The COMPASS Complex Regulates Fungal Development and Virulence through Histone Crosstalk in the Fungal Pathogen Cryptococcus neoformans
Abstract
:1. Importance
2. Introduction
3. Materials and Methods
3.1. Strains, Growth Conditions, and Microscopic Examination
3.2. Gene Manipulation
3.3. Protein Domain Prediction and Phylogenetic Analysis
3.4. Protein Extraction, Western Blotting, and Co-Immunoprecipitation Assay
3.5. 3D Modeling
3.6. RNA Extraction, RNA-Seq, and Quantitative Real-Time PCR Analyses
3.7. Virulence Trait Assays
3.8. Galleria mellonella Larvae Model System
3.9. Murine Model of Cryptococcosis
3.10. Data Availability
4. Results
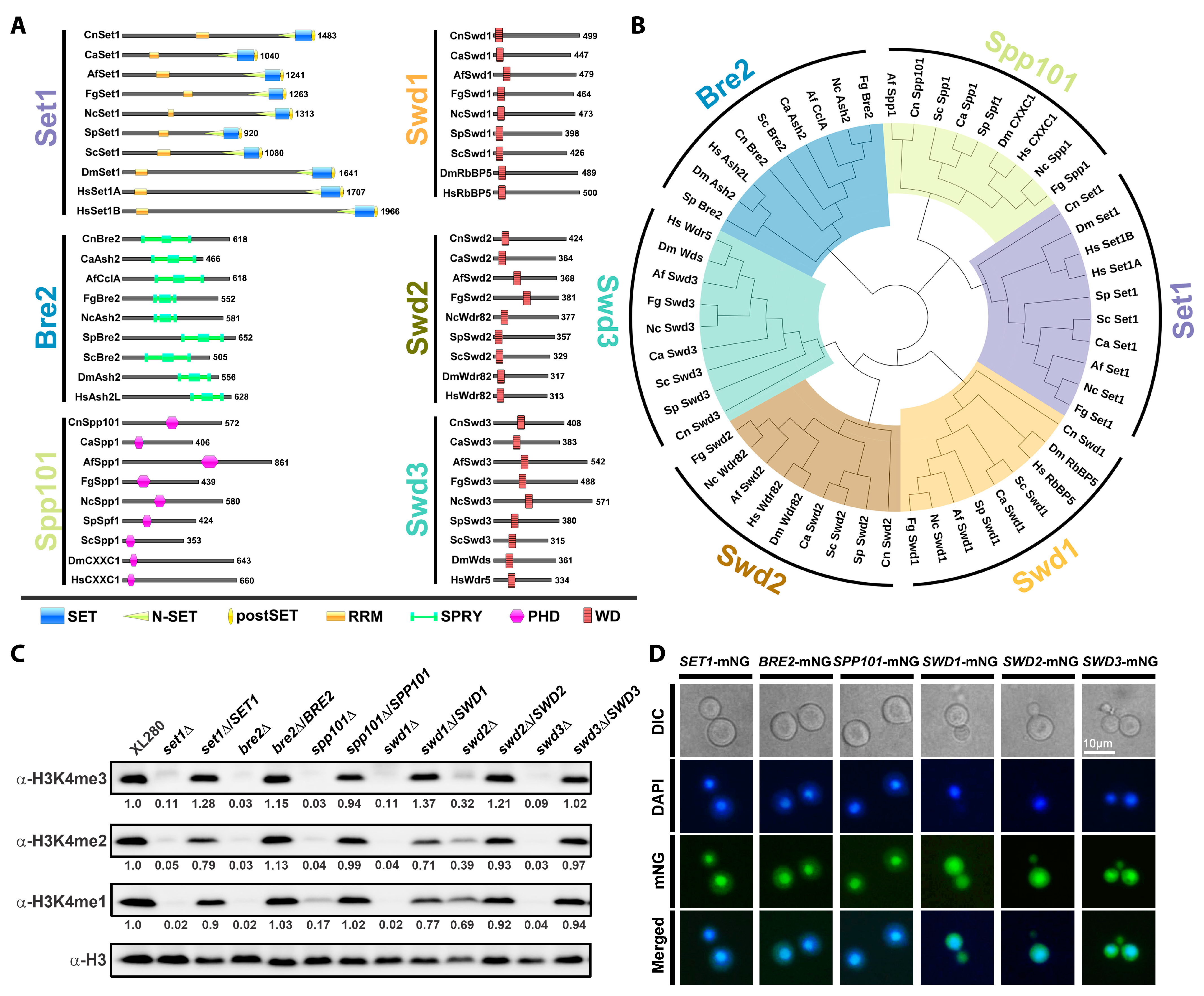
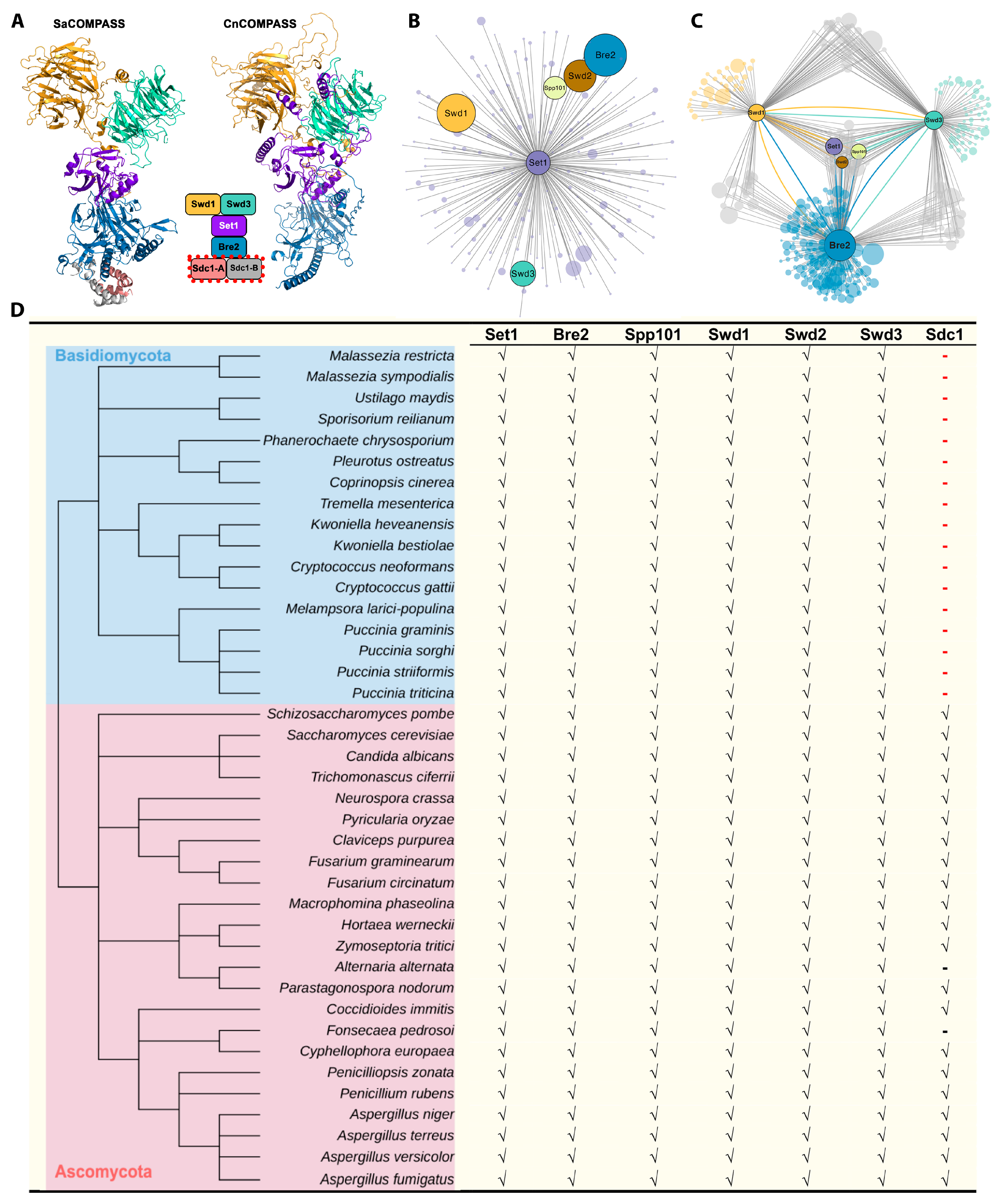
4.1. Identification of COMPASS Subunits in C. neoformans
4.2. Interaction between COMPASS Subunits in C. neoformans
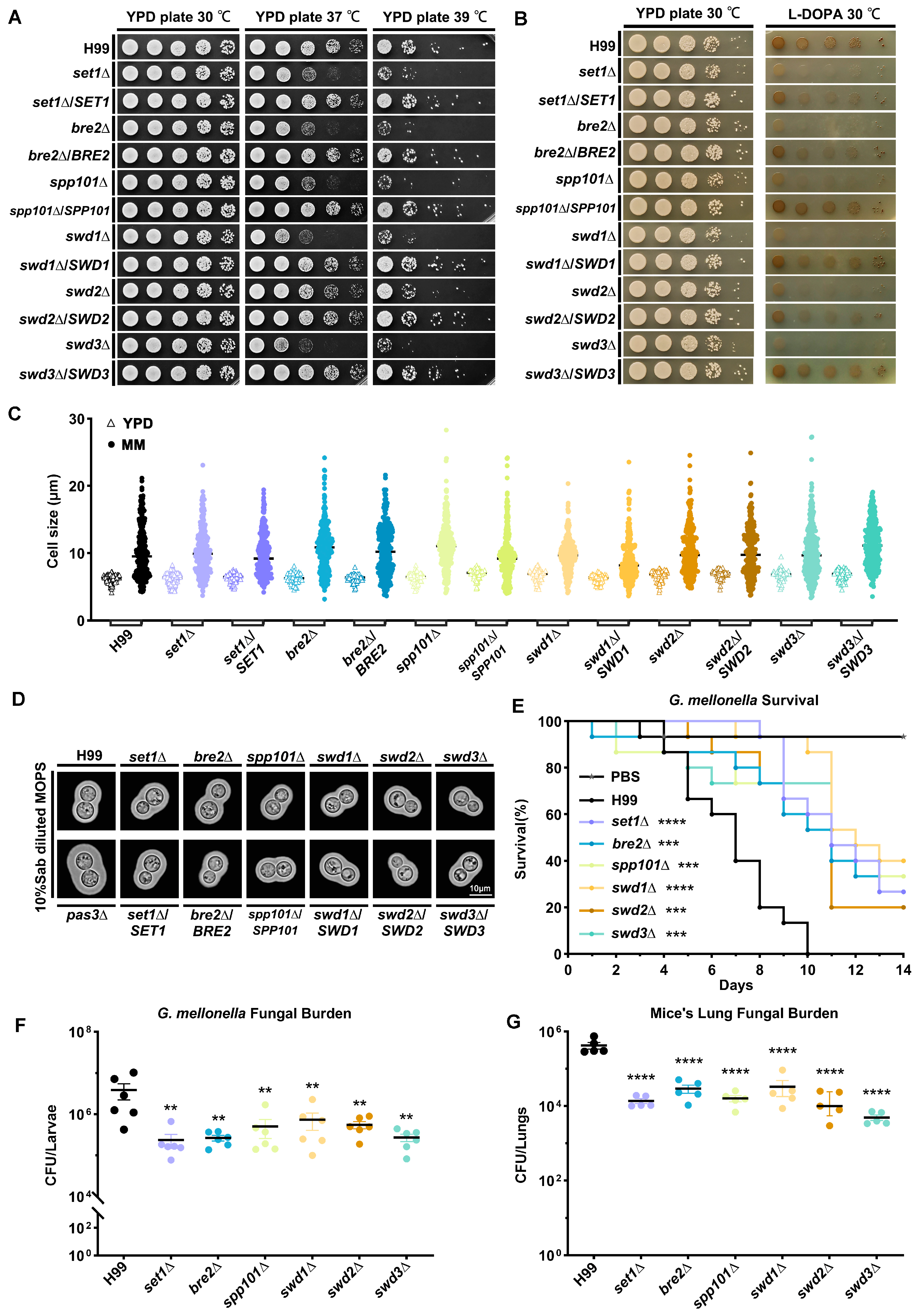
4.3. COMPASS Subunits Regulate Virulence Traits in C. neoformans
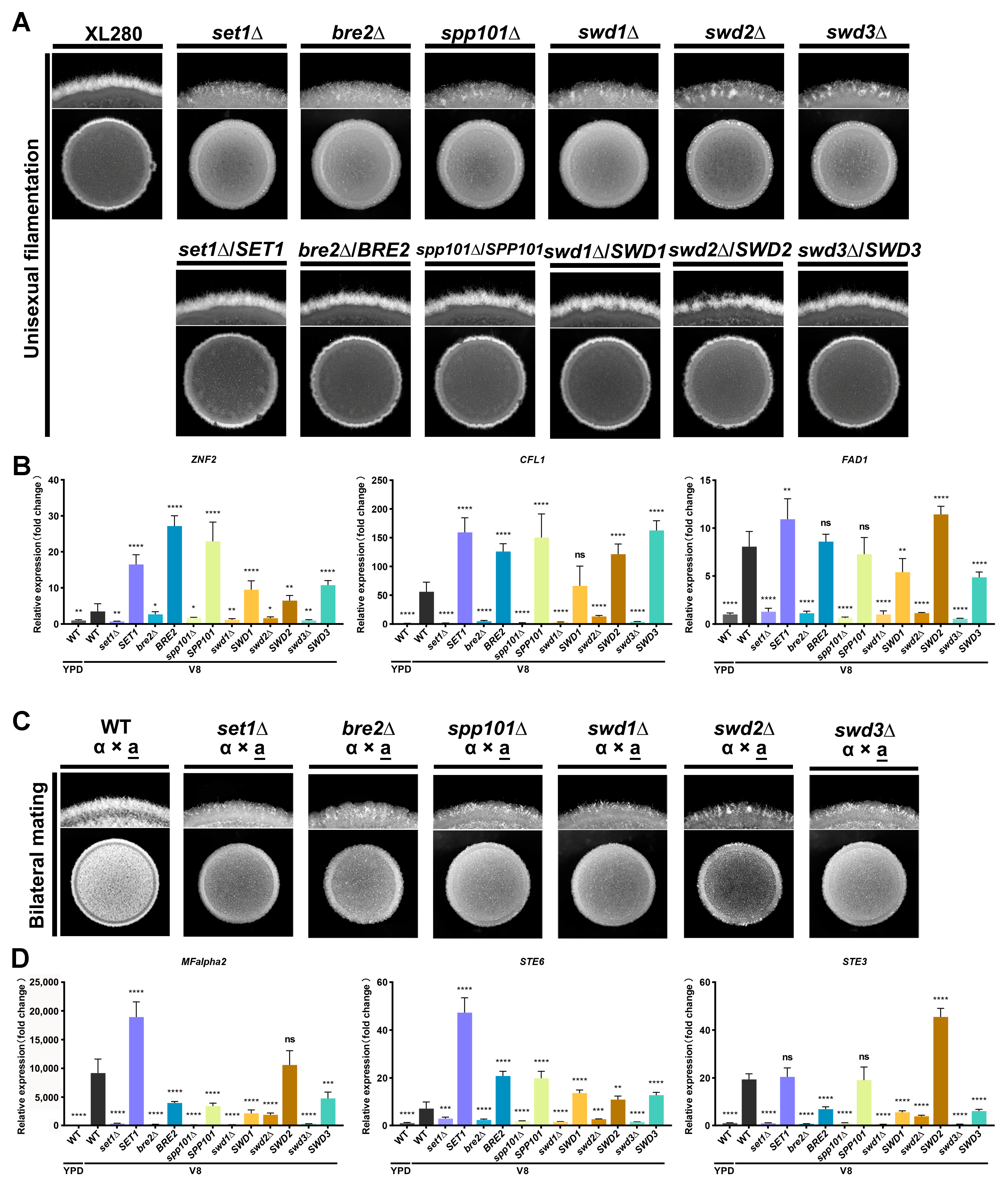
4.4. COMPASS Subunits Regulate Yeast-to-Hyphal Transition in C. deneoformans
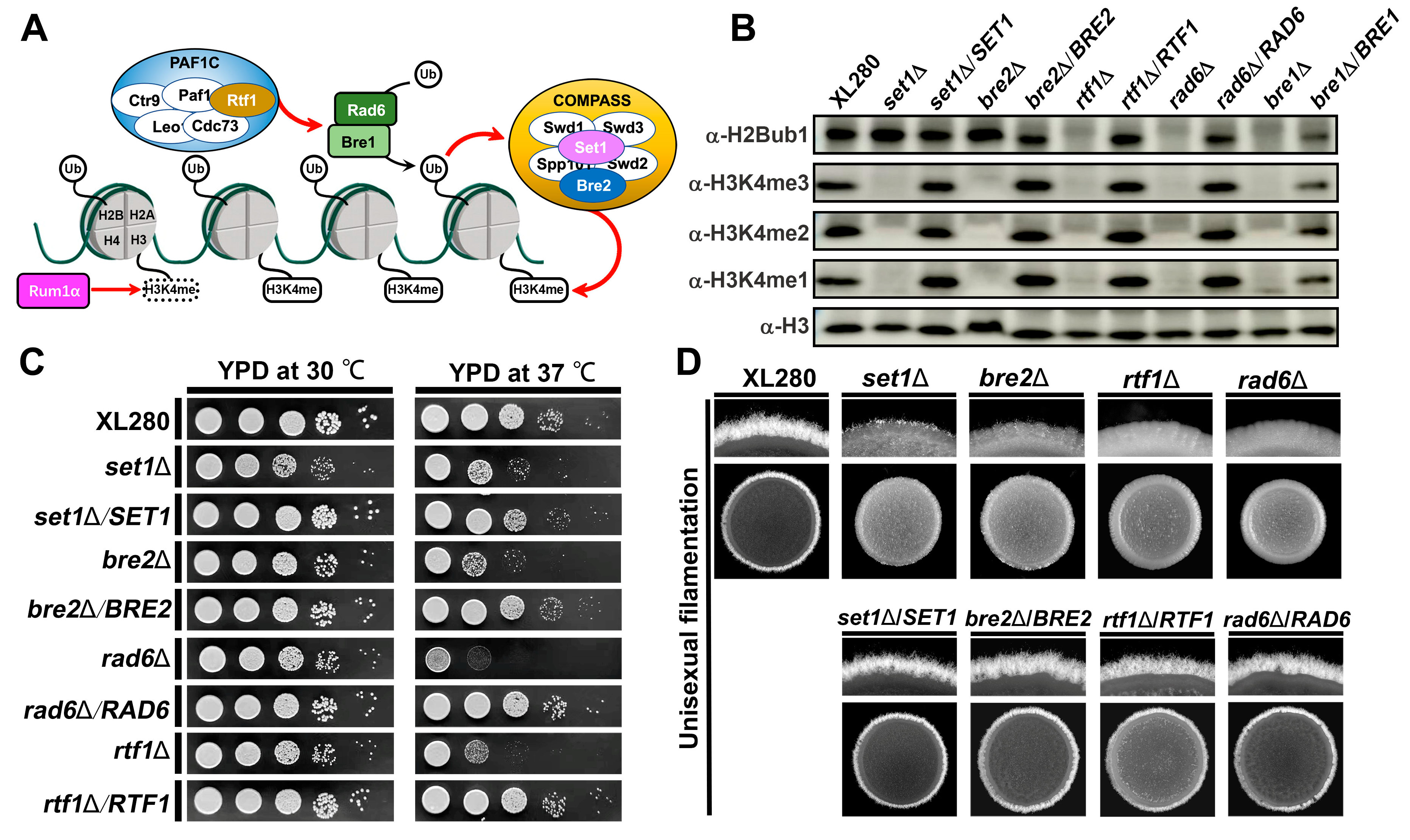
4.5. COMPASS-Mediated H3K4 Methylation Requires H2Bub1 in C. deneoformans
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, J.; Wood, A.; Lee, J.-S.; Schuster, R.; Dueker, J.; Maguire, C.; Swanson, S.K.; Florens, L.; Washburn, M.; Shilatifard, A. Molecular Regulation of Histone H3 Trimethylation by COMPASS and the Regulation of Gene Expression. Mol. Cell 2005, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Roguev, A.; Schaft, D.; Shevchenko, A.; Pijnappel, W.; Wilm, M.; Aasland, R.; Stewart, A. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 2001, 20, 7137–7148. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.-H.; Westfield, G.H.; Oleskie, A.N.; Trievel, R.C.; Shilatifard, A.; Skiniotis, G. Structural analysis of the core COMPASS family of histone H3K4 methylases from yeast to human. Proc. Natl. Acad. Sci. USA 2011, 108, 20526–20531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Liu, N.; Xie, D.; Liu, Y.; Xiao, Y.; Li, F. Structural basis for histone H3K4me3 recognition by the N-terminal domain of the PHD finger protein Spp1. Biochem. J. 2019, 476, 1957–1973. [Google Scholar] [CrossRef]
- Qu, Q.; Takahashi, Y.H.; Yang, Y.; Hu, H.; Zhang, Y.; Brunzelle, J.S.; Couture, J.F.; Shilatifard, A.; Skiniotis, G. Structure and Conformational Dynamics of a COMPASS Histone H3K4 Methyltransferase Complex. Cell 2018, 174, 1117–1126.e12. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.L.; Li, H.; Lau, H.-T.; Leonen, C.; Dhall, A.; Ong, S.-E.; Chatterjee, C.; Zheng, N. Crystal Structure of the COMPASS H3K4 Methyltransferase Catalytic Module. Cell 2018, 174, 1106–1116.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilatifard, A. The COMPASS Family of Histone H3K4 Methylases: Mechanisms of Regulation in Development and Disease Pathogenesis. Annu. Rev. Biochem. 2012, 81, 65–95. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.L.; He, X.Y.; Moore, C. The essential WD repeat protein Swd2 has dual functions in RNA polymerase II transcription termination and lysine 4 methylation of histone H3. Mol. Cell. Biol. 2004, 24, 2932–2943. [Google Scholar] [CrossRef] [Green Version]
- Soares, L.M.; Buratowski, S. Yeast Swd2 Is Essential Because of Antagonism between Set1 Histone Methyltransferase Complex and APT (Associated with Pta1) Termination Factor. J. Biol. Chem. 2012, 287, 15219–15231. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.L.; Westfield, G.H.; Takahashi, Y.H.; Cook, M.; Gao, X.; Woodfin, A.R.; Lee, J.S.; Morgan, M.A.; Jackson, J.; Smith, E.R.; et al. Context dependency of Set1/COMPASS-mediated histone H3 Lys4 trimethylation. Genes Dev. 2014, 28, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Vlaming, H.; van Welsem, T.; de Graaf, E.L.; Ontoso, D.; Altelaar, A.F.M.; San-Segundo, P.A.; Heck, A.J.R.; van Leeuwen, F. Flexibility in crosstalk between H2B ubiquitination and H3 methylation in vivo. EMBO Rep. 2014, 15, 1077–1084. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; McGinty, R.K.; Muir, T.W.; Kim, J.A.; Kim, J. Crosstalk among Set1 complex subunits involved in H2B ubiquitylation-dependent H3K4 methylation. Nucleic Acids Res. 2018, 46, 11129–11143. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.E.; Canze, M.; Bryk, M. The Requirements for COMPASS and Paf1 in Transcriptional Silencing and Methylation of Histone H3 in Saccharomyces cerevisiae. Genetics 2006, 173, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Halbach, A.; Zhang, H.; Wengi, A.; Jablonska, Z.; Gruber, I.M.; Halbeisen, R.E.; Dehe, P.M.; Kemmeren, P.; Holstege, F.; Geli, V.; et al. Cotranslational assembly of the yeast SET1C histone methyltransferase complex. EMBO J. 2009, 28, 2959–2970. [Google Scholar] [CrossRef] [Green Version]
- Cenik, B.K.; Shilatifard, A. COMPASS and SWI/SNF complexes in development and disease. Nat. Rev. Genet. 2021, 22, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, X.; Sun, W.; Zhang, M.; Yin, Y.; Pan, S.; He, D.; Shen, M.; Yang, J.; Zheng, Q.; et al. The COMPASS-like complex modulates fungal development and pathogenesis by regulating H3K4me3-mediated targeted gene expression in Magnaporthe oryzae. Mol. Plant Pathol. 2021, 22, 422–439. [Google Scholar] [CrossRef]
- Meng, S.; Huang, S.; Liu, J.; Gai, Y.; Li, M.; Duan, S.; Zhang, S.; Sun, X.; Yang, Q.; Wang, Y.; et al. Histone Methylation Is Required for Virulence, Conidiation, and Multi-Stress Resistance of Alternaria alternata. Front. Microbiol. 2022, 13, 924476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, N.; Yin, Y.; Chen, Y.; Jiang, J.; Ma, Z. Histone H3K4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in Fusarium graminearum. Environ. Microbiol. 2015, 17, 4615–4630. [Google Scholar] [CrossRef]
- Palmer, J.M.; Bok, J.W.; Lee, S.; Dagenais, T.R.T.; Andes, D.R.; Kontoyiannis, D.P.; Keller, N.P. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 2013, 1, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachleitner, S.; Sørensen, J.L.; Gacek-Matthews, A.; Sulyok, M.; Studt, L.; Strauss, J. Evidence of a Demethylase-Independent Role for the H3K4-Specific Histone Demethylases in Aspergillus nidulans and Fusarium graminearum Secondary Metabolism. Front. Microbiol. 2019, 10, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, Y.; Kawatani, M.; Futamura, Y.; Osada, H.; Koyama, Y. An overproduction of astellolides induced by genetic disruption of chromatin-remodeling factors in Aspergillus oryzae. J. Antibiot. 2015, 69, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Chiang, Y.M.; Szewczyk, E.; Reyes-Dominguez, Y.; Davidson, A.D.; Sanchez, J.F.; Lo, H.C.; Watanabe, K.; Strauss, J.; Oakley, B.R.; et al. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 2009, 5, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Dallery, J.; Adelin, É.; Le Goff, G.; Pigné, S.; Auger, A.; Ouazzani, J.; O’Connell, R.J. H3K4 trimethylation by CclA regulates pathogenicity and the production of three families of terpenoid secondary metabolites in Colletotrichum higginsianum. Mol. Plant Pathol. 2019, 20, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, S.B.; Nguyen, M.H.; Zhang, Z.; Cheng, S.; Jia, H.Y.; Weisner, N.; Iczkowski, K.; Clancy, C.J. Candida albicans SET1 encodes a histone 3 lysine 4 methyltransferase that contributes to the pathogenesis of invasive candidiasis. Mol. Microbiol. 2006, 60, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Kwon, S.; Lee, E.-J.; Lee, J.-S. Set1-mediated H3K4 methylation is required for Candida albicans virulence by regulating intracellular level of reactive oxygen species. Virulence 2021, 12, 2648–2658. [Google Scholar] [CrossRef]
- Baker, K.M.; Hoda, S.; Saha, D.; Gregor, J.B.; Georgescu, L.; Serratore, N.D.; Zhang, Y.; Cheng, L.; Lanman, N.A.; Briggs, S.D. The Set1 Histone H3K4 Methyltransferase Contributes to Azole Susceptibility in a Species-Specific Manner by Differentially Altering the Expression of Drug Efflux Pumps and the Ergosterol Gene Pathway. Antimicrob. Agents Chemother. 2022, 66, e0225021. [Google Scholar] [CrossRef]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Johnston, S.A.; Voelz, K.; May, R.C. Cryptococcus neoformans Thermotolerance to Avian Body Temperature Is Sufficient for Extracellular Growth But Not Intracellular Survival In Macrophages. Sci. Rep. 2016, 6, 20977. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.L.M.; Jin, R.M.; Leipheimer, J.; Bard, J.E.; Yergeau, D.; Wohlfert, E.A.; Panepinto, J.C. Thermotolerance in the pathogen Cryptococcus neoformans is linked to antigen masking via mRNA decay-dependent reprogramming. Nat. Commun. 2019, 10, 4950. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A.; Rosas, A.L.; Nosanchuk, J.D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2000, 3, 354–358. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans Capsule: A Sword and a Shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Huang, Y.; Zhou, Y.; Zang, X.; Deng, H.; Liu, Y.; Shen, D.; Xue, X. Cryptococcus escapes host immunity: What do we know? Front. Cell Infect. Microbiol. 2022, 12, 1041036. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhai, B.; Lin, X. The Link between Morphotype Transition and Virulence in Cryptococcus neoformans. PLoS Pathog. 2012, 8, e1002765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Lin, X. Multiple Applications of a Transient CRISPR-Cas9 Coupled with Electroporation (TRACE) System in the Cryptococcus neoformans Species Complex. Genetics 2018, 208, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Fan, Y.; Lin, X. Transformation of Cryptococcus neoformans by electroporation using a transient CRISPR-Cas9 expression (TRACE) system. Fungal Genet. Biol. 2020, 138, 103364. [Google Scholar] [CrossRef]
- Xia, Y.; Li, K.; Li, J.; Wang, T.; Gu, L.; Xun, L. T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis. Nucleic Acids Res. 2019, 47, e15. [Google Scholar] [CrossRef] [Green Version]
- So, Y.-S.; Yang, D.-H.; Jung, K.-W.; Huh, W.-K.; Bahn, Y.-S. Molecular Characterization of Adenylyl Cyclase Complex Proteins Using Versatile Protein-Tagging Plasmid Systems in Cryptococcus neoformans. J. Microbiol. Biotechnol. 2017, 27, 357–364. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, X. An intergenic “safe haven” region in Cryptococcus neoformans serotype D genomes. Fungal Genet. Biol. 2020, 144, 103464. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Maybruck, B.T.; Donlin, M.J.; Chang, A.L.; Kayode, S.; Ormerod, K.L.; Fraser, J.A.; Doering, T.L.; Lodge, J.K. A fluorogenic C. neoformans reporter strain with a robust expression of m-cherry expressed from a safe haven site in the genome. Fungal Genet. Biol. 2017, 108, 13–25. [Google Scholar] [CrossRef]
- Basenko, E.Y.; Pulman, J.A.; Shanmugasundram, A.; Harb, O.S.; Crouch, K.; Starns, D.; Warrenfeltz, S.; Aurrecoechea, C.; Stoeckert, J.C.J.; Kissinger, J.C.; et al. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J. Fungi 2018, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Federhen, S. The NCBI Taxonomy database. Nucleic Acids Res. 2012, 40, D136–D143. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, X. A PAS Protein Directs Metabolic Reprogramming during Cryptococcal Adaptation to Hypoxia. mBio 2021, 12, e03602-20. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for Visualization and Analysis of Biological Networks. Comput.-Aided Tissue Eng. 2011, 696, 291–303. [Google Scholar]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Zídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, K.; Zhang, G. Improved model quality assessment using sequence and structural information by enhanced deep neural networks. Briefings Bioinform. 2023, 24, bbac507. [Google Scholar] [CrossRef]
- Guo, S.-S.; Liu, J.; Zhou, X.-G.; Zhang, G.-J. DeepUMQA: Ultrafast shape recognition-based protein model quality assessment using deep learning. Bioinformatics 2022, 38, 1895–1903. [Google Scholar] [CrossRef]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevijano-Contador, N.; de Oliveira, H.C.; García-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, Á.; Janbon, G.; Ariño, J.; Zaragoza, Ó. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef] [Green Version]
- Dambuza, I.M.; Drake, T.; Chapuis, A.; Zhou, X.; Correia, J.; Taylor-Smith, L.; LeGrave, N.; Rasmussen, T.; Fisher, M.C.; Bicanic, T.; et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog. 2018, 14, e1006978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclère, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stempinski, P.R.; Smith, D.F.Q.; Casadevall, A. Cryptococcus neoformans Virulence Assay Using a Galleria mellonella Larvae Model System. Bio-Protocol 2022, 12, e4480. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Pham, T.; Hipsher, K.; Glueck, N.; Fan, Y.; Lin, X. Immunoprotection against Cryptococcosis Offered by Znf2 Depends on Capsule and the Hyphal Morphology. mBio 2022, 13, e0278521. [Google Scholar] [CrossRef]
- Zhai, B.; Zhu, P.; Foyle, D.; Upadhyay, S.; Idnurm, A.; Lin, X. Congenic Strains of the Filamentous Form of Cryptococcus neoformans for Studies of Fungal Morphogenesis and Virulence. Infect. Immun. 2013, 81, 2626–2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The Antidepressant Sertraline Provides a Promising Therapeutic Option for Neurotropic Cryptococcal Infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Y.; Upadhyay, S.; Xue, C.; Lin, X. Activation of Meiotic Genes Mediates Ploidy Reduction during Cryptococcal Infection. Curr. Biol. 2020, 30, 1387–1396.e5. [Google Scholar] [CrossRef]
- Lin, X.; Huang, J.C.; Mitchell, T.G.; Heitman, J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS Genet. 2006, 2, e187. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Wang, L.; Zheng, W.; Wang, S. Regulatory Roles of Histone Modifications in Filamentous Fungal Pathogens. J. Fungi 2022, 8, 565. [Google Scholar] [CrossRef]
- Van Oss, S.B.; Shirra, M.K.; Bataille, A.R.; Wier, A.D.; Yen, K.Y.; Vinayachandran, V.; Byeon, I.J.L.; Cucinotta, C.E.; Heroux, A.; Jeon, J.; et al. The Histone Modification Domain of Paf1 Complex Subunit Rtf1 Directly Stimulates H2B Ubiquitylation through an Interaction with Rad6. Mol. Cell 2016, 64, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Upadhyay, S.; Lin, X. PAS Domain Protein Pas3 Interacts with the Chromatin Modifier Bre1 in Regulating Cryptococcal Morphogenesis. mBio 2018, 9, e02135-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life Cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar]
- Arturo Casadevall, J.P. Cryptococcus Neoformans; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan Cell Production Enhances the Virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaragoza, O.; Nielsen, K. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr. Opin. Microbiol. 2013, 16, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ballou, E.R. The Cryptococcus neoformans Titan Cell: From In Vivo Phenomenon to In Vitro Model. Curr. Clin. Microbiol. Rep. 2018, 5, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tian, X.; Gyawali, R.; Lin, X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc. Natl. Acad. Sci. USA 2013, 110, 11571–11576. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Y.; Li, J.; Liu, Y.; Cui, B. H3K4me3 and Wdr82 are associated with tumor progression and a favorable prognosis in human colorectal cancer. Oncol. Lett. 2018, 16, 2125–2134. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, S.-B.; Ma, G.; Tang, X.-F.; Chen, S.-P.; He, J.; Liu, W.-L.; Xie, D.; Zeng, Y.-X.; Li, J. The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma. J. Transl. Med. 2013, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.; Krogan, N.J.; Dover, J.; Erdjument-Bromage, H.; Tempst, P.; Johnston, M.; Greenblatt, J.F.; Shilatifard, A. COMPASS: A complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 2001, 98, 12902–12907. [Google Scholar] [CrossRef] [Green Version]
- South, P.F.; Fingerman, I.M.; Mersman, D.P.; Du, H.-N.; Briggs, S.D. A Conserved Interaction between the SDI Domain of Bre2 and the Dpy-30 Domain of Sdc1 Is Required for Histone Methylation and Gene Expression. J. Biol. Chem. 2010, 285, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, V.; Zhang, P.; Chaturvedi, C.-P.; Thornton, J.; Brunzelle, J.S.; Skiniotis, G.; Shilatifard, A.; Brand, M.; Couture, J.-F. Molecular Basis for DPY-30 Association to COMPASS-like and NURF Complexes. Structure 2014, 22, 1821–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, R.C.; Moore, T.D.; Odom, A.R.; Heitman, J. Characterization of the MFalpha pheromone of the human fungal pathogen cryptococcus neoformans. Mol. Microbiol. 2000, 38, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Vallim, M.A.; Fernandes, L.; Alspaugh, J.A. The RAM1 gene encoding a protein-farnesyltransferase beta-subunit homologue is essential in Cryptococcus neoformans. Microbiology 2014, 150, 1925–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Chen, X.; Zhao, F.; Jiang, Y.; Lu, Z.; Ji, H.; Feng, Y.; Li, J.; Zhang, H.; Zheng, J.; et al. The COMPASS Complex Regulates Fungal Development and Virulence through Histone Crosstalk in the Fungal Pathogen Cryptococcus neoformans. J. Fungi 2023, 9, 672. https://doi.org/10.3390/jof9060672
Liu R, Chen X, Zhao F, Jiang Y, Lu Z, Ji H, Feng Y, Li J, Zhang H, Zheng J, et al. The COMPASS Complex Regulates Fungal Development and Virulence through Histone Crosstalk in the Fungal Pathogen Cryptococcus neoformans. Journal of Fungi. 2023; 9(6):672. https://doi.org/10.3390/jof9060672
Chicago/Turabian StyleLiu, Ruoyan, Xiaoyu Chen, Fujie Zhao, Yixuan Jiang, Zhenguo Lu, Huining Ji, Yuanyuan Feng, Junqiang Li, Heng Zhang, Jianting Zheng, and et al. 2023. "The COMPASS Complex Regulates Fungal Development and Virulence through Histone Crosstalk in the Fungal Pathogen Cryptococcus neoformans" Journal of Fungi 9, no. 6: 672. https://doi.org/10.3390/jof9060672




