Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens
Abstract
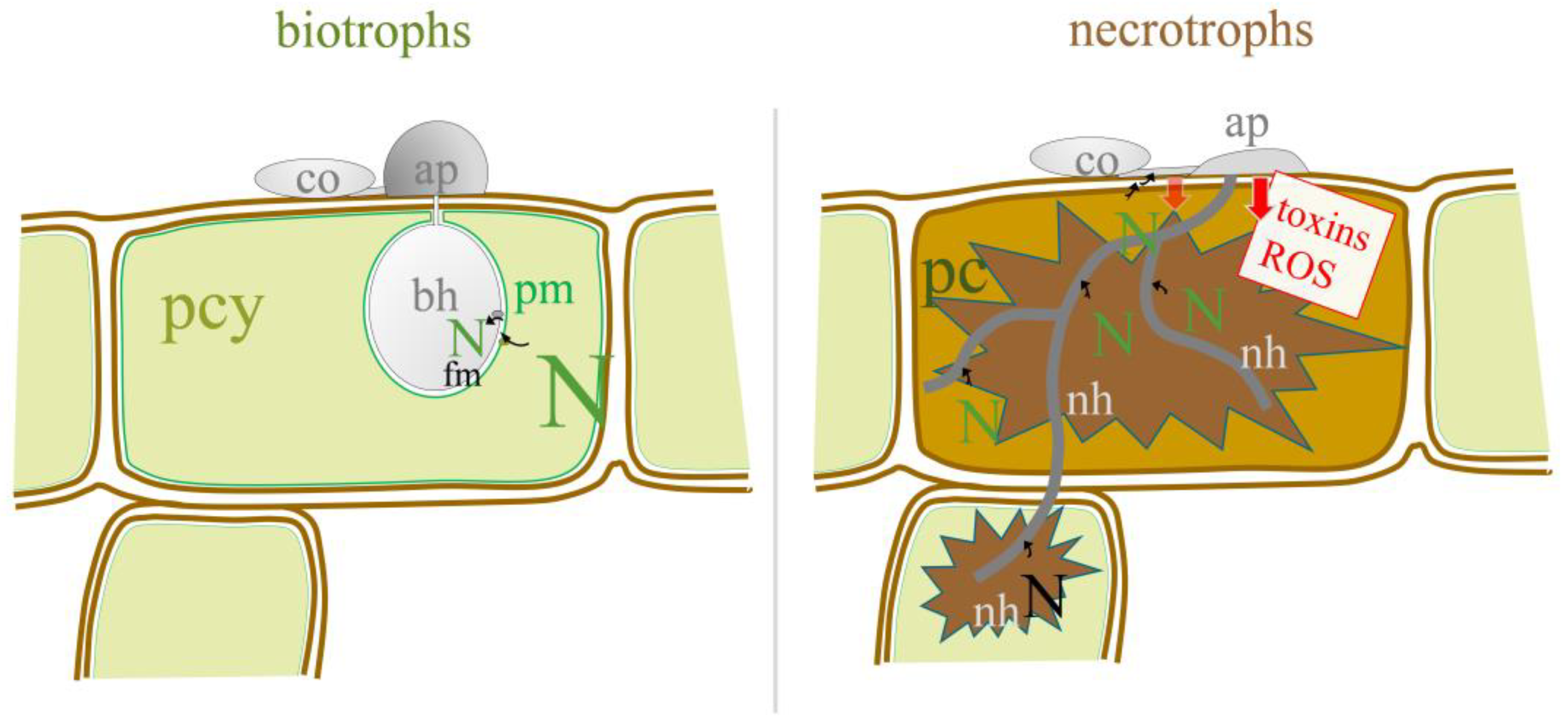
:1. Introduction
2. Materials and Methods
2.1. Fungal and Plant Material and Infection Experiments
2.2. Inhibition of Urease Activity
2.3. Microscopy
2.4. Statistical Analysis
3. Results
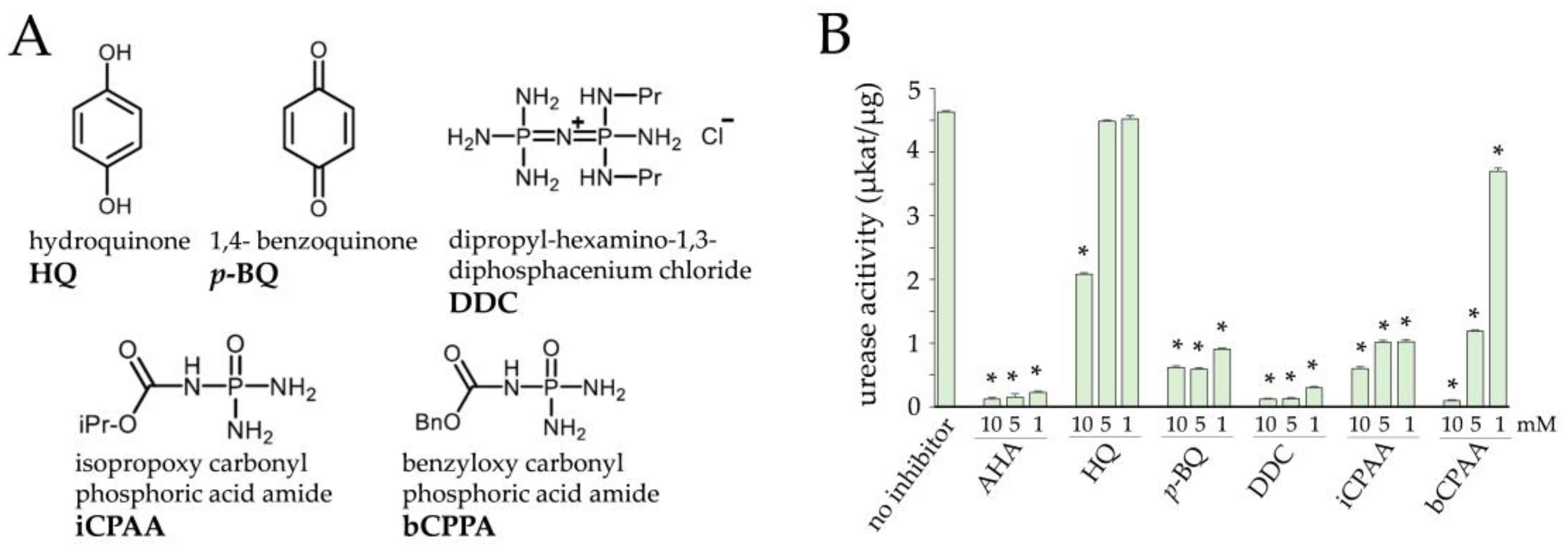
3.1. Inhibition of Urease Activity by Two Natural and Three Novel Synthetic Compounds
3.2. Efficacy of Urease Inhibitors against the Obligate Biotrophs Blumeria graminis f. sp. tritici and Uromyces viciae-fabae
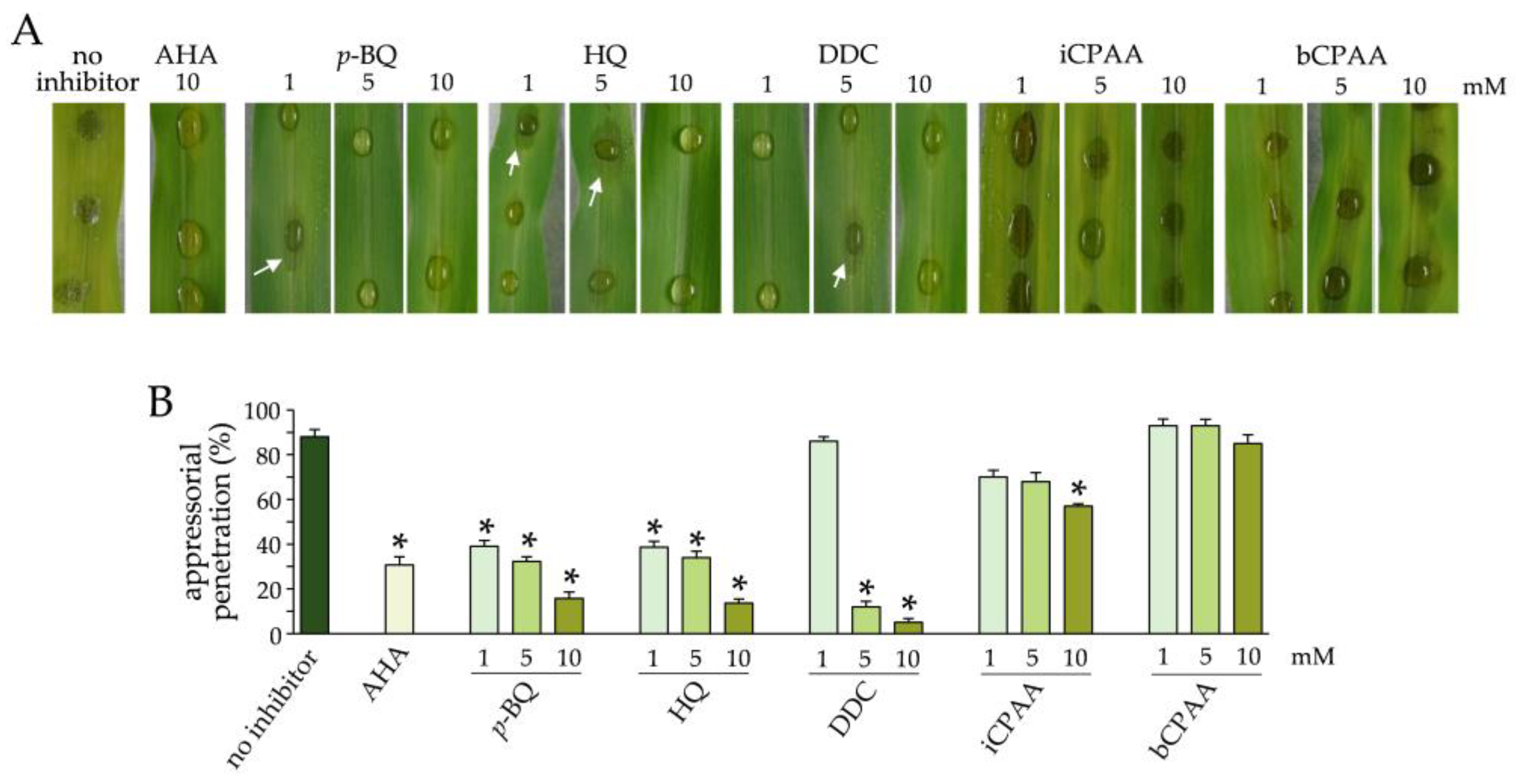
3.3. Effect of Urease Inhibitors on Infection of Maize Leaves by C. graminicola
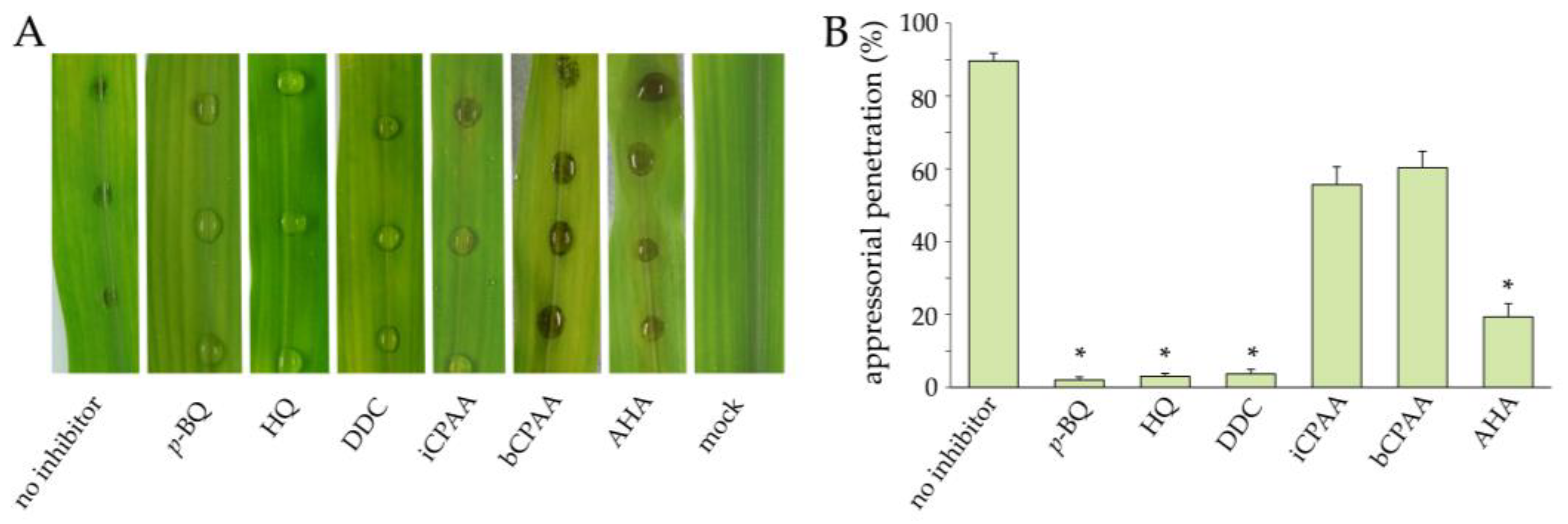
3.4. Effect of Urease Inhibitors on Necrotrophic Plant Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mora, D.; Arioli, S. Microbial urease in health and disease. PLoS Pathog. 2014, 10, e1004472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skouloubris, S.; Thiberge, J.M.; Labigne, A.; De Reuse, H. The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect. Immun. 1998, 66, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Moshfegh, A.P.; Sachs, G.; Scott, D.R. The periplasmic alphacarbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 2005, 187, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeks, D.L.; Eskandari, S.; Scott, D.R.; Sachs, G. A H+-gated urea channel: The link between Helicobacter pylori urease and gastric colonization. Science 2000, 287, 482–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherford, J.C. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014, 10, e1004062. [Google Scholar] [CrossRef] [Green Version]
- Smoot, D.T.; Mobley, H.L.; Chippendale, G.R.; Lewison, J.F.; Resau, J.H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect. Immun. 1990, 58, 1992–1994. [Google Scholar] [CrossRef] [Green Version]
- Burne, R.A.; Chen, Y.Y. Bacterial ureases in infectious diseases. Microbes. Infect. 2000, 2, 533–542. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Surana, R.; Milam, J.E.; Montano, G.T.; Chen, G.H.; Sonstein, J.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B.; Olszewski, M.A. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am. J. Pathol. 2009, 174, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, M.A.; Noverr, M.C.; Chen, G.H.; Toews, G.B.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004, 164, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Munch, S.; Ludwig, N.; Floss, D.S.; Sugui, J.A.; Koszucka, A.M.; Voll, L.M.; Sonnewald, U.; Deising, H.B. Identification of virulence genes in the corn pathogen Colletotrichum graminicola by Agrobacterium tumefaciens-mediated transformation. Mol. Plant. Pathol. 2011, 12, 43–55. [Google Scholar] [CrossRef]
- Benatto Perino, E.H.; Glienke, C.; Silva, A.D.; Deising, H.B. Molecular characterization of the purine degradation pathway genes ALA1 and URE1 of the maize anthracnose fungus Colletotrichum graminicola Identified urease as a novel target for plant disease control. Phytopathology 2020, 110, 1530–1540. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Podila, G.K.; Rogers, L.M.; Kolattukudy, P.E. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 1993, 103, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.; Neef, U.; Struck, C.; Gottfert, M.; Mendgen, K. A putative amino acid transporter is specifically expressed in haustoria of the rust fungus Uromyces fabae. Mol. Plant Microbe Interact. 1997, 10, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Voegele, R.T.; Struck, C.; Hahn, M.; Mendgen, K. The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc. Natl. Acad. Sci. USA 2001, 98, 8133–8138. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, I.; Collemare, J.; Mehrabi, R.; De Wit, P.J.G.M. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS. Microbiol. Rev. 2013, 37, 67–93. [Google Scholar] [CrossRef] [Green Version]
- Deising, H.; Frittrang, A.K.; Kunz, S.; Mendgen, K. Regulation of pectin methylesterase and polygalacturonate lyase activity during differentiation of infection structures in Uromyces viciae-fabae. Microbiology 1995, 141, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 2020, 10, 8503. [Google Scholar] [CrossRef]
- Sugui, J.A.; Deising, H.B. Isolation of infection-specific sequence tags expressed during early stages of maize anthracnose disease development. Mol. Plant Path. 2002, 3, 197–203. [Google Scholar] [CrossRef]
- Deising, H.; Jungblut, P.R.; Mendgen, K. Differentiation-related proteins of the broad bean rust fungus Uromyces viciae-fabae, as revealed by high-resolution 2-dimensional polyacrylamide-gel electrophoresis. Arch. Microbiol. 1991, 155, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Horbach, R.; Graf, A.; Weihmann, F.; Antelo, L.; Mathea, S.; Liermann, J.C.; Opatz, T.; Thines, E.; Aguirre, J.; Deising, H.B. Sfp-type 4′-phosphopantetheinyl transferase is indispensable for fungal pathogenicity. Plant Cell 2009, 21, 3379–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bzura, J.; Koncki, R. A mechanized urease activity assay. Enzyme Microb. Technol. 2019, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Kohmoto, K. Host-specific toxins and chemical structures from alternaria species. Annu. Rev. Phytopathol. 1983, 21, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Cianci, M.; Musiani, F.; Lente, G.; Palombo, M.; Ciurli, S. Inactivation of urease by catechol: Kinetics and structure. J. Inorg. Biochem. 2017, 166, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, L.; Cianci, M.; Musiani, F.; Ciurli, S. Inactivation of urease by 1,4-benzoquinone: Chemistry at the protein surface. Dalton. Trans. 2016, 45, 5455–5459. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, L.; Cianci, M.; Benini, S.; Ciurli, S. The structure of the elusive urease-urea complex unveils the mechanism of a paradigmatic nickel-dependent enzyme. Angew. Chem. Int. Ed. Engl. 2019, 58, 7415–7419. [Google Scholar] [CrossRef]
- Alfonso, C.; Raposo, R.; Melgarejo, P. Genetic diversity in Botrytis cinerea populations on vegetable crops in greenhouses in south-eastern Spain. Plant Pathol. 2000, 49, 243–251. [Google Scholar] [CrossRef]
- Holz, G.; Coertze, S.; Williamson, B. The ecology of Botrytis on plant surfaces. In Botrytis: Biology, Pathology and Control; Springer: Berlin/Heidelberg, Germany, 2007; pp. 9–27. [Google Scholar]
- Münch, S.; Lingner, U.; Floss, D.S.; Ludwig, N.; Sauer, N.; Deising, H.B. The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 2008, 165, 41–51. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Panstruga, R. Tête à tête inside a plant cell: Establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol. 2006, 171, 699–718. [Google Scholar] [CrossRef] [Green Version]
- Chethana, K.W.T.; Jayawardena, R.S.; Chen, Y.J.; Konta, S.; Tibpromma, S.; Abeywickrama, P.D.; Gomdola, D.; Balasuriya, A.; Xu, J.; Lumyong, S.; et al. Diversity and function of appressoria. Pathogens 2021, 10, 746. [Google Scholar] [CrossRef]
- Talbot, N.J.; McCafferty, H.R.K.; Ma, M.; Moore, K.; Hamer, J.E. Nitrogen starvation of the rice blast fungus Magnaporthe grisea may act as an environmental cue for disease symptom expression. Physiol. Mol. Plant Pathol. 1997, 50, 179–195. [Google Scholar] [CrossRef]
- Tiedemann, A.V. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol. Mol. Plant Pathol. 1997, 50, 151–166. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. Rsc. Advances 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Heyno, E.; Alkan, N.; Fluhr, R. A dual role for plant quinone reductases in host-fungus interaction. Physiol. Plant 2013, 149, 340–353. [Google Scholar] [CrossRef]
- Oliceira-Garcia, E.; von Tiedemann, A.; Deising, H. The measure mix matters: Multiple-component plant protection is indispensable for coping with the enormous genome plasticity and mutation rates in pathogenic microorganisms. J. Plant Dis. Prot. 2020, 128, 3–6. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliyeva-Schnorr, L.; Schuster, C.; Deising, H.B. Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens. J. Fungi 2023, 9, 708. https://doi.org/10.3390/jof9070708
Aliyeva-Schnorr L, Schuster C, Deising HB. Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens. Journal of Fungi. 2023; 9(7):708. https://doi.org/10.3390/jof9070708
Chicago/Turabian StyleAliyeva-Schnorr, Lala, Carola Schuster, and Holger B. Deising. 2023. "Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens" Journal of Fungi 9, no. 7: 708. https://doi.org/10.3390/jof9070708
APA StyleAliyeva-Schnorr, L., Schuster, C., & Deising, H. B. (2023). Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens. Journal of Fungi, 9(7), 708. https://doi.org/10.3390/jof9070708





