MAP Kinase FgHog1 and Importin β FgNmd5 Regulate Calcium Homeostasis in Fusarium graminearum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Construction of Gene Deletion Mutants and Complemented Strains
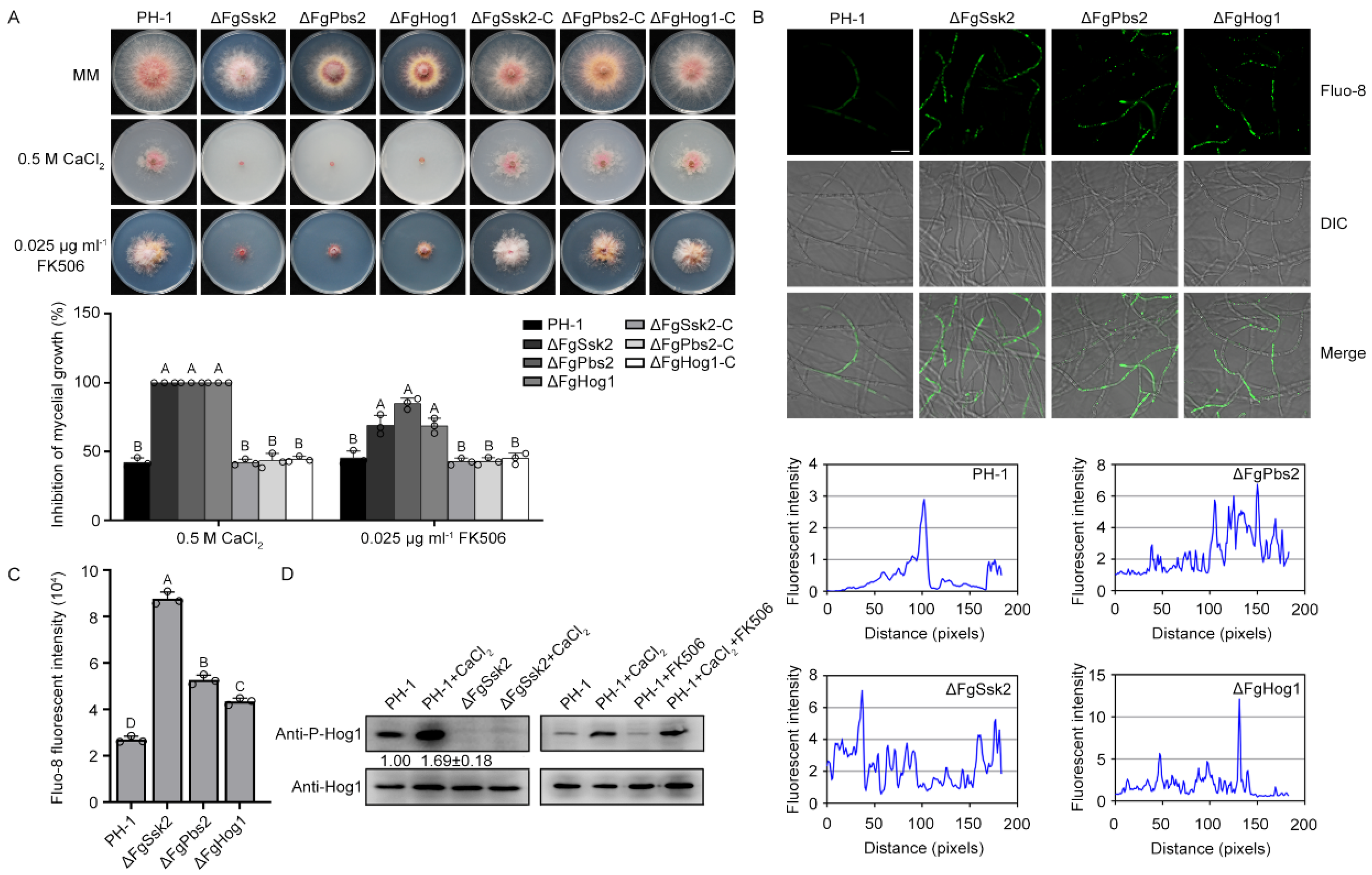
2.3. Ca2+ Signals Measurement
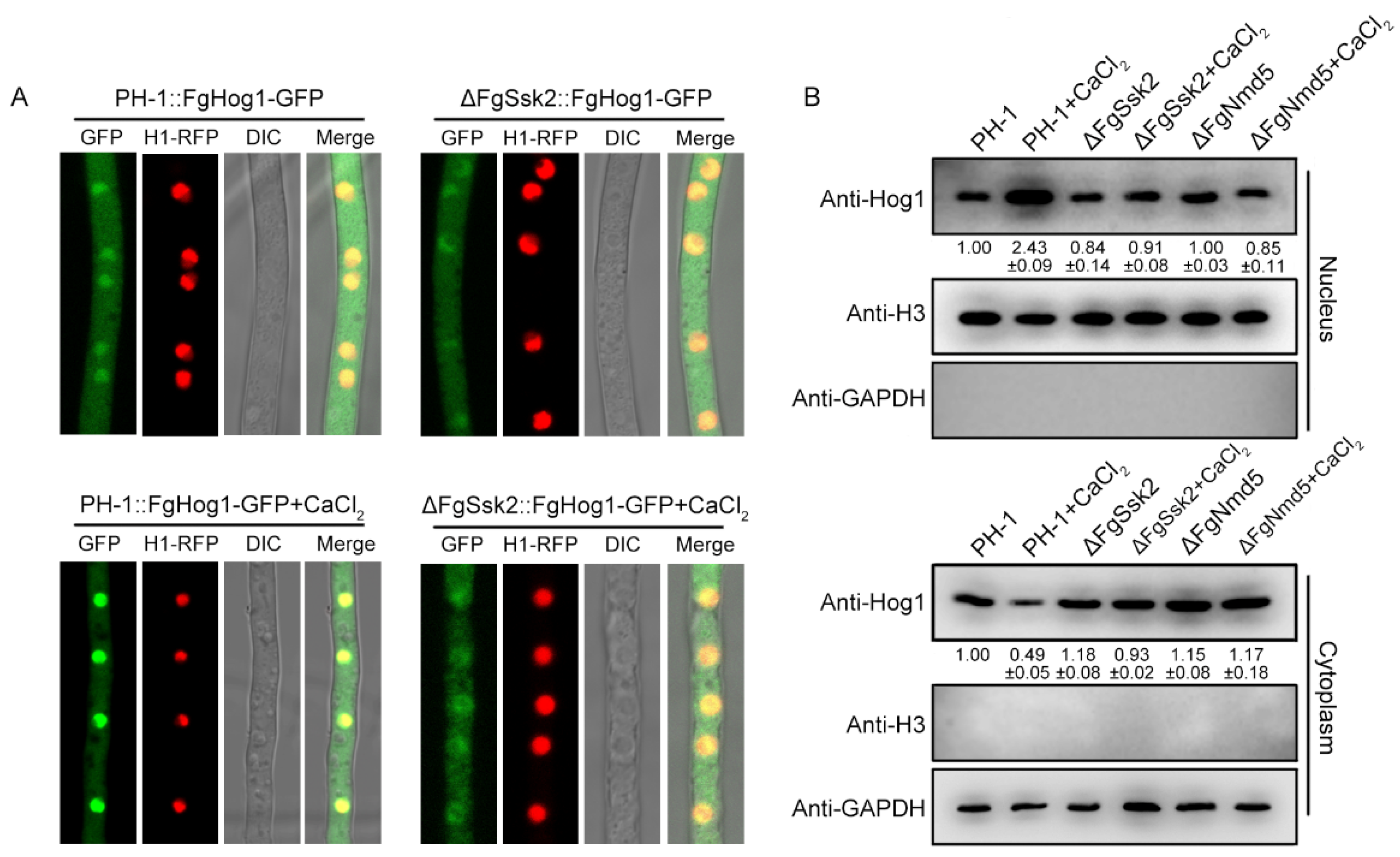
2.4. Western Blotting Assay
2.5. Nuclear–Cytoplasmic Fractionation
2.6. Yeast Two-Hybrid (Y2H) Assays
2.7. Co-Immunoprecipitation (Co-IP) Assay
2.8. Bimolecular Fluorescence Complementation (BiFC) Assay
2.9. Conidiation, Virulence and DON Production
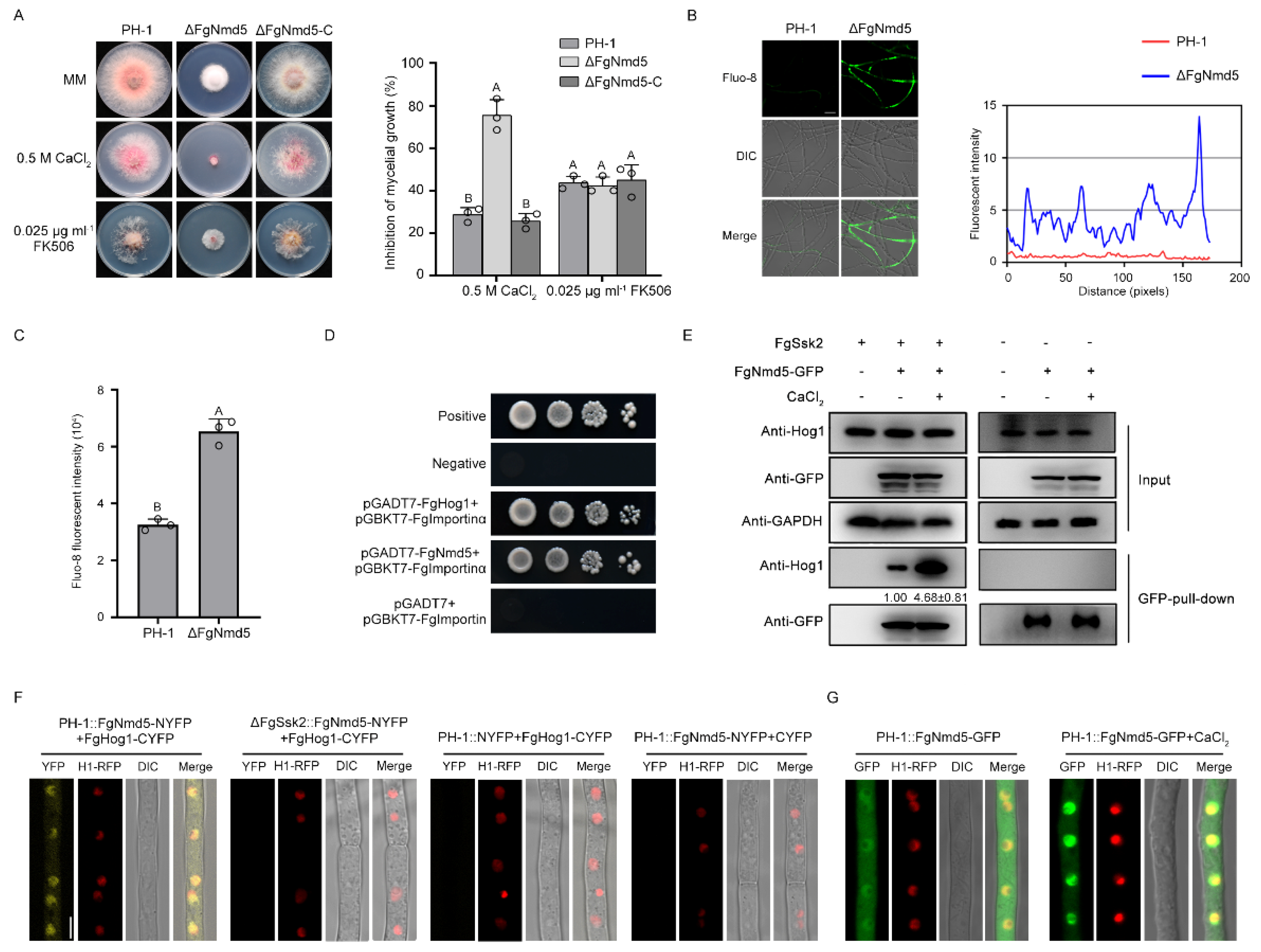
3. Results
3.1. MAPK FgHog1 Is Phosphorylated upon Ca2+ Treatment
3.2. Phosphorylated FgHog1 Is Transported into the Nucleus under Ca2+ Treatment
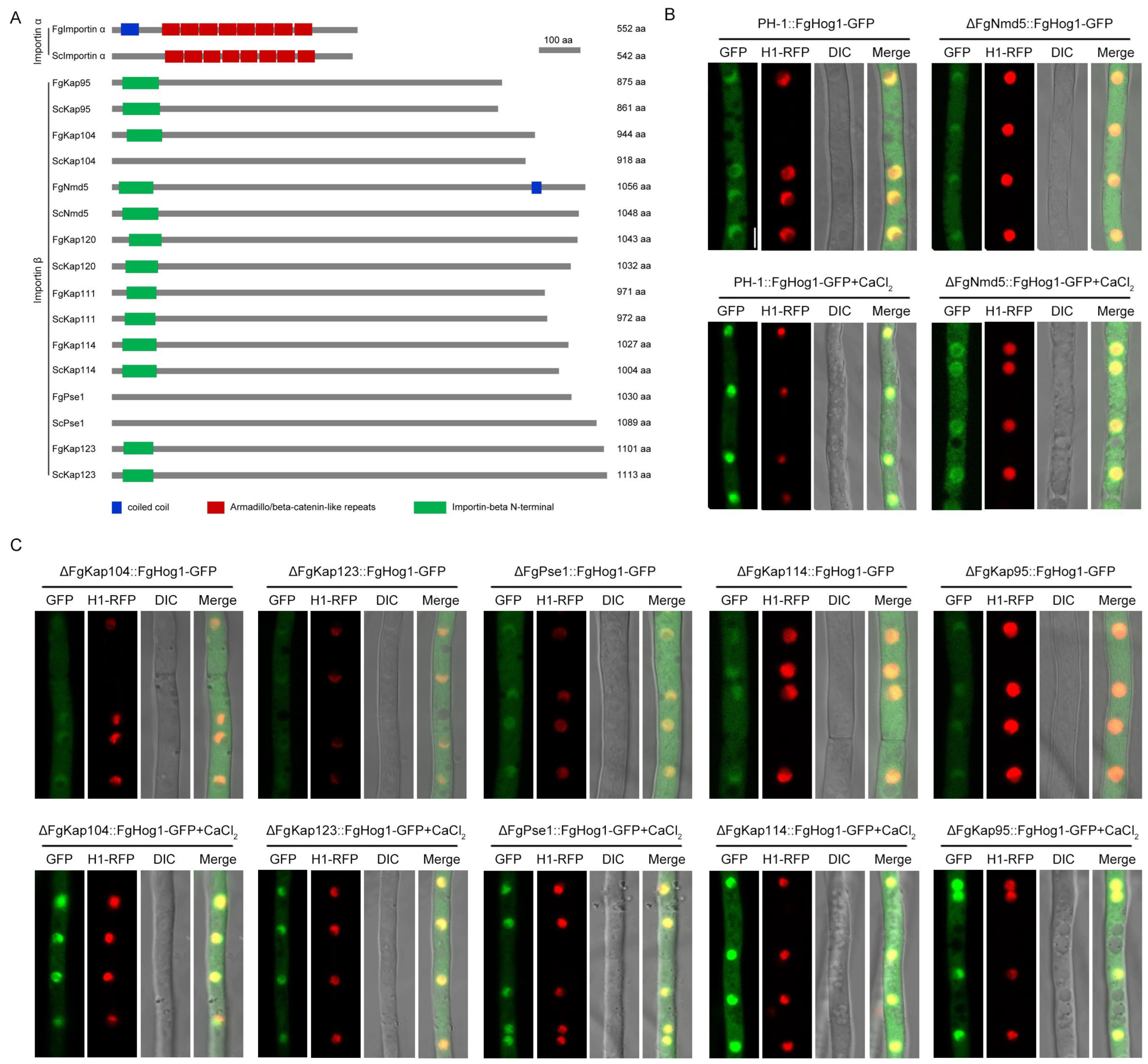
3.3. Identification of Nuclear Importin α and β in F. graminearum
3.4. The Importin β FgNmd5 Is Responsible for Transporting the Phosphorylated FgHog1 into Nuclear
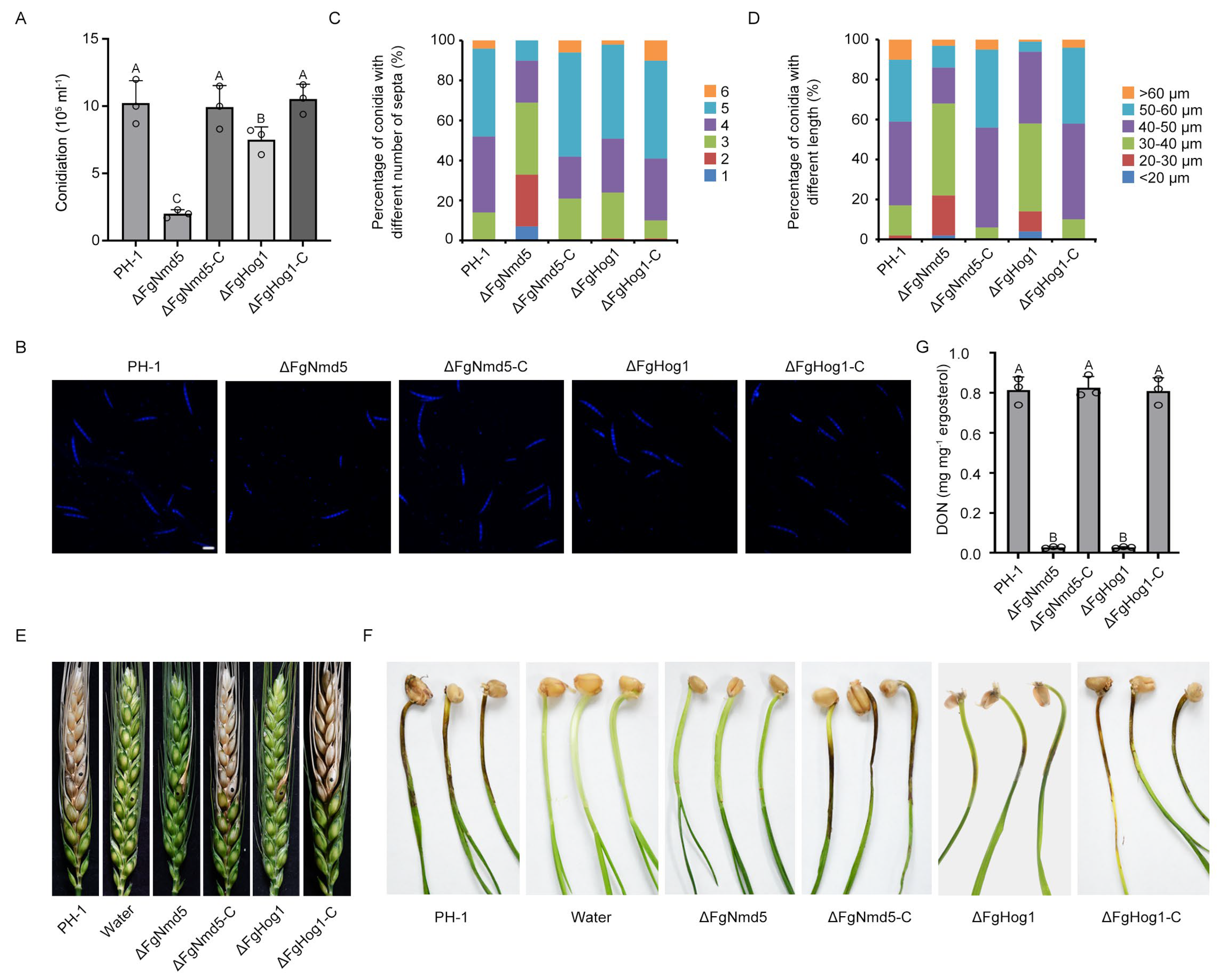
3.5. Ca2+ Homeostasis Plays Important Roles in Development and Virulence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhao, T.; Zhuo, J.; Zhan, C.; Zhang, F.; Linhardt, R.J.; Bai, Z.; Yang, Y. MAPK/HOG signaling pathway induced stress-responsive damage repair is a mechanism for Pichia pastoristo survive from hyperosmoticstress. J. Chem. Technol. Biotechnol. 2020, 96, 412–422. [Google Scholar] [CrossRef]
- Day, A.M.; Quinn, J. Stress-activated protein kinases in human fungal pathogens. Front. Cell. Infect. Microbiol. 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- de Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, E.; Molin, C.; Swaminathan, S.; Ramne, A.; Sunnerhagen, P. Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 2004, 53, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, E.; An, J.; Lee, Y.; Choi, E.; Choi, W.; Moon, E.; Kim, W. Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Env. Microbiol. 2017, 19, 584–597. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, D.; Weng, F.; Wang, Y. Activation of a mitogen-activated protein kinase Hog1 by DNA damaging agent methyl methanesulfonate in Yeast. Front. Mol. Biosci. 2020, 7, 581095. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Yao, S.; Chen, D.; Zhang, L.; Huang, J.; Zhang, L. The Hog1 positive regulated YCT1 gene expression under cadmium tolerance of budding yeast. FEMS Microbiol. Lett. 2018, 365, fny170. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, J.; Liu, Y.; Deng, Y. Roles of high osmolarity glycerol and cell wall integrity pathways in cadmium toxicity in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 6169. [Google Scholar] [CrossRef]
- Kanshin, E.; Kubiniok, P.; Thattikota, Y.; D’Amours, D.; Thibault, P. Phosphoproteome dynamics of Saccharomyces cerevisiae under heat shock and cold stress. Mol. Syst. Biol. 2015, 11, 813. [Google Scholar] [CrossRef]
- Dunayevich, P.; Baltanas, R.; Clemente, J.A.; Couto, A.; Sapochnik, D.; Vasen, G.; Colman-Lerner, A. Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci. Rep. 2018, 8, 15168. [Google Scholar] [CrossRef]
- Furukawa, K.; Randhawa, A.; Kaur, H.; Mondal, A.K.; Hohmann, S. Fungal fludioxonil sensitivity is diminished by a constitutively active form of the group III histidine kinase. FEBS Lett. 2012, 586, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jian, Y.; Chen, Y.; Kistler, H.C.; He, P.; Ma, Z.; Yin, Y. A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum. Nat. Commun. 2019, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 2001, 35, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Denis, V.; Cyert, M.S. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell. Biol. 2002, 156, 29–34. [Google Scholar] [CrossRef]
- O’Day, D.H. CaMBOT: Profiling and characterizing calmodulin-binding proteins. Cell. Signal. 2003, 15, 347–354. [Google Scholar] [CrossRef]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Takatsume, Y.; Ohdate, T.; Maeta, K.; Nomura, W.; Izawa, S.; Inoue, Y. Calcineurin/Crz1 destabilizes Msn2 and Msn4 in the nucleus in response to Ca(2+) in Saccharomyces cerevisiae. Biochem. J. 2010, 427, 275–287. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Sung, G.H. Regulation of MAP kinase Hog1 by calmodulin during hyperosmotic stress. Biochim. Biophys. Acta 2016, 1863, 2551–2559. [Google Scholar] [CrossRef]
- Kim, Y.H.; Han, M.E.; Oh, S.O. The molecular mechanism for nuclear transport and its application. Anat. Cell. Biol. 2017, 50, 77–85. [Google Scholar] [CrossRef]
- Kimura, M.; Imamoto, N. Biological significance of the importin-beta family-dependent nucleocytoplasmic transport pathways. Traffic 2014, 15, 727–748. [Google Scholar] [CrossRef]
- Pemberton, L.F.; Paschal, B.M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 2005, 6, 187–198. [Google Scholar] [CrossRef]
- Cairo, L.V.; Wozniak, R.W. The nuclear transport factor kap121 is required for stability of the Dam1 complex and mitotic kinetochore bi-orientation. Cell. Rep. 2016, 14, 2440–2450. [Google Scholar] [CrossRef][Green Version]
- Wing, C.E.; Fung, H.Y.J.; Chook, Y.M. Karyopherin-mediated nucleocytoplasmic transport. Nat. Rev. Mol. Cell. Biol. 2022, 23, 307–328. [Google Scholar] [CrossRef]
- Ferrigno, P.; Posas, F.; Koepp, D.; Saito, H.; Silver, P.A. Regulated nucleo cytoplasmic exchange of HOG1 MAPK requires the importin homologs. EMBO J. 1998, 5606–5614. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, A.A.; Tourtellotte, J.; Niwa, M. Late phase of the endoplasmic reticulum stress response pathway is regulated by Hog1 MAP kinase. J. Biol. Chem. 2010, 285, 17545–17555. [Google Scholar] [CrossRef] [PubMed]
- Stepien, L.; Chelkowski, J. Fusarium head blight of wheat: Pathogenic species and their mycotoxins. World Mycotoxin J. 2010, 3, 107–119. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Dominguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef]
- Liu, X.H.; Lu, J.P.; Zhang, L.; Dong, B.; Min, H.; Lin, F.C. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot. Cell 2007, 6, 997–1005. [Google Scholar] [CrossRef]
- Yu, F.; Gu, Q.; Yun, Y.; Yin, Y.; Xu, J.R.; Shim, W.B.; Ma, Z. The TOR signaling pathway regulates vegetative development and virulence in Fusarium graminearum. New. Phytol. 2014, 203, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Liu, Z.; Yin, Y.; Jiang, J.; Chen, Y.; Xu, J.R.; Ma, Z. Functional analysis of the Fusarium graminearum phosphatome. New. Phytol. 2015, 207, 119–134. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, C.; Yu, F.; Yin, Y.; Shim, W.B.; Ma, Z. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. Env. Microbiol. 2015, 17, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ye, R.; Xin, Y.; Fang, X.; Li, C.; Shi, H.; Zhou, X.; Qi, Y. An importin beta protein negatively regulates microRNA activity in Arabidopsis. Plant Cell 2011, 23, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, N.E.; Fukuda, C.A.; da Silva, T.D.; de Oliveira, H.C.; de Barros, A.C.; Dreyer, T.R.; Bertolini, M.C.; Fontes, M.R.M. Comparative study of the interactions between fungal transcription factor nuclear localization sequences with mammalian and fungal importin-alpha. Sci. Rep. 2020, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.Y.; Zhao, X.; Xu, J.R.; Guldener, U.; Kistler, H.C. Conidial germination in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 2008, 45, 389–399. [Google Scholar] [CrossRef]
- Tisi, R.; Rigamonti, M.; Groppi, S.; Belotti, F. Calcium homeostasis and signaling in fungi and their relevance for pathogenicity of yeasts and filamentous fungi. AIMS Mol. Sci. 2016, 3, 505–549. [Google Scholar] [CrossRef]
- Kozubowski, L.; Aboobakar, E.F.; Cardenas, M.E.; Heitman, J. Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryot. Cell 2011, 10, 1396–1402. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Zhang, B.; Zheng, W.; Xing, L.; Li, M. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res. 2011, 11, 430–439. [Google Scholar] [CrossRef]
- Teng, J.; Iida, K.; Imai, A.; Nakano, M.; Tada, T.; Iida, H. Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit. Microbiology 2013, 159, 970–979. [Google Scholar] [CrossRef]
- Kraus, P.R.; Nichols, C.B.; Heitman, J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot. Cell 2005, 4, 1079–1087. [Google Scholar] [CrossRef]
- Kozubowski, L.; Lee, S.C.; Heitman, J. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 2009, 11, 370–380. [Google Scholar] [CrossRef]
- Anjago, W.M.; Biregeya, J.; Shi, M.; Chen, Y.; Wang, Y.; Wang, Z.; Hong, Y.; Chen, M. The calcium chloride responsive type 2C protein phosphatases play synergistic roles in regulating MAPK pathways in Magnaporthe oryzae. J. Fungi 2022, 8, 1287. [Google Scholar] [CrossRef]
- Shitamukai, A.; Hirata, D.; Sonobe, S.; Miyakawa, T. Evidence for antagonistic regulation of cell growth by the calcineurin and high osmolarity glycerol pathways in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 3651–3661. [Google Scholar] [CrossRef] [PubMed]
- de Castro, P.A.; Chen, C.; de Almeida, R.S.; Freitas, F.Z.; Bertolini, M.C.; Morais, E.R.; Brown, N.A.; Ramalho, L.N.; Hagiwara, D.; Mitchell, T.K.; et al. ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Mol. Microbiol. 2014, 94, 655–674. [Google Scholar] [CrossRef]
- Maeta, K.; Izawa, S.; Inoue, Y. Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 253–260. [Google Scholar] [CrossRef]
- Zehorai, E.; Seger, R. Beta-like importins mediate the nuclear translocation of mitogen-activated protein kinases. Mol. Cell. Biol. 2014, 34, 259–270. [Google Scholar] [CrossRef]
- Wosika, V.; Pelet, S. Single-particle imaging of stress-promoters induction reveals the interplay between MAPK signaling, chromatin and transcription factors. Nat. Commun. 2020, 11, 3171. [Google Scholar] [CrossRef]
- Chachami, G.; Paraskeva, E.; Mingot, J.M.; Braliou, G.G.; Gorlich, D.; Simos, G. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem. Biophys. Res. Commun. 2009, 390, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Rassadi, R.; Rabie, B.; Matusiewicz, N.; Stochaj, U. Regulated nuclear accumulation of the yeast hsp70 Ssa4p in ethanol-stressed cells is mediated by the N-terminal domain, requires the nuclear carrier Nmd5p and protein kinase C. FASEB J. 2004, 18, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, C.; Zhou, T.; Zhang, L.H.; Deng, Y.Z. Karyopherin MoKap119-mediated nuclear import of cyclin-dependent kinase regulator MoCks1 is essential for Magnaporthe oryzae pathogenicity. Cell. Microbiol. 2020, 22, e13114. [Google Scholar] [CrossRef] [PubMed]
- Peiter, E.; Fischer, M.; Sidaway, K.; Roberts, S.K.; Sanders, D. The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 2005, 579, 5697–5703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, H.; Zhang, Y.; Jiang, L. Vcx1-D1 (M383I), the Vcx1 mutant with a calcineurin-independent vacuolar Ca(2+)/H(+) exchanger activity, confers calcineurin-independent Mn(2+) tolerance in Saccharomyces cerevisiae. Can. J. Microbiol. 2016, 62, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Fang, T.; Xu, H.; Jiang, L. The pH-sensing Rim101 pathway positively regulates the transcriptional expression of the calcium pump gene PMR1 to affect calcium sensitivity in budding yeast. Biochem. Biophys. Res. Commun. 2020, 532, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Poletto, N.P.; Henriques, J.A.; Bonatto, D. Relationship between endoplasmic reticulum- and Golgi-associated calcium homeostasis and 4-NQO-induced DNA repair in Saccharomyces cerevisiae. Arch. Microbiol. 2010, 192, 247–257. [Google Scholar] [CrossRef]
- LaFayette, S.L.; Collins, C.; Zaas, A.K.; Schell, W.A.; Betancourt-Quiroz, M.; Gunatilaka, A.A.; Perfect, J.R.; Cowen, L.E. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010, 6, e1001069. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Ying, S.H.; Feng, M.G. Five vacuolar Ca(2+) exchangers play different roles in calcineurin-dependent Ca(2+)/Mn(2+) tolerance, multistress responses and virulence of a filamentous entomopathogen. Fungal Genet. Biol. 2014, 73, 12–19. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Zhu, X.; Dong, B.; Liu, X.; Lu, J.; Lin, F. The P5-type ATPase Spf1 is required for development and virulence of the rice blast fungus Pyricularia oryzae. Curr. Genet. 2020, 66, 385–395. [Google Scholar] [CrossRef]
- Wu, C.; Guo, Z.; Zhang, M.; Chen, H.; Peng, M.; Abubakar, Y.S.; Zheng, H.; Yun, Y.; Zheng, W.; Wang, Z.; et al. Golgi-localized calcium/manganese transporters FgGdt1 and FgPmr1 regulate fungal development and virulence by maintaining Ca(2+) and Mn(2+) homeostasis in Fusarium graminearum. Env. Microbiol. 2022, 24, 4623–4640. [Google Scholar] [CrossRef]
- Thewes, S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot. Cell 2014, 13, 694–705. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell. Host Microbe 2019, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, Y.; Dong, L.; Sun, K.; Liu, H.; Ma, Z.; Yan, L.; Yin, Y. MAP Kinase FgHog1 and Importin β FgNmd5 Regulate Calcium Homeostasis in Fusarium graminearum. J. Fungi 2023, 9, 707. https://doi.org/10.3390/jof9070707
Zhang L, Li Y, Dong L, Sun K, Liu H, Ma Z, Yan L, Yin Y. MAP Kinase FgHog1 and Importin β FgNmd5 Regulate Calcium Homeostasis in Fusarium graminearum. Journal of Fungi. 2023; 9(7):707. https://doi.org/10.3390/jof9070707
Chicago/Turabian StyleZhang, Lixin, Yiqing Li, Lanlan Dong, Kewei Sun, Hao Liu, Zhonghua Ma, Leiyan Yan, and Yanni Yin. 2023. "MAP Kinase FgHog1 and Importin β FgNmd5 Regulate Calcium Homeostasis in Fusarium graminearum" Journal of Fungi 9, no. 7: 707. https://doi.org/10.3390/jof9070707
APA StyleZhang, L., Li, Y., Dong, L., Sun, K., Liu, H., Ma, Z., Yan, L., & Yin, Y. (2023). MAP Kinase FgHog1 and Importin β FgNmd5 Regulate Calcium Homeostasis in Fusarium graminearum. Journal of Fungi, 9(7), 707. https://doi.org/10.3390/jof9070707







