Epidemiology, Modern Diagnostics, and the Management of Mucorales Infections
Abstract
:1. Introduction
2. Taxonomy of Mucorales
3. Epidemiology of Mucormycosis
3.1. Update on Epidemiology: Burden and Causative Pathogens
3.2. Host Risk Factors
COVID-19-Associated Mucormycosis
3.3. Healthcare-Associated Mucormycosis
3.4. Natural Disaster-Associated Mucormycosis
4. Diagnostics
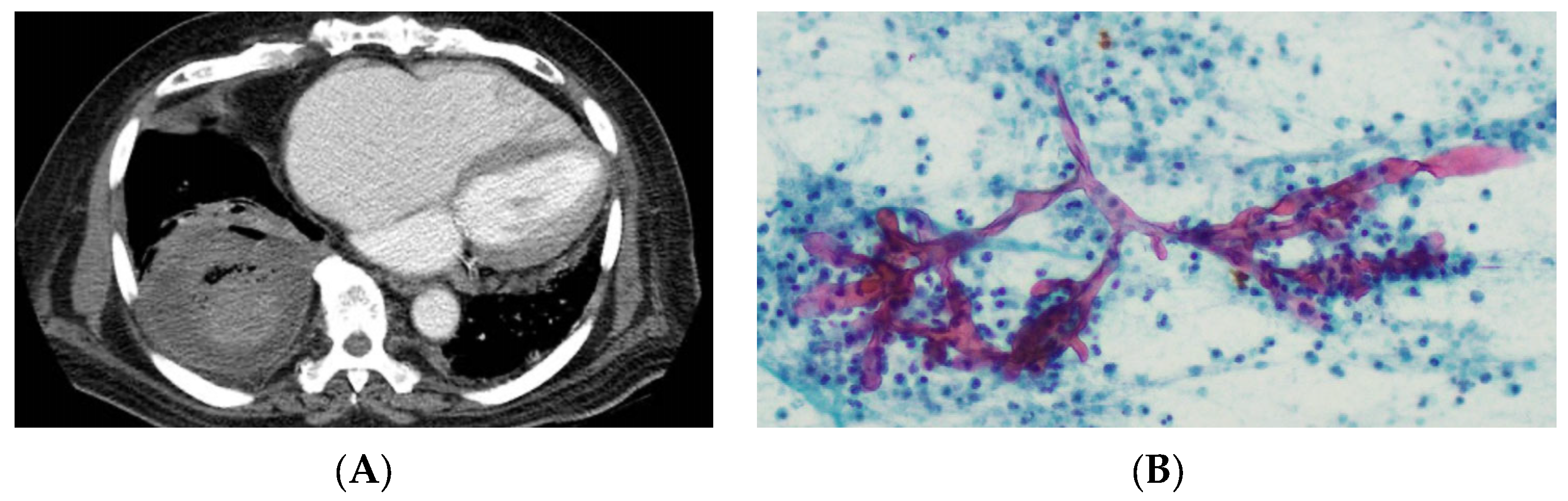
4.1. Radiology
4.2. Microscopy and Histology
4.3. Culture-Based Diagnostics
4.4. Molecular Diagnostics
4.5. Serological Diagnosis
4.6. Emerging Diagnostic Approaches
4.7. Susceptibility Testing
4.8. Whole-Genome Sequencing
5. Management and New Antifungal Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMB: | Amphotericin B |
| ATCC: | American Type Culture Collection |
| BAL: | Bronchoalveolar lavage |
| c-AmB: | Amphotericin B deoxycholate |
| CAM: | COVID-19-associated mucormycosis |
| CBP: | Clinical breakpoint |
| CI: | Confidence interval |
| CLSI: | Clinical and Laboratory Standards Institute |
| CT: | Computed tomography |
| COVID-19: | Coronavirus disease 2019 |
| DNA: | Deoxyribonucleic acid |
| ECV/ECOFF: | Epidemiological cutoff value |
| EUCAST: | European Committee on Antimicrobial Susceptibility Testing |
| FDG-PET: | Fluorodeoxyglucose positron emission tomography |
| FFPE: | Formalin fixed paraffin-embedded |
| GI: | Gastrointestinal |
| GMS: | Grocott–Gomori methenamine silver |
| H&E: | Haematoxylin and eosin |
| HRMA: | High-resolution melting analysis |
| IA: | Invasive aspergillosis |
| ICU: | Intensive care unit |
| IHC: | Immunohistochemistry |
| ITS: | Internal transcribed spacer |
| L-AmB: | Liposomal Amphotericin B |
| LFIA: | Lateral flow immunoassay |
| MALDI TOF | Matrix assisted laser desorption ionisation-time of flight |
| MIC: | Minimum inhibitory concentration |
| MRI: | Magnetic Resonance Imaging |
| PAS: | Periodic acid-Schiff |
| PCR: | Polymerase chain reaction |
| POS: | Posaconazole |
| RNA: | Ribonucleic acid |
| ROCM: | Rhino-orbital-cerebral mucormycosis |
| SNP: | Single nucleotide polymorphism |
| TDM: | Therapeutic drug monitoring |
| ITR: | Itraconazole |
| WGS: | Whole genome sequencing |
References
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The Epidemiology and Clinical Manifestations of Mucormycosis: A Systematic Review and Meta-Analysis of Case Reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Bernal-Martinez, L.; Isla, G.; Gomez-Lopez, A.; Alcazar-Fuoli, L.; Buitrago, M.J. Incidence of Zygomycosis in Transplant Recipients. Clin. Microbiol. Infect. 2009, 15, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes Mellitus as the Major Risk Factor for Mucormycosis in Mexico: Epidemiology, Diagnosis, and Outcomes of Reported Cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.-P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The Emergence of COVID-19 Associated Mucormycosis: A Review of Cases from 18 Countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Outbreaks of Mucorales and the Species Involved. Mycopathologia 2019, 185, 765–781. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Chen, S.C.-A.; Kong, D.C.M. Contemporary Management and Clinical Outcomes of Mucormycosis: A Systematic Review and Meta-Analysis of Case Reports. Int. J. Antimicrob. Agents 2019, 53, 589–597. [Google Scholar] [CrossRef]
- Vaughan, C.; Bartolo, A.; Vallabh, N.; Leong, S.C. A Meta-Analysis of Survival Factors in Rhino-Orbital-Cerebral Mucormycosis-Has Anything Changed in the Past 20 Years? Clin. Otolaryngol. 2018, 43, 1454–1464. [Google Scholar] [CrossRef]
- Muthu, V.; Agarwal, R.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Aggarwal, A.N.; Chakrabarti, A. Has the Mortality from Pulmonary Mucormycosis Changed over Time? A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 538–549. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A Higher-Level Phylogenetic Classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A Phylum-Level Phylogenetic Classification of Zygomycete Fungi Based on Genome-Scale Data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, K.; Pawłowska, J.; Walther, G.; Wrzosek, M.; de Hoog, G.S.; Benny, G.L.; Kirk, P.M.; Voigt, K. The Family Structure of the Mucorales: A Synoptic Revision Based on Comprehensive Multigene-Genealogies. Persoonia-Mol. Phylogeny Evol. Fungi 2013, 30, 57–76. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J. Taxonomy of Fungi Causing Mucormycosis and Entomophthoramycosis (Zygomycosis) and Nomenclature of the Disease: Molecular Mycologic Perspectives. Clin. Infect. Dis. 2012, 54, S8–S15. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.; Stielow, J.B.; de Hoog, G.S.; Bensch, K.; Schwartze, V.U.; Voigt, K.; Alastruey-Izquierdo, A.; Kurzai, O.; Walther, G. A New Species Concept for the Clinically Relevant Mucor circinelloides Complex. Persoonia-Mol. Phylogeny Evol. Fungi 2020, 44, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, S.; de Hoog, G.S.; Meis, J.F.; Walther, G. Species Boundaries and Nomenclature of Rhizopus arrhizus (syn. R. oryzae). Mycoses 2014, 57, 108–127. [Google Scholar] [CrossRef]
- Hallur, V.; Prakash, H.; Sable, M.; Preetam, C.; Purushotham, P.; Senapati, R.; Shankarnarayan, S.A.; Bag, N.D.; Rudramurthy, S.M. Cunninghamella arunalokei a New Species of Cunninghamella from India Causing Disease in an Immunocompetent Individual. J. Fungi 2021, 7, 670. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Daveson, K.; Slavin, M.A.; van Hal, S.J.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Chapman, B.; Halliday, C.L.; Hajkowicz, K.; et al. Mucormycosis in Australia: Contemporary Epidemiology and Outcomes. Clin. Microbiol. Infect. 2016, 22, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Stemler, J.; Hamed, K.; Salmanton-García, J.; Rezaei-Matehkolaei, A.; Gräfe, S.K.; Sal, E.; Zarrouk, M.; Seidel, D.; Abdelaziz Khedr, R.; Ben-Ami, R.; et al. Mucormycosis in the Middle East and North Africa: Analysis of the FungiScope® Registry and Cases from the Literature. Mycoses 2020, 63, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, S.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Zarrinfar, H.; Skiada, A.; Mirhendi, H.; Nashibi, R.; Niknejad, F.; Nazeri, M.; Rafiei, A.; et al. Mucormycosis in Iran: A Six-Year Retrospective Experience. J. Mycol. Med. 2018, 28, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A Prospective Multicenter Study on Mucormycosis in India: Epidemiology, Diagnosis, and Treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef]
- Nucci, M.; Engelhardt, M.; Hamed, K. Mucormycosis in South America: A Review of 143 Reported Cases. Mycoses 2019, 62, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, Clinical and Economic Burden of Mucormycosis-Related Hospitalizations in the United States: A Retrospective Study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef] [Green Version]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 Cases Accrued by the Registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective Antifungal Therapy (PATH) Alliance®: Focus on Mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A Multicentre Observational Study on the Epidemiology, Risk Factors, Management and Outcomes of Mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.-W.; Zhu, P.-Q.; Chen, X.-Q.; Yu, J. Mucormycosis in Mainland China: A Systematic Review of Case Reports. Mycopathologia 2022, 187, 1–14. [Google Scholar] [CrossRef]
- Bretagne, S.; Sitbon, K.; Desnos-Ollivier, M.; Garcia-Hermoso, D.; Letscher-Bru, V.; Cassaing, S.; Millon, L.; Morio, F.; Gangneux, J.-P.; Hasseine, L.; et al. Active Surveillance Program to Increase Awareness on Invasive Fungal Diseases: The French RESSIF Network (2012 to 2018). mBio 2022, 13, e00920-22. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Drogari-Apiranthitou, M.; Pavleas, I.; Daikou, E.; Petrikkos, G. Global Cutaneous Mucormycosis: A Systematic Review. J. Fungi 2022, 8, 194. [Google Scholar] [CrossRef]
- Dang, J.; Goel, P.; Choi, K.J.; Massenzio, E.; Landau, M.J.; Pham, C.H.; Huang, S.; Yenikomshian, H.A.; Spellberg, B.; Gillenwater, T.J. Mucormycosis Following Burn Injuries: A Systematic Review. Burns 2023, 49, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Didehdar, M.; Chegini, Z.; Moradabadi, A.; Anoushirvani, A.A.; Tabaeian, S.P.; Yousefimashouf, M.; Shariati, A. Gastrointestinal Mucormycosis: A Periodic Systematic Review of Case Reports from 2015 to 2021. Microb. Pathog. 2022, 163, 105388. [Google Scholar] [CrossRef]
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.-C.; Lortholary, O. Increasing Incidence of Zygomycosis (Mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and Diagnosis of Mucormycosis: An Update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Epidemiology of Mucormycosis in India. Microorganisms 2021, 9, 523. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive Zygomycosis in India: Experience in a Tertiary Care Hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The Rising Trend of Invasive Zygomycosis in Patients with Uncontrolled Diabetes Mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten Years’ Experience in Zygomycosis at a Tertiary Care Centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef]
- Jabeen, K.; Farooqi, J.; Mirza, S.; Denning, D.; Zafar, A. Serious Fungal Infections in Pakistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, A.; Shrimali, T.; Sharma, A. Mucormycosis Caused by Apophysomyces Species: An Experience from a Tertiary Care Hospital in Western India and Systematic Review of Global Cases. Mycoses 2023, 66, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Paul, R.A.; Gupta, S.; Negi, V.; Chakrabarti, A. The Environmental Source of Emerging Apophysomyces variabilis Infection in India. Med. Mycol. 2016, 54, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Marak, R.S.K.; Shivaprakash, M.R.; Gupta, S.; Garg, R.; Sakhuja, V.; Singhal, S.; Baghela, A.; Dixit, A.; Garg, M.K.; et al. Cavitary Pulmonary Zygomycosis Caused by Rhizopus homothallicus. J. Clin. Microbiol. 2010, 48, 1965–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkayil, P.; Pandey, M.; Agarwal, R.; Kale, P.; Singh, G.; Xess, I. Rhizopus homothallicus Causing Invasive Infections: Series of Three Cases from a Single Centre in North India. Mycopathologia 2017, 182, 921–926. [Google Scholar] [CrossRef]
- Compain, F.; Aït-Ammar, N.; Botterel, F.; Gibault, L.; Le Pimpec Barthes, F.; Dannaoui, E. Fatal Pulmonary Mucormycosis Due to Rhizopus homothallicus. Mycopathologia 2017, 182, 907–913. [Google Scholar] [CrossRef]
- Taneja, J.; Agrawal, V.K.; Jamal, D.; Abbas, Z. A Rare Case of Pulmonary Mucormycosis Caused By Rhizopus homothallicus in Post Heart Transplant Patient. J. Intensive Crit. Care 2020, 6, 23. [Google Scholar]
- Chander, J.; Kaur, M.; Singla, N.; Punia, R.; Singhal, S.; Attri, A.; Alastruey-Izquierdo, A.; Stchigel, A.; Cano-Lira, J.; Guarro, J. Mucormycosis: Battle with the Deadly Enemy over a Five-Year Period in India. J. Fungi 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kanaujia, R.; Rudramurthy, S.M. Rhizopus homothallicus: An Emerging Pathogen in Era of COVID-19 Associated Mucormycosis. Indian. J. Med. Microbiol. 2021, 39, 473–474. [Google Scholar] [CrossRef]
- Gupta, M.K.; Selvaraj, S.; Tilak, R.; Kumar, N.; Kumar, R.; Chakravarty, J. Rhizopus homothallicus Rhino-orbital-cerebral Mucormycosis: Six Cases from a Tertiary Care Centre, North India. Trop. Med. Int. Health 2023, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Z.; Shen, Y.; She, X.; Lu, G.; Zhan, P.; Fu, M.; Zhang, X.; Ge, Y.; Liu, W. Primary Cutaneous Zygomycosis Caused by Rhizomucor variabilis: A New Endemic Zygomycosis? A Case Report and Review of 6 Cases Reported from China. Clin. Infect. Dis. 2009, 49, e39–e43. [Google Scholar] [CrossRef] [Green Version]
- Hemashettar, B.M.; Patil, R.N.; O’Donnell, K.; Chaturvedi, V.; Ren, P.; Padhye, A.A. Chronic Rhinofacial Mucormycosis Caused by Mucor irregularis (Rhizomucor variabilis) in India. J. Clin. Microbiol. 2011, 49, 2372–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Guo, P.; Wong, H.; Xie, J.; Han, J.; Xu, Y.; Zhou, H. Vacuum-Assisted Closure and Skin Grafting Combined with Amphotericin B for Successful Treatment of an Immunocompromised Patient with Cutaneous Mucormycosis Caused by Mucor irregularis: A Case Report and Literature Review. Mycopathologia 2021, 186, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Joichi, Y.; Chijimatsu, I.; Yarita, K.; Kamei, K.; Miki, M.; Onodera, M.; Harada, M.; Yokozaki, M.; Kobayashi, M.; Ohge, H. Detection of Mucor velutinosus in a Blood Culture after Autologous Peripheral Blood Stem Cell Transplantation: A Pediatric Case Report. Med. Mycol. J. 2014, 55, E43–E48. [Google Scholar] [CrossRef] [Green Version]
- Sugui, J.A.; Christensen, J.A.; Bennett, J.E.; Zelazny, A.M.; Kwon-Chung, K.J. Hematogenously Disseminated Skin Disease Caused by Mucor velutinosus in a Patient with Acute Myeloid Leukemia. J. Clin. Microbiol. 2011, 49, 2728–2732. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Jantausch, B.; Torres, C.; Campos, J.; Zelazny, A. Central Line–Associated Mucor velutinosus Bloodstream Infection in an Immunocompetent Pediatric Patient. J. Pediatr. Infect. Dis. Soc. 2018, 7, e55–e57. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, E.; Cano, J.; Stchigel, A.M.; Sutton, D.A.; Fothergill, A.W.; Salas, V.; Rinaldi, M.G.; Guarro, J. Two New Species of Mucor from Clinical Samples. Med. Mycol. 2011, 49, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Hospenthal, D.R.; Chung, K.K.; Lairet, K.; Thompson, E.H.; Guarro, J.; Renz, E.M.; Sutton, D.A. Saksenaea erythrospora Infection Following Combat Trauma. J. Clin. Microbiol. 2011, 49, 3707–3709. [Google Scholar] [CrossRef] [Green Version]
- Troiano, G.; Nante, N. Emerging Fungal Infections: Focus on Saksenaea erythrospora. J. Prev. Med. Hyg. 2021, 62, E382–E385. [Google Scholar] [CrossRef]
- Chander, J.; Singla, N.; Kaur, M.; Punia, R.S.; Attri, A.; Alastruey-Izquierdo, A.; Cano-Lira, J.F.; Stchigel, A.M.; Guarro, J. Saksenaea erythrospora, an Emerging Mucoralean Fungus Causing Severe Necrotizing Skin and Soft Tissue Infections—A Study from a Tertiary Care Hospital in North India. Infect. Dis. 2017, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xess, I.; Mohapatra, S.; Shivaprakash, M.R.; Chakrabarti, A.; Benny, G.L.; O’Donnell, K.; Padhye, A.A. Evidence Implicating Thamnostylum lucknowense as an Etiological Agent of Rhino-Orbital Mucormycosis. J. Clin. Microbiol. 2012, 50, 1491–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Tsui, C.; Li, M.; Xiao, K.; de Hoog, G.S.; Verweij, P.E.; Cao, Y.; Lu, H.; Jiang, Y. First Case of Rhinocerebral Mucormycosis Caused by Lichtheimia ornata, with a Review of Lichtheimia Infections. Mycopathologia 2020, 185, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Stchigel, A.M.; Guarro, J.; Guevara, E.; Pintos, L.; Sanchis, M.; Cano-Lira, J.F. Primary Cutaneous Mucormycosis Produced by the New Species Apophysomyces mexicanus. J. Clin. Microbiol. 2014, 52, 4428–4431. [Google Scholar] [CrossRef] [Green Version]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Poirée, S.; Kania, R.; Lortholary, O. Diabetes and Mucormycosis: A Complex Interplay. Diabetes Metab. 2012, 38, 193–204. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V.I. Zygomycosis in the 1990s in a Tertiary-Care Cancer Center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Maertens, J.; Demuynck, H.; Verbeken, E.; Zachée, P.; Verhoef, G.; Vandenberghe, P.; Boogaerts, M. Mucormycosis in Allogeneic Bone Marrow Transplant Recipients: Report of Five Cases and Review of the Role of Iron Overload in the Pathogenesis. Bone Marrow Transpl. Transplant. 1999, 24, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Zygomycosis in Solid Organ Transplant Recipients: A Prospective, Matched Case-Control Study to Assess Risks for Disease and Outcome. J. Infect. Dis. 2009, 200, 1002–1011. [Google Scholar] [CrossRef]
- Moreira, J.; Varon, A.; Galhardo, M.C.; Santos, F.; Lyra, M.; Castro, R.; Oliveira, R.; Lamas, C.C. The Burden of Mucormycosis in HIV-Infected Patients: A Systematic Review. J. Infect. 2016, 73, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Boelaert, J.R.; Fenves, A.Z.; Coburn, J.W. Deferoxamine Therapy and Mucormycosis in Dialysis Patients: Report of an International Registry. Am. J. Kidney Dis. 1991, 18, 660–667. [Google Scholar] [CrossRef]
- McNab, A.A. Iron Overload Is a Risk Factor for Zygomycosis. Arch. Ophthalmol. 1997, 115, 919. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Sanchez, S.; Mansour, M.K.; Triant, V.A.; Goldberg, M.B. Isolated Cerebral Mucormycosis in Immunocompetent Adults Who Inject Drugs: Case Reports and Systematic Review of the Literature. Open. Forum Infect. Dis. 2020, 7, ofaa552. [Google Scholar] [CrossRef]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.-C.; Lantieri, L.; Lortholary, O.; Lanternier, F. Posttraumatic Mucormycosis. Medicine 2014, 93, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A Systematic Review of Cases Reported Worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Singh, R. Mucormycosis in India: Unique Features. Mycoses 2014, 57, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Özbek, L.; Topçu, U.; Manay, M.; Esen, B.H.; Bektas, S.N.; Aydın, S.; Özdemir, B.; Khostelidi, S.N.; Klimko, N.; Cornely, O.; et al. COVID-19-Associated Mucormycosis: A Systematic Review and Meta-Analysis of 958 Cases. Clin. Microbiol. Infect. 2023, 29, 722–731. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-Associated Fungal Infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Joshi, S.; Telang, R.; Tambe, M.; Havaldar, R.; Sane, M.; Shaikh, A.; Roy, C.; Yathati, K.; Sonawale, S.; Borkar, R.; et al. Outbreak of Mucormycosis in Coronavirus Disease Patients, Pune, India. Emerg. Infect. Dis. 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Almyroudi, M.P.; Akinosoglou, K.; Rello, J.; Blot, S.; Dimopoulos, G. Clinical Phenotypes of COVID-19 Associated Mucormycosis (CAM): A Comprehensive Review. Diagnostics 2022, 12, 3092. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Mora-Montes, H.M. Mucormycosis and COVID-19-Associated Mucormycosis: Insights of a Deadly but Neglected Mycosis. J. Fungi 2022, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, P.; Rauf, A.; Chaudhary, A.; Prajapati, P.K.; Emran, T.B.; Gonçalves Lima, C.M.; Conte-Junior, C.A. Mucormycosis in the COVID-19 Environment: A Multifaceted Complication. Front. Cell Infect. Microbiol. 2022, 12, 964. [Google Scholar] [CrossRef]
- Radotra, B.; Challa, S. Pathogenesis and Pathology of COVID-Associated Mucormycosis: What Is New and Why. Curr. Fungal Infect. Rep. 2022, 16, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Arora, A.; Aggarwal, A.; Passi, G.; Sharma, A.; Singh, G.; Barnwal, R.P. Mucormycosis Amid COVID-19 Crisis: Pathogenesis, Diagnosis, and Novel Treatment Strategies to Combat the Spread. Front. Microbiol. 2022, 12, 4005. [Google Scholar] [CrossRef]
- Krishna, V.; Bansal, N.; Morjaria, J.; Kaul, S. COVID-19-Associated Pulmonary Mucormycosis. J. Fungi 2022, 8, 711. [Google Scholar] [CrossRef]
- Narayanan, S.; Chua, J.V.; Baddley, J.W. Coronavirus Disease 2019–Associated Mucormycosis: Risk Factors and Mechanisms of Disease. Clin. Infect. Dis. 2022, 74, 1279–1283. [Google Scholar] [CrossRef]
- Ghazi, B.K.; Rackimuthu, S.; Wara, U.U.; Mohan, A.; Khawaja, U.A.; Ahmad, S.; Ahmad, S.; Hasan, M.M.; Dos Santos Costa, A.C.; Ahmad, S.; et al. Rampant Increase in Cases of Mucormycosis in India and Pakistan: A Serious Cause for Concern during the Ongoing COVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2021, 105, 1144–1147. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-Associated Mucormycosis. Clin. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef] [Green Version]
- Antoniadou, A. Outbreaks of Zygomycosis in Hospitals. Clin. Microbiol. Infect. 2009, 15, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, H.; Rutala, W.A.; Sickbert-Bennett, E.E.; Weber, D.J. Review of Fungal Outbreaks and Infection Prevention in Healthcare Settings during Construction and Renovation. Clin. Infect. Dis. 2015, 61, 433–444. [Google Scholar] [CrossRef]
- Krasinski, K.; Holzman, R.S.; Hanna, B.; Greco, M.A.; Graff, M.; Bhogal, M. Nosocomial Fungal Infection during Hospital Renovation. Infect. Control 1985, 6, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Rickerts, V.; Böhme, A.; Viertel, A.; Behrendt, G.; Jacobi, V.; Tintelnot, K.; Just-Nübling, G. Cluster of Pulmonary Infections Caused by Cunninghamella Bertholletiae in Immunocompromised Patients. Clin. Infect. Dis. 2000, 31, 910–913. [Google Scholar] [CrossRef] [Green Version]
- Abzug, M.J.; Gardner, S.; Glode, M.P.; Cymanski, M.; Roe, M.H.; Odom, L.F. Heliport-Associated Nosocomial Mucormycoses. Infect. Control Hosp. Epidemiol. 1992, 13, 325–326. [Google Scholar] [CrossRef] [PubMed]
- El-Mahallawy, H.A.; Khedr, R.; Taha, H.; Shalaby, L.; Mostafa, A. Investigation and Management of a Rhizomucor Outbreak in a Pediatric Cancer Hospital in Egypt. Pediatr. Blood Cancer 2016, 63, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Levy, V.; Rio, B.; Bazarbachi, A.; Hunault, M.; Delmer, A.; Zittoun, R.; Blanc, V.; Wolff, M. Two Cases of Epidemic Mucormycosis Infection in Patients with Acute Lymphoblastic Leukemia. Am. J. Hematol. 1996, 52, 64–65. [Google Scholar] [CrossRef]
- del Palacio Hernanz, A.; Fereres, J.; Garraus, S.L.; Rodriguez-Noriega, A.; Sanz, F.S. Nosocomial Infection by Rhizomucor pusillus in a Clinical Haematology Unit. J. Hosp. Infect. 1983, 4, 45–49. [Google Scholar] [CrossRef]
- Hammond, D.E. Cutaneous Phycomycosis. Report of Three Cases with Identification of Rhizopus. Arch. Dermatol. 1979, 115, 990–992. [Google Scholar] [CrossRef]
- Sheldon, D.L. Cutaneous Mucormycosis. Two Documented Cases of Suspected Nosocomial Cause. JAMA J. Am. Med. Assoc. 1979, 241, 1032–1034. [Google Scholar] [CrossRef]
- Gartenberg, G.; Bottone, E.J.; Keusch, G.T.; Weitzman, I. Hospital-Acquired Mucormycosis (Rhizopus rhizopodiformis) of Skin and Subcutaneous Tissue. N. Engl. J. Med. 1978, 299, 1115–1118. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Nosocomial Outbreak of Rhizopus Infections Associated with Elastoplast Wound Dressings-Minnesota. Morb. Mortal. Wkly. Rep. Report. 1978, 27, 33–34.
- Mitchell, S.; Gray, J.; Morgan, M.; Hocking, M.; Durbin, G. Nosocomial Infection with Rhizopus microsporus in Preterm Infants: Association with Wooden Tongue Depressors. Lancet 1996, 348, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Maraví-Poma, E.; Rodríguez-Tudela, J.L.; de Jalón, J.G.; Manrique-Larralde, A.; Torroba, L.; Urtasun, J.; Salvador, B.; Montes, M.; Mellado, E.; Rodríguez-Albarrán, F.; et al. Outbreak of Gastric Mucormycosis Associated with the Use of Wooden Tongue Depressors in Critically Ill Patients. Intensive Care Med. 2004, 30, 724–728. [Google Scholar] [CrossRef]
- LeMaile-Williams, M.; Burwell, L.A.; Salisbury, D.; Noble-Wang, J.; Arduino, M.; Lott, T.; Brandt, M.E.; Iiames, S.; Srinivasan, A.; Fridkin, S.K. Outbreak of Cutaneous Rhizopus arrhizus Infection Associated with Karaya Ostomy Bags. Clin. Infect. Dis. 2006, 43, e83–e88. [Google Scholar] [CrossRef] [Green Version]
- Gamarra, S.; Chaves, M.S.; Cabeza, M.S.; Macedo, D.; Leonardelli, F.; Franco, D.; Boleas, M.; Garcia-Effron, G. Mucormycosis Outbreak Due to Rhizopus microsporus after Arthroscopic Anterior Cruciate Ligament Reconstruction Surgery Evaluated by RAPD and MALDI-TOF Mass Spectrometry. J. Mycol. Med. 2018, 28, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.J.; Clancy, C.J.; Pasculle, A.W.; Liu, G.; Cheng, S.; Cumbie, R.B.; Driscoll, E.; Ayres, A.; Donahue, L.; Buck, M.; et al. Remediation of Mucorales-Contaminated Healthcare Linens at a Laundry Facility Following an Investigation of a Case Cluster of Hospital-Acquired Mucormycosis. Clin. Infect. Dis. 2022, 74, 1401–1407. [Google Scholar] [CrossRef]
- Duffy, J.; Harris, J.; Gade, L.; Sehulster, L.; Newhouse, E.; O’Connell, H.; Noble-Wang, J.; Rao, C.; Balajee, S.A.; Chiller, T. Mucormycosis Outbreak Associated with Hospital Linens. Pediatr. Infect. Dis. J. 2014, 33, 472–476. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chen, J.H.K.; Wong, S.C.Y.; Leung, S.S.M.; So, S.Y.C.; Lung, D.C.; Lee, W.-M.; Trendell-Smith, N.J.; Chan, W.-M.; Ng, D.; et al. Hospital Outbreak of Pulmonary and Cutaneous Zygomycosis Due to Contaminated Linen Items from Substandard Laundry. Clin. Infect. Dis. 2016, 62, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Sundermann, A.J.; Clancy, C.J.; Pasculle, A.W.; Liu, G.; Cumbie, R.B.; Driscoll, E.; Ayres, A.; Donahue, L.; Pergam, S.A.; Abbo, L.; et al. How Clean Is the Linen at My Hospital? The Mucorales on Unclean Linen Discovery Study of Large United States Transplant and Cancer Centers. Clin. Infect. Dis. 2019, 68, 850–853. [Google Scholar] [CrossRef]
- Jordan, A.; James, A.E.; Gold, J.A.W.; Wu, K.; Glowicz, J.; Wolfe, F.; Vyas, K.; Litvintseva, A.; Gade, L.; Liverett, H.; et al. Investigation of a Prolonged and Large Outbreak of Healthcare-Associated Mucormycosis Cases in an Acute Care Hospital—Arkansas, June 2019–May 2021. Open. Forum Infect. Dis. 2022, 9, ofac510. [Google Scholar] [CrossRef]
- Garner, D.; Machin, K. Investigation and Management of an Outbreak of Mucormycosis in a Paediatric Oncology Unit. J. Hosp. Infect. 2008, 70, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Walker, T.A.; Lockhart, S.R.; Ng, D.; Chiller, T.; Melchreit, R.; Brandt, M.E.; Smith, R.M. Centers for Disease Control and Prevention (CDC) Notes from the Field: Fatal Gastrointestinal Mucormycosis in a Premature Infant Associated with a Contaminated Dietary Supplement—Connecticut, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 155–156. [Google Scholar] [PubMed]
- Lee, S.C.; Billmyre, R.B.; Li, A.; Carson, S.; Sykes, S.M.; Huh, E.Y.; Mieczkowski, P.; Ko, D.C.; Cuomo, C.A.; Heitman, J. Analysis of a Food-Borne Fungal Pathogen Outbreak: Virulence and Genome of a Mucor Circinelloides Isolate from Yogurt. mBio 2014, 5, e01390-14. [Google Scholar] [CrossRef] [Green Version]
- Bouakline, A.; Lacroix, C.; Roux, N.; Gangneux, J.P.; Derouin, F. Fungal Contamination of Food in Hematology Units. J. Clin. Microbiol. 2000, 38, 4272–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of Intestinal Infection Due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef] [Green Version]
- Poirier, P.; Nourrisson, C.; Gibold, L.; Chalus, E.; Guelon, D.; Descamp, S.; Traore, O.; Cambon, M.; Aumeran, C. Three Cases of Cutaneous Mucormycosis with Lichtheimia spp. (Ex Absidia/Mycocladus) in ICU. Possible Cross-Transmission in an Intensive Care Unit between 2 Cases. J. Mycol. Med. 2013, 23, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Thatipelli, S.; Santoiemma, P.; Echenique, I.A.; Green, R.; Ison, M.G.; Ladner, D.; Kanwar, Y.S.; Stosor, V. Donor-derived Renal Allograft Mucormycosis in a Combined Liver and Kidney Transplantation: Case Report and Review of the Literature. Transpl. Transplant. Infect. Dis. 2021, 23, e13534. [Google Scholar] [CrossRef]
- Zhan, H.X.; Lv, Y.; Zhang, Y.; Liu, C.; Wang, B.; Jiang, Y.Y.; Liu, X.M. Hepatic and Renal Artery Rupture Due to Aspergillus and Mucor Mixed Infection after Combined Liver and Kidney Transplantation: A Case Report. Transpl. Transplant. Proc. 2008, 40, 1771–1773. [Google Scholar] [CrossRef]
- Davari, H.R.; Malekhossini, S.A.; Salahi, H.; Bahador, A.; Saberifirozi, M.; Geramizadeh, B.; Lahsaee, S.M.; Khosravi, M.B.; Imanieh, M.H.; Bagheri, M.H. Outcome of Mucormycosis in Liver Transplantation: Four Cases and a Review of Literature. Exp. Clin. Transpl. Transplant. 2003, 1, 147–152. [Google Scholar]
- Devauchelle, P.; Jeanne, M.; Fréalle, E. Mucormycosis in Burn Patients. J. Fungi 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Littlehales, E.; Teague, R.; Andrew, D.; Yassaie, E. Mucormycosis in Burns: A Review. J. Burn. Care Res. 2022, 43, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, G.; Hayette, M.P.; Jacquemin, D.; Melin, P.; Mutsers, J.; De Mol, P. An Outbreak of Absidia corymbifera Infection Associated with Bandage Contamination in a Burns Unit. J. Hosp. Infect. 2005, 61, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fréalle, E.; Rocchi, S.; Bacus, M.; Bachelet, H.; Pasquesoone, L.; Tavernier, B.; Mathieu, D.; Millon, L.; Jeanne, M. Real-Time Polymerase Chain Reaction Detection of Lichtheimia Species in Bandages Associated with Cutaneous Mucormycosis in Burn Patients. J. Hosp. Infect. 2018, 99, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, K.P.; Jackson, B.R.; Perkins, K.M.; Glowicz, J.; Kerins, J.L.; Black, S.R.; Lockhart, S.R.; Christensen, B.E.; Beer, K.D. A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. J. Fungi 2019, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Davoudi, S.; Graviss, L.S.; Kontoyiannis, D.P. Healthcare-Associated Outbreaks Due to Mucorales and Other Uncommon Fungi. Eur. J. Clin. Investig. 2015, 45, 767–773. [Google Scholar] [CrossRef]
- Foster, C.E.; Revell, P.A.; Campbell, J.R.; Marquez, L. Healthcare-Associated Pediatric Cutaneous Mucormycosis at Texas Children’s Hospital, 2012–2019. Pediatr. Infect. Dis. J. 2021, 40, 746–748. [Google Scholar] [CrossRef]
- Marek, C.; Croxen, M.A.; Dingle, T.C.; Bharat, A.; Schwartz, I.S.; Wiens, R.; Smith, S. The Use of Genome Sequencing to Investigate an Outbreak of Hospital-acquired Mucormycosis in Transplant Patients. Transpl. Transplant. Infect. Dis. 2019, 21, e13163. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, D.; Criscuolo, A.; Lee, S.C.; Legrand, M.; Chaouat, M.; Denis, B.; Lafaurie, M.; Rouveau, M.; Soler, C.; Schaal, J.-V.; et al. Outbreak of Invasive Wound Mucormycosis in a Burn Unit Due to Multiple Strains of Mucor circinelloides f. circinelloides Resolved by Whole-Genome Sequencing. mBio 2018, 9, e00573-18. [Google Scholar] [CrossRef] [Green Version]
- Bowers, J.R.; Monroy-Nieto, J.; Gade, L.; Travis, J.; Refojo, N.; Abrantes, R.; Santander, J.; French, C.; Dignani, M.C.; Hevia, A.I.; et al. Rhizopus microsporus Infections Associated with Surgical Procedures, Argentina, 2006–2014. Emerg. Infect. Dis. 2020, 26, 937–944. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Kaul, D.; Muto, C.; Cheng, S.J.; Richter, R.A.; Bruno, V.M.; Liu, G.; Beyhan, S.; Sundermann, A.J.; Mounaud, S.; et al. Genetic Diversity of Clinical and Environmental Mucorales Isolates Obtained from an Investigation of Mucormycosis Cases among Solid Organ Transplant Recipients. Microb. Genom. 2020, 6, mgen000473. [Google Scholar] [CrossRef]
- Holzel, H.; Macqueen, S.; MacDonald, A.; Alexander, S.; Campbell, C.K.; Johnson, E.M.; Warnock, D.W. Rhizopus microsporus in Wooden Tongue Depressors: A Major Threat or Minor Inconvenience? J. Hosp. Infect. 1998, 38, 113–118. [Google Scholar] [CrossRef]
- Verweij, P.E.; Voss, A.; Donnelly, J.P.; de Pauw, B.E.; Meis, J.F. Wooden Sticks as the Source of a Pseudoepidemic of Infection with Rhizopus microsporus var. rhizopodiformis among Immunocompromised Patients. J. Clin. Microbiol. 1997, 35, 2422–2423. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.; Coulter, C.; Lye, G.; Nimmo, G. Rhizopus and Tongue Depressors. Lancet 1996, 348, 1250. [Google Scholar] [CrossRef]
- Benedict, K.; Park, B.J. Invasive Fungal Infections after Natural Disasters. Emerg. Infect. Dis. 2014, 20, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Floret, N.; Viel, J.-F.; Mauny, F.; Hoen, B.; Piarroux, R. Negligible Risk for Epidemics after Geophysical Disasters. Emerg. Infect. Dis. 2006, 12, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.F.; Castro, D.; Valencia, A.; Morales, P. Necrotizing Soft Tissue Lesions after a Volcanic Cataclysm. World J. Surg. 1991, 15, 240–247. [Google Scholar] [CrossRef]
- Snell, B.J.; Tavakoli, K. Necrotizing Fasciitis Caused by Apophysomyces elegans Complicating Soft-Tissue and Pelvic Injuries in a Tsunami Survivor from Thailand. Plast. Reconstr. Surg. 2007, 119, 448–449. [Google Scholar] [CrossRef]
- Andresen, D.; Donaldson, A.; Choo, L.; Knox, A.; Klaassen, M.; Ursic, C.; Vonthethoff, L.; Krilis, S.; Konecny, P. Multifocal Cutaneous Mucormycosis Complicating Polymicrobial Wound Infections in a Tsunami Survivor from Sri Lanka. Lancet 2005, 365, 876–878. [Google Scholar] [CrossRef]
- Maegele, M.; Gregor, S.; Yuecel, N.; Simanski, C.; Paffrath, T.; Rixen, D.; Heiss, M.; Rudroff, C.; Saad, S.; Perbix, W.; et al. One Year Ago Not Business as Usual: Wound Management, Infection and Psychoemotional Control during Tertiary Medical Care Following the 2004 Tsunami Disaster in Southeast Asia. Crit. Care 2006, 10, R50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.-C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.-J.; et al. Necrotizing Cutaneous Mucormycosis after a Tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef] [Green Version]
- Wurster, S.; Tatara, A.M.; Albert, N.D.; Ibrahim, A.S.; Heitman, J.; Lee, S.C.; Shetty, A.C.; McCracken, C.; Graf, K.T.; Mikos, A.G.; et al. Tornadic Shear Stress Induces a Transient, Calcineurin-Dependent Hypervirulent Phenotype in Mucorales Molds. mBio 2020, 11, e01414-20. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Smith, J.M.; Hink, E.M.; Durairaj, V.D. Increased Incidence of Rhino-Orbital-Cerebral Mucormycosis after Colorado Flooding. Ophthalmic Plast. Reconstr. Surg. 2017, 33, S148–S151. [Google Scholar] [CrossRef] [PubMed]
- Wurster, S.; Paraskevopoulos, T.; Toda, M.; Jiang, Y.; Tarrand, J.J.; Williams, S.; Chiller, T.M.; Jackson, B.R.; Kontoyiannis, D.P. Invasive Mould Infections in Patients from Floodwater-Damaged Areas after Hurricane Harvey—A Closer Look at an Immunocompromised Cancer Patient Population. J. Infect. 2022, 84, 701–709. [Google Scholar] [CrossRef]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.-H. Comparison of Computed Tomographic Findings in Pulmonary Mucormycosis and Invasive Pulmonary Aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzai, L.; Anglani, M.; Giraudo, C.; Martucci, M.; Cester, G.; Causin, F. Imaging Features of Rhinocerebral Mucormycosis: From Onset to Vascular Complications. Acta Radiol. 2022, 63, 232–244. [Google Scholar] [CrossRef]
- Pruthi, H.; Muthu, V.; Bhujade, H.; Sharma, A.; Baloji, A.; Ratnakara, R.G.; Bal, A.; Singh, H.; Sandhu, M.S.; Negi, S.; et al. Pulmonary Artery Pseudoaneurysm in COVID-19-Associated Pulmonary Mucormycosis: Case Series and Systematic Review of the Literature. Mycopathologia 2022, 187, 31–37. [Google Scholar] [CrossRef]
- Stanzani, M.; Sassi, C.; Lewis, R.E.; Tolomelli, G.; Bazzocchi, A.; Cavo, M.; Vianelli, N.; Battista, G. High Resolution Computed Tomography Angiography Improves the Radiographic Diagnosis of Invasive Mold Disease in Patients with Hematological Malignancies. Clin. Infect. Dis. 2015, 60, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Douglas, A.; Lau, E.; Thursky, K.; Slavin, M. What, Where and Why: Exploring Fluorodeoxyglucose-PET’s Ability to Localise and Differentiate Infection from Cancer. Curr. Opin. Infect. Dis. 2017, 30, 552–564. [Google Scholar] [CrossRef]
- Eibschutz, L.S.; Rabiee, B.; Asadollahi, S.; Gupta, A.; Assadi, M.; Alavi, A.; Gholamrezanezhad, A. FDG-PET/CT of COVID-19 and Other Lung Infections. Semin. Nucl. Med. 2022, 52, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, A.; Alipour, R.; Khot, A.; Bajel, A.; Antippa, P.; Slavin, M.; Thursky, K. The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG PET/CT) in Assessment of Complex Invasive Fungal Disease and Opportunistic Co-infections in Patients with Acute Leukemia Prior to Allogeneic Hematopoietic Cell Transplant. Transpl. Transplant. Infect. Dis. 2021, 23, e13547. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Zygomycosis: Conventional Laboratory Diagnosis. Clin. Microbiol. Infect. 2009, 15, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, S.; Ahmed, T.I.; Happa, K.; Sultan, M.; Manhas, S.; Shamas, S. Spectrum of Mucormycosis before and during COVID-19: Epidemiology, Diagnosis, and Current Therapeutic Interventions. Curr. Fungal Infect. Rep. 2022, 16, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Burnham-Marusich, A.R.; Hubbard, B.; Kvam, A.J.; Gates-Hollingsworth, M.; Green, H.R.; Soukup, E.; Limper, A.H.; Kozel, T.R. Conservation of Mannan Synthesis in Fungi of the Zygomycota and Ascomycota Reveals a Broad Diagnostic Target. mSphere 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.E.; Salonen, J.; Ekfors, T.O. The Use of Immunohistochemistry to Improve Sensitivity and Specificity in the Diagnosis of Systemic Mycoses in Patients with Haematological Malignancies. J. Pathol. 1997, 181, 100–105. [Google Scholar] [CrossRef]
- Jung, J.; Park, Y.S.; Sung, H.; Song, J.S.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.-H. Assessment of the Accuracy of Histomorphologic Diagnosis of Aspergillosis and Mucormycosis by Immunohistochemical Tests. Clin. Infect. Dis. 2015, 61, civ660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Song, J.S.; Kim, J.Y.; Cha, H.H.; Yun, J.H.; Park, J.W.; Jung, K.H.; Jo, K.M.; Jung, J.; Kim, M.J.; et al. Diagnostic Performance of Immunohistochemistry for the Aspergillosis and Mucormycosis. Mycoses 2019, 62, 1006–1014. [Google Scholar] [CrossRef]
- Walsh, T.J.; Gamaletsou, M.N.; McGinnis, M.R.; Hayden, R.T.; Kontoyiannis, D.P. Early Clinical and Laboratory Diagnosis of Invasive Pulmonary, Extrapulmonary, and Disseminated Mucormycosis (Zygomycosis). Clin. Infect. Dis. 2012, 54, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M.; Caramalho, R.; Lass-Flörl, C. Laboratory Diagnosis of Mucormycosis: Current Status and Future Perspectives. Future Microbiol. 2014, 9, 683–695. [Google Scholar] [CrossRef]
- Kidd, S.; Halliday, C.; Ellis, D. Descriptions of Medical Fungi, 4th ed.; CAB International: Wallingford, UK, 2023. [Google Scholar]
- Becker, P.T.; de Bel, A.; Martiny, D.; Ranque, S.; Piarroux, R.; Cassagne, C.; Detandt, M.; Hendrickx, M. Identification of Filamentous Fungi Isolates by MALDI-TOF Mass Spectrometry: Clinical Evaluation of an Extended Reference Spectra Library. Med. Mycol. 2014, 52, 826–834. [Google Scholar] [CrossRef] [Green Version]
- De Carolis, E.; Posteraro, B.; Lass-Flörl, C.; Vella, A.; Florio, A.R.; Torelli, R.; Girmenia, C.; Colozza, C.; Tortorano, A.M.; Sanguinetti, M.; et al. Species Identification of Aspergillus, Fusarium and Mucorales with Direct Surface Analysis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Clin. Microbiol. Infect. 2012, 18, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an Environmental DNA Barcode for Fungi: An in Silico Approach Reveals Potential PCR Biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.H.; Ryberg, M.; Abarenkov, K.; Sjökvist, E.; Kristiansson, E. The ITS Region as a Target for Characterization of Fungal Communities Using Emerging Sequencing Technologies. FEMS Microbiol. Lett. 2009, 296, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sparks, R.; Halliday, C.; Chen, S.C.-A. Panfungal PCR on Formalin Fixed Paraffin Embedded Tissue—To Proceed or Not Proceed? Pathology 2023, 55, S46. [Google Scholar] [CrossRef]
- Trubiano, J.; Dennison, A.; Morrissey, C.; Chua, K.; Halliday, C.; Chen, S.-A.; Spelman, D. Clinical Utility of Panfungal Polymerase Chain Reaction for the Diagnosis of Invasive Fungal Disease: A Single Center Experience. Med. Mycol. 2016, 54, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An Integrated Genomic and Transcriptomic Survey of Mucormycosis-Causing Fungi. Nat. Commun. 2016, 7, 12218. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 Mediates Fungal Invasion of Host Cells during Mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef] [Green Version]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early Diagnosis and Monitoring of Mucormycosis by Detection of Circulating DNA in Serum: Retrospective Analysis of 44 Cases Collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales Polymerase Chain Reaction (PCR) for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef]
- Rocchi, S.; Scherer, E.; Mengoli, C.; Alanio, A.; Botterel, F.; Bougnoux, M.E.; Bretagne, S.; Cogliati, M.; Cornu, M.; Dalle, F.; et al. Interlaboratory Evaluation of Mucorales PCR Assays for Testing Serum Specimens: A Study by the Fungal PCR Initiative and the Modimucor Study Group. Med. Mycol. 2021, 59, 126–138. [Google Scholar] [CrossRef]
- Mercier, T.; Reynders, M.; Beuselinck, K.; Guldentops, E.; Maertens, J.; Lagrou, K. Serial Detection of Circulating Mucorales DNA in Invasive Mucormycosis: A Retrospective Multicenter Evaluation. J. Fungi 2019, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Guegan, H.; Iriart, X.; Bougnoux, M.-E.; Berry, A.; Robert-Gangneux, F.; Gangneux, J.-P. Evaluation of MucorGenius® Mucorales PCR Assay for the Diagnosis of Pulmonary Mucormycosis. J. Infect. 2020, 81, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Shokouhi, S.; Mirzaei, J.; Mohseni Sajadi, M.; Javadi, A. Comparison of Serum PCR Assay and Histopathology for the Diagnosis of Invasive Aspergillosis and Mucormycosis in Immunocompromised Patients with Sinus Involvement. Curr. Med. Mycol. 2016, 2, 46–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolatabadi, S.; Najafzadeh, M.J.; de Hoog, G.S. Rapid Screening for Human-Pathogenic Mucorales Using Rolling Circle Amplification. Mycoses 2014, 57, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Lengerova, M.; Racil, Z.; Hrncirova, K.; Kocmanova, I.; Volfova, P.; Ricna, D.; Bejdak, P.; Moulis, M.; Pavlovsky, Z.; Weinbergerova, B.; et al. Rapid Detection and Identification of Mucormycetes in Bronchoalveolar Lavage Samples from Immunocompromised Patients with Pulmonary Infiltrates by Use of High-Resolution Melt Analysis. J. Clin. Microbiol. 2014, 52, 2824–2828. [Google Scholar] [CrossRef] [Green Version]
- Huppler, A.R.; Fisher, B.T.; Lehrnbecher, T.; Walsh, T.J.; Steinbach, W.J. Role of Molecular Biomarkers in the Diagnosis of Invasive Fungal Diseases in Children. J. Pediatr. Infect. Dis. Soc. 2017, 6, S32–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Oinuma, K.-I.; Niki, M.; Yamagoe, S.; Miyazaki, Y.; Asai, K.; Yamada, K.; Hirata, K.; Kaneko, Y.; Kakeya, H. Identification of a Novel Rhizopus-Specific Antigen by Screening with a Signal Sequence Trap and Evaluation as a Possible Diagnostic Marker of Mucormycosis. Med. Mycol. 2017, 55, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, L.; Turner, L.F.; McLaughlin, D.W. Indirect Enzyme-Linked Immunosorbent Assay for Zygomycosis. J. Clin. Microbiol. 1989, 27, 1979–1982. [Google Scholar] [CrossRef] [Green Version]
- Wysong, D.R.; Waldorf, A.R. Electrophoretic and Immunoblot Analyses of Rhizopus arrhizus Antigens. J. Clin. Microbiol. 1987, 25, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Heldt, S.; Hoenigl, M. Lateral Flow Assays for the Diagnosis of Invasive Aspergillosis: Current Status. Curr. Fungal Infect. Rep. 2017, 11, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Orne, C.; Burnham-Marusich, A.; Baldin, C.; Gebremariam, T.; Ibrahim, A.; Kvam, A.; Kozel, T. Cell Wall Fucomannan Is a Biomarker for Diagnosis of Invasive Murine Mucormycosis. In Proceedings of the 28th ECCMID, Madrid, Spain, 21–24 April 2018; pp. 21–24. [Google Scholar]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Forghieri, F.; Zanetti, E.; Quadrelli, C.; Candoni, A.; Maertens, J.; Rossi, G.; et al. Mucorales-Specific T Cells Emerge in the Course of Invasive Mucormycosis and May Be Used as a Surrogate Diagnostic Marker in High-Risk Patients. Blood 2011, 118, 5416–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacher, P.; Steinbach, A.; Kniemeyer, O.; Hamprecht, A.; Assenmacher, M.; Vehreschild, M.J.G.T.; Vehreschild, J.J.; Brakhage, A.A.; Cornely, O.A.; Scheffold, A. Fungus-Specific CD4+ T Cells for Rapid Identification of Invasive Pulmonary Mold Infection. Am. J. Respir. Crit. Care Med. 2015, 191, 348–352. [Google Scholar] [CrossRef]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Gilioli, A.; Forghieri, F.; Candoni, A.; Cesaro, S.; Quadrelli, C.; Maertens, J.; et al. Mucorales-Specific T Cells in Patients with Hematologic Malignancies. PLoS ONE 2016, 11, e0149108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying Amphotericin B–Based Frontline Therapy Significantly Increases Mortality among Patients with Hematologic Malignancy Who Have Zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Koshy, S.; Ismail, N.; Astudillo, C.L.; Haeger, C.M.; Aloum, O.; Acharige, M.T.; Farmakiotis, D.; Baden, L.R.; Marty, F.M.; Kontoyiannis, D.P.; et al. Breath-Based Diagnosis of Invasive Mucormycosis (IM). Open. Forum Infect. Dis. 2017, 4, S53–S54. [Google Scholar] [CrossRef]
- Hussain, K.K.; Malavia, D.; MJohnson, E.; Littlechild, J.; Winlove, C.P.; Vollmer, F.; Gow, N.A. Biosensors and Diagnostics for Fungal Detection. J. Fungi 2020, 6, 349. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Li, R. The Use of Combined PCR, Fluorescence in Situ Hybridisation and Immunohistochemical Staining to Diagnose Mucormycosis from Formalin-fixed Paraffin-embedded Tissues. Mycoses 2021, 64, 1460–1470. [Google Scholar] [CrossRef]
- Mery, A.; Sendid, B.; François, N.; Cornu, M.; Poissy, J.; Guerardel, Y.; Poulain, D. Application of Mass Spectrometry Technology to Early Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2016, 54, 2786–2797. [Google Scholar] [CrossRef] [Green Version]
- Guinea, J.; Meletiadis, J.; Arikan-Akdagli, S.; Muehlethaler, K.; Kahlmeter, G.; Arendrup, M.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID; European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST Definitive Document E.DEF 9.4. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_EDef_9.4_method_for_susceptibility_testing_of_moulds.pdf (accessed on 16 April 2023).
- Alexander, B.; Procop, G.; Dufresne, P.; Espinel-Ingroff, A.; Fuller, J.; Ghannoum, M.; Hanson, K.; Holliday, D.; Ostrosky-Zeichner, L.; Schuetz, A.; et al. M38—Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Chowdhary, A.; Singh, P.K.; Kathuria, S.; Hagen, F.; Meis, J.F. Comparison of the EUCAST and CLSI Broth Microdilution Methods for Testing Isavuconazole, Posaconazole, and Amphotericin B against Molecularly Identified Mucorales Species. Antimicrob. Agents Chemother. 2015, 59, 7882–7887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Jensen, R.H.; Meletiadis, J. In Vitro Activity of Isavuconazole and Comparators against Clinical Isolates of the Mucorales Order. Antimicrob. Agents Chemother. 2015, 59, 7735–7742. [Google Scholar] [CrossRef] [Green Version]
- Dannaoui, E. In Vitro Susceptibilities of Zygomycetes to Conventional and New Antifungals. J. Antimicrob. Chemother. 2003, 51, 45–52. [Google Scholar] [CrossRef]
- Vidal, P.; Schwarz, P.; Dannaoui, E. Evaluation of the Gradient Concentration Strip Method for Antifungal Susceptibility Testing of Isavuconazole and Comparators for Mucorales Species. Antimicrob. Agents Chemother. 2019, 63, e00838-19. [Google Scholar] [CrossRef] [Green Version]
- Escribano, P.; Mesquida, A.; López-Montesinos, S.; Reigadas, E.; Muñoz, P.; Guinea, J. Amphotericin B, Itraconazole, Posaconazole, and Isavuconazole MICs against Clinical Mucorales Isolates Obtained by Visual Inspection and Spectrophotometric Reading According to the EUCAST 9.4 Procedure. Med. Mycol. 2023, 61, myad045. [Google Scholar] [CrossRef]
- Wagner, L.; de Hoog, S.; Alastruey-Izquierdo, A.; Voigt, K.; Kurzai, O.; Walther, G. A Revised Species Concept for Opportunistic Mucor Species Reveals Species-Specific Antifungal Susceptibility Profiles. Antimicrob. Agents Chemother. 2019, 63, e00653-19. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter Evaluation of MIC Distributions for Epidemiologic Cutoff Value Definition To Detect Amphotericin B, Posaconazole, and Itraconazole Resistance among the Most Clinically Relevant Species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; van de Sande, W.W.J.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal Susceptibility and Phylogeny of Opportunistic Members of the Order Mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In Vitro Susceptibilities of 217 Clinical Isolates of Zygomycetes to Conventional and New Antifungal Agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alastruey-Izquierdo, A.; Castelli, M.V.; Cuesta, I.; Monzon, A.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Activity of Posaconazole and Other Antifungal Agents against Mucorales Strains Identified by Sequencing of Internal Transcribed Spacers. Antimicrob. Agents Chemother. 2009, 53, 1686–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badali, H.; Cañete-Gibas, C.; McCarthy, D.; Patterson, H.; Sanders, C.; David, M.P.; Mele, J.; Fan, H.; Wiederhold, N.P. Epidemiology and Antifungal Susceptibilities of Mucoralean Fungi in Clinical Samples from the United States. J. Clin. Microbiol. 2021, 59, e0123021. [Google Scholar] [CrossRef]
- Borman, A.M.; Fraser, M.; Patterson, Z.; Palmer, M.D.; Johnson, E.M. In Vitro Antifungal Drug Resistance Profiles of Clinically Relevant Members of the Mucorales (Mucoromycota) Especially with the Newer Triazoles. J. Fungi 2021, 7, 271. [Google Scholar] [CrossRef]
- Lamoth, F.; Damonti, L.; Alexander, B.D. Role of Antifungal Susceptibility Testing in Non-Aspergillus Invasive Mold Infections. J. Clin. Microbiol. 2016, 54, 1638–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinel-Ingroff, A.; Arthington-Skaggs, B.; Iqbal, N.; Ellis, D.; Pfaller, M.A.; Messer, S.; Rinaldi, M.; Fothergill, A.; Gibbs, D.L.; Wang, A. Multicenter Evaluation of a New Disk Agar Diffusion Method for Susceptibility Testing of Filamentous Fungi with Voriconazole, Posaconazole, Itraconazole, Amphotericin B, and Caspofungin. J. Clin. Microbiol. 2007, 45, 1811–1820. [Google Scholar] [CrossRef] [Green Version]
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and Exposure–Response Relationships of Isavuconazole Plasma Concentrations in the Phase 3 SECURE Trial of Patients with Invasive Mould Diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Rhomberg, P.R.; Wiederhold, N.P.; Gibas, C.; Sanders, C.; Fan, H.; Mele, J.; Kovanda, L.L.; Castanheira, M. In Vitro Activity of Isavuconazole against Opportunistic Fungal Pathogens from Two Mycology Reference Laboratories. Antimicrob. Agents Chemother. 2018, 62, e01230-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.N.; Fothergill, A.W.; McCarthy, D.I.; Rinaldi, M.G.; Graybill, J.R. In Vitro Activities of Posaconazole, Itraconazole, Voriconazole, Amphotericin B, and Fluconazole against 37 Clinical Isolates of Zygomycetes. Antimicrob. Agents Chemother. 2002, 46, 1581–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalhaes, C.G.; Rhomberg, P.R.; Huband, M.D.; Pfaller, M.A.; Castanheira, M. Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific. J. Fungi 2023, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Cornely, O.A.; Dannaoui, E. Antifungal Combinations in Mucorales: A Microbiological Perspective. Mycoses 2019, 62, myc.12909. [Google Scholar] [CrossRef]
- Dannaoui, E.; Afeltra, J.; Meis, J.F.G.M.; Verweij, P.E. In Vitro Susceptibilities of Zygomycetes to Combinations of Antimicrobial Agents. Antimicrob. Agents Chemother. 2002, 46, 2708–2711. [Google Scholar] [CrossRef] [Green Version]
- Biswas, C.; Sorrell, T.C.; Djordjevic, J.T.; Zuo, X.; Jolliffe, K.A.; Chen, S.C.-A. In Vitro Activity of Miltefosine as a Single Agent and in Combination with Voriconazole or Posaconazole against Uncommon Filamentous Fungal Pathogens. J. Antimicrob. Chemother. 2013, 68, 2842–2846. [Google Scholar] [CrossRef]
- Arikan, S.; Sancak, B.; Alp, S.; Hascelik, G.; Mcnicholas, P. Comparative in vitro Activities of Posaconazole, Voriconazole, Itraconazole, and Amphotericin B against Aspergillus and Rhizopus, and Synergy Testing for Rhizopus. Med. Mycol. 2008, 46, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, R.; Yu, J. Drug Combinations against Mucor irregularis in vitro. Antimicrob. Agents Chemother. 2013, 57, 3395–3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Narbona, M.; Guinea, J.; Martínez-Alarcón, J.; Peláez, T.; Bouza, E. In Vitro Activities of Amphotericin B, Caspofungin, Itraconazole, Posaconazole, and Voriconazole against 45 Clinical Isolates of Zygomycetes: Comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob. Agents Chemother. 2007, 51, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Lamoth, F.; Alexander, B.D. Comparing Etest and Broth Microdilution for Antifungal Susceptibility Testing of the Most-Relevant Pathogenic Molds. J. Clin. Microbiol. 2015, 53, 3176–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caramalho, R.; Maurer, E.; Binder, U.; Araújo, R.; Dolatabadi, S.; Lass-Flörl, C.; Lackner, M. Etest Cannot Be Recommended for in vitro Susceptibility Testing of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 3663–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, D.; Leonardelli, F.; Dudiuk, C.; Theill, L.; Cabeza, M.S.; Gamarra, S.; Garcia-Effron, G. Molecular Confirmation of the Linkage between the Rhizopus oryzae CYP51A Gene Coding Region and Its Intrinsic Voriconazole and Fluconazole Resistance. Antimicrob. Agents Chemother. 2018, 62, e00224-18. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.-J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T.; et al. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLoS Genet. 2009, 5, e1000549. [Google Scholar] [CrossRef]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic Short-Tailed Azole Resistance in Mucormycetes Is Due to an Evolutionary Conserved Aminoacid Substitution of the Lanosterol 14α-Demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Hermoso, D.; Hoinard, D.; Gantier, J.-C.; Grenouillet, F.; Dromer, F.; Dannaoui, E. Molecular and Phenotypic Evaluation of Lichtheimia corymbifera (Formerly Absidia corymbifera) Complex Isolates Associated with Human Mucormycosis: Rehabilitation of L. ramosa. J. Clin. Microbiol. 2009, 47, 3862–3870. [Google Scholar] [CrossRef] [Green Version]
- Etienne, K.A.; Gillece, J.; Hilsabeck, R.; Schupp, J.M.; Colman, R.; Lockhart, S.R.; Gade, L.; Thompson, E.H.; Sutton, D.A.; Neblett-Fanfair, R.; et al. Whole Genome Sequence Typing to Investigate the Apophysomyces Outbreak Following a Tornado in Joplin, Missouri, 2011. PLoS ONE 2012, 7, e49989. [Google Scholar] [CrossRef] [Green Version]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic Diversity of Fungal and Bacterial Communities in Human Skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Shelburne, S.A.; Ajami, N.J.; Chibucos, M.C.; Beird, H.C.; Tarrand, J.; Galloway-Peña, J.; Albert, N.; Chemaly, R.F.; Ghantoji, S.S.; Marsh, L.; et al. Implementation of a Pan-Genomic Approach to Investigate Holobiont-Infecting Microbe Interaction: A Case Report of a Leukemic Patient with Invasive Mucormycosis. PLoS ONE 2015, 10, e0139851. [Google Scholar] [CrossRef] [PubMed]
- Stiller, J.W.; Hall, B.D. The Origin of Red Algae: Implications for Plastid Evolution. Proc. Natl. Acad. Sci. USA 1997, 94, 4520–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A. ECMM/ISHAM Recommendations for Clinical Management of COVID-19 Associated Mucormycosis in Low- and Middle-income Countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Bupha-Intr, O.; Butters, C.; Reynolds, G.; Kennedy, K.; Meyer, W.; Patil, S.; Bryant, P.; Morrissey, C.O.; Slavin, M.A.; Thursky, K.A.; et al. Consensus Guidelines for the Diagnosis and Management of Invasive Fungal Disease Due to Moulds Other than Aspergillus in the Haematology/Oncology Setting, 2021. Intern. Med. J. 2021, 51, 177–219. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Lee, S.C. Current Treatments against Mucormycosis and Future Directions. PLoS Pathog. 2022, 18, e1010858. [Google Scholar] [CrossRef]
- Ganesan, P.; Ganapathy, D.; Sekaran, S.; Murthykumar, K.; Sundramoorthy, A.K.; Pitchiah, S.; Shanmugam, R. Molecular Mechanisms of Antifungal Resistance in Mucormycosis. Biomed. Res. Int. 2022, 2022, 6722245. [Google Scholar] [CrossRef]
- Hoekstra, W.J.; Garvey, E.P.; Moore, W.R.; Rafferty, S.W.; Yates, C.M.; Schotzinger, R.J. Design and Optimization of Highly-Selective Fungal CYP51 Inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 3455–3458. [Google Scholar] [CrossRef]
- Gebremariam, T.; Wiederhold, N.P.; Fothergill, A.W.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. VT-1161 Protects Immunosuppressed Mice from Rhizopus arrhizus var. arrhizus Infection. Antimicrob. Agents Chemother. 2015, 59, 7815–7817. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.; Alkhazraji, S.; Lin, L.; Wiederhold, N.P.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Prophylactic Treatment with VT-1161 Protects Immunosuppressed Mice from Rhizopus arrhizus var. arrhizus Infection. Antimicrob. Agents Chemother. 2017, 61, e00390-17. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.; Gu, Y.; Alkhazraji, S.; Youssef, E.; Shaw, K.J.; Ibrahim, A.S. The Combination Treatment of Fosmanogepix and Liposomal Amphotericin B Is Superior to Monotherapy in Treating Experimental Invasive Mold Infections. Antimicrob. Agents Chemother. 2022, 66, e0038022. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Pulmonary Murine Mucormycosis Due to Rhizopus arrhizus. Antimicrob. Agents Chemother. 2020, 64, e00178-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Estoppey, D.; Roggo, S.; Pistorius, D.; Fuchs, F.; Studer, C.; Ibrahim, A.S.; Aust, T.; Grandjean, F.; Mihalic, M.; et al. Jawsamycin Exhibits in vivo Antifungal Properties by Inhibiting Spt14/Gpi3-Mediated Biosynthesis of Glycosylphosphatidylinositol. Nat. Commun. 2020, 11, 3387. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Schwarz, P.; Lortholary, O. In Vitro Interactions between Antifungals and Immunosuppressive Drugs against Zygomycetes. Antimicrob. Agents Chemother. 2009, 53, 3549–3551. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, F.; Kontoyiannis, D.P. The Calcineurin Pathway Inhibitor Tacrolimus Enhances the in vitro Activity of Azoles against Mucorales via Apoptosis. Eukaryot. Cell 2013, 12, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, P.; Schwarz, P.V.; Felske-Zech, H.; Dannaoui, E. In Vitro Interactions between Isavuconazole and Tacrolimus, Cyclosporin A or Sirolimus against Mucorales. J. Antimicrob. Chemother. 2019, 74, 1921–1927. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Shertz, C.A.; Lee, S.C.; Heitman, J.; Cardenas, M.E. Rapamycin Exerts Antifungal Activity in vitro and in vivo against Mucor circinelloides via FKBP12-Dependent Inhibition of Tor. Eukaryot. Cell 2012, 11, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus Enhances the Potency of Posaconazole Against Rhizopus oryzae in vitro and in an Experimental Model of Mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Colley, T.; Alanio, A.; Kelly, S.L.; Sehra, G.; Kizawa, Y.; Warrilow, A.G.S.; Parker, J.E.; Kelly, D.E.; Kimura, G.; Anderson-Dring, L.; et al. In vitro and in vivo Antifungal Profile of a Novel and Long-Acting Inhaled Azole, PC945, on Aspergillus fumigatus Infection. Antimicrob. Agents Chemother. 2017, 61, e02280-16. [Google Scholar] [CrossRef] [Green Version]
- Koselny, K.; Green, J.; DiDone, L.; Halterman, J.P.; Fothergill, A.W.; Wiederhold, N.P.; Patterson, T.F.; Cushion, M.T.; Rappelye, C.; Wellington, M.; et al. The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity in vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis. Antimicrob. Agents Chemother. 2016, 60, 7115–7127. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Messer, S.A.; Georgopapadakou, N.; Martell, L.A.; Besterman, J.M.; Diekema, D.J. Activity of MGCD290, a Hos2 Histone Deacetylase Inhibitor, in Combination with Azole Antifungals against Opportunistic Fungal Pathogens. J. Clin. Microbiol. 2009, 47, 3797–3804. [Google Scholar] [CrossRef] [Green Version]
- Galgóczy, L.; Lukács, G.; Nyilasi, I.; Papp, T.; Vágvölgyi, C. Antifungal Activity of Statins and Their Interaction with Amphotericin B against Clinically Important Zygomycetes. Acta Biol. Hung. 2010, 61, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Pesti, M.; Papp, T.; Vágvölgyi, C. In Vitro Synergistic Interactions of the Effects of Various Statins and Azoles against Some Clinically Important Fungi. FEMS Microbiol. Lett. 2010, 307, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremariam, T.; Alkhazraji, S.; Soliman, S.S.M.; Gu, Y.; Jeon, H.H.; Zhang, L.; French, S.W.; Stevens, D.A.; Edwards, J.E.; Filler, S.G.; et al. Anti-CotH3 Antibodies Protect Mice from Mucormycosis by Prevention of Invasion and Augmenting Opsonophagocytosis. Sci. Adv. 2019, 5, eaaw1327. [Google Scholar] [CrossRef] [Green Version]
- Goel, P.; Jain, V.; Sengar, M.; Mohta, A.; Das, P.; Bansal, P. Gastrointestinal Mucormycosis: A Success Story and Appraisal of Concepts. J. Infect. Public Health 2013, 6, 58–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Ami, R.; Lewis, R.E.; Tarrand, J.; Leventakos, K.; Kontoyiannis, D.P. Antifungal Activity of Colistin against Mucorales Species in vitro and in a Murine Model of Rhizopus oryzae Pulmonary Infection. Antimicrob. Agents Chemother. 2010, 54, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; Taneja, J.; Khullar, S.; Pandey, A.K. Antifungal Activity of Silver Nanoparticles on Fungal Isolates from Patients of Suspected Mucormycosis. Int. Microbiol. 2022, 26, 143–147. [Google Scholar] [CrossRef]
- Elfiky, A.A. Dual Targeting of RdRps of SARS-CoV-2 and the Mucormycosis-Causing Fungus: An in silico Perspective. Future Microbiol. 2022, 17, 755–762. [Google Scholar] [CrossRef]

| Family | Genus | Clinically Relevant Species |
|---|---|---|
| Mucoraceae | Actinomucor | A. elegans |
| Saksenaeaceae | Apophysomyces | A. mexicanus A. ossiformis A. trapeziformis A. variabilis |
| Mucoraceae | Cokeromyces | C. recurvatus |
| Cunninghamellaceae | Cunninghamella | C. arunalokei [20] C. bertholletiae C. blakesleeana C. echinulata C. elegans |
| Lichtheimiaceae | Lichtheimia | L. corymbifera L. ornata L. ramosa |
| Mucoraceae | Mucor | M. amphibiorum M. circinelloides * M. griseocyanus * M. indicus M. irregularis M. janssenii * M. lusitanicus * M. plumbeus M. racemosus M. ramosissimus * M. variicolumellatus * M. velutinosus * |
| Lichtheimiaceae | Rhizomucor | R. miehei R. pusillus |
| Rhizopodaceae | Rhizopus | R. arrhizus (including var. arrhizus and var. delemar) R. homothallicus R. microsporus R. schipperae |
| Saksenaeaceae | Saksenaea | S. erythrospora S. loutrophoriformis S. trapezispora S. vasiformis |
| Syncephalastraceae | Syncephalastrum | S. racemosum |
| Lichtheimiaceae | Thamnostylum | T. lucknowense |
| Host Factor | Associated Clinical Syndrome | References |
|---|---|---|
| Diabetes mellitus, particularly with ketoacidosis | ROCM | [67] |
| Corticosteroid use | ROCM | [68] |
| Haematologic malignancies | Pulmonary or disseminated infection | [68] |
| COVID-19 | ROCM | [6] |
| Haematopoietic cell transplantation | Pulmonary | [69] |
| Solid organ transplantation | Disseminated infection | [70] |
| HIV/AIDS | Disseminated infection | [71] |
| Treatment with deferoxamine | ROCM | [72] |
| Iron overload | ROCM | [73] |
| Injection drug use | Isolated cerebral | [74] |
| Major trauma | Cutaneous | [75] |
| Burns | Cutaneous | [34] |
| Species | Antifungal | MIC (mg/L) | Calculated ECV | ||
|---|---|---|---|---|---|
| Range | Mode | ≥95% | ≥97.5% | ||
| L. corymbifera | AMB | 0.06–16 | 0.5 | 1 | 2 |
| POS | 0.06–4 | 0.5 | 1 | 2 | |
| ITR | 0.06–8 | 0.25 | Not determined | ||
| M. circinelloides | AMB | 0.03–4 | 0.25 | 1 | 2 |
| POS | 0.06–16 | 1 | 4 | 4 | |
| ITR | 0.25–16 | 4 | Not determined | ||
| R. arrhizus | AMB | 0.03–4 | 1 | 2 | 4 |
| POS | 0.03–32 | 0.5 | 1 | 2 | |
| ITR | 0.06–16 | 0.5 | 2 | 2 | |
| R. microsporus | AMB | 0.06–4 | 0.5 | 2 | 2 |
| POS | 0.06–16 | 0.5 | 1 | 2 | |
| ITR | 0.25–32 | 1 | Not determined | ||
| Agent | Mechanism of Action | Efficacy | References |
|---|---|---|---|
| Opelconazole (inhaled) | Inhibition of formation of ergosterol in fungal cell membrane | In vitro activity against R. arrhizus (syn. oryzae) (ATCC 11145) (MIC 2 mg/L); poor activity against R. pusillus, M. circinelloides, and L. corymbifera | [243] |
| AR-12 | Celecoxib derivative which inhibits fungal acetyl coenzyme A (acetyl-CoA) synthetase | In vitro activity against R. arrhizus (MIC 4 mg/L) | [244] |
| MGCD290 | Inhibits fungal histone deacetylase 2 | In vitro synergy with triazoles | [245] |
| Statins (fluvastatin, rosuvastatin or atorvastatin) | Affect the synthesis of ergosterol by inhibiting 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase | In vitro synergy with either amphotericin B or various azoles in combination | [246,247] |
| Anti-CotH3 antibodies | Monoclonal or polyclonal antibodies that inhibit CotH3, a fungal cell protein which binds to glucose regulated protein 78 on endothelial cells | In vivo activity in murine models with improved outcomes. | [248] |
| Colistin | Polymyxin antibiotic | In vitro and in vivo activity in murine models Single case report of GI mucormycosis demonstrated treatment success when used in combination with standard of care | [249,250] |
| Silver nanoparticles | Small particles with known antimicrobial activity | Potent in vitro activity against some R. arrhizus isolates (MIC < 8–64 mg/L) | [251] |
| Sofosbuvir | Antiviral targeting RNA-dependent RNA polymerase | In silico experiments demonstrating inhibition of R. arrhizus | [252] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.; Howard-Jones, A.R.; Sparks, R.; Stefani, M.; Sivalingam, V.; Halliday, C.L.; Beardsley, J.; Chen, S.C.-A. Epidemiology, Modern Diagnostics, and the Management of Mucorales Infections. J. Fungi 2023, 9, 659. https://doi.org/10.3390/jof9060659
Pham D, Howard-Jones AR, Sparks R, Stefani M, Sivalingam V, Halliday CL, Beardsley J, Chen SC-A. Epidemiology, Modern Diagnostics, and the Management of Mucorales Infections. Journal of Fungi. 2023; 9(6):659. https://doi.org/10.3390/jof9060659
Chicago/Turabian StylePham, David, Annaleise R. Howard-Jones, Rebecca Sparks, Maurizio Stefani, Varsha Sivalingam, Catriona L. Halliday, Justin Beardsley, and Sharon C.-A. Chen. 2023. "Epidemiology, Modern Diagnostics, and the Management of Mucorales Infections" Journal of Fungi 9, no. 6: 659. https://doi.org/10.3390/jof9060659
APA StylePham, D., Howard-Jones, A. R., Sparks, R., Stefani, M., Sivalingam, V., Halliday, C. L., Beardsley, J., & Chen, S. C.-A. (2023). Epidemiology, Modern Diagnostics, and the Management of Mucorales Infections. Journal of Fungi, 9(6), 659. https://doi.org/10.3390/jof9060659







