biol. 2013, 53, 59–70. [Google Scholar] [CrossRef]
- Sáenz, V.; Alvarez-Moreno, C.; Pape, P.L.; Restrepo, S.; Guarro, J.; Ramírez, A.M.C. A One health perspective to recognize Fusarium as important in clinical practice. J. Fungi 2020, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Carranco, A.S.; Gillingham, M.A.F.; Wilhelm, K.; Torres, M.d.L.; Sommer, S.; Romo, D. Transcending sea turtles: First report of hatching failure in eggs of an Amazonian freshwater turtle with symptoms of the fungal emerging disease fusariosis. Transbound Emerg. Dis. 2022, 69, e3282–e3288. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ríos, M.; Martín-Torrijos, L.; Diéguez-Uribeondo, J. The invasive alien Red-eared slider turtle, Trachemys scripta, as a carrier of STEF-disease pathogens. Fungal Biol. 2022, 126, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gleason, F.H.; Allerstorfer, M.; Lilje, O. Newly emerging diseases of marine turtles, especially sea turtle egg fusariosis (SEFT), caused by species in the Fusarium solani complex (FSSC). Mycology 2020, 11, 184–194. [Google Scholar] [CrossRef]
- Phillott, A.D.; Parmenter, C.J. The distribution of failed eggs and the appearance of fungi in artificial nests of green (Chelonia mydas) and loggerhead (Caretta caretta) sea turtles. Aust. J. Zool 2001, 49, 713. [Google Scholar] [CrossRef]
- Phillott, A.D.; Parmenter, C.J.; McKillup, S.C. Calcium dof eggshell after fungal invasion of sea turtle eggs. Chelonian Conserv. Biol. 2006, 5, 146. [Google Scholar] [CrossRef]
- Das, M.P.; Kumar, S. Microbial deterioration of low density polyethylene by Aspergillus and Fusarium sp. Int. J. ChemTech Res. 2014, 6, 299–305. [Google Scholar]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551. [Google Scholar] [CrossRef]
- Sarmiento-Ramírez, J.M.; van der Voort, M.; Raaijmakers, J.M.; Diéguez-Uribeondo, J. Unravelling the microbiome of eggs of the endangered sea turtle Eretmochelys imbricata identifies bacteria with activity against the emerging pathogen Fusarium falciforme. PLoS ONE 2014, 9, e95206. [Google Scholar] [CrossRef]
- Kerwin, A.H.; Nyholm, S.V. Reproductive system symbiotic bacteria are conserved between two distinct populations of Euprymna scolopes from Oahu, Hawaii. mSphere 2018, 3, e00531-17. [Google Scholar] [CrossRef]
- Suria, A.M.; Tan, K.C.; Kerwin, A.H.; Gitzel, L.; Abini-Agbomson, L.; Bertenshaw, J.M.; Sewell, J.; Nyholm, S.V.; Balunas, M.J. Hawaiian Bobtail squid symbionts inhibit marine bacteria via production of specialized metabolites, including new bromoalterochromides BAC-D/D’. mSphere 2020, 5, e00166-20. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, D.B.; Miller, A.N.; Allender, M.C.; Maddox, C.W.; Terio, K.A. Emydomyces testavorans, a new genus and species of Onygenalean fungus isolated from shell lesions of freshwater aquatic turtles. J. Clin. Microbiol. 2019, 57, e00628-18. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.R.; Hernández-Gómez, O.; Krohn, A.R.; Mutlow, A.; Patterson, L.; Rosenblum, E.B.; Timmer, M.; Willis, J.; Bushell, J. Turtle shell disease fungus (Emydomyces testavorans): First documented occurrence in California and prevalence in free-living turtles. Ichthyol. Herpetol. 2021, 109, 958–962. [Google Scholar] [CrossRef]
- Haman, K.; Hallock, L.; Schmidt, T.; Holman, E.; Murphie, B. Shell disease in Northwestern Pond Turtles (Actinemys marmorata) in Washington State, USA. Herpetol. Rev. 2019, 50, 495–502. [Google Scholar]
- Woodburn, D.B.; Kinsel, M.J.; Poll, C.P.; Langan, J.N.; Haman, K.; Gamble, K.C.; Maddox, C.; Jeon, A.B.; Wellehan, J.F.X.; Ossiboff, R.J.; et al. Shell lesions associated with Emydomyces testavorans infection in freshwater aquatic turtles. Vet. Pathol. 2021, 58, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Adamovicz, L.; Allender, M.C.; Gibbons, P.M. Emerging infectious diseases of chelonians: An update. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 263–283. [Google Scholar] [CrossRef]
- Lee, S.C.; Corradi, N.; Byrnes, E.J.; Torres-Martinez, S.; Dietrich, F.S.; Keeling, P.J.; Heitman, J. Microsporidia Evolved from Ancestral Sexual Fungi. Curr. Biol. 2008, 18, 1675–1679. [Google Scholar] [CrossRef]
- Vergneau-Grosset, C.; Larrat, S. Microsporidiosis in Vertebrate Companion Exotic Animals. J. Fungi 2015, 2, 3. [Google Scholar] [CrossRef]
- Eydner, M.; Donhauser, J.; Beineke, A.; Guenther, P.; Blahak, S. Microsporidiosis in Four Tortoises (Testudo hermanni Boettgeri). Vet. Pathol. 2017, 54, 704–709. [Google Scholar] [CrossRef]
- Shibasaki, K.; Tokiwa, T.; Sukegawa, A.; Kondo, H.; Tamukai, K.; Haga, Y.; Ike, K. First Report of Fatal Disseminated Microsporidiosis in Two Inland Bearded Dragons Pogona Vitticeps in Japan. JMM Case Rep. 2017, 4, e005089. [Google Scholar] [CrossRef]
- Wünschmann, A.; Armién, A.G.; Childress, A.L.; Wellehan, J.F.X.; Giannitti, F. Intrapericardial Encephalitozoon Pogonae-Associated Arteritis with Fatal Hemopericardium in Two Juvenile Central Bearded Dragons. J. Vet. Diagn. Investig. 2019, 31, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Dovč, A.; Stvarnik, M.; Lindtner Knific, R.; Gregurić Gračner, G.; Klobučar, I.; Zorman Rojs, O. Monitoring of unhatched eggs in Hermann’s Tortoise (Testudo hermanni) after artificial incubation and possible improvements in hatching. Animals 2021, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Loncaric, I.; Richter, B.; Spergser, J. Fatal Purpureocillium lilacinum pneumonia in a green tree python. J. Vet. Diagn. Investig 2018, 30, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Klasen, L.; Schneider, J.; Hübel, J.; Cramer, K. Pulmonary fungal granulomas and fibrinous pneumonia caused by different hypocrealean fungi in reptiles. Vet. Microbiol. 2018, 225, 58–63. [Google Scholar] [CrossRef]
| Species | Etiologic Agent | Epidemiological Pattern * | Transmission Route § | Pathogenetic Features | Lesions | Prevention Strategies | Ref. |
|---|---|---|---|---|---|---|---|
| Gopherus berlendieri | Fusarium semitectum | Group infection © | Environment | fungal keratinases | superficial | [20] | |
| Testudo hermanni | Fusarium semitectum | Group infection © | Environment | fungal keratinases | superficial | moving | [21] |
| Graptemys ouachitensis | Fusarium solani | Single case © | fungal keratinases and poor management | superficial | improved management practices | [22] | |
| Chelonoidis nigra | Aphanoascella galapagosensis | Single case and group © | superficial | none | [23] | ||
| Geochelone gigantea | Exophiala oligosperma | Single case © | superficial | none | [24] | ||
| Testudo hermanni | Alternaria sp. | Single case © | superficial | none | [25] | ||
| Carettocheylis insculpta | Purpureocillium lilacinum | Group infection (W) | immunosuppression, stress of shipment, poor management | superficial | water changes and good management practices | [26] | |
| Caretta caretta | Fusarium spp | Group infection © (R) | Environment | stressors, poor management | superficial pneumonia | improved management practices | [27] |
| Caretta caretta | Fusarium solani | Group infection © (R) | cold stunning | superficial | [28] | ||
| Apalone ferox | Mucorales | Group infection © | mixed bacterial infection | superficial | [29] | ||
| Trionyx sinensis | Purpureocillium lilacinum | Group infection © | poor management | superficial | improved management practices | [34] | |
| Caretta caretta | Fusarium solani | Single case © (R) | Environment | immunosuppression for trauma, surgery, rehabilitation | superficial | improved management practices | [36] |
| Caretta caretta | Fusarium solani | Group infection © | Environment through wound | fungal keratinases, stress for stranding, and poor management | superficial | improved management practices | [37] |
| Lepidochelys kempii | Fusarium solani | Single case © (R) | Through wound | cold stunning, mixed bacterial infection | superficial | [38] | |
| Caretta caretta | FSSC | Group infection (W) (R) | stranded | superficial | preventing pathogen introduction and diffusion | [44] | |
| T. hermanni | Trichosporon jirovecii | Single case © | Through wound | Infection of a skin lesion | superficial | preventing infections of open wounds | [46] |
| Caretta caretta | Purpureocillium lilacinum | Single case © | Water | poor management, introduction of infected hosts | pneumonia | improved management practices | [51] |
| Caretta caretta | Purpureocillium lilacinum | Group infection © | captive stress, nutritional deficits, poor management | superficial pneumonia | improved management practices | [52] | |
| Lepidochelys kempii | Colletotrichum acutatum | Single case © (R) | Cold stunning | [57] | |||
| Megalocheylis gigantea | Penicillium griseofulvum | Single case © | stress consequent to burns | systemic | [58] | ||
| Chelonia mydas | Veronaea botryosa | Group infection (W) | Respiratory | stranded animals | obstructive tracheitis | [64] | |
| Chelonia mydas | Candida palmioleophila | Single case © R | shell fracture | systemic | [71] |
| Animal Species | Agent | Country | Disease | Reference |
|---|---|---|---|---|
| Gopherus berlandieri | Fusarium semitectum | Texas | Necrotizing scute disease | [20] |
| Testudo hermani | F. semitectum | Italy | Shell mycosis | [21] |
| Graptemys ouachitensis | Fusarium solani | Portugal | Shell mycosis | [57] |
| Caretta caretta | FSSC * | Italy | Skin and shell | [27] |
| Caretta caretta | F. solani | Italy | Skin, shell and bone | [28] |
| Caretta caretta | F. solani | Spain | Skin | [36] |
| Caretta caretta | F. solani | France | Skin and systemic | [37] |
| Caretta caretta | FSSC ** | South Africa | Skin | [44] |
| Lepidochels kempii | F. solani | USA | Abscess | [38] |
| Emys orbicularis | Fusarium sp. and Mucor | Serbia | Systemic | [66] |
| Caretta caretta | F. solani | Cape Verde | STEF | [76] |
| Chelonia mydas | FSSC | Australia, Ecuador | STEF | [3] |
| Eretmochelys imbricata | FSSC | Ecuador | STEF | [3] |
| Lepidochelys olivacea | FSSC | Ecuador | STEF | [3] |
| Dermochelys coriacea | FSSC | Colombia, Costa Rica | STEF | [3] |
| Natator depressus | FSSC | Australia | STEF | [3] |
| Podocnemis unifilis | FSSC | Ecuador | FTEF | [79] |
| Podocnemis unifilis | FSSC | Ecuador | FTEF | [83] |
| Animal Species | Agent | Disease | Antimycotic Drugs | Outcome | Reference |
|---|---|---|---|---|---|
| Aldabrachelys gigantea | P. lilacinum | systemic | Ketoconazole 10 mg/kg PO | death | [48] |
| Aldabrachelys gigantea | C. krusei | systemic | Itraconazole 5 mg/kg/die PO | death | [70] |
| Lepidochelys kempi | Co. acutatum | systemic | Fluconazole 0.75 mg/kg SC/48 h, then Itraconazole 5 mg/kg/die PO | death | [57] |
| Caretta caretta | F. solani | shell | Topical 10% iodine in alcohol and topical ketoconazole | recovery | [36] |
| Caretta caretta | F. solani | Skin and systemic | Posaconazole 0.2 mg/kg/48 h PO | recovery by 33% | [37] |
| Testudo graeca | C. albicans | Pneumonia | Amphotericin B 0.1 mg/kg/die intrapulmonary | recovery | [65] |
| A. marmarota | E. testavorans | Skin shell | Topical iodine, terbinafine | recovery | [97] |
| Carettochelys insculpta | P. lilacinum | shell | Malachite green and formaldehyde dips, itraconazole 10 mg/kg/48 h PO | recovery | [25] |
| T. hermanni | F. semitectum | shell | Povidone iodine and Iruxol ointment/daily | recovery | [21] |
| T. hermanni | T. jirovecii | skin | Povidone iodine and Iruxol ointment/daily | recovery | [45] |
| T. hermanni | Alternaria sp | shell | Topical 10% iodine in alcohol | recovery | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardoni, S.; Mancianti, F. Mycotic Diseases in Chelonians. J. Fungi 2023, 9, 518. https://doi.org/10.3390/jof9050518
Nardoni S, Mancianti F. Mycotic Diseases in Chelonians. Journal of Fungi. 2023; 9(5):518. https://doi.org/10.3390/jof9050518
Chicago/Turabian StyleNardoni, Simona, and Francesca Mancianti. 2023. "Mycotic Diseases in Chelonians" Journal of Fungi 9, no. 5: 518. https://doi.org/10.3390/jof9050518






