Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Aerobiological Methodology
2.3. Immunochemical Methodology
2.4. Meteorological Data
2.5. Statistical Analysis
3. Results
3.1. Aerobiological and Meteorological Results
3.2. Airborne Fungal Aeroallergens vs. Spores and vs. Meteorology
4. Discussion
- (a)
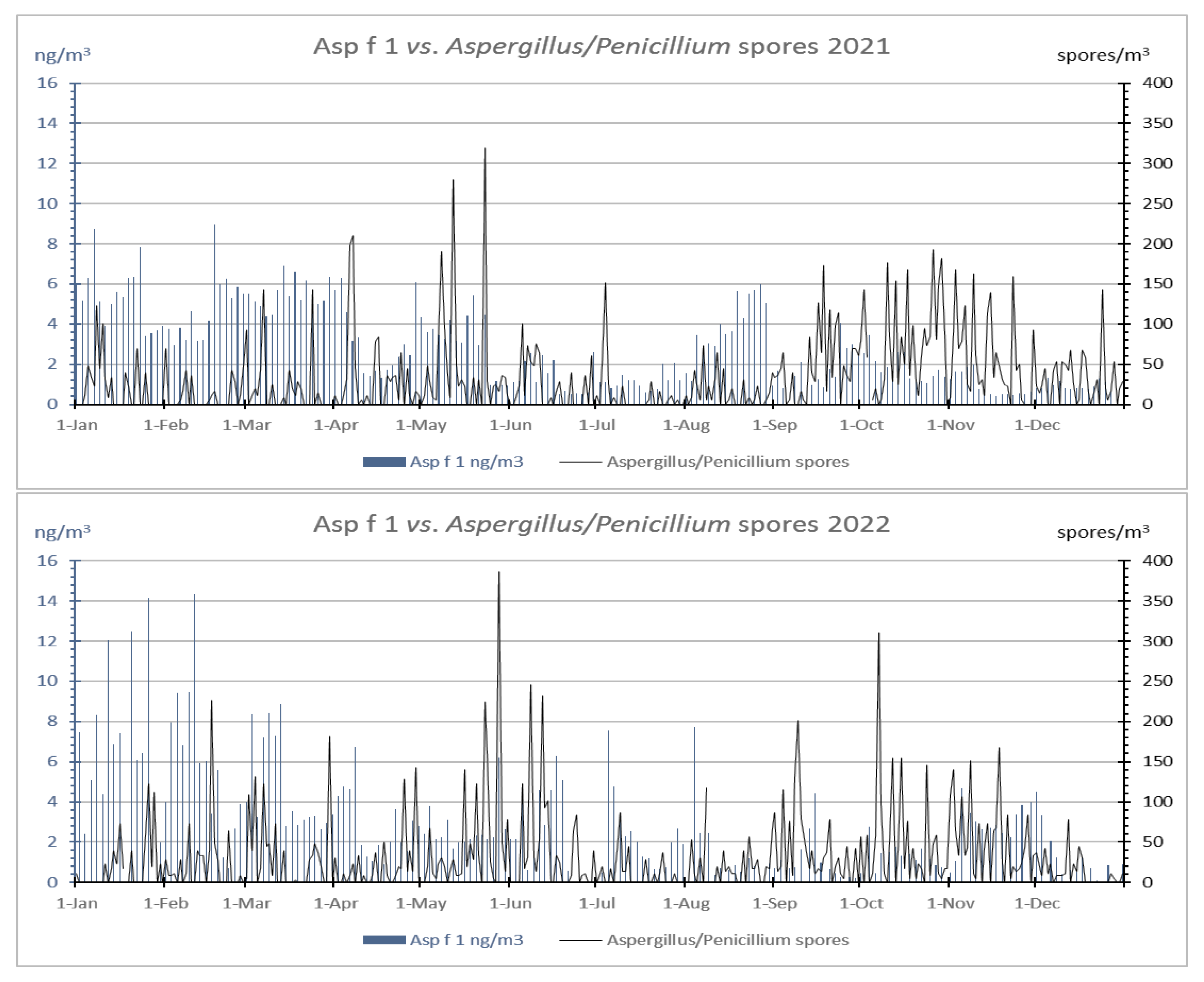
- The coincidence of the values of ASIn, AAIn, and the dates of the peaks of spores and allergens, as well as the dynamic found during the two years studied, confirm that the detection of the Aspergillus/Penicillium spore type and Asp f 1 allergen is possible with both aerobiological techniques, and follow a defined behavior along the time;
- (b)
- (c)
- Aspergillus/Penicillium spore type is conditioned to precipitation and relative humidity, which promote the production and release of the spores of these fungi. On the other hand, the aeroallergens registered in winter could be derived from hyphal fragments, which, under the light microscope, are extremely difficult to distinguish but can be detected through the allergens with immunological techniques.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Mandrioli, P.; Caneva, G.; Sabbioni, C. Cultural Heritage and Aerobiology. Methods and Measurement Techniques for Biodeterioration Monitoring; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Stetzenbach, L.D.; Buttner, M.P.; Cruz, P. Detection and enumeration of airborne biocontaminants. Curr. Opin. Biotechnol. 2004, 15, 170–174. [Google Scholar] [CrossRef]
- Luo, W.; Hu, H.; Wu, Z.; Wei, N.; Huang, H.; Zheng, P.; Liu, Y.; Sun, B. Molecular allergen sensitization of Aspergillus fumigatus between allergic bronchopulmonary aspergillosis and A. fumigatus-sensitized asthma in Guangzhou, Southern China. J. Clin. Lab. Anal. 2020, 34, e23448. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Frew, A.J.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bresciani, M.; Carlsen, K.H.; Van Cauwenberge, P.; Darsow, U.; Fokkens, W.J.; et al. Standard skin prick testing and sensitization to inhalant allergens across Europe—A survey from the GA2LEN network. Allergy 2005, 60, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Curin, M.; Valenta, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef]
- Chowdhary, A.; Agarwal, K.; Kathuria, S.; Gaur, S.N.; Randhawa, H.S.; Meis, J.F. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: A global overview. Crit. Rev. Microbiol. 2014, 40, 30–48. [Google Scholar] [CrossRef]
- Crameri, R.; Weichel, M.; Flückiger, S.; Glaser, A.G.; Rhyner, C. Fungal allergies: A yet unsolved problem. Allergy Asthma Mod. Soc. A Sci. Approach 2006, 91, 121–133. [Google Scholar]
- Hope, W.W.; Walsh, T.J.; Denning, D.W. The invasive and saprophytic syndromes due to Aspergillus spp. Med. Mycol. 2005, 43 (Suppl. 1), S207–S238. [Google Scholar] [CrossRef]
- Banerjee, B.; Greenberger, P.A.; Fink, J.N.; Kurup, V.P. Molecular characterization of Aspergillus fumigatus allergens. Indian J. Chest Dis. Allied Sci. 2000, 42, 239–248. [Google Scholar]
- Grinn-Gofroń, A. Airborne Aspergillus and Penicillium in the atmosphere of Szczecin (Poland) (2004–2009). Aerobiologia 2011, 27, 67–76. [Google Scholar] [CrossRef]
- Iglesias Fernández, I.; Seijo Coello, M.C.; Fernández González, M.; Escuredo Pérez, O. Aerobiological monitoring of Aspergillus/Penicillium spores during the potato storage. Aerobiologia 2012, 28, 213–219. [Google Scholar] [CrossRef]
- Prester, L.; Macan, J.; Matković, K.; Vučemilo, M. Determination of Allergen 1 in Poultry Farms Using the Enzyme Immunoassay. Arch. Ind. Hyg. Toxicol. 2010, 61, 167–173. [Google Scholar]
- Dananché, C.; Gustin, M.P.; Cassier, P.; Loeffert, S.T.; Thibaudon, M.; Bénet, T.; Vanhems, P. Evaluation of hirst-type spore trap to monitor environmental fungal load in hospital. PLoS ONE 2017, 12, e0177263. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Pereira, A.M.; De Linares, C.; Canela, M.; Belmonte, J. Spatial distribution of fungi from the analysis of aerobiological data with a gamma function. Aerobiologia 2021, 37, 461–477. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore-trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Galán, C.; Cariñanos, P.; Alcázar, P.; Domínguez, E. Manual de Calidad y Gestión de la Red Española de Aerobiología; [Quality Manual and Management Spanish Aerobiology Network]; Servicio de Publicaciones de la Universidad de Córdoba: Córdoba, Spain, 2007. [Google Scholar]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R.; The EAS QC Working Group. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2017, 30, 385–395. [Google Scholar] [CrossRef]
- Galán, C.; Ariatti, A.; Bonini, M.; Clot, B.; Crouzy, B.; Dahl, A.; Fernández-González, D.; Frenguelli, G.; Gehrig, R.; Isard, S.; et al. Recommended terminology for aerobiological studies. Aerobiología 2017, 33, 293–295. [Google Scholar] [CrossRef]
- O’Gorman, C.M. Airborne Aspergillus fumigatus conidia: A risk factor for aspergillosis. Fungal Biol. Rev. 2011, 25, 151–157. [Google Scholar] [CrossRef]
- De Linares, C.; Navarro, D.; Puigdemunt, R.; Belmonte, J. Airborne Alt a 1 dynamic and its relationship with the airborne dynamics of Alternaria conidia and Pleosporales spores. J. Fungi 2022, 8, 125. [Google Scholar] [CrossRef]
- Indoor Biotechnologies. Available online: https://inbio.com/elisa-2.0/elisa-2.0-kits-mold (accessed on 25 April 2023).
- Oliveira, M.; Ribeiro, H.; Delgado, J.L.; Abreu, I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanization level. Int. J. Biometeorol. 2009, 53, 61–73. [Google Scholar] [CrossRef]
- Rosas, I.; Calderon, C.; Ulloa, M.; Lacey, J. Abundance of airborne Penicillium CFU in relation to urbanization in Mexico City. Appl. Environ. Microbiol. 1993, 59, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Antón, S.F.; de la Cruz, D.R.; Sánchez, J.S.; Sánchez Reyes, E. Analysis of the airborne fungal spores present in the atmosphere of Salamanca (MW Spain): A preliminary survey. Aerobiologia 2019, 35, 447–462. [Google Scholar] [CrossRef]
- O’Gorman, C.M.; Fuller, H.T. Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmos. Environ. 2008, 42, 4355–4368. [Google Scholar] [CrossRef]
- Adhikari, A.; Reponen, T.; Grinshpun, S.A.; Martuzevicius, D.; LeMasters, G. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: A two-year study. Environ. Pollut. 2006, 140, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, K.; Käärik, A. A study of airborne fungal spores with the aid of the FOA slit-sampler. Grana 1977, 16, 133–137. [Google Scholar] [CrossRef]
- Larsen, L.; Gravesen, S. Seasonal variation of outdoor airborne viable microfungi in Copenhagen, Denmark. Grana 1991, 30, 467–471. [Google Scholar] [CrossRef]
- Sporik, R.B.; Arruda, L.K.; Woodfolk, J.; Chapman, M.D.; Platts-Mills, T.A.E. Environmental exposure to Aspergillus fumigatus allergen (Asp f I). Clin. Exp. Allergy 1993, 23, 326–331. [Google Scholar] [CrossRef]
- Buss Cezar Fontana, M. Estudio Epidemiológico de Alergia a Hongos y Otros Neumoalergenos, en Estudiantes de Medicina de la Universitat Autònoma de Barcelona, con Relación a Los Niveles Fúngicos Ambientales; Universitat Autònoma de Barcelona: Barcelona, Spain, 2009. [Google Scholar]
- Rúa Giraldo, A.L. Aerobiología de las Esporas de Pleosporales en Ambientes intra y Extradomiciliarios de Barcelona: Aplicación a la Clínica en Población Alérgica; Universitat Autònoma de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Vermani, M.; Vijayan, V.K.; Kausar, M.A.; Agarwal, M.K. Quantification of airborne Aspergillus allergens: Redefining the approach. J. Asthma 2010, 47, 754–761. [Google Scholar] [CrossRef]
- Almaguer, M.; Fernández-González, M.; Díaz, L.; Sánchez, K.C.; Rodríguez-Rajo, F.J.; Aira, M.J. Aspergillus and Penicillium spores as urban pathogens of the Havana atmosphere, Cuba. Aerobiologia 2021, 37, 767–783. [Google Scholar] [CrossRef]
- Millington, W.M.; Corden, J.M. Long term trends in outdoor Aspergillus/Penicillium spore concentrations in Derby, UK from 1970 to 2003 and a comparative study in 1994 and 1996 with the indoor air of two local houses. Aerobiologia 2005, 21, 105–113. [Google Scholar] [CrossRef]
- Brito, F.F.; Alonso, A.M.; Carnés, J.; Martín-Martín, R.; Fernández-Caldas, E.; Galindo, P.A.; Alfaya, T.; Amo-Salas, M. Correlation between Alt a 1 levels and clinical symptoms in Alternaria alternata-monosensitized patients. J. Investig. Allergol. Clin. Immunol. 2012, 22, 154–159. [Google Scholar]
- Grewling, Ł.; Bogawski, P.; Szymańska, A.; Nowak, M.; Kostecki, Ł.; Smith, M. Particle size distribution of the major Alternaria alternata allergen, Alt a 1, derived from airborne spores and subspore fragments. Fungal Biol. 2020, 124, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Peláez, T.; Alcalá, L.; Bouza, E. Outdoor environmental levels of Aspergillus spp. Conidia Over A Wide Geogr. Area. Sabouraudia 2006, 44, 349–356. [Google Scholar]
- Fernández-Rodríguez, S.; Tormo-Molina, R.; Lemonis, N.; Clot, B.; O’Connor, D.J.; Sodeau, J.R. Comparison of fungal spores concentrations measured with wideband integrated bioaerosol sensor and Hirst methodology. Atmos. Environ. 2018, 175, 1–14. [Google Scholar] [CrossRef]
- Sadyś, M.; Adams-Groom, B.; Herbert, R.J.; Kennedy, R. Comparisons of fungal spore distributions using air sampling at Worcester, England (2006–2010). Aerobiologia 2016, 32, 619–634. [Google Scholar] [CrossRef]
- Akgül, H.; Yılmazkaya, D.; Akata, I.; Tosunoğlu, A.; Bıçakçı, A. Determination of airborne fungal spores of Gaziantep (SE Turkey). Aerobiologia 2016, 32, 441–452. [Google Scholar] [CrossRef]
- Lee, S.A.; Liao, C.H. Size-selective assessment of agricultural workers’ personal exposure to airborne fungi and fungal fragments. Sci. Total Environ. 2014, 466, 725–732. [Google Scholar] [CrossRef]
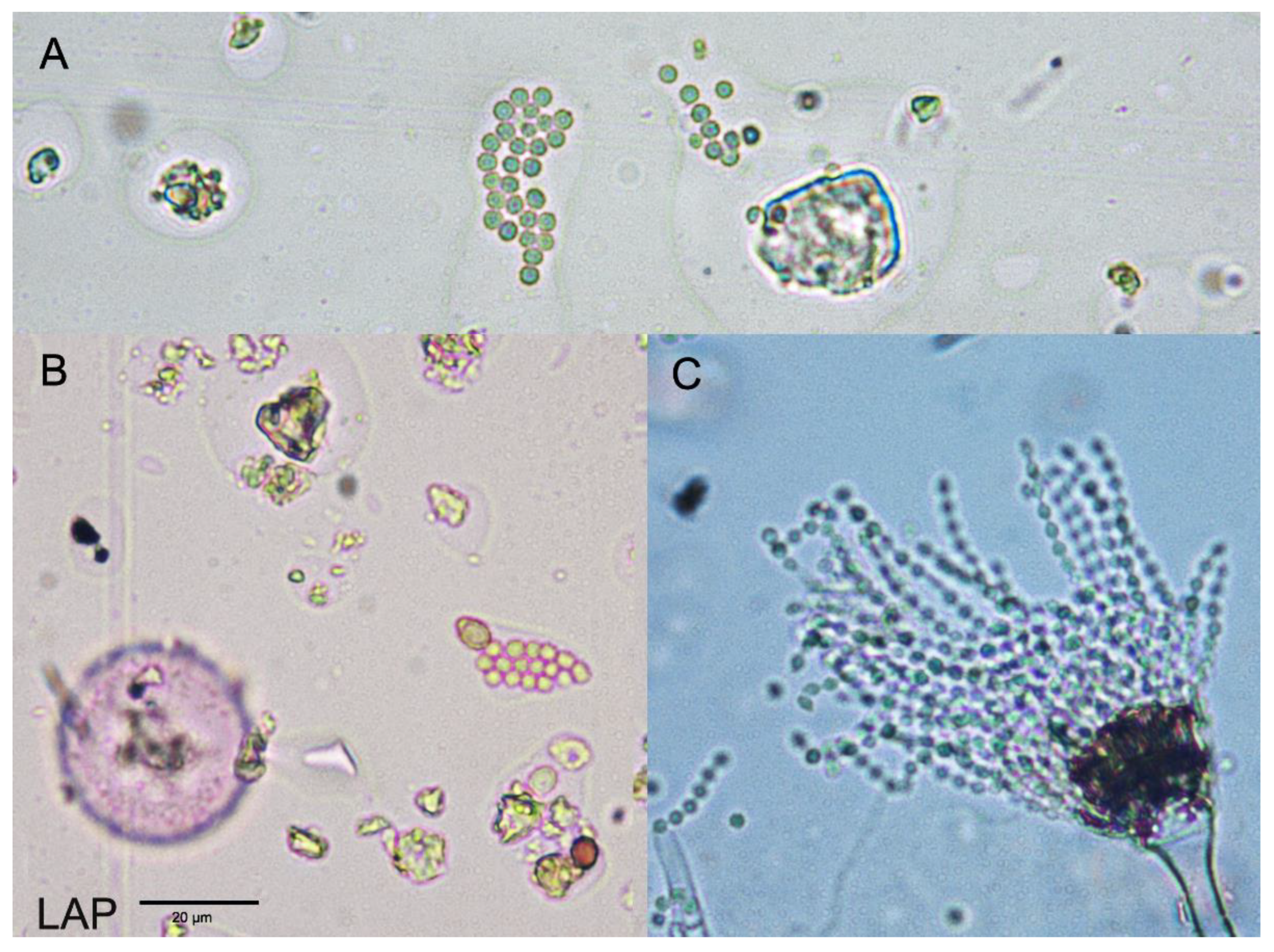

| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Mean 2011–2020 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASIn (spore * day/m3) | 16,106 | 9730 | 8462 | 11,421 | 9817 | 8660 | 6964 | 9724 | 6759 | 8812 | 9645 |
| Peak (spores/m3) | 624 | 591 | 918 | 395 | 361 | 252 | 207 | 392 | 274 | 708 | 472 |
| Peak day | 21-nov | 28-sep | 31-oct | 26-nov | 09-apr | 13-mar | 04-oct | 17-jun | 29-may | 05-apr | -- |
| Nr of days with spores | 222 | 190 | 178 | 246 | 259 | 249 | 237 | 250 | 227 | 209 | 227 |
| Nr of analyzed days | 364 | 364 | 357 | 362 | 359 | 360 | 361 | 364 | 361 | 340 | 359 |
| ATSIN (spore * day/m3) | 1,873,455 | 2,065,498 | 1,986,474 | 2,567,732 | 1,427,132 | 1,898,641 | 1,401,089 | 2,867,906 | 1,259,152 | 1,644,947 | 1,899,202 |
| % vs. Total Spores | 0.86 | 0.47 | 0.43 | 0.44 | 0.69 | 0.46 | 0.50 | 0.34 | 0.54 | 0.54 | 0.52 |
| T Max (°C) | 21.1 | 21.0 | 20.6 | 21.2 | 21.8 | 21.5 | 21.7 | 21.2 | 21.7 | 21.4 | 21.3 |
| T Min (°C) | 9.6 | 8.9 | 8.8 | 9.7 | 9.7 | 9.7 | 9.4 | 9.9 | 9.3 | 9.6 | 9.5 |
| T Mean (°C) | 15.3 | 15.0 | 147 | 15.5 | 15.7 | 15.6 | 15.6 | 15.6 | 15.5 | 15.5 | 15.4 |
| P 0–24 h (mm) | 809.3 | 445.6 | 563.7 | 639.0 | 326.6 | 441.6 | 468.8 | 919.4 | 553.0 | 847.2 | 601.4 |
| RH (%) | 74.2 | 69.6 | 71.8 | 74.4 | 68.9 | 69.7 | 68.8 | 73.3 | 68.6 | 73.8 | 71.3 |
| 2021 | 2022 | ||
|---|---|---|---|
| Hirst sampler | Aspergillus/Penicillium spores. ASIn (spore * day/m3) | 10,912 | 9957 |
| Peak (spore/m3) | 319 | 386 | |
| Peak day | 23-may | 28-may | |
| Nr of days with Aspergillus/Penicillium spore type | 237 | 235 | |
| Nr of analyzed days | 364 | 356 | |
| Total spores. ASIn (spore * day/m3) | 1,380,260 | 1,329,525 | |
| % vs. Total spores | 0.79 | 0.75 | |
| High-volume sampler | Asp f 1. AAIn (ng * day/m3) | 506,7 | 505,0 |
| Peak (ng/m3) | 9.0 | 14.4 | |
| Peak day | 18-feb | 11-feb | |
| Nr of days with Asp f 1 | 179 | 163 | |
| Nr of analyzed days | 179 | 163 | |
| Meteorological data | T Max (°C) | 21.1 | 22.7 |
| T Min (°C) | 9.4 | 10.3 | |
| T Mean (°C) | 15.3 | 16.5 | |
| P 0–24h (mm) | 417.9 | 393.7 | |
| RH (%) | 72.6 | 70.6 |
| 2021 | 2022 | |||||||
|---|---|---|---|---|---|---|---|---|
| Asp./Pen. Spore Type | Asp f 1 | Asp./Pen. Spore Type | Asp f 1 | |||||
| Spearman T. | N | Spearman T. | N | Spearman T. | N | Spearman T. | N | |
| Asp./Pen. spore type | 1 | 367 | −0.098 | 181 | 1 | 359 | −0.008 | 163 |
| Asp f 1 | −0.098 | 181 | 1 | 182 | −0.008 | 163 | 1 | 166 |
| TMax (°C) | −0.043 | 367 | −0.186 * | 182 | 0.057 | 359 | −0.348 ** | 166 |
| TMin (°C) | −0.021 | 367 | −0.207 ** | 182 | 0.043 | 359 | −0.408 ** | 166 |
| TMean (°C) | −0.033 | 367 | −0.189 * | 182 | 0.050 | 359 | −0.409 ** | 166 |
| P 0–24 (mm) | 0.234 ** | 367 | 0.040 | 182 | 0.039 | 359 | 0.061 | 166 |
| RH (%) | 0.182 ** | 367 | 0.104 | 182 | 0.101 | 359 | −0.067 | 166 |
| Spearman T. | N | |||||||
| Asp./Pen. spore type (2021) vs. Asp./Pen. spore type (2022) | 0.145 ** | 358 | ||||||
| Asp f 1 (2021) vs. Asp f 1 (2022) | −0.022 | 31 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Linares, C.; Navarro, D.; Puigdemunt, R.; Belmonte, J. Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard? J. Fungi 2023, 9, 624. https://doi.org/10.3390/jof9060624
De Linares C, Navarro D, Puigdemunt R, Belmonte J. Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard? Journal of Fungi. 2023; 9(6):624. https://doi.org/10.3390/jof9060624
Chicago/Turabian StyleDe Linares, Concepción, David Navarro, Rut Puigdemunt, and Jordina Belmonte. 2023. "Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard?" Journal of Fungi 9, no. 6: 624. https://doi.org/10.3390/jof9060624
APA StyleDe Linares, C., Navarro, D., Puigdemunt, R., & Belmonte, J. (2023). Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard? Journal of Fungi, 9(6), 624. https://doi.org/10.3390/jof9060624







