Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Inoculation Assay, and Drought Treatment
2.2. Microscopic Observation of Root Colonization
2.3. Measurement of Seedling Relative Water Content and Growth Variables
2.4. Tissue Structure Observation
2.5. Measurement of Osmolytes and Antioxidant Enzyme Activities
2.6. Photosynthesis Analysis
2.7. Determination of Nutrient Elements in Seedlings and Rhizosphere Soil
2.8. DNA Extraction, High-Throughput Sequencing, and Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
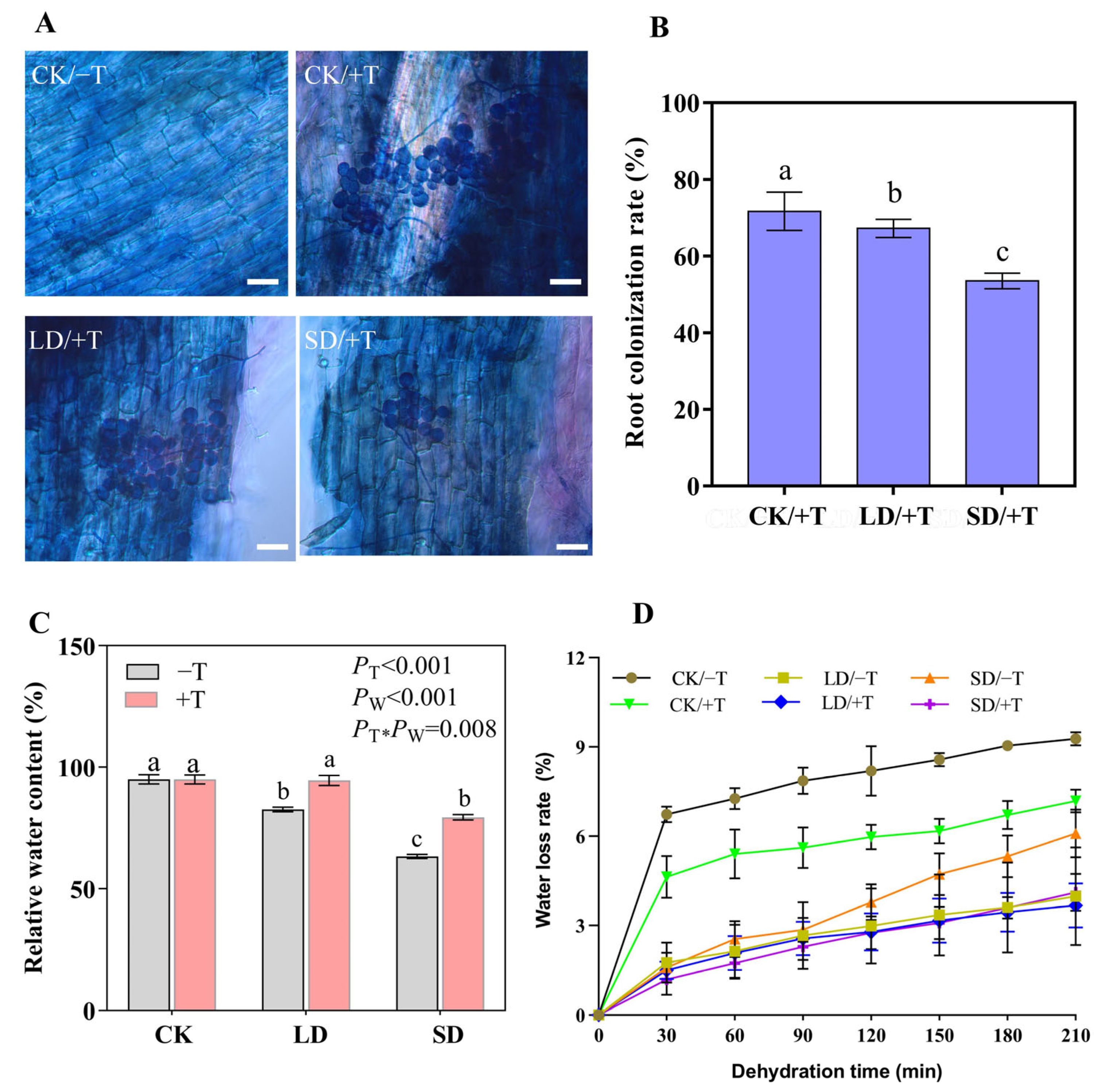
3.1. Trichoderma longibrachiatum Colonization of P. massoniana Roots Reduced Seedling Water Loss under Drought Stress
3.2. Trichoderma longibrachiatum Promoted the Growth and Nutrient Absorption of P. massoniana Seedlings in Response to Drought Stress
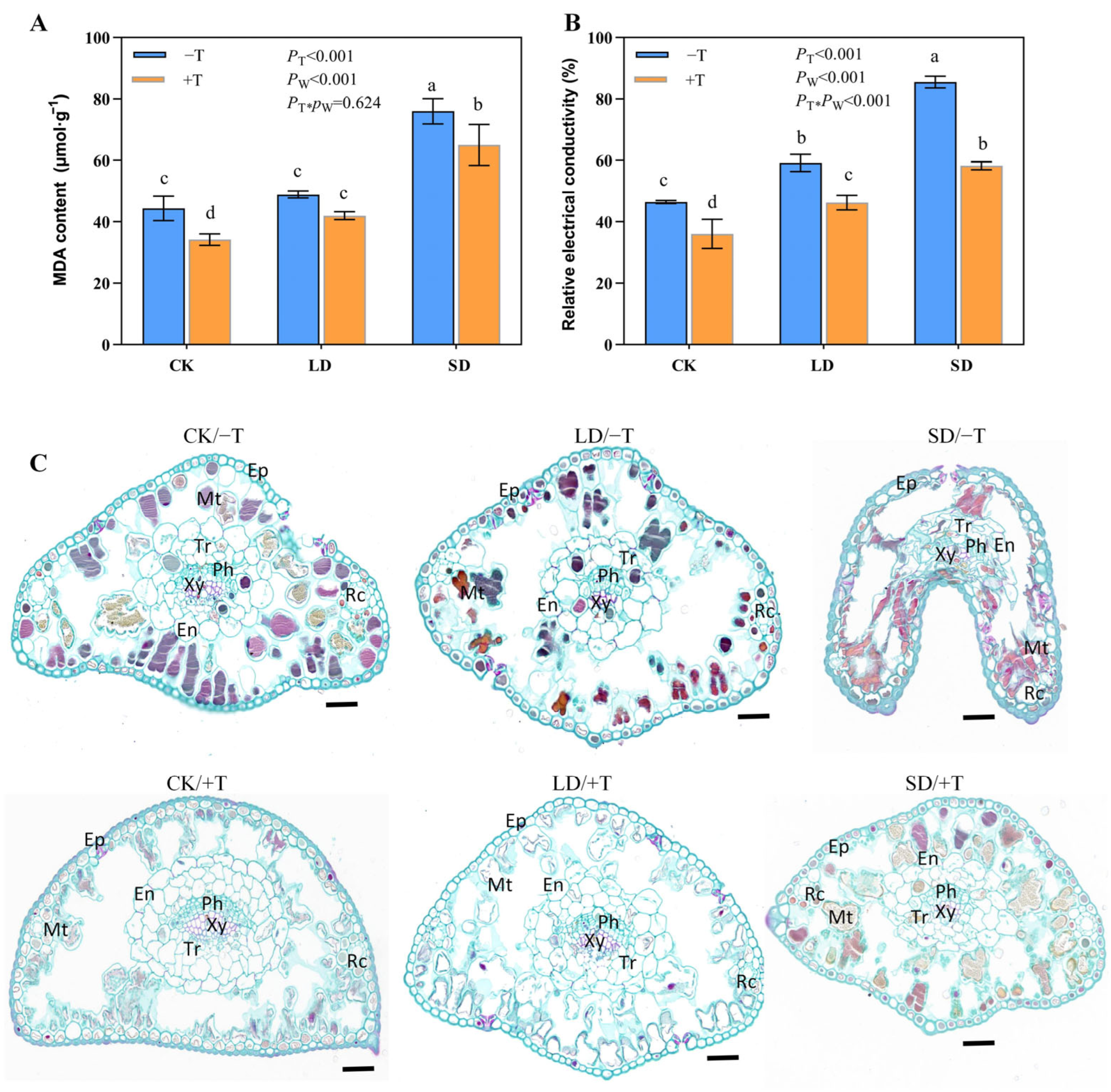
3.3. Drought-Induced Damage to the Needle and Root Tissues Were Alleviated by T. longibrachiatum Inoculation
3.4. Trichoderma longibrachiatum Treatment Changed Osmolytes and Antioxidant Metabolism in P. massoniana under Drought Stress
3.5. Drought-Induced Photosynthetic Inhibition Was Mitigated by T. longibrachiatum Inoculation
3.6. Rhizosphere Soil Nutrient Contents and Enzyme Activities Were Improved by T. longibrachiatum Inoculation
3.7. Composition of the Rhizosphere Soil Microbial Community
3.8. Co-Occurrence Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Shen, T.; Yang, Z.; Tan, J.; Xu, K.; Chen, X.; Xu, M. Identification of genes involved in oleoresin biosynthesis in Pinus massoniana through the combination of SMRT and Illumina sequencing. Ind. Crops Prod. 2022, 188, 115553. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Q.; Shi, T.; Wu, G. Developing Growth Models of Stand Volume for Subtropical Forests in Karst Areas: A case study in the Guizhou plateau. Forests 2021, 12, 83. [Google Scholar] [CrossRef]
- Song, X.; Lyu, S.; Wen, X. Limitation of soil moisture on the response of transpiration to vapor pressure deficit in a subtropical coniferous plantation subjected to seasonal drought. J. Hydrol. 2020, 591, 125301. [Google Scholar] [CrossRef]
- Du, M.; Ding, G.; Cai, Q. The Transcriptomic responses of Pinus massoniana to drought stress. Forests 2018, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Shalizi, M.N.; Goldfarb, B.; Burney, O.T.; Shear, T.H. Effects of five growing media and two fertilizer levels on polybag–raised camden whitegum (Eucalyptus benthamii Maiden & Cambage) seedling morphology and drought hardiness. Forests 2019, 10, 543. [Google Scholar] [CrossRef] [Green Version]
- Bockstette, S.W.; Mata, R.D.L.; Thomas, B.R. Best of both worlds: Hybrids of two commercially important pines (Pinus contorta × Pinus banksiana) combine increased growth potential and high drought tolerance. Can. J. Forest Res. 2021, 51, 1410–1418. [Google Scholar] [CrossRef]
- Sun, S.; Chen, H.; Yang, Z.; Lu, J.; Wu, D.; Luo, Q.; Jia, J.; Tan, J. Identification of WRKY transcription factor family genes in Pinus massoniana Lamb. and their expression patterns and functions in response to drought stress. BMC Plant Biol. 2022, 22, 424. [Google Scholar] [CrossRef]
- Lu, Z.K. Mechanisms of Drought-Tolerance Ectomycorrhizal Fungi Enhancing Drought Resistance of Pinus massoniana Seedlings. Master’s Thesis, Gui Zhou University, Guiyang, China, 2020. Volume 6. pp. 1–80. (In Chinese) [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, X.; Lu, Z.; Sun, X.; Ding, G. Role of Suillus placidus in improving the drought tolerance of masson pine (Pinus massoniana Lamb.) seedlings. Forests 2021, 12, 332. [Google Scholar] [CrossRef]
- Hohmann, P.; Jones, E.E.; Hill, R.A.; Stewart, A. Understanding Trichoderma in the root system of Pinus radiata: Associations between rhizosphere colonisation and growth promotion for commercially grown seedlings. Fungal Biol. 2011, 115, 759–767. [Google Scholar] [CrossRef]
- Regliński, T.; Rodenburg, N.; Taylor, J.T.; Northcott, G.L.; Chee, A.A.; Spiers, T.M.; Hill, R.A. Trichoderma atroviride promotes growth and enhances systemic resistance to Diplodia pinea in radiata pine (Pinus radiata) seedlings. Forest Pathol. 2012, 42, 75–78. [Google Scholar] [CrossRef]
- Babu, A.G.; Shea, P.J.; Oh, B.T. Trichoderma sp. PDR1-7 promotes Pinus sylvestris reforestation of lead-contaminated mine tailing sites. Sci Total Environ. 2014, 476, 561–567. [Google Scholar] [CrossRef]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of two trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Bio. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Estévez-Geffriaud, V.; Vicente, R.; Vergara-Díaz, O.; Jesús, J.; Reinaldo, N.; Trillas, M.I. Application of Trichoderma asperellum T34 on maize (Zea mays) seeds protects against drought stress. Planta 2020, 252, 8. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.; He, C.; Yao, J.; Wei, M.; He, X. Complementary effects of dark septate endophytes and Trichoderma strains on growth and active ingredient accumulation of astragalus mongholicus under drought stress. J. Fungi 2022, 8, 920. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Liu, H.; Wang, W.; Hou, J.; Li, X. Dual inoculation of dark septate endophytes and Trichoderma viride drives plant performance and rhizosphere microbiome adaptations of Astragalus mongholicus to drought. Environ. Microbiol. 2022, 24, 324–340. [Google Scholar] [CrossRef]
- Rui, Z.; Jiang, X.; Yu, C. Effects of polyethylene glycol 6000 stress on the growth and physiology of Trichoderma longibrachiatum. J. For. Environ. 2023, 43, 210–216. (In Chinese) [Google Scholar]
- Wei, Y.Q.; Zhao, Y.; Shi, M.Z.; Cao, Z.Y.; Lu, Q.; Yang, T.X.; Fan, Y.Y.; Wei, Z.M. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresource Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.H.; Yang, K.J. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil 2019, 436, 211–227. [Google Scholar] [CrossRef]
- He, F.; Zhang, H.Q.; Tang, M. Aquaporin gene expression and physiological responses of Robinia pseudoacacial L. to the mycorrhizal fungus rhizophagus irregularis and drought stress. Mycorrhiza 2016, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.J.; Cheng, M.C.; Lin, T.P. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radical Bio. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Fan, L.; Zheng, S.; Wang, X. Antisense suppression of phospholipase D alpha retards abscisic acid- and ethylene promoted senescence of postharvest Arabidopsis leaves. Plant Cell 1997, 9, 2183–2196. [Google Scholar] [CrossRef]
- Wood, N.J.; Baker, A.; Quinnell, R.J.; Camargo-Valero, M.A. A Simple and Non-destructive Method for Chlorophyll Quantification of Chlamydomonas Cultures Using Digital Image Analysis. Front. Bioeng. Biotechnol. 2020, 8, 746. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Et. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Till Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Mao, Y.; Jiang, W.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Effects of Trichoderma asperellum 6S-2 on Apple Tree Growth and Replanted Soil Microbial Environment. J. Fungi 2022, 8, 63. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; Loópez-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Yuan, J.; Yang, X.; Cui, Y.; Chen, L.; Ran, W.; Shen, Q. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Li, S.; Hui, L.; Li, Y.; Chen, K.; Meng, T.; Yu, C.; Leng, F.; Ma, J. Comparative analysis of carbon and nitrogen metabolism, antioxidant indexes, polysaccharides and lobetyolin changes of different tissues from Codonopsis pilosula co-inoculated with Trichoderma. J. Plant Physiol. 2021, 267, 153546. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-Biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Yadav, A.N. Bacterial mitigation of drought stress in plants: Current perspectives and future challenges. Curr. Microbiol. 2022, 79, 248. [Google Scholar] [CrossRef]
- Ali, F.; Bano, A.; Fazal, A. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017, 82, 363–375. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofifi, N.R.; Shikari, A.B.; Salgotra, R. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Gusain, Y.S.; Singh, U.; Sharma, A. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L). Afr. J. Biotechnol. 2015, 14, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. BBA-Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Cornejo-Ríos, K.; OsornoSuárez, M.D.P.; Hernández-León, S.; Reyes-Santamaría, M.I.; Juárez-Díaz, J.A.; Pérez-España, V.H.; Peláez-Acero, A.; Madariaga-Navarrete, A.; Saucedo García, M. Impact of Trichoderma asperellum on chilling and drought stress in tomato (Solanum lycopersicum). Horticulturae 2021, 7, 385. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Pan, L.; Wang, X.; Tu, K. Assessment of the optical properties of peaches with fungal infection using spatially-resolved diffuse reflectance technique and their relationships with tissue structural and biochemical properties. Food Chem. 2020, 321, 126704. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.A. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Spor, A.; Edel-Hermann, V.; Heraud, C.; Breuil, M.C.; Bizouard, F.; Toyoda, S.; Yoshida, N.; Steinberg, C.; Philippot, L. N2O production, a widespread trait in fungi. Sci. Rep. 2015, 5, 9697. [Google Scholar] [CrossRef] [Green Version]
- Kersters, K.; De Vos, P.; Gillis, M.; Swings, J.; Vandamme, P.; Stackebrandt, E. Introduction to the Proteobacteria. In The Prokaryotes, 3rd ed.; Springer: New York, NY, USA, 2006; Volume 5, pp. 3–37. [Google Scholar]
- Chodak, M.; Gołębiewski, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Soil chemical properties affect the reaction of forest soil bacteria to drought and rewetting stress. Ann. Microbiol. 2015, 65, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Bu, X.; Gu, X.; Zhou, X.; Zhang, M.; Guo, Z.; Zhang, J.; Zhou, X.; Chen, X.; Wang, X. Extreme drought slightly decreased soil labile organic C and N contents and altered microbial community structure in a subtropical evergreen forest. Forest Ecol. Manag. 2018, 429, 18–27. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbime. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Sahu, P.K.; Pandey, A.; White, J.F.; Verma, S.K. Endophytic Burkholderia: Multifunctional roles in plant growth promotion and stress tolerance. Microbiol. Res. 2022, 265, 127201. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. mBio 2015, 6, e00621-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinjo, R.; Uesaka, K.; Ihara, K.; Sakazaki, S.; Yano, K.; Kondo, M.; Tanaka, A. Draft genome sequence of Burkholderia vietnamiensis strain RS1, a nitrogen-fixing endophyte isolated from sweet potato. Microbiol. Resour. Announc. 2018, 7, e00820-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, M.; Fang, S.; MacDonald, J.; Xu, J.; Yuan, Z.C. Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol Res. 2020, 233, 126395. [Google Scholar] [CrossRef]
- Van den Heuvel, R.N.; van der Biezen, E.; Jetten, M.S.; Hefting, M.M.; Kartal, B. Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ. Microbiol. 2010, 12, 3264–3271. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wei, G. Core microbiota drive functional stability of soil microbiome in reforestation ecosystems. Glob. Chang. Biol. 2022, 28, 1038–1047. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Lee, I.J. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J. Plant Res. 2015, 128, 259–268. [Google Scholar] [CrossRef]
- Wei, D.P.; Wanasinghe, D.N.; Hyde, K.D.; Mortimer, P.E.; Xu, J.; Xiao, Y.P.; Bhunjun, C.S.; To-Anun, C. The genus Simplicillium. MycoKeys 2019, 60, 69–92. [Google Scholar] [CrossRef] [Green Version]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Reckling, M.; Wirth, S. Biochar-based Bradyrhizobium inoculum improves growth of lupin (Lupinus angustifolius L.) under drought stress. Eur. J. Soil Biol. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Chinachanta, K.; Shutsrirung, A.; Herrmann, L.; Lesueur, D. Isolation and characterization of KDML105 aromatic rice rhizobacteria producing indole-3-acetic acid: Impact of organic and conventional paddy rice practices. Lett. Appl. Microbiol. 2022, 74, 354–366. [Google Scholar] [CrossRef]
- Karmakar, J.; Goswami, S.; Pramanik, K.; Maiti, T.K.; Kar, R.K.; Dey, N. Growth promoting properties of Mycobacterium and Bacillus on rice plants under induced drought. Plant Sci. Today 2021, 8, 49–57. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Jiang, X.; Xu, H.; Ding, G. Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community. J. Fungi 2023, 9, 694. https://doi.org/10.3390/jof9070694
Yu C, Jiang X, Xu H, Ding G. Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community. Journal of Fungi. 2023; 9(7):694. https://doi.org/10.3390/jof9070694
Chicago/Turabian StyleYu, Cun, Xian Jiang, Hongyun Xu, and Guijie Ding. 2023. "Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community" Journal of Fungi 9, no. 7: 694. https://doi.org/10.3390/jof9070694
APA StyleYu, C., Jiang, X., Xu, H., & Ding, G. (2023). Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community. Journal of Fungi, 9(7), 694. https://doi.org/10.3390/jof9070694





