Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection
Abstract
:1. Introduction
2. The Infectious Agent and Infection Chain of PCP
3. Increased Incidence of PCP in Patients without HIV/AIDS
4. Immunodeficient Conditions and Risk Factors for PCP
4.1. Immunosuppressive Agents
| Drugs | Mechanisms of Action | Chemical Properties | Approved Applications | References * |
|---|---|---|---|---|
| Corticosteroids ** | Suppression of inflammation, leukocyte migration and activation; induction of apoptosis | Steroid hormones | Various diseases | [4,32,37] |
| Inhibitors of DNA/RNA synthesis | ||||
| Temozolomide | Inhibit DNA and cellular replication | Alkylating agent/imidazotetrazine | Brain cancer, astrocytoma, and glioblastoma multiforme | [38,39] |
| Cyclophosphamide | Inhibit DNA and cellular replication | Alkylating agent/phosphoramide mustard | Lymphoma, multiple myeloma, leukemia, ovarian cancer, breast cancer, small cell lung cancer, neuroblastoma, and sarcoma; organ transplant rejection | [40,41] |
| Bleomycin | Induce DNA strand breaks | Nonribosomal peptide | Lymphoma, testicular cancer, ovarian cancer, and cervical cancer | [42,43] |
| Fluorouracil | DNA synthesis inhibitor | Antimetabolite/pyrimidine analog | Various cancers | [43,44] |
| Cytarabine | DNA synthesis inhibitor | Antimetabolite | Leukemia and lymphoma | [43,45] |
| Methotrexate | DNA/RNA synthesis inhibitor | Antimetabolite/antifolate | Cancers, autoimmune diseases, and ectopic pregnancy | [43,46] |
| Azathioprine | Purine synthesis inhibitor | Antimetabolite/Purine analog | Rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and kidney transplant rejection | [43,47,48] |
| Cladribine | Purine synthesis inhibitor | Antimetabolite/Purine analog | Leukemia and lymphoma | [43,49] |
| Fludarabine | Purine synthesis inhibitor | Antimetabolite/Purine analog | Leukemia and lymphoma | [43,50,51,52] |
| Inhibitors of immune functions | ||||
| Rituximab | B-cell signaling inhibitor | Anti-CD20 monoclonal antibody | Autoimmune diseases, Hematological cancers | [53,54] |
| Alemtuzumab | Deplete CD52-bearing B and T cells | Anti-CD52 monoclonal antibody | Hematological cancers, multiple sclerosis, and organ transplant rejection | [43,52,55] |
| Abatacept | T-cell signaling inhibitor | Recombinant protein | Rheumatoid arthritis, juvenile idiopathic arthritis, and psoriatic arthritis | [56,57] |
| Belatacept | T-cell signaling inhibitor | Recombinant protein | Organ transplant rejection | [58,59,60] |
| Tocilizumab | Anti–IL-6 receptor | Anti-IL6 receptor monoclonal antibody | Rheumatoid arthritis, juvenile rheumatoid arthritis | [61,62,63] |
| Adalimumab | TNFα inhibitor | Anti-TNFα monoclonal antibody | Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, chronic psoriasis, hidradenitis suppurativa, and juvenile idiopathic arthritis | [64,65] |
| Etanercept | TNFα inhibitor | Recombinant protein | Rheumatoid arthritis, juvenile rheumatoid arthritis and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis | [43,66,67] |
| Golimumab | TNFα inhibitor | Anti-TNFα monoclonal antibody | Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and rheumatoid arthritis | [68,69] |
| Infliximab | TNFα inhibitor | Anti-TNFα monoclonal antibody | Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, and ankylosing spondylitis | [43,70,71] |
| Cyclosporine | Calcineurin inhibitor | Anticalcineurin | Autoimmune diseases, and organ transplant rejection | [43,72,73,74] |
| Tacrolimus | Calcineurin inhibitor | Anticalcineurin/macrolide lactone | Organ transplant rejection, eczema, uveitis, and vitiligo | [75,76,77] |
| Everolimus | Inhibitor of mammalian target of rapamycin (mTOR) | Derivative of sirolimus | Organ transplant rejection, kidney cancer, breast cancer, and subependymal giant cell astrocytoma | [78,79,80] |
| Sirolimus (rapamycin) | Inhibitor of mammalian target of rapamycin (mTOR) | Macrolide compound | Organ transplant rejection, lymphangioleiomyomatosis | [43,81,82,83] |
4.2. Cancers
4.3. Solid Organ and Stem Cell Transplantation
4.4. Autoimmune and Inflammatory Diseases
4.5. Primary or Congenital Immunodeficiencies in Children
4.6. COVID-19
4.7. Other Underlying Conditions
4.8. Effects of Different Immunocompromised Conditions on Immune Functions
5. PCP Outbreaks
6. Pneumocystis Colonization
7. Reactivation of a Latent Infection Versus Acquisition of a New Infection
8. Prevention of PCP
9. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajdusek, D.C. Pneumocystis carinii as the cause of human disease: Historical perspective and magnitude of the problem: Introductory remarks. Natl. Cancer Inst. Monogr. 1976, 43, 1–11. [Google Scholar] [PubMed]
- Walzer, P.D.; Perl, D.P.; Krogstad, D.J.; Rawson, P.G.; Schultz, M.G. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann. Intern. Med. 1974, 80, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sepkowitz, K.A. Pneumocystis carinii pneumonia among patients with neoplastic disease. Semin. Respir. Infect. 1992, 7, 114–121. [Google Scholar]
- Yale, S.H.; Limper, A.H. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: Associated illness and prior corticosteroid therapy. Mayo Clin. Proc. 1996, 71, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Arend, S.M.; Kroon, F.P.; van’t Wout, J.W. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch. Intern. Med. 1995, 155, 2436–2441. [Google Scholar] [CrossRef]
- Mansharamani, N.G.; Garland, R.; Delaney, D.; Koziel, H. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: Comparison of HIV-associated cases to other immunocompromised states. Chest 2000, 118, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Rayens, E.; Norris, K.A. Prevalence and healthcare burden of fungal infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Cordonnier, C.; Cesaro, S.; Maschmeyer, G.; Einsele, H.; Donnelly, J.P.; Alanio, A.; Hauser, P.M.; Lagrou, K.; Melchers, W.J.; Helweg-Larsen, J.; et al. Pneumocystis jirovecii pneumonia: Still a concern in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 2016, 71, 2379–2385. [Google Scholar] [CrossRef] [Green Version]
- Bollee, G.; Sarfati, C.; Thiery, G.; Bergeron, A.; de Miranda, S.; Menotti, J.; de Castro, N.; Tazi, A.; Schlemmer, B.; Azoulay, E. Clinical picture of Pneumocystis jiroveci pneumonia in cancer patients. Chest 2007, 132, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Motojima, S.; Ohkuni, Y.; Matsunuma, R.; Nakashima, K.; Iwasaki, T.; Nakashita, T.; Otsuka, Y.; Kaneko, N. Early diagnosis and treatment are crucial for the survival of Pneumocystis pneumonia patients without human immunodeficiency virus infection. J. Infect. Chemother. 2012, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Festic, E.; Gajic, O.; Limper, A.H.; Aksamit, T.R. Acute respiratory failure due to Pneumocystis pneumonia in patients without human immunodeficiency virus infection: Outcome and associated features. Chest 2005, 128, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, A.L.; Traore, K.; Plekhanova, I.; Bouchrik, M.; Bossard, C.; Picot, S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int. J. Infect. Dis. 2016, 46, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, K.K.; Cherukuri, B.; Anne, S.; Shankar, T.U.; Mohan Reddy, G.M.; Guttikonda, N. An unusual case of severe Pneumocystis jiroveci pneumonia (PJP) presenting as “recurrent cytokine storm’’ following COVID-19 infection. J. Assoc. Physicians India 2021, 69, 78. [Google Scholar]
- Ma, L.; Cisse, O.H.; Kovacs, J.A. A molecular window into the biology and epidemiology of Pneumocystis spp. Clin. Microbiol. Rev. 2018, 31, e00009–e00018. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, J.K. Pneumocystis jiroveci n. sp. from man: Morphology, physiology, and immunology in relation to pathology. Natl. Cancer Inst. Monogr. 1976, 43, 13–30. [Google Scholar]
- Redhead, S.A.; Cushion, M.T.; Frenkel, J.K.; Stringer, J.R. Pneumocystis and Trypanosoma cruzi: Nomenclature and typifications. J. Eukaryot. Microbiol. 2006, 53, 2–11. [Google Scholar] [CrossRef]
- Keely, S.P.; Fischer, J.M.; Cushion, M.T.; Stringer, J.R. Phylogenetic identification of Pneumocystis murina sp. nov., a new species in laboratory mice. Microbiology 2004, 150, 1153–1165. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, J.K. Pneumocystis pneumonia, an immunodeficiency-dependent disease (IDD): A critical historical overview. J. Eukaryot. Microbiol. 1999, 46, 89S–92S. [Google Scholar]
- Cushion, M.T.; Keely, S.P.; Stringer, J.R. Molecular and phenotypic description of Pneumocystis wakefieldiae sp. nov., a new species in rats. Mycologia 2004, 96, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Dei-Cas, E.; Chabe, M.; Moukhlis, R.; Durand-Joly, I.; Aliouat, E.M.; Stringer, J.R.; Cushion, M.; Noel, C.; de Hoog, G.S.; Guillot, J.; et al. Pneumocystis oryctolagi sp. nov., an uncultured fungus causing pneumonia in rabbits at weaning: Review of current knowledge, and description of a new taxon on genotypic, phylogenetic and phenotypic bases. FEMS Microbiol. Rev. 2006, 30, 853–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Cissé, O.H.; Kovacs, J.A.; Pneumocystis canis C. Weissenbacher-Lang ex L. Ma, O.H. Cissé & J.A. Kovacs, sp. nov. Index Fungorum no. 450 at 2020. Available online: http://www.indexfungorum.org/Publications/Index%20Fungorum%20no.450.pdf (accessed on 20 July 2023).
- Cushion, M.T.; Linke, M.J.; Ashbaugh, A.; Sesterhenn, T.; Collins, M.S.; Lynch, K.; Brubaker, R.; Walzer, P.D. Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS ONE 2010, 5, e8524. [Google Scholar] [CrossRef]
- Cisse, O.H.; Ma, L.; Jiang, C.; Snyder, M.; Kovacs, J.A. Humans are selectively exposed to Pneumocystis jirovecii. MBio 2020, 11, e03138-19. [Google Scholar] [CrossRef] [Green Version]
- Choukri, F.; Menotti, J.; Sarfati, C.; Lucet, J.C.; Nevez, G.; Garin, Y.J.; Derouin, F.; Totet, A. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin. Infect. Dis. 2010, 51, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maini, R.; Henderson, K.L.; Sheridan, E.A.; Lamagni, T.; Nichols, G.; Delpech, V.; Phin, N. Increasing Pneumocystis pneumonia, England, UK, 2000-2010. Emerg. Infect. Dis. 2013, 19, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbrink, B.; Scheikholeslami-Sabzewari, J.; Borzikowsky, C.; von Samson-Himmelstjerna, F.A.; Ullmann, A.J.; Kunzendorf, U.; Schulte, K. Evolving epidemiology of Pneumocystis pneumonia: Findings from a longitudinal population-based study and a retrospective multi-center study in Germany. Lancet Reg. Health-Eur. 2022, 18, 100400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, L.; Jiang, S.J.; Qu, H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: A meta-analysis. Oncotarget 2017, 8, 59729–59739. [Google Scholar] [CrossRef] [Green Version]
- Reid, A.B.; Chen, S.C.; Worth, L.J. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: New risks and diagnostic tools. Curr. Opin. Infect. Dis. 2011, 24, 534–544. [Google Scholar] [CrossRef]
- Roux, A.; Gonzalez, F.; Roux, M.; Mehrad, M.; Menotti, J.; Zahar, J.R.; Tadros, V.X.; Azoulay, E.; Brillet, P.Y.; Vincent, F.; et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med. Mal. Infect. 2014, 44, 185–198. [Google Scholar] [CrossRef]
- Ghembaza, A.; Vautier, M.; Cacoub, P.; Pourcher, V.; Saadoun, D. Risk factors and prevention of Pneumocystis jirovecii neumonia in patients with autoimmune and inflammatory diseases. Chest 2020, 158, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Norris, K.A. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 2012, 25, 297–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepkowitz, K.A.; Brown, A.E.; Telzak, E.E.; Gottlieb, S.; Armstrong, D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 1992, 267, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, M.L.; Martin-Garrido, I.; Donazar-Ezcurra, M.; Limper, A.H.; Carmona, E.M. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can. Respir. J. 2016, 2016, 2464791. [Google Scholar] [CrossRef] [Green Version]
- Baddley, J.W.; Cantini, F.; Goletti, D.; Gomez-Reino, J.J.; Mylonakis, E.; San-Juan, R.; Fernandez-Ruiz, M.; Torre-Cisneros, J. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (Soluble immune effector molecules [I]: Anti-tumor necrosis factor-alpha agents). Clin. Microbiol. Infect. 2018, 24 (Suppl. 2), S10–S20. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, D.; Zhai, K.; Tong, Z. Transcriptomic analysis reveals significant B lymphocyte suppression in corticosteroid-treated hosts with Pneumocystis pneumonia. Am. J. Respir. Cell Mol. Biol. 2017, 56, 322–331. [Google Scholar] [CrossRef]
- Climans, S.A.; Grunfeld, E.; Mason, W.P.; Chan, K.K.W. Effectiveness and safety of Pneumocystis pneumonia prophylaxis for patients receiving temozolomide chemoradiotherapy. Neuro Oncol. 2022, 24, 1738–1748. [Google Scholar] [CrossRef]
- De Vos, F.Y.; Gijtenbeek, J.M.; Bleeker-Rovers, C.P.; van Herpen, C.M. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit. Rev. Oncol. Hematol. 2013, 85, 373–382. [Google Scholar] [CrossRef]
- Sen, R.P.; Walsh, T.E.; Fisher, W.; Brock, N. Pulmonary complications of combination therapy with cyclophosphamide and prednisone. Chest 1991, 99, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kang, M.; Suh, K.J.; Kim, J.W.; Kim, S.H.; Kim, J.W.; Kim, Y.J.; Song, K.H.; Kim, E.S.; Kim, H.B.; et al. Pneumocystis jirovecii pneumonia in diffuse large B-cell lymphoma treated with R-CHOP. Mycoses 2021, 64, 60–65. [Google Scholar] [CrossRef]
- Browne, M.J.; Hubbard, S.M.; Longo, D.L.; Fisher, R.; Wesley, R.; Ihde, D.C.; Young, R.C.; Pizzo, P.A. Excess prevalence of Pneumocystis carinii pneumonia in patients treated for lymphoma with combination chemotherapy. Ann. Intern. Med. 1986, 104, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.M.; Limper, A.H. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther. Adv. Respir. Dis. 2011, 5, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cummings, C.; Faulkner, M.; Obianyo, I. Pneumocystis carinii pneumonia following 5-fluorouracil administration. J. Natl. Med. Assoc. 1987, 79, 1205–1209. [Google Scholar] [PubMed]
- Hughes, W.T.; Feldman, S.; Aur, R.J.; Verzosa, M.S.; Hustu, H.O.; Simone, J.V. Intensity of immunosuppressive therapy and the incidence of Pneumocystis carinii pneumonitis. Cancer 1975, 36, 2004–2009. [Google Scholar] [CrossRef]
- Havele, S.A.; Ellis, A.; Chaitoff, A.; Khanna, U.; Parambil, J.; Langford, C.A.; Fernandez, A.P. Safety of trimethoprim-sulfamethoxazole for Pneumocystis jirovecii pneumonia prophylaxis in patients taking methotrexate. J. Am. Acad. Dermatol. 2021, 84, 166–168. [Google Scholar] [CrossRef]
- Rifkind, D.; Starzl, T.E.; Marchioro, T.L.; Waddell, W.R.; Rowlands, D.T., Jr.; Hill, R.B., Jr. Transplantation Pneumonia. JAMA 1964, 189, 808–812. [Google Scholar] [CrossRef] [Green Version]
- Seddik, M.; Melliez, H.; Seguy, D.; Viget, N.; Cortot, A.; Colombel, J.F. Pneumocystis jiroveci (carinii) pneumonia after initiation of infliximab and azathioprine therapy in a patient with Crohn’s disease. Inflamm. Bowel Dis. 2005, 11, 618–620. [Google Scholar] [CrossRef]
- Obeid, K.M.; Aguilar, J.; Szpunar, S.; Sharma, M.; del Busto, R.; Al-Katib, A.; Johnson, L.B. Risk factors for Pneumocystis jirovecii pneumonia in patients with lymphoproliferative disorders. Clin. Lymphoma Myeloma Leuk. 2012, 12, 66–69. [Google Scholar] [CrossRef]
- Schilling, P.J.; Vadhan-Raj, S. Concurrent cytomegalovirus and Pneumocystis pneumonia after fludarabine therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 1990, 323, 833–834. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hargis, J.B.; Kester, K.E.; Hospenthal, D.R.; Knutson, S.W.; Diehl, L.F. Opportunistic pulmonary infections with fludarabine in previously treated patients with low-grade lymphoid malignancies: A role for Pneumocystis carinii pneumonia prophylaxis. Am. J. Hematol. 1995, 49, 135–142. [Google Scholar] [CrossRef]
- Maertens, J.; Cesaro, S.; Maschmeyer, G.; Einsele, H.; Donnelly, J.P.; Alanio, A.; Hauser, P.M.; Lagrou, K.; Melchers, W.J.; Helweg-Larsen, J.; et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 2016, 71, 2397–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Garrido, I.; Carmona, E.M.; Specks, U.; Limper, A.H. Pneumocystis pneumonia in patients treated with rituximab. Chest 2013, 144, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, M.; Chakravorty, M.; Lanyon, P.C.; Courtney, P. Pneumocystis jirovecii following rituximab. Rheumatology 2021, 60, iii70–iii72. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.I.; Marty, F.M.; Fiumara, K.; Treon, S.P.; Gribben, J.G.; Baden, L.R. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin. Infect. Dis. 2006, 43, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ospina, F.E.; Agualimpia, A.; Bonilla-Abadia, F.; Canas, C.A.; Tobon, G.J. Pneumocystis jirovecii pneumonia in a patient with rheumatoid arthritis treated with abatacept. Case Rep. Rheumatol. 2014, 2014, 835050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P.J.; Gottlieb, A.B.; van der Heijde, D.; FitzGerald, O.; Johnsen, A.; Nys, M.; Banerjee, S.; Gladman, D.D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 2017, 76, 1550–1558. [Google Scholar] [CrossRef]
- Azar, M.M.; Cohen, E.; Ma, L.; Cisse, O.H.; Gan, G.; Deng, Y.; Belfield, K.; Asch, W.; Grant, M.; Gleeson, S.; et al. Genetic and epidemiologic analyses of an outbreak of Pneumocystis jirovecii pneumonia among kidney transplant recipients in the United States. Clin. Infect. Dis. 2022, 74, 639–647. [Google Scholar] [CrossRef]
- Haidinger, M.; Hecking, M.; Memarsadeghi, M.; Weichhart, T.; Werzowa, J.; Horl, W.H.; Saemann, M.D. Late onset Pneumocystis pneumonia in renal transplantation after long-term immunosuppression with belatacept. Transpl. Infect. Dis. 2009, 11, 171–174. [Google Scholar] [CrossRef]
- Bertrand, D.; Chavarot, N.; Gatault, P.; Garrouste, C.; Bouvier, N.; Grall-Jezequel, A.; Jaureguy, M.; Caillard, S.; Lemoine, M.; Colosio, C.; et al. Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol. Dial. Transplant. 2020, 35, 336–345. [Google Scholar] [CrossRef]
- Kameda, H.; Tokuda, H.; Sakai, F.; Johkoh, T.; Mori, S.; Yoshida, Y.; Takayanagi, N.; Taki, H.; Hasegawa, Y.; Hatta, K.; et al. Clinical and radiological features of acute-onset diffuse interstitial lung diseases in patients with rheumatoid arthritis receiving treatment with biological agents: Importance of Pneumocystis pneumonia in Japan revealed by a multicenter study. Intern. Med. 2011, 50, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Schiff, M.H.; Kremer, J.M.; Jahreis, A.; Vernon, E.; Isaacs, J.D.; van Vollenhoven, R.F. Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 2011, 13, R141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhaneni, S.; Chiller, T.M. Fungal Infections and New Biologic Therapies. Curr. Rheumatol. Rep. 2016, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sakai, R.; Koike, R.; Sakai, F.; Sugiyama, H.; Tanaka, M.; Komano, Y.; Akiyama, Y.; Mimura, T.; Kaneko, M.; et al. Clinical characteristics and risk factors for Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis receiving adalimumab: A retrospective review and case-control study of 17 patients. Mod. Rheumatol. 2013, 23, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Desales, A.L.; Mendez-Navarro, J.; Mendez-Tovar, L.J.; Ortiz-Olvera, N.X.; Cullen, G.; Ocampo, J.; Lemus, W.; Tun, A.E.; Mayoral-Zavala, A.; Dehesa-Violante, M. Pneumocystosis in a patient with Crohn’s disease treated with combination therapy with adalimumab. J. Crohn’s Colitis 2012, 6, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Sakai, R.; Koike, R.; Komano, Y.; Nanki, T.; Sakai, F.; Sugiyama, H.; Matsushima, H.; Kojima, T.; Ohta, S.; et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: A retrospective review of 15 cases and analysis of risk factors. Mod. Rheumatol. 2012, 22, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kameda, H. The Japanese experience with biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2010, 6, 644–652. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Kyriakidis, I.; Zundorf, I.; Groll, A.H. Invasive fungal infections in pediatric patients treated with tumor necrosis alpha (TNF-alpha) inhibitors. Mycoses 2017, 60, 222–229. [Google Scholar] [CrossRef]
- Tanaka, Y.; Senoo, A.; Fujii, H.; Baker, D. Evaluation of golimumab for the treatment of patients with active rheumatoid arthritis. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 319–326. [Google Scholar] [CrossRef]
- Kaur, N.; Mahl, T.C. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: A review of 84 cases. Dig. Dis. Sci. 2007, 52, 1481–1484. [Google Scholar] [CrossRef]
- Harigai, M.; Koike, R.; Miyasaka, N. Pneumocystis Pneumonia Under Anti-Tumor Necrosis Factor Therapy (PAT) Study, Group. Pneumocystis pneumonia associated with infliximab in Japan. N. Engl. J. Med. 2007, 357, 1874–1876. [Google Scholar] [CrossRef]
- Markus, M.B. Cyclosporin A and Pneumocystis pneumonia. Med. J. Aust. 1985, 143, 91. [Google Scholar] [CrossRef] [PubMed]
- Franson, T.R.; Kauffman, H.M., Jr.; Adams, M.B.; Lemann, J., Jr.; Cabrera, E.; Hanacik, L. Cyclosporine therapy and refractory Pneumocystis carinii pneumonia. A potential association. Arch. Surg. 1987, 122, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Lionet, A.; Kipnis, E.; Noel, C.; Hazzan, M. Risk factors for Pneumocystis pneumonia after the first 6 months following renal transplantation. Transpl. Infect. Dis. 2017, 19, e12735. [Google Scholar] [CrossRef]
- Matsuoka, K.; Saito, E.; Fujii, T.; Takenaka, K.; Kimura, M.; Nagahori, M.; Ohtsuka, K.; Watanabe, M. Tacrolimus for the Treatment of Ulcerative Colitis. Intest. Res. 2015, 13, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escher, M.; Stange, E.F.; Herrlinger, K.R. Two cases of fatal Pneumocystis jirovecii pneumonia as a complication of tacrolimus therapy in ulcerative colitis—A need for prophylaxis. J. Crohn’s Colitis 2010, 4, 606–609. [Google Scholar] [CrossRef] [Green Version]
- Miguel Montanes, R.; Elkrief, L.; Hajage, D.; Houssel, P.; Fantin, B.; Francoz, C.; Dreyfuss, D.; Ricard, J.D.; Durand, F. An outbreak of Pneumocytis jirovecii pneumonia among liver transplant recipients. Transpl. Infect. Dis. 2018, 20, e12956. [Google Scholar] [CrossRef]
- Hu, Y.N.; Lee, N.Y.; Roan, J.N.; Hsu, C.H.; Luo, C.Y. High-dose calcineurin inhibitor-free everolimus as a maintenance regimen for heart transplantation may be a risk factor for Pneumocystis pneumonia. Transpl. Infect. Dis. 2017, 19, e12709. [Google Scholar] [CrossRef]
- Loron, M.C.; Grange, S.; Guerrot, D.; Di Fiore, F.; Freguin, C.; Hanoy, M.; Le Roy, F.; Poussard, G.; Etienne, I.; Legallicier, B.; et al. Pneumocystis jirovecii pneumonia in everolimus-treated renal cell carcinoma. J. Clin. Oncol. 2015, 33, e45–e47. [Google Scholar] [CrossRef]
- Nakamura, M.; Matsunuma, R.; Yamaguchi, K.; Hayami, R.; Tsuneizumi, M. Pneumocystis pneumonia and acute kidney injury induced by everolimus treatment in a patient with metastatic breast cancer. Case Rep. Oncol. 2020, 13, 170–175. [Google Scholar] [CrossRef]
- Shetty, A.K. Pneumocystis jirovecii pneumonia: A potential complication of sirolimus therapy. J. Paediatr. Child. Health 2019, 55, 484. [Google Scholar] [CrossRef] [Green Version]
- Russell, T.B.; Rinker, E.K.; Dillingham, C.S.; Givner, L.B.; McLean, T.W. Pneumocystis jirovecii pneumonia during sirolimus therapy for kaposiform hemangioendothelioma. Pediatrics 2018, 141, S421–S424. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, M.; Mohammadpour, Z.; Dashti-Khavidaki, S.; Milajerdi, A. m-TOR inhibitors and risk of Pneumocystis pneumonia after solid organ transplantation: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2019, 75, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, A.; Holte, H.; Fossa, A.; Lauritzsen, G.F.; Gaustad, P.; Torfoss, D. Pneumocystis jirovecii pneumonia in B-cell lymphoma patients treated with the rituximab-CHOEP-14 regimen. Haematologica 2007, 92, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Curtis, J.R.; Jun, K.I.; Kim, T.M.; Heo, D.S.; Ha, J.; Suh, K.S.; Lee, K.W.; Lee, H.; Yang, J.; et al. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients receiving rituximab. Chest 2022, 161, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Robak, T.; Brown, J.R.; Awan, F.T.; Badoux, X.; Coutre, S.; Loscertales, J.; Taylor, K.; Vandenberghe, E.; Wach, M.; et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: An open-label, randomised phase 3 trial. Lancet Haematol. 2017, 4, e114–e126. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.N.; Ice, L.L.; Thompson, C.A.; Tosh, P.K.; Osmon, D.R.; Dierkhising, R.A.; Plevak, M.F.; Limper, A.H. Low incidence of pneumocystis pneumonia utilizing PCR-based diagnosis in patients with B-cell lymphoma receiving rituximab-containing combination chemotherapy. Am. J. Hematol. 2016, 91, 1113–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, F.E.; Hollifield, M.; Schuer, K.; Lines, J.L.; Randall, T.D.; Garvy, B.A. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J. Immunol. 2006, 176, 6147–6154. [Google Scholar] [CrossRef] [Green Version]
- Mikulska, M.; Lanini, S.; Gudiol, C.; Drgona, L.; Ippolito, G.; Fernandez-Ruiz, M.; Salzberger, B. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin. Microbiol. Infect. 2018, 24 (Suppl. 2), S71–S82. [Google Scholar] [CrossRef] [Green Version]
- Lau, A.Y.; Lui, G.C.Y.; Chan, K.P.; Au, C.; Mok, V.C.T.; Ziemssen, T. Pneumocystis pneumonia in a patient treated with alemtuzumab for relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101503. [Google Scholar] [CrossRef]
- Dustin, P., Jr.; Maurus, R. Fatal pneumopathy caused by “Pneumocystis carinii” in a leukemic child treated with prednisone. Bull. L’academie R. Med. Belg. 1959, 24, 566–579. [Google Scholar]
- Symmers, W.S. Generalized cytomegalic inclusion-body disease associated with Pneumocystis pneumonia in adults. A report of three cases. with Wegener’s granulomatosis. thrombotic purpura, and Hodgkin’s disease as predisposing conditions. J. Clin. Pathol. 1960, 13, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemiale, V.; Debrumetz, A.; Delannoy, A.; Alberti, C.; Azoulay, E. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir. Res. 2013, 14, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaborit, B.J.; Tessoulin, B.; Lavergne, R.A.; Morio, F.; Sagan, C.; Canet, E.; Lecomte, R.; Leturnier, P.; Deschanvres, C.; Khatchatourian, L.; et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: A prospective observational study. Ann. Intensive Care 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Hardak, E.; Neuberger, A.; Yigla, M.; Berger, G.; Finkelstein, R.; Sprecher, H.; Oren, I. Outcome of Pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology 2012, 17, 681–686. [Google Scholar] [CrossRef]
- Roblot, F.; Godet, C.; Le Moal, G.; Garo, B.; Faouzi Souala, M.; Dary, M.; De Gentile, L.; Gandji, J.A.; Guimard, Y.; Lacroix, C.; et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 523–531. [Google Scholar] [CrossRef]
- Fillatre, P.; Decaux, O.; Jouneau, S.; Revest, M.; Gacouin, A.; Robert-Gangneux, F.; Fresnel, A.; Guiguen, C.; Le Tulzo, Y.; Jego, P.; et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am. J. Med. 2014, 127, 1242.e11–1242.e17. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Lee, J.; Cho, Y.J.; Park, Y.S.; Lee, C.H.; Yoon, H.I.; Lee, S.M.; Yim, J.J.; Lee, J.H.; Yoo, C.G.; et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J. Infect. 2014, 69, 88–95. [Google Scholar] [CrossRef]
- Roblot, F.; Le Moal, G.; Kauffmann-Lacroix, C.; Bastides, F.; Boutoille, D.; Verdon, R.; Godet, C.; Tattevin, P.; Groupe d’Etudes et de Recherche en Infectiologie Clinique du Centre Ouest. Pneumocystis jirovecii pneumonia in HIV-negative patients: A prospective study with focus on immunosuppressive drugs and markers of immune impairment. Scand. J. Infect. Dis. 2014, 46, 210–214. [Google Scholar] [CrossRef]
- Kofteridis, D.P.; Valachis, A.; Velegraki, M.; Antoniou, M.; Christofaki, M.; Vrentzos, G.E.; Andrianaki, A.M.; Samonis, G. Predisposing factors, clinical characteristics and outcome of Pneumonocystis jirovecii pneumonia in HIV-negative patients. J. Infect. Chemother. 2014, 20, 412–416. [Google Scholar] [CrossRef]
- Takeda, K.; Harada, S.; Hayama, B.; Hoashi, K.; Enokida, T.; Sasaki, T.; Okamoto, K.; Nakano, K.; Ohkushi, D. Clinical characteristics and risk factors associated with Pneumocystis jirovecii infection in patients with solid tumors: Study of thirteen-year medical records of a large cancer center. BMC Cancer 2021, 21, 987. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, E.Y.; Lee, S.H.; Roh, Y.H.; Leem, A.Y.; Song, J.H.; Kim, S.Y.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; et al. Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci. Rep. 2019, 9, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, W.T.; Kuhn, S.; Chaudhary, S.; Feldman, S.; Verzosa, M.; Aur, R.J.; Pratt, C.; George, S.L. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. N. Engl. J. Med. 1977, 297, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Prevention of infection due to Pneumocystis carinii. Antimicrob. Agents Chemother. 1998, 42, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.M.; LaRosa, S.P.; Kalmadi, S.; Arroliga, A.C.; Avery, R.K.; Truesdell-LaRosa, L.; Longworth, D.L. Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin. Infect. Dis. 1999, 28, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.I.; Fishman, J.A.; Practice ASTIDCo. Pneumocystis pneumonia in solid organ transplantation. Am. J. Transplant. 2013, 13 (Suppl. 4), 272–279. [Google Scholar] [CrossRef]
- Vindrios, W.; Argy, N.; Le Gal, S.; Lescure, F.X.; Massias, L.; Le, M.P.; Wolff, M.; Yazdanpanah, Y.; Nevez, G.; Houze, S.; et al. Outbreak of Pneumocystis jirovecii Infection Among Heart Transplant Recipients: Molecular Investigation and Management of an Interhuman Transmission. Clin. Infect. Dis. 2017, 65, 1120–1126. [Google Scholar] [CrossRef]
- Hosseini-Moghaddam, S.M.; Shokoohi, M.; Singh, G.; Dufresne, S.F.; Boucher, A.; Jevnikar, A.; Prasad, G.V.R.; Shoker, A.; Kabbani, D.; Hebert, M.J.; et al. A multicenter case-control study of the effect of acute rejection and cytomegalovirus infection on Pneumocystis pneumonia in solid organ transplant recipients. Clin. Infect. Dis. 2019, 68, 1320–1326. [Google Scholar] [CrossRef]
- Permpalung, N.; Kittipibul, V.; Mekraksakit, P.; Rattanawong, P.; Nematollahi, S.; Zhang, S.X.; Steinke, S.M. A comprehensive evaluation of risk factors for Pneumocystis jirovecii pneumonia in adult solid organ transplant recipients: A systematic review and meta-analysis. Transplantation 2021, 105, 2291–2306. [Google Scholar] [CrossRef]
- Brakemeier, S.; Pfau, A.; Zukunft, B.; Budde, K.; Nickel, P. Prophylaxis and treatment of Pneumocystis jirovecii pneumonia after solid organ transplantation. Pharmacol. Res. 2018, 134, 61–67. [Google Scholar] [CrossRef]
- Fishman, J.A.; Gans, H.A.S.T. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13587. [Google Scholar] [CrossRef]
- Bruce, E.S.; Kearsley-Fleet, L.; Watson, K.D.; Symmons, D.P.; Hyrich, K.L. Risk of Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis treated with inhibitors of tumour necrosis factor alpha: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology 2016, 55, 1336–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louie, G.H.; Wang, Z.; Ward, M.M. Trends in hospitalizations for Pneumocystis jiroveci pneumonia among patients with rheumatoid arthritis in the US: 1996–2007. Arthritis Rheum. 2010, 62, 3826–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.M.; Donald, F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: The role of hospital experience in diagnosis and mortality. Arthritis Rheum. 1999, 42, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Sugimoto, M. Pneumocystis jirovecii pneumonia in rheumatoid arthritis patients: Risks and prophylaxis recommendations. Clin. Med. Insights Circ. Respir. Pulm. Med. 2015, 9, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sonomoto, K.; Tanaka, H.; Nguyen, T.M.; Yoshinari, H.; Nakano, K.; Nakayamada, S.; Tanaka, Y. Prophylaxis against pneumocystis pneumonia in rheumatoid arthritis patients treated with b/tsDMARDs: Insights from 3,787 cases in FIRST registry. Rheumatology 2021, 61, 1831–1840. [Google Scholar] [CrossRef]
- Mimori, T.; Harigai, M.; Atsumi, T.; Fujii, T.; Kuwana, M.; Matsuno, H.; Momohara, S.; Takei, S.; Tamura, N.; Takasaki, Y.; et al. Safety and effectiveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: Interim analysis of a post-marketing surveillance study of 2679 patients in Japan. Mod. Rheumatol. 2017, 27, 755–765. [Google Scholar] [CrossRef]
- Hashimoto, A.; Suto, S.; Horie, K.; Fukuda, H.; Nogi, S.; Iwata, K.; Tsuno, H.; Ogihara, H.; Kawakami, M.; Komiya, A.; et al. Incidence and Risk Factors for Infections Requiring Hospitalization, Including Pneumocystis Pneumonia, in Japanese Patients with Rheumatoid Arthritis. Int. J. Rheumatol. 2017, 2017, 6730812. [Google Scholar] [CrossRef]
- Yukawa, K.; Nagamoto, Y.; Watanabe, H.; Funaki, M.; Iwahashi, M.; Yamana, J.; Sasaki, R.; Yamana, S. Risk Factors for Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis and a prophylactic indication of trimethoprim/sulfamethoxazole. J. Clin. Rheumatol. 2018, 24, 355–360. [Google Scholar] [CrossRef]
- Tokuda, H.; Sakai, F.; Yamada, H.; Johkoh, T.; Imamura, A.; Dohi, M.; Hirakata, M.; Yamada, T.; Kamatani, N.; Kikuchi, Y.; et al. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: A multicenter study. Intern. Med. 2008, 47, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Mecoli, C.A.; Saylor, D.; Gelber, A.C.; Christopher-Stine, L. Pneumocystis jiroveci pneumonia in rheumatic disease: A 20-year single-centre experience. Clin. Exp. Rheumatol. 2017, 35, 671–673. [Google Scholar]
- Hsu, H.C.; Chang, Y.S.; Hou, T.Y.; Chen, L.F.; Hu, L.F.; Lin, T.M.; Chiou, C.S.; Tsai, K.L.; Lin, S.H.; Kuo, P.I.; et al. Pneumocystis jirovecii pneumonia in autoimmune rheumatic diseases: A nationwide population-based study. Clin. Rheumatol. 2021, 40, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ghannoum, M.; Deng, C.; Gao, Y.; Zhu, H.; Yu, X.; Lavergne, V. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: Usefulness of lymphocyte subtyping. Int. J. Infect. Dis. 2017, 57, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godeau, B.; Coutant-Perronne, V.; Le Thi Huong, D.; Guillevin, L.; Magadur, G.; De Bandt, M.; Dellion, S.; Rossert, J.; Rostoker, G.; Piette, J.C.; et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: Report of 34 cases. J. Rheumatol. 1994, 21, 246–251. [Google Scholar] [PubMed]
- Lertnawapan, R.; Totemchokchyakarn, K.; Nantiruj, K.; Janwityanujit, S. Risk factors of Pneumocystis jeroveci pneumonia in patients with systemic lupus erythematosus. Rheumatol. Int. 2009, 29, 491–496. [Google Scholar] [CrossRef]
- Wang, Z.G.; Liu, X.M.; Wang, Q.; Chen, N.F.; Tong, S.Q. A retrospective study of patients with systemic lupus erythematosus combined with Pneumocystis jiroveci pneumonia treated with caspofungin and trimethoprim/sulfamethoxazole. Medicine 2019, 98, e15997. [Google Scholar] [CrossRef]
- Gupta, D.; Zachariah, A.; Roppelt, H.; Patel, A.M.; Gruber, B.L. Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: A survey of US rheumatologists and the review of literature. J. Clin. Rheumatol. 2008, 14, 267–272. [Google Scholar] [CrossRef]
- Yeo, K.J.; Chen, H.H.; Chen, Y.M.; Lin, C.H.; Chen, D.Y.; Lai, C.M.; Chao, W.C. Hydroxychloroquine may reduce risk of Pneumocystis pneumonia in lupus patients: A Nationwide, population-based case-control study. BMC Infect. Dis. 2020, 20, 112. [Google Scholar] [CrossRef] [Green Version]
- Lahiff, C.; Khiaron, O.B.; Nolan, N.; Chadwick, G.A. Pneumocystis carinii pneumonia in a patient on etanercept for psoriatic arthritis. Ir. J. Med. Sci. 2007, 176, 309–311. [Google Scholar] [CrossRef]
- Jobanputra, P. Polyarteritis nodosa. Diagnostic challenges in a patient with cutaneous vasculitis, psoriasis, psoriatic arthritis and pancytopenia: Fatal progression after treatment with G-CSF. Oxf. Med. Case Reports. 2016, 2016, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.K.; Chen, M.L. Remission of nephrotic membranous glomerulonephritis after high-dose trimethoprim-sulfamethoxazole treatment for pneumocystis jiroveci pneumonia. Clin. Nephrol. 2007, 68, 99–103. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yang, A.H.; Yang, W.C.; Lin, C.C. Risk factors for Pneumocystis jiroveci pneumonia in glomerulonephritis patients receiving immunosuppressants. Intern. Med. 2012, 51, 2869–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez Roca, J.J.; Pelaez Ballesta, A.; Lara, G.; Soto, S.; Mene Fenor, E. Diffuse alveolar hemorrhage associated with Henoch-Schonlein purpura and Pneumocystis jirovecii infection: A case report. Rev. Clin. Esp. 2015, 215, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Bungo, M.W.; Beetham, W.P., Jr. Arthritis rounds. Pneumocystis carinii associated with polyarteritis and immunosuppressive therapy. Arthritis Rheum. 1977, 20, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.E.; Neely, J.; Chow, A.; DeGuzman, M.; Lai, J.; Lvovich, S.; McGrath, T.; Pereira, M.; Pinal-Fernandez, I.; Roberts, J.; et al. Risk factors associated with Pneumocystis jirovecii pneumonia in juvenile myositis in North America. Rheumatology 2021, 60, 829–836. [Google Scholar] [CrossRef]
- Cotter, T.G.; Gathaiya, N.; Catania, J.; Loftus, E.V., Jr.; Tremaine, W.J.; Baddour, L.M.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J.; Limper, A.H.; et al. Low risk of pneumonia from Pneumocystis jirovecii infection in patients with inflammatory bowel disease receiving immune suppression. Clin. Gastroenterol. Hepatol. 2017, 15, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, S.J.; Sadarangani, M.; Jacobson, K. Pneumocystis jirovecii pneumonia in pediatric inflammatory bowel disease: A case report and literature review. Front. Pediatr. 2017, 5, 161. [Google Scholar] [CrossRef]
- Schwartz, J.; Stein, D.J.; Feuerstein, J.D. Comprehensive national inpatient Sample data reveals low but rising Pneumocystis jiroveci pneumonia risk in inflammatory bowel disease patients. Ann. Gastroenterol. 2022, 35, 260–266. [Google Scholar] [CrossRef]
- Yoshida, A.; Kamata, N.; Yamada, A.; Yokoyama, Y.; Omori, T.; Fujii, T.; Hayashi, R.; Kinjo, T.; Matsui, A.; Fukata, N.; et al. Risk factors for mortality in Pneumocystis jirovecii pneumonia in patients with inflammatory bowel disease. Inflamm. Intest. Dis. 2019, 3, 167–172. [Google Scholar] [CrossRef]
- Long, M.D.; Farraye, F.A.; Okafor, P.N.; Martin, C.; Sandler, R.S.; Kappelman, M.D. Increased risk of pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.; Park, S.H.; Lee, J.; Jo, S.; Kim, S.O.; Noh, S.; Park, J.C.; Kim, J.Y.; Kim, J.; Ham, N.S.; et al. Incidence and risk factors of Pneumocystis jirovecii pneumonia in Korean patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2020, 35, 218–224. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Radford-Smith, G.L.; Bampton, P.A.; Andrews, J.M.; Tan, P.K.; Croft, A.; Gearry, R.B.; Florin, T.H. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: An Australian and New Zealand experience. J. Gastroenterol. Hepatol. 2010, 25, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Baulier, G.; Issa, N.; Gabriel, F.; Accoceberry, I.; Camou, F.; Duffau, P. Guidelines for prophylaxis of Pneumocystis pneumonia cannot rely solely on CD4-cell count in autoimmune and inflammatory diseases. Clin. Exp. Rheumatol. 2018, 36, 490–493. [Google Scholar] [PubMed]
- Falagas, M.E.; Manta, K.G.; Betsi, G.I.; Pappas, G. Infection-related morbidity and mortality in patients with connective tissue diseases: A systematic review. Clin. Rheumatol. 2007, 26, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sowden, E.; Carmichael, A.J. Autoimmune inflammatory disorders, systemic corticosteroids and Pneumocystis pneumonia: A strategy for prevention. BMC Infect. Dis. 2004, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Ognibene, F.P.; Shelhamer, J.H.; Hoffman, G.S.; Kerr, G.S.; Reda, D.; Fauci, A.S.; Leavitt, R.Y. Pneumocystis carinii pneumonia: A major complication of immunosuppressive therapy in patients with Wegener’s granulomatosis. Am. J. Respir. Crit. Care Med. 1995, 151, 795–799. [Google Scholar] [CrossRef]
- Decker, J.L.; Klippel, J.H.; Plotz, P.H.; Steinberg, A.D. Cyclophosphamide or azathioprine in lupus glomerulonephritis. A controlled trial: Results at 28 months. Ann. Intern. Med. 1975, 83, 606–615. [Google Scholar]
- Harder, M.Z.; Razzaque, M.A.; Shazzad, M.N.; Haq, S.A.; Ahmed, S.; Ahmed, S.N. Pneumocystis jiroveci pnemonia in systemic lupus erythematosus: A case report. Int. J. Med. Health Res. 2017, 3, 84–86. [Google Scholar]
- Weng, C.T.; Liu, M.F.; Weng, M.Y.; Lee, N.Y.; Wang, M.C.; Lin, W.C.; Ou, C.Y.; Lai, W.W.; Hsu, S.C.; Chao, S.C.; et al. Pneumocystis jirovecii pneumonia in systemic lupus erythematosus from southern Taiwan. J. Clin. Rheumatol. 2013, 19, 252–258. [Google Scholar] [CrossRef]
- Mori, S.; Sugimoto, M. Pneumocystis jirovecii infection: An emerging threat to patients with rheumatoid arthritis. Rheumatology 2012, 51, 2120–2130. [Google Scholar] [CrossRef] [Green Version]
- Iikuni, N.; Kitahama, M.; Ohta, S.; Okamoto, H.; Kamatani, N.; Nishinarita, M. Evaluation of Pneumocystis pneumonia infection risk factors in patients with connective tissue disease. Mod. Rheumatol. 2006, 16, 282–288. [Google Scholar] [CrossRef]
- Gajdusek, D.C. Pneumocystis carinii; etiologic agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics 1957, 19, 543–565. [Google Scholar] [PubMed]
- Goldman, A.S.; Goldman, L.R.; Goldman, D.A. What caused the epidemic of Pneumocystis pneumonia in European premature infants in the mid-20th century? Pediatrics 2005, 115, e725–e736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmes, E.C.; Chen, J.L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding early-life adaptive immunity to guide interventions for pediatric health. Front. Immunol. 2020, 11, 595297. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023. [Google Scholar] [CrossRef]
- Montes-Cano, M.A.; Chabe, M.; Fontillon-Alberdi, M.; de-Lahorra, C.; Respaldiza, N.; Medrano, F.J.; Varela, J.M.; Dei-Cas, E.; Calderon, E.J. Vertical transmission of Pneumocystis jirovecii in humans. Emerg. Infect. Dis. 2009, 15, 125–127. [Google Scholar] [CrossRef]
- Szydlowicz, M.; Krolak-Olejnik, B.; Vargas, S.L.; Zajaczkowska, Z.; Paluszynska, D.; Szczygiel, A.; Matos, O.; Hendrich, A.B.; Kicia, M. Pneumocystis jirovecii colonization in preterm newborns with respiratory distress syndrome. J. Infect. Dis. 2022, 225, 1807–1810. [Google Scholar] [CrossRef]
- Rojas, P.; Friaza, V.; Garcia, E.; de la Horra, C.; Vargas, S.L.; Calderon, E.J.; Pavon, A. early acquisition of Pneumocystis jirovecii colonization and potential association with respiratory distress syndrome in preterm newborn infants. Clin. Infect. Dis. 2017, 65, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Basiaga, M.L.; Ross, M.E.; Gerber, J.S.; Ogdie, A. Incidence of Pneumocystis jirovecii and adverse events associated with Pneumocystis prophylaxis in children receiving glucocorticoids. J. Pediatric Infect. Dis. Soc. 2018, 7, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Terblanche, A.J.; Green, R.J.; Rheeder, P.; Wittenberg, D.F. Adjunctive corticosteroid treatment of clinical Pneumocystis jiroveci pneumonia in infants less than 18 months of age—A randomised controlled trial. S. Afr. Med. J. 2008, 98, 287–290. [Google Scholar]
- Peglow, S.L.; Smulian, A.G.; Linke, M.J.; Pogue, C.L.; Nurre, S.; Crisler, J.; Phair, J.; Gold, J.W.; Armstrong, D.; Walzer, P.D. Serologic responses to Pneumocystis carinii antigens in health and disease. J. Infect. Dis. 1990, 161, 296–306. [Google Scholar] [CrossRef]
- Wakefield, A.E.; Stewart, T.J.; Moxon, E.R.; Marsh, K.; Hopkin, J.M. Infection with Pneumocystis carinii is prevalent in healthy Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Fox, M.R.; Lawrence, G.G.; Guarner, J.; Hanzlick, R.L.; Huang, L.; del Rio, C.; Rimland, D.; Duchin, J.S.; Colley, D.G. Genetic differences in Pneumocystis isolates recovered from immunocompetent infants and from adults with AIDS: Epidemiological Implications. J. Infect. Dis. 2005, 192, 1815–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walzer, P.D.; Schultz, M.G.; Western, K.A.; Robbins, J.F. Pneumocystis carinii pneumonia and primary immune deficiency diseases. Natl. Cancer Inst. Monogr. 1976, 43, 65–74. [Google Scholar] [PubMed]
- Gray, P.E.; Logan, G.J.; Alexander, I.E.; Poulton, S.; Roscioli, T.; Ziegler, J. A novel intronic splice site deletion of the IL-2 receptor common gamma chain results in expression of a dysfunctional protein and T-cell-positive X-linked Severe combined immunodeficiency. Int. J. Immunogenet. 2015, 42, 11–14. [Google Scholar] [CrossRef]
- Sato, T.; Okano, T.; Tanaka-Kubota, M.; Kimura, S.; Miyamoto, S.; Ono, S.; Yamashita, M.; Mitsuiki, N.; Takagi, M.; Imai, K.; et al. Novel compound heterozygous mutations in a Japanese girl with Janus kinase 3 deficiency. Pediatr. Int. 2016, 58, 1076–1080. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, H.; Lian, C.; Wu, B.; Lin, J.; Huang, G.; Cui, B. Case Report: Mutations in JAK3 causing severe combined immunodeficiency complicated by disseminated Bacille Calmette-Guerin disease and Pneumocystis pneumonia. Front. Immunol. 2022, 13, 1055607. [Google Scholar] [CrossRef]
- Hershfield. Adenosine Deaminase Deficiency. In GeneReviews((R)); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2017. [Google Scholar]
- Stepensky, P.; Keller, B.; Buchta, M.; Kienzler, A.K.; Elpeleg, O.; Somech, R.; Cohen, S.; Shachar, I.; Miosge, L.A.; Schlesier, M.; et al. Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects. J. Allergy Clin. Immunol. 2013, 131, 477–485.e1. [Google Scholar] [CrossRef]
- Fuchs, S.; Rensing-Ehl, A.; Pannicke, U.; Lorenz, M.R.; Fisch, P.; Jeelall, Y.; Rohr, J.; Speckmann, C.; Vraetz, T.; Farmand, S.; et al. Omenn syndrome associated with a functional reversion due to a somatic second-site mutation in CARD11 deficiency. Blood. 2015, 126, 1658–1669. [Google Scholar] [CrossRef] [Green Version]
- Al-Saud, B.K.; Al-Sum, Z.; Alassiri, H.; Al-Ghonaium, A.; Al-Muhsen, S.; Al-Dhekri, H.; Arnaout, R.; Alsmadi, O.; Borrero, E.; Abu-Staiteh, A.; et al. Clinical, immunological, and molecular characterization of hyper-IgM syndrome due to CD40 deficiency in eleven patients. J. Clin. Immunol. 2013, 33, 1325–1335. [Google Scholar] [CrossRef]
- Levy, J.; Espanol-Boren, T.; Thomas, C.; Fischer, A.; Tovo, P.; Bordigoni, P.; Resnick, I.; Fasth, A.; Baer, M.; Gomez, L.; et al. Clinical spectrum of X-linked hyper-IgM syndrome. J. Pediatr. 1997, 131, 47–54. [Google Scholar] [CrossRef]
- Brunet, B.A.; Rodriguez, R. Unusual presentation of combined immunodeficiency in a child with homozygous DOCK8 mutation. Ann. Allergy Asthma Immunol. 2017, 119, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Jakobsen, M.A.; Larsen, M.J.; Muller, A.C.; Hansen, S.; Lillevang, S.T.; Fisker, N.; Barington, T. Immunodeficiency associated with a nonsense mutation of IKBKB. J. Clin. Immunol. 2014, 34, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Salt, B.H.; Niemela, J.E.; Pandey, R.; Hanson, E.P.; Deering, R.P.; Quinones, R.; Jain, A.; Orange, J.S.; Gelfand, E.W. IKBKG (nuclear factor-kappa B essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J. Allergy Clin. Immunol. 2008, 121, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutboul, D.; Kuehn, H.S.; Van de Wyngaert, Z.; Niemela, J.E.; Callebaut, I.; Stoddard, J.; Lenoir, C.; Barlogis, V.; Farnarier, C.; Vely, F.; et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J. Clin. Invest. 2018, 128, 3071–3087. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, H.S.; Boisson, B.; Cunningham-Rundles, C.; Reichenbach, J.; Stray-Pedersen, A.; Gelfand, E.W.; Maffucci, P.; Pierce, K.R.; Abbott, J.K.; Voelkerding, K.V.; et al. Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. N. Engl. J. Med. 2016, 374, 1032–1043. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, M.; Morio, T. Inborn errors of IKAROS and AIOLOS. Curr. Opin. Immunol. 2021, 72, 239–248. [Google Scholar] [CrossRef]
- Deal, C.; Thauland, T.J.; Stiehm, E.R.; Garcia-Lloret, M.I.; Butte, M.J. Intact B-Cell Signaling and Function With Host B-Cells 47 Years After Transplantation for X-SCID. Front. Immunol. 2020, 11, 415. [Google Scholar] [CrossRef]
- Sonoda, M.; Ishimura, M.; Eguchi, K.; Yada, Y.; Lenhartova, N.; Shiraishi, A.; Tanaka, T.; Sakai, Y.; Ohga, S. Progressive B cell depletion in human MALT1 deficiency. Clin. Exp. Immunol. 2021, 206, 237–247. [Google Scholar] [CrossRef]
- Clarridge, K.; Leitenberg, D.; Loechelt, B.; Picard, C.; Keller, M. Major Histocompatibility Complex Class II Deficiency due to a Novel Mutation in RFXANK in a Child of Mexican Descent. J. Clin. Immunol. 2016, 36, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Sharifinejad, N.; Jamee, M.; Zaki-Dizaji, M.; Lo, B.; Shaghaghi, M.; Mohammadi, H.; Jadidi-Niaragh, F.; Shaghaghi, S.; Yazdani, R.; Abolhassani, H.; et al. Clinical, Immunological, and Genetic Features in 49 Patients With ZAP-70 Deficiency: A Systematic Review. Front. Immunol. 2020, 11, 831. [Google Scholar] [CrossRef]
- Barata, L.T.; Henriques, R.; Hivroz, C.; Jouanguy, E.; Paiva, A.; Freitas, A.M.; Coimbra, H.B.; Fischer, A.; da Mota, H.C. Primary immunodeficiency secondary to ZAP-70 deficiency. Acta Med. Port. 2001, 14, 413–417. [Google Scholar]
- Walkovich, K.; Vander Lugt, M. ZAP70-Related Combined Immunodeficiency. In GeneReviews((R)); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Schroeder, M.L.; Triggs-Raine, B.; Zelinski, T. Genotyping an immunodeficiency causing c.1624-11G>A ZAP70 mutation in Canadian Mennonites. BMC Med Genet. 2016, 17, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossling, K.L.; Schipp, C.; Fischer, U.; Babor, F.; Koch, G.; Schuster, F.R.; Dietzel-Dahmen, J.; Wieczorek, D.; Borkhardt, A.; Meisel, R.; et al. Hematopoietic Stem Cell Transplantation in an Infant with Immunodeficiency, Centromeric Instability, and Facial Anomaly Syndrome. Front. Immunol. 2017, 8, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banday, A.Z.; Jindal, A.K.; Kaur, A.; Kumar, Y.; Nameirakpam, J.; Patra, P.K.; Rawat, A. A young girl with hypogammaglobulinemia and granulomatous hepatitis caused by a novel mutation in ZBTB24 gene: A case based analysis. Immunobiology. 2020, 225, 151912. [Google Scholar] [CrossRef] [PubMed]
- Garty, B.Z.; Ben-Baruch, A.; Rolinsky, A.; Woellner, C.; Grimbacher, B.; Marcus, N. Pneumocystis jirovecii pneumonia in a baby with hyper-IgE syndrome. Eur. J. Pediatr. 2010, 169, 35–37. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, Y.; Song, M.; Cai, S.; Luo, H.; OuYang, R.; Yang, P.; Shi, X.; Long, Y.; Chen, Y. Omalizumab for STAT3 Hyper-IgE Syndromes in Adulthood: A Case Report and Literature Review. Front Med 2022, 9, 835257. [Google Scholar] [CrossRef]
- Keller, M.D.; Ganesh, J.; Heltzer, M.; Paessler, M.; Bergqvist, A.G.; Baluarte, H.J.; Watkins, D.; Rosenblatt, D.S.; Orange, J.S. Severe combined immunodeficiency resulting from mutations in MTHFD1. Pediatrics. 2013, 131, e629–e634. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, K.; Kobayashi, R.; Sano, H.; Suzuki, D.; Maruoka, H.; Yasuda, K.; Chida, N.; Yamada, M.; Kobayashi, K. Impact of folate therapy on combined immunodeficiency secondary to hereditary folate malabsorption. Clin. Immunol. 2014, 153, 17–22. [Google Scholar] [CrossRef]
- Pongphitcha, P.; Sirachainan, N.; Khongkraparn, A.; Tim-Aroon, T.; Songdej, D.; Wattanasirichaigoon, D. A novel TCN2 mutation with unusual clinical manifestations of hemolytic crisis and unexplained metabolic acidosis: Expanding the genotype and phenotype of transcobalamin II deficiency. BMC Pediatr. 2022, 22, 233. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Corradini, N.; Hadj-Rabia, S.; Fournet, J.C.; Faivre, L.; Le Deist, F.; Durand, P.; Doffinger, R.; Smahi, A.; Israel, A.; et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002, 109, e97. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Echevarria, A.; Gonzalez-Granado, L.I.; Allende, L.M.; De Felipe, B.; Teresa, D.R.; Calvo, C.; Perez-Martinez, A.; Raquel, R.G.; Neth, O. Fatal Pneumocystis jirovecii and Cytomegalovirus Infections in an Infant With Normal TRECs Count: Pitfalls of Newborn Screening for Severe Combined Immunodeficiency. Pediatr. Infect. Dis. 2019, 38, 157–160. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Chang, J.; Yamashita, M.; Niemela, J.E.; Zou, C.; Okuyama, K.; Harada, J.; Stoddard, J.L.; Nunes-Santos, C.J.; Boast, B.; et al. T and B cell abnormalities, pneumocystis pneumonia, and chronic lymphocytic leukemia associated with an AIOLOS defect in patients. J. Exp. Med. 2021, 218, e20211118. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.B.; McLean, T.W.; Gelber, R.D.; Donnelly, M.J.; Gilliland, D.G.; Tarbell, N.J.; Sallan, S.E. Intensified therapy for infants with acute lymphoblastic leukemia: Results from the Dana-Farber Cancer Institute Consortium. Cancer. 1997, 80, 2285–2295. [Google Scholar] [CrossRef]
- Hosking, L.M.; Bannister, E.G.; Cook, M.C.; Choo, S.; Kumble, S.; Cole, T.S. Trichohepatoenteric Syndrome Presenting with Severe Infection and Later Onset Diarrhoea. J. Clin. Immunol. 2018, 38, 1–3. [Google Scholar] [CrossRef]
- Wang, T.; Ong, P.; Roscioli, T.; Cliffe, S.T.; Church, J.A. Hepatic veno-occlusive disease with immunodeficiency (VODI): First reported case in the U.S. and identification of a unique mutation in Sp110. Clin. Immunol. 2012, 145, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Marquardsen, F.A.; Baldin, F.; Wunderer, F.; Al-Herz, W.; Mikhael, R.; Lefranc, G.; Baz, Z.; Rezaee, F.; Hanna, R.; Kfir-Erenfeld, S.; et al. Detection of Sp110 by Flow Cytometry and Application to Screening Patients for Veno-occlusive Disease with Immunodeficiency. J. Clin. Immunol. 2017, 37, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Alibrahim, A.; Lepore, M.; Lierl, M.; Filipovich, A.; Assaad, A. Pneumocystis carinii pneumonia in an infant with X-linked agammaglobulinemia. J. Allergy Clin. Immunol. 1998, 101, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Mandola, A.B.; Sharfe, N.; Nagdi, Z.; Dadi, H.; Vong, L.; Merico, D.; Ngan, B.; Reid, B.; Roifman, C.M. Combined immunodeficiency caused by a novel homozygous NFKB1 mutation. J. Allergy Clin. Immunol. 2021, 147, 727–733.e2. [Google Scholar] [CrossRef]
- Siddiqi, A.E.; Liu, A.Y.; Charville, G.W.; Kunder, C.A.; Uzel, G.; Sadighi Akha, A.A.; Oak, J.; Martin, B.; Sacha, J.; Lewis, D.B.; et al. Disseminated Pneumocystis jirovecii Infection with Osteomyelitis in a Patient with CTLA-4 Haploinsufficiency. J. Clin. Immunol. 2020, 40, 412–414. [Google Scholar] [CrossRef]
- Ferec, C.; Cutting, G.R. Assessing the Disease-Liability of Mutations in CFTR. Cold Spring Harb. Perspect. Med. 2012, 2, a009480. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, F.K.; Johansen, K.S.; Rosenkvist, J.; Tygstrup, I.; Valerius, N.H. Refractory Pneumocystis carinii infection in chronic granulomatous disease: Successful treatment with granulocytes. Pediatrics. 1979, 64, 935–938. [Google Scholar] [CrossRef]
- Lu, Y.W.; Chen, T.C. Use of trimethoprim-sulfamethoxazole in a patient with G6PD deficiency for treating Pneumocystis jirovecii pneumonia without haemolysis: Case report and literature review. J. Clin. Pharm. Ther. 2020, 45, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Shen, X.; Liao, L.; Wang, X.; Song, M.; Zheng, X.; Zhu, Y.; Yang, Y. The medication for pneumocystis pneumonia with glucose-6-phosphate dehydrogenase deficiency patients. Front. Pharmacol. 2022, 13, 957376. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lara, M.F.; Wisniowski-Yanez, A.; Perez-Patrigeon, S.; Hsu, A.P.; Holland, S.M.; Cuellar-Rodriguez, J.M. Pneumocystis jiroveci pneumonia and GATA2 deficiency: Expanding the spectrum of the disease. J. Infect. 2017, 74, 425–427. [Google Scholar] [CrossRef]
- Drutman, S.B.; Mansouri, D.; Mahdaviani, S.A.; Neehus, A.L.; Hum, D.; Bryk, R.; Hernandez, N.; Belkaya, S.; Rapaport, F.; Bigio, B.; et al. Fatal Cytomegalovirus Infection in an Adult with Inherited NOS2 Deficiency. N. Engl. J. Med. 2020, 382, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Somekh, I.; Lev, A.; Barel, O.; Lee, Y.N.; Hendel, A.; Simon, A.J.; Somech, R. Exploring genetic defects in adults who were clinically diagnosed as severe combined immune deficiency during infancy. Immunol. Res. 2021, 69, 145–152. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, C.; Wu, Y.; Cao, K.; Li, X.; Cao, W.; Cao, L.; Zhang, S.; Ba, Y.; Zheng, Y.; et al. Unusual Talaromyces marneffei and Pneumocystis jirovecii coinfection in a child with a STAT1 mutation: A case report and literature review. Front. Immunol. 2023, 14, 1103184. [Google Scholar] [CrossRef]
- Consortium, I.R.F.I.; Fornes, O.; Jia, A.; Kuehn, H.S.; Min, Q.; Pannicke, U.; Schleussner, N.; Thouenon, R.; Yu, Z.; de Los Angeles Astbury, M.; et al. A multimorphic mutation in IRF4 causes human autosomal dominant combined immunodeficiency. Sci. Immunol. 2023, 8, eade7953. [Google Scholar] [CrossRef]
- Zeleznik, M.; Soltirovska Salamon, A.; Debeljak, M.; Goropevsek, A.; Sustar, N.; Kljucevsek, D.; Ihan, A.; Avcin, T. Case report: Pneumocystis jirovecii pneumonia in a severe case of Aicardi-Goutieres syndrome with an IFIH1 gain-of-function mutation mimicking combined immunodeficiency. Front. Immunol. 2022, 13, 1033513. [Google Scholar] [CrossRef]
- Podlipnik, S.; de la Mora, L.; Alsina, M.; Mascaro, J.M., Jr. Pneumocystis jirovecii pneumonia in a patient with pustular psoriasis with an IL-36RN deficiency treated with infliximab: Case report and review of the literature. Australas. J. Dermatol. 2017, 58, e44–e47. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Huyck, H.L.; Bhagwat, S.; O’Reilly, M.A.; Finkelstein, J.N.; Gigliotti, F.; Wright, T.W. Parenchymal cell TNF receptors contribute to inflammatory cell recruitment and respiratory failure in Pneumocystis carinii-induced pneumonia. J. Immunol. 2008, 181, 1409–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillant, D.; Albanese, J.; Tomachot, L.; Durbec, O.; Granthil, C. Purpura fulminans and C7 deficiency complicated by Pneumocystis carinii pneumonia. Ann. Fr. Anesth. Reanim. 1991, 10, 394–397. [Google Scholar] [CrossRef]
- Ziv, A.; Werner, L.; Konnikova, L.; Awad, A.; Jeske, T.; Hastreiter, M.; Mitsialis, V.; Stauber, T.; Wall, S.; Kotlarz, D.; et al. An RTEL1 Mutation Links to Infantile-Onset Ulcerative Colitis and Severe Immunodeficiency. J. Clin. Immunol. 2020, 40, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Borie, R.; Kannengiesser, C.; Sicre de Fontbrune, F.; Boutboul, D.; Tabeze, L.; Brunet-Possenti, F.; Lainey, E.; Debray, M.P.; Cazes, A.; Crestani, B. Pneumocystosis revealing immunodeficiency secondary to TERC mutation. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharman, J.P.; Brander, D.M.; Mato, A.R.; Ghosh, N.; Schuster, S.J.; Kambhampati, S.; Burke, J.M.; Lansigan, F.; Schreeder, M.T.; Lunin, S.D.; et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): A phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021, 8, e254–e266. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Brander, D.M.; Kim, H.T.; Tyekucheva, S.; Bsat, J.; Savell, A.; Hellman, J.M.; Bazemore, J.; Francoeur, K.; Alencar, A.; et al. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019, 6, e419–e428. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Inagaki, K.; Blackshear, C.; Hobbs, C.V. Pneumocystis Infection in Children: National trends and characteristics in the United States, 1997-2012. Pediatr. Infect. Dis. J. 2019, 38, 241–247. [Google Scholar] [CrossRef]
- Menu, E.; Driouich, J.S.; Luciani, L.; Morand, A.; Ranque, S.; L’Ollivier, C. Detection of Pneumocystis jirovecii in hospitalized children less than 3 years of age. J. Fungi 2021, 7, 546. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Chopra, A. Narrative review of the relationship between COVID-19 and PJP: Does it represent coinfection or colonization? Infection 2021, 49, 1079–1090. [Google Scholar] [CrossRef]
- Gentile, I.; Viceconte, G.; Lanzardo, A.; Zotta, I.; Zappulo, E.; Pinchera, B.; Scotto, R.; Schiano Moriello, N.; Foggia, M.; Giaccone, A.; et al. Pneumocystis jirovecii pneumonia in non-HIV patients recovering from COVID-19: A single-center experience. Int. J. Environ. Res. Public. Health 2021, 18, 11399. [Google Scholar] [CrossRef] [PubMed]
- Viceconte, G.; Buonomo, A.R.; Lanzardo, A.; Pinchera, B.; Zappulo, E.; Scotto, R.; Schiano Moriello, N.; Vargas, M.; Iacovazzo, C.; Servillo, G.; et al. Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect. Dis. 2021, 53, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Gioia, F.; Albasata, H.; Hosseini-Moghaddam, S.M. Concurrent infection with SARS-CoV-2 and Pneumocystis jirovecii in immunocompromised and immunocompetent individuals. J. Fungi 2022, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Ahmadpour, E.; Nami, S.; Mohammadi, R.; Hosseini, H.; Behravan, M.; Morovati, H. Global prevalence, mortality, and main risk factors for COVID-19 associated pneumocystosis: A systematic review and meta-analysis. Asian Pac. J. Trop. Med. 2022, 15, 431–441. [Google Scholar] [CrossRef]
- Alanio, A.; Delliere, S.; Voicu, S.; Bretagne, S.; Megarbane, B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Luyt, C.E.; Lampros, A.; Fekkar, A. COVID-19-related respiratory failure and lymphopenia do not seem associated with pneumocystosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1734–1736. [Google Scholar] [CrossRef]
- Bretagne, S.; Sitbon, K.; Botterel, F.; Delliere, S.; Letscher-Bru, V.; Chouaki, T.; Bellanger, A.P.; Bonnal, C.; Fekkar, A.; Persat, F.; et al. COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: A retrospective multicenter observational cohort during the first French pandemic wave. Microbiol. Spectr. 2021, 9, e0113821. [Google Scholar] [CrossRef]
- Gerber, V.; Ruch, Y.; Chamaraux-Tran, T.N.; Oulehri, W.; Schneider, F.; Lindner, V.; Greigert, V.; Denis, J.; Brunet, J.; Danion, F. Detection of Pneumocystis jirovecii in patients with severe COVID-19: Diagnostic and therapeutic challenges. J Fungi 2021, 7, 585. [Google Scholar] [CrossRef]
- Mouren, D.; Goyard, C.; Catherinot, E.; Givel, C.; Chabrol, A.; Tcherakian, C.; Longchampt, E.; Vargaftig, J.; Farfour, E.; Legal, A.; et al. COVID-19 and Pneumocystis jirovecii pneumonia: Back to the basics. Respir. Med. Res. 2021, 79, 100814. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, F.; Tiecco, G.; Storti, S.; Fumarola, B.; Brianese, N.; Bertelli, D.; Castelli, F. Pneumocystis jirovecii pneumonia in breast cancer mimicking SARS-CoV-2 pneumonia during pandemic. Infez. Med. 2021, 29, 614–617. [Google Scholar] [CrossRef]
- Cattaneo, L.; Buonomo, A.R.; Iacovazzo, C.; Giaccone, A.; Scotto, R.; Viceconte, G.; Mercinelli, S.; Vargas, M.; Roscetto, E.; Cacciatore, F.; et al. Invasive fungal infections in hospitalized patients with COVID-19: A non-intensive care single-centre experience during the first pandemic waves. J. Fungi. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.A.; Alberici, F.; Bossini, N.; Scolari, F.; Pascucci, F.; Tomasoni, G.; Caruso, A. Pneumocystis jirevocii and SARS-CoV-2 co-infection: A common feature in transplant recipients? Vaccines 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, F.; Airoldi, C.; Falaschi, Z.; Bellan, M.; Castello, L.M.; Filippone, F.; Matranga, S.; Masellis, S.; Smeriglia, A.; Solidoro, P.; et al. Mycotic infection prevalence among patients undergoing bronchoalveolar lavage with search of SARS-CoV-2 after two negative nasopharyngeal swabs. J. Breath. Res. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Farinacci, D.; Ciccullo, A.; Borghetti, A.; Visconti, E.; Tamburrini, E.; Izzi, I.M.; Cauda, R.; Di Giambenedetto, S.; Pallavicini, F. People living with HIV in the COVID-19 era: A case report. AIDS Res. Hum. Retroviruses. 2021, 37, 253–254. [Google Scholar] [CrossRef]
- Niamatullah, H.; Nasir, N.; Jabeen, K.; Rattani, S.; Farooqi, J.; Ghanchi, N.; Irfan, M. Post-COVID-19 Pneumocystis pneumonia cases from Pakistan: An observational study. Access Microbiol. 2023, 5, acmi000406. [Google Scholar] [CrossRef]
- Baraboutis, I.G.; Gargalianos, P.; Aggelonidou, E.; Adraktas, A.; Collaborators. Initial real-life experience from a designated COVID-19 centre in Athens, Greece: A proposed therapeutic algorithm. SN Compr. Clin. Med. 2020, 2, 689–693. [Google Scholar] [CrossRef]
- Sreenath, K.; Batra, P.; Vinayaraj, E.V.; Bhatia, R.; SaiKiran, K.; Singh, V.; Singh, S.; Verma, N.; Singh, U.B.; Mohan, A.; et al. Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol. Spectr. 2021, 9, e0016321. [Google Scholar] [CrossRef]
- Alebna, P.L.; Bellamy, S.; Tabur, T.A.; Mangia, A. Rare case of persistently depressed T lymphocyte subsets after SARS-CoV-2 infection. Am. J. Case Rep. 2022, 23, e937760. [Google Scholar] [CrossRef]
- Bhat, P.; Noval, M.; Doub, J.B.; Heil, E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int. J. Infect. Dis. 2020, 99, 119–121. [Google Scholar] [CrossRef]
- Menon, A.A.; Berg, D.D.; Brea, E.J.; Deutsch, A.J.; Kidia, K.K.; Thurber, E.G.; Polsky, S.B.; Yeh, T.; Duskin, J.A.; Holliday, A.M.; et al. A case of COVID-19 and Pneumocystis jirovecii coinfection. Am. J. Respir. Crit. Care Med. 2020, 202, 136–138. [Google Scholar] [CrossRef]
- Merchant, E.A.; Flint, K.; Barouch, D.H.; Blair, B.M. Co-infection with coronavirus disease 2019, previously undiagnosed human immunodeficiency virus, Pneumocystis jirovecii pneumonia and cytomegalovirus pneumonitis, with possible immune reconstitution inflammatory syndrome. IDCases 2021, 24, e01153. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, C.; Tompkins, K.; Sellers, S.A.; Bramson, B.; Eron, J.; Parr, J.B.; Schranz, A.J. Pneumocystis and severe acute respiratory syndrome Coronavirus 2 coinfection: A case report and review of an emerging diagnostic dilemma. Open Forum Infect. Dis. 2021, 8, ofaa633. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Dahi, P.B.; Ponce, D.M.; Sauter, C.S.; Shaffer, B.C.; Chung, D.J.; Politikos, I.; Lin, R.J.; Giralt, S.A.; Papanicolaou, G.; et al. Hematopoietic cell transplantation is feasible in patients with prior COVID-19 infection. Transplant. Cell Ther. 2022, 28, 55.e51–55.e55. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, W.; Li, M.; Dong, L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020, 39, 2797–2802. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Ming, F.; Tang, W.; Liang, K. The diagnostic trap occurred in two COVID-19 cases combined Pneumocystis pneumonia in patient with AIDS. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Peng, J.; Ni, M.; Du, D.; Lu, Y.; Song, J.; Liu, W.; Shen, N.; Wang, X.; Zhu, Y.; Vallance, B.A.; et al. Successful treatment of a kidney transplant patient with COVID-19 and late-onset Pneumocystis jirovecii pneumonia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.; Ruiz-Mateos, E.; Juiz-Gonzalez, P.M.; Vitalle, J.; Vieitez, I.; Vazquez-Friol, M.D.C.; Torres-Beceiro, I.; Perez-Gomez, A.; Gallego-Garcia, P.; Estevez-Gomez, N.; et al. SARS-CoV-2 evolution and spike-specific CD4+ T-cell response in persistent COVID-19 with severe HIV immune suppression. Microorganisms. 2022, 10. [Google Scholar] [CrossRef]
- Amparo-Vicente, M.; Morte, E. Pneumonia by Pneumocystis jirovecii after COVID-19 in non-HIV patient. Rev. Chilena Infectol. 2022, 39, 357–360. [Google Scholar] [CrossRef]
- Blanco, J.L.; Ambrosioni, J.; Garcia, F.; Martinez, E.; Soriano, A.; Mallolas, J.; Miro, J.M.; Investigators, C.-I.H. COVID-19 in patients with HIV: Clinical case series. Lancet HIV. 2020, 7, e314–e316. [Google Scholar] [CrossRef]
- Quintana-Ortega, C.; Remesal, A.; Ruiz de Valbuena, M.; de la Serna, O.; Laplaza-Gonzalez, M.; Alvarez-Rojas, E.; Udaondo, C.; Alcobendas, R.; Murias, S. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: A case report. Mod. Rheumatol. Case Rep. 2021, 5, 101–107. [Google Scholar] [CrossRef]
- Nguyen, H.; Salkeld, J.; Agarwal, S.; Goodman, A. compassionate use of REGN-COV2 in the treatment of COVID-19 in a patient with impaired humoral immunity. Clin. Infect. Pract. 2021, 12, 100089. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.; Snell, L.B.; Simons, R.; Douthwaite, S.T.; Lee, M.J. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: A diagnostic dilemma in HIV. AIDS. 2020, 34, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Waters, L.; Cevik, M.; Collins, S.; Lewis, J.; Wu, M.S.; Blanchard, T.J.; Geretti, A.M. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin. Med. 2020, 20, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Wenlock, R.D.; Brown, C.S.; Iwuji, C.; Vera, J.H. Can I go back to work? A case of persistent SARS-CoV-2 with advanced untreated HIV infection. Int. J. STD AIDS. 2022, 33, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.R.; Talib, W.; Al-Dulaimi, A.; Daoud, S.; Al Maqbali, M. The first detection of Pneumocystis jirovecii in asthmatic patients post-COVID-19 in Jordan. Bosn. J. Basic. Med. Sci. 2022, 22, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Adachi, E.; Saito, M.; Koga, M.; Tsutsumi, T.; Yotsuyanagi, H. Favorable Outcome following sotrovimab monoclonal antibody in a patient with prolonged SARS-CoV-2 omicron infection with HIV/AIDS. Intern. Med. 2022, 61, 3459–3462. [Google Scholar] [CrossRef]
- Takahashi, T.; Saito, A.; Kuronuma, K.; Nishikiori, H.; Chiba, H. Pneumocystis jirovecii pneumonia associated with COVID-19 in patients with interstitial pneumonia. Medicina 2022, 58, 1151. [Google Scholar] [CrossRef]
- Moradians, V.; Shateri Amiri, B.; Bahadorizadeh, L.; Gholizadeh Mesgarha, M.; Sadeghi, S. Concurrent COVID-19 and Pneumocystis carinii pneumonia in a patient subsequently found to have underlying hairy cell leukemia. Radiol. Case Rep. 2022, 17, 3238–3242. [Google Scholar] [CrossRef]
- Tehrani, S.; Ziaie, S.; Kashefizadeh, A.; Fadaei, M.; Najafiarab, H.; Keyvanfar, A. Case Report: Pneumonia in a patient with combined variable immunodeficiency: COVID-19 or Pneumocystis pneumonia? Front. Med. 2022, 9, 814300. [Google Scholar] [CrossRef]
- Broadhurst, A.G.B.; Lalla, U.; Taljaard, J.J.; Louw, E.H.; Koegelenberg, C.F.N.; Allwood, B.W. The diagnostic challenge of Pneumocystis pneumonia and COVID-19 co-infection in HIV. Respirol. Case Rep. 2021, 9, e00725. [Google Scholar] [CrossRef]
- Parker, A.; Shaw, J.; Karamchand, S.; Lahri, S.; Schrueder, N.; Chothia, M.Y.; Mowlana, A.; Lalla, U.; Allwood, B.W.; Koegelenberg, C.F.N.; et al. HIV and SARS-CoV-2 co-infection: The diagnostic challenges of dual pandemics. S Afr. Med. J. 2020, 110, 473–475. [Google Scholar] [CrossRef]
- Jeican, I.I.; Inisca, P.; Gheban, D.; Tabaran, F.; Aluas, M.; Trombitas, V.; Cristea, V.; Crivii, C.; Junie, L.M.; Albu, S. COVID-19 and Pneumocystis jirovecii pulmonary coinfection-the first case confirmed through autopsy. Medicina 2021, 57. [Google Scholar] [CrossRef] [PubMed]
- Skonieczny, P.; Heleniak, Z.; Szostakiewicz, M.; Kuziemski, K.; Debska-Slizien, A. Coinfection of COVID-19 and pneumocystosis in a patient after kidney transplantation. Pol. Arch. Intern. Med. 2021, 131, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Yehouenou Tessi, R.T.; Onka, B.; El Bakkari, A.; Jerguigue, H.; Latib, R.; Omor, Y. An etiology of ground–glass images during COVID-19: Pneumocystis jiroveci pneumonia. SAGE Open Med. Case Rep. 2022, 10, 2050313X221091391. [Google Scholar] [CrossRef] [PubMed]
- Mang, S.; Kaddu-Mulindwa, D.; Metz, C.; Becker, A.; Seiler, F.; Smola, S.; Massmann, A.; Becker, S.L.; Papan, C.; Bals, R.; et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome Coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin. Infect. Dis. 2021, 72, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Larzabal, F.J.; Vilela, A.; Brusca, S.; Saluzzi, I.; Ghergo, G.E.; Angiono, M.A. Simultaneous diagnosis and favorable evolution of infection with Pneumocystis jirovecii, SARS-CoV-2 and advanced HIV. Medicina 2020, 80, 554–556. [Google Scholar] [PubMed]
- Connolly, S.P.; McGrath, J.; Sui, J.; Muldoon, E.G. Rare, disseminated Kaposi sarcoma in advanced HIV with high-burden pulmonary and skeletal involvement. BMJ Case Rep. 2021, 14, e245448. [Google Scholar] [CrossRef]
- Anggraeni, A.T.; Soedarsono, S.; Soeprijanto, B. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia: The importance of radiological diagnostic and HIV testing. Radiol. Case Rep. 2021, 16, 3685–3689. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Li, J.; Han, H.; Xia, Z.; Liu, F.; Wu, K.; Yang, L.; Liu, X.; Zhu, C. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta 2020, 508, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.A.; Ribeiro, L.R.; Lima, K.V.B.; Lima, L. Adaptive immunity to SARS-CoV-2 infection: A systematic review. Front. Immunol. 2022, 13, 1001198. [Google Scholar] [CrossRef] [PubMed]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Kruger, K.; Baumgart, S.; Drube, S.; Lemhofer, C.; Reuken, P.; Stallmach, A.; Muller, M.; et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients—Indications for their potential involvement in the development of Long COVID? Front. Immunol. 2022, 13, 1070994. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Chattopadhyay, S.; Hazra, S.; Hansda, A.K.; Goswami, R. COVID-19 pandemic: The delta variant, T-cell responses, and the efficacy of developing vaccines. Inflamm. Res. 2022, 71, 377–396. [Google Scholar] [CrossRef]
- Liu, K.; Yang, T.; Peng, X.F.; Lv, S.M.; Ye, X.L.; Zhao, T.S.; Li, J.C.; Shao, Z.J.; Lu, Q.B.; Li, J.Y.; et al. A systematic meta-analysis of immune signatures in patients with COVID-19. Rev. Med. Virol. 2021, 31, e2195. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, Y.; Luo, Q.; Huang, Z.; Zhao, R.; Liu, S.; Le, A.; Li, J.; Wan, L. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J. Infect. Dis. 2020, 222, 198–202. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Westmeier, J.; Paniskaki, K.; Karakose, Z.; Werner, T.; Sutter, K.; Dolff, S.; Overbeck, M.; Limmer, A.; Liu, J.; Zheng, X.; et al. Impaired cytotoxic CD8(+) T cell response in elderly COVID-19 patients. MBio 2020, 11, e02243-20. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.R.; Geng, R.; Li, Q.; Chen, Y.; Li, S.F.; Wang, Q.; Min, J.; Yang, Y.; Li, B.; Jiang, R.D.; et al. ACE2-independent infection of T lymphocytes by SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Szydlowicz, M.; Matos, O. Pneumocystis pneumonia in the COVID-19 pandemic era: Similarities and challenges. Trends Parasitol. 2021, 37, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive fungal infections complicating COVID-19: A Narrative Review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef] [PubMed]
- Mina, S.; Yaakoub, H.; Annweiler, C.; Dubee, V.; Papon, N. COVID-19 and fungal infections: A double debacle. Microbes Infect. 2022, 24, 105039. [Google Scholar] [CrossRef] [PubMed]
- Burghi, G.; Biard, L.; Roux, A.; Valade, S.; Robert-Gangneux, F.; Hamane, S.; Maubon, D.; Debourgogne, A.; Le Gal, S.; Dalle, F.; et al. Characteristics and outcome according to underlying disease in non-AIDS patients with acute respiratory failure due to Pneumocystis pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1191–1198. [Google Scholar] [CrossRef]
- Ling, C.; Qian, S.; Wang, Q.; Zeng, J.; Jia, X.; Liu, J.; Li, Z. Pneumocystis pneumonia in non-HIV children: A 10-year retrospective study. Clin. Respir. J. 2018, 12, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Shoji, K.; Michihata, N.; Miyairi, I.; Matsui, H.; Fushimi, K.; Yasunaga, H. Recent epidemiology of Pneumocystis pneumonia in Japan. J. Infect. Chemother. 2020, 26, 1260–1264. [Google Scholar] [CrossRef]
- Charpentier, E.; Menard, S.; Marques, C.; Berry, A.; Iriart, X. Immune response in Pneumocystis infections according to the host immune system status. J. Fungi 2021, 7, 625. [Google Scholar] [CrossRef]
- Otieno-Odhiambo, P.; Wasserman, S.; Hoving, J.C. The contribution of host cells to Pneumocystis immunity: An Update. Pathogens 2019, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Burke, B.A.; Good, R.A. Pneumocystis carinii infection. Medicine 1973, 52, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Masur, H.; Ognibene, F.P.; Yarchoan, R.; Shelhamer, J.H.; Baird, B.F.; Travis, W.; Suffredini, A.F.; Deyton, L.; Kovacs, J.A.; Falloon, J.; et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann. Intern. Med. 1989, 111, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacs, D. Infectious risks associated with biologics. Adv. Exp. Med. Biol. 2013, 764, 151–158. [Google Scholar] [CrossRef]
- Roberts, M.B.; Fishman, J.A. Immunosuppressive agents and infectious risk in transplantation: Managing the “Net State of Immunosuppression”. Clin. Infect. Dis. 2021, 73, e1302–e1317. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Kasai, M.; Fukuda, T.; Ichimura, T.; Yasui, T.; Sumi, T. Chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. Anticancer. Drugs 2015, 26, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Classen, A.Y.; Henze, L.; von Lilienfeld-Toal, M.; Maschmeyer, G.; Sandherr, M.; Graeff, L.D.; Alakel, N.; Christopeit, M.; Krause, S.W.; Mayer, K.; et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO). Ann. Hematol. 2021, 100, 1603–1620. [Google Scholar] [CrossRef]
- Ruckert, M.; Flohr, A.S.; Hecht, M.; Gaipl, U.S. Radiotherapy and the immune system: More than just immune suppression. Stem Cells 2021, 39, 1155–1165. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Mansouri, D.; Khosravi, A.; Garssen, J.; Velayati, A.; Adcock, I.M. Cancers related to immunodeficiencies: Update and perspectives. Front. Immunol. 2016, 7, 365. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes. Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, M.D.; Wilkes, D.S. Transplant-related immunosuppression: A review of immunosuppression and pulmonary infections. Proc. Am. Thorac. Soc. 2005, 2, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Al-Ozairi, E.; Al-Baqsumi, Z.; Ahmad, R.; Al-Mulla, F. Cellular and humoral immune responses in COVID-19 and immunotherapeutic approaches. Immunotargets Ther. 2021, 10, 63–85. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Annunziato, F.; Cosmi, L. Hallmarks of immune response in COVID-19: Exploring dysregulation and exhaustion. Semin. Immunol. 2021, 55, 101508. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.O.; Griffiths, J.S.; Loeffler, J.; Orr, S.; White, P.L. Defective antifungal immunity in patients with COVID-19. Front. Immunol. 2022, 13, 1080822. [Google Scholar] [CrossRef]
- Delliere, S.; Gits-Muselli, M.; Bretagne, S.; Alanio, A. Outbreak-causing fungi: Pneumocystis jirovecii. Mycopathologia 2020, 185, 783–800. [Google Scholar] [CrossRef]
- Hardy, A.M.; Wajszczuk, C.P.; Suffredini, A.F.; Hakala, T.R.; Ho, M. Pneumocystis carinii pneumonia in renal-transplant recipients treated with cyclosporine and steroids. J. Infect. Dis. 1984, 149, 143–147. [Google Scholar] [CrossRef]
- Hennequin, C.; Page, B.; Roux, P.; Legendre, C.; Kreis, H. Outbreak of Pneumocystis carinii pneumonia in a renal transplant unit. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 122–126. [Google Scholar] [CrossRef]
- Olsson, M.; Eriksson, B.M.; Elvin, K.; Strandberg, M.; Wahlgren, M. Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scand. J. Infect. Dis. 2001, 33, 285–289. [Google Scholar] [CrossRef]
- Hocker, B.; Wendt, C.; Nahimana, A.; Tonshoff, B.; Hauser, P.M. Molecular evidence of Pneumocystis transmission in pediatric transplant unit. Emerg. Infect. Dis. 2005, 11, 330–332. [Google Scholar] [CrossRef] [Green Version]
- de Boer, M.G.; Bruijnesteijn van Coppenraet, L.E.; Gaasbeek, A.; Berger, S.P.; Gelinck, L.B.; van Houwelingen, H.C.; van den Broek, P.; Kuijper, E.J.; Kroon, F.P.; Vandenbroucke, J.P. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: Interhuman transmission or a common environmental source? Clin. Infect. Dis. 2007, 44, 1143–1149. [Google Scholar] [CrossRef]
- Schmoldt, S.; Schuhegger, R.; Wendler, T.; Huber, I.; Sollner, H.; Hogardt, M.; Arbogast, H.; Heesemann, J.; Bader, L.; Sing, A. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J. Clin. Microbiol. 2008, 46, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazaki, H.; Goto, N.; Uchida, K.; Kobayashi, T.; Gatanaga, H.; Oka, S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation 2009, 88, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Arichi, N.; Kishikawa, H.; Mitsui, Y.; Kato, T.; Nishimura, K.; Tachikawa, R.; Tomii, K.; Shiina, H.; Igawa, M.; Ichikawa, Y. Cluster outbreak of Pneumocystis pneumonia among kidney transplant patients within a single center. Transplant. Proc. 2009, 41, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Gianella, S.; Haeberli, L.; Joos, B.; Ledergerber, B.; Wuthrich, R.P.; Weber, R.; Kuster, H.; Hauser, P.M.; Fehr, T.; Mueller, N.J. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl. Infect. Dis. 2010, 12, 1–10. [Google Scholar] [CrossRef]
- Wynckel, A.; Toubas, D.; Noel, N.; Toupance, O.; Rieu, P. Outbreak of pneumocystis pneumonia occurring in late post-transplantation period. Nephrol. Dial. Transplant. 2011, 26, 2417. [Google Scholar] [CrossRef] [Green Version]
- Phipps, L.M.; Chen, S.C.; Kable, K.; Halliday, C.L.; Firacative, C.; Meyer, W.; Wong, G.; Nankivell, B.J. Nosocomial Pneumocystis jirovecii pneumonia: Lessons from a cluster in kidney transplant recipients. Transplantation. 2011, 92, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Vivancos, R.; Corless, C.; Wood, G.; Beeching, N.J.; Beadsworth, M.B. Increasing frequency of Pneumocystis jirovecii pneumonia in renal transplant recipients in the United Kingdom: Clonal variability, clusters, and geographic location. Clin. Infect. Dis. 2011, 53, 307–308. [Google Scholar] [CrossRef] [Green Version]
- Pliquett, R.U.; Asbe-Vollkopf, A.; Hauser, P.M.; Presti, L.L.; Hunfeld, K.P.; Berger, A.; Scheuermann, E.H.; Jung, O.; Geiger, H.; Hauser, I.A. A Pneumocystis jirovecii pneumonia outbreak in a single kidney-transplant center: Role of cytomegalovirus co-infection. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2429–2437. [Google Scholar] [CrossRef]
- Brunot, V.; Pernin, V.; Chartier, C.; Garrigue, V.; Vetromile, F.; Szwarc, I.; Delmas, S.; Portales, P.; Basset, D.; Mourad, G. An epidemic of Pneumocystis jiroveci pneumonia in a renal transplantation center: Role of T-cell lymphopenia. Transplant. Proc. 2012, 44, 2818–2820. [Google Scholar] [CrossRef]
- Chapman, J.R.; Marriott, D.J.; Chen, S.C.; MacDonald, P.S. Post-transplant Pneumocystis jirovecii pneumonia--a re-emerged public health problem? Kidney Int. 2013, 84, 240–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nankivell, B.J.; Firacative, C.; Kable, K.; Chen, S.C.; Meyer, W. Molecular epidemiology linking multihospital clusters of opportunistic Pneumocystis jirovecii pneumonia. Clin. Infect. Dis. 2013, 57, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Rostved, A.A.; Sassi, M.; Kurtzhals, J.A.; Sorensen, S.S.; Rasmussen, A.; Ross, C.; Gogineni, E.; Huber, C.; Kutty, G.; Kovacs, J.A.; et al. Outbreak of Pneumocystis pneumonia in renal and liver transplant patients caused by genotypically distinct strains of Pneumocystis jirovecii. Transplantation 2013, 96, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Debourgogne, A.; Favreau, S.; Ladriere, M.; Bourry, S.; Machouart, M. Characteristics of Pneumocystis pneumonia in Nancy from January 2007 to April 2011 and focus on an outbreak in nephrology. J. Mycol. Med. 2014, 24, 19–24. [Google Scholar] [CrossRef]
- Chandola, P.; Lall, M.; Sen, S.; Bharadwaj, R. Outbreak of Pneumocystis jirovecii pneumonia in renal transplant recipients on prophylaxis: Our observation and experience. Indian. J. Med. Microbiol. 2014, 32, 333–336. [Google Scholar] [CrossRef]
- Gits-Muselli, M.; Peraldi, M.N.; de Castro, N.; Delcey, V.; Menotti, J.; Guigue, N.; Hamane, S.; Raffoux, E.; Bergeron, A.; Valade, S.; et al. New short tandem repeat-based molecular typing method for Pneumocystis jirovecii reveals intrahospital transmission between patients from different wards. PLoS ONE 2015, 10, e0125763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desoubeaux, G.; Dominique, M.; Morio, F.; Thepault, R.A.; Franck-Martel, C.; Tellier, A.C.; Ferrandiere, M.; Hennequin, C.; Bernard, L.; Salame, E.; et al. epidemiological outbreaks of Pneumocystis jirovecii pneumonia are not limited to kidney transplant recipients: Genotyping confirms common source of transmission in a liver transplantation unit. J. Clin. Microbiol. 2016, 54, 1314–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulpuru, S.; Knoll, G.; Weir, C.; Desjardins, M.; Johnson, D.; Gorn, I.; Fairhead, T.; Bissonnette, J.; Bruce, N.; Toye, B.; et al. Pneumocystis pneumonia outbreak among renal transplant recipients at a North American transplant center: Risk factors and implications for infection control. Am. J. Infect. Control 2016, 44, 425–431. [Google Scholar] [CrossRef]
- Urabe, N.; Ishii, Y.; Hyodo, Y.; Aoki, K.; Yoshizawa, S.; Saga, T.; Murayama, S.Y.; Sakai, K.; Homma, S.; Tateda, K. Molecular epidemiologic analysis of a Pneumocystis pneumonia outbreak among renal transplant patients. Clin. Microbiol. Infect. 2016, 22, 365–371. [Google Scholar] [CrossRef]
- Inkster, T.; Dodd, S.; Gunson, R.; Imrie, L.; Spalding, E.; Packer, S.; Deighan, C.; Daly, C.; Coia, J.; Imtiaz, T.; et al. Investigation of outbreaks of Pneumocystis jirovecii pneumonia in two Scottish renal units. J. Hosp. Infect. 2017, 96, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Robin, C.; Alanio, A.; Gits-Muselli, M.; la Martire, G.; Schlemmer, F.; Botterel, F.; Angebault, C.; Leclerc, M.; Beckerich, F.; Redjoul, R.; et al. Molecular demonstration of a Pneumocystis outbreak in stem cell transplant patients: Evidence for transmission in the daycare center. Front. Microbiol. 2017, 8, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frealle, E.; Valade, S.; Guigue, N.; Hamane, S.; Chabe, M.; Le Gal, S.; Damiani, C.; Totet, A.; Aliouat, E.M.; Nevez, G.; et al. Diffusion of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis colonization: Frequency and putative risk factors. Med. Mycol. 2017, 55, 568–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanio, A.; Gits-Muselli, M.; Guigue, N.; Desnos-Ollivier, M.; Calderon, E.J.; Di Cave, D.; Dupont, D.; Hamprecht, A.; Hauser, P.M.; Helweg-Larsen, J.; et al. Diversity of Pneumocystis jirovecii across Europe: A multicentre observational study. EBioMedicine 2017, 22, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintenberger, C.; Maubon, D.; Charpentier, E.; Rendu, J.; Pavese, P.; Augier, C.; Malvezzi, P.; Camara, B.; Mallaret, M.R.; Bouillet, L.; et al. Grouped cases of pulmonary pneumocystosis after solid organ transplantation: Advantages of coordination by an infectious diseases unit for overall management and epidemiological monitoring. Infect. Control Hosp. Epidemiol. 2017, 38, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E.; Garnaud, C.; Wintenberger, C.; Bailly, S.; Murat, J.B.; Rendu, J.; Pavese, P.; Drouet, T.; Augier, C.; Malvezzi, P.; et al. Added Value of Next-generation sequencing for multilocus sequence typing analysis of a Pneumocystis jirovecii pneumonia outbreak1. Emerg. Infect. Dis. 2017, 23, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Nevez, G.; Le Gal, S.; Noel, N.; Wynckel, A.; Huguenin, A.; Le Govic, Y.; Pougnet, L.; Virmaux, M.; Toubas, D.; Bajolet, O. Investigation of nosocomial Pneumocystis infections: Usefulness of longitudinal screening of epidemic and post-epidemic Pneumocystis genotypes. J. Hosp. Infect. 2018, 99, 332–345. [Google Scholar] [CrossRef]
- Ricci, G.; Santos, D.W.; Kovacs, J.A.; Nishikaku, A.S.; de Sandes-Freitas, T.V.; Rodrigues, A.M.; Kutty, G.; Affonso, R.; Silva, H.T.; Medina-Pestana, J.O.; et al. Genetic diversity of Pneumocystis jirovecii from a cluster of cases of pneumonia in renal transplant patients: Cross-sectional study. Mycoses 2018, 61, 845–852. [Google Scholar] [CrossRef]
- Veronese, G.; Ammirati, E.; Moioli, M.C.; Baldan, R.; Orcese, C.A.; De Rezende, G.; Veronese, S.; Masciocco, G.; Perna, E.; Travi, G.; et al. Single-center outbreak of Pneumocystis jirovecii pneumonia in heart transplant recipients. Transpl. Infect. Dis. 2018, 20, e12880. [Google Scholar] [CrossRef]
- Szydlowicz, M.; Jakuszko, K.; Szymczak, A.; Piesiak, P.; Kowal, A.; Kopacz, Z.; Wesolowska, M.; Lobo, M.L.; Matos, O.; Hendrich, A.B.; et al. Prevalence and genotyping of Pneumocystis jirovecii in renal transplant recipients-preliminary report. Parasitol. Res. 2019, 118, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Moghaddam, S.M.; Dufresne, P.J.; Hunter Gutierrez, E.; Dufresne, S.F.; House, A.A.; Humar, A.; Kumar, D.; Jevnikar, A.M. Long-lasting cluster of nosocomial pneumonia with a single Pneumocystis jirovecii genotype involving different organ allograft recipients. Clin. Transplant. 2020, 34, e14108. [Google Scholar] [CrossRef]
- Yiannakis, E.P.; Boswell, T.C. Systematic review of outbreaks of Pneumocystis jirovecii pneumonia: Evidence that P. jirovecii is a transmissible organism and the implications for healthcare infection control. J. Hosp. Infect. 2016, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.; Rabodonirina, M.; Nevez, G. Pneumocystis jirovecii genotypes involved in Pneumocystis pneumonia outbreaks among renal transplant recipients. Clin. Infect. Dis. 2013, 56, 165–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, M.; Ripamonti, C.; Mueller, N.J.; Yazaki, H.; Kutty, G.; Ma, L.; Huber, C.; Gogineni, E.; Oka, S.; Goto, N.; et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: Implications for transmission and virulence. Clin. Infect. Dis. 2012, 54, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pifer, L.L.; Niell, H.B.; Morrison, B.J.; Counce, J.D., Jr.; Freeman, J.M.; Woods, D.R.; Neely, C.L. Pneumocystis carinii antigenemia in adults with malignancy, infection, or pulmonary disease. J. Clin. Microbiol. 1984, 20, 887–890. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.L.; Welsh, D.A.; Beard, C.B.; Jones, J.L.; Lawrence, G.G.; Fox, M.R.; Crothers, K.; Morris, A.; Charbonnet, D.; Swartzman, A.; et al. Pneumocystis colonisation is common among hospitalised HIV infected patients with non-Pneumocystis pneumonia. Thorax 2008, 63, 329–334. [Google Scholar] [CrossRef]
- Medrano, F.J.; Montes-Cano, M.; Conde, M.; de la Horra, C.; Respaldiza, N.; Gasch, A.; Perez-Lozano, M.J.; Varela, J.M.; Calderon, E.J. Pneumocystis jirovecii in general population. Emerg. Infect. Dis. 2005, 11, 245–250. [Google Scholar] [CrossRef]
- Ponce, C.A.; Gallo, M.; Bustamante, R.; Vargas, S.L. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin. Infect. Dis. 2010, 50, 347–353. [Google Scholar] [CrossRef] [Green Version]
- de la Horra, C.; Varela, J.M.; Fernandez-Alonso, J.; Medrano, F.J.; Respaldiza, N.; Montes-Cano, M.A.; Calderon, E.J. Association between human-Pneumocystis infection and small-cell lung carcinoma. Eur. J. Clin. Invest. 2004, 34, 229–235. [Google Scholar] [CrossRef]
- Morris, A.; Sciurba, F.C.; Lebedeva, I.P.; Githaiga, A.; Elliott, W.M.; Hogg, J.C.; Huang, L.; Norris, K.A. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am. J. Respir. Crit. Care Med. 2004, 170, 408–413. [Google Scholar] [CrossRef]
- Vidal, S.; de la Horra, C.; Martin, J.; Montes-Cano, M.A.; Rodriguez, E.; Respaldiza, N.; Rodriguez, F.; Varela, J.M.; Medrano, F.J.; Calderon, E.J. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin. Microbiol. Infect. 2006, 12, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.L.; Hughes, W.T.; Santolaya, M.E.; Ulloa, A.V.; Ponce, C.A.; Cabrera, C.E.; Cumsille, F.; Gigliotti, F. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 2001, 32, 855–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, C.; Aguilar, Y.A.; Velez, L.A.; Rueda, Z.V. High transient colonization by Pneumocystis jirovecii between mothers and newborn. Eur. J. Pediatr. 2017, 176, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, Z.; Huangda, W.; Kutty, G.; Ishihara, M.; Wang, H.; Abouelleil, A.; Bishop, L.; Davey, E.; Deng, R.; et al. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat. Commun. 2016, 7, 10740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Chen, Z.; Huang, D.W.; Cisse, O.H.; Rothenburger, J.L.; Latinne, A.; Bishop, L.; Blair, R.; Brenchley, J.M.; Chabe, M.; et al. Diversity and complexity of the large surface protein family in the compacted genomes of multiple Pneumocystis species. MBio 2020, 11, e02878-19. [Google Scholar] [CrossRef] [Green Version]
- Vestereng, V.H.; Bishop, L.R.; Hernandez, B.; Kutty, G.; Larsen, H.H.; Kovacs, J.A. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J. Infect. Dis. 2004, 189, 1540–1544. [Google Scholar] [CrossRef] [Green Version]
- Weissenbacher-Lang, C.; Blasi, B.; Bauer, P.; Binanti, D.; Bittermann, K.; Ergin, L.; Hogler, C.; Hogler, T.; Klier, M.; Matt, J.; et al. Detection of Pneumocystis and morphological description of fungal distribution and severity of infection in thirty-six mammal species. J. Fungi 2023, 9, 220. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar] [CrossRef]
- Chen, W.; Gigliotti, F.; Harmsen, A.G. Latency is not an inevitable outcome of infection with Pneumocystis carinii. Infect. Immun. 1993, 61, 5406–5409. [Google Scholar] [CrossRef] [Green Version]
- Keely, S.P.; Stringer, J.R.; Baughman, R.P.; Linke, M.J.; Walzer, P.D.; Smulian, A.G. Genetic variation among Pneumocystis carinii hominis isolates in recurrent pneumocystosis. J. Infect. Dis. 1995, 172, 595–598. [Google Scholar] [CrossRef]
- Tsolaki, A.G.; Miller, R.F.; Underwood, A.P.; Banerji, S.; Wakefield, A.E. Genetic diversity at the internal transcribed spacer regions of the rRNA operon among isolates of Pneumocystis carinii from AIDS patients with recurrent pneumonia. J. Infect. Dis. 1996, 174, 141–156. [Google Scholar] [CrossRef] [Green Version]
- de la Horra, C.; Friaza, V.; Morilla, R.; Delgado, J.; Medrano, F.J.; Miller, R.F.; de Armas, Y.; Calderon, E.J. Update on dihydropteroate synthase (DHPS) mutations in Pneumocystis jirovecii. J. Fungi 2021, 7, 856. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhuo, L.; Lan, Y.; Dai, Z.; Chen, W.S.; Cai, W.; Kovacs, J.A.; Ma, L.; Tang, X. Mutational analysis of Pneumocystis jirovecii dihydropteroate synthase and dihydrofolate reductase genes in HIV-infected patients in China. J. Clin. Microbiol. 2014, 52, 4017–4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Borio, L.; Masur, H.; Kovacs, J.A. Pneumocystis carinii dihydropteroate synthase but not dihydrofolate reductase gene mutations correlate with prior trimethoprim-sulfamethoxazole or dapsone use. J. Infect. Dis. 1999, 180, 1969–1978. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Health Care Infection Control Practices Advisory, C. 2007 guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Mok, C.C.; Hamijoyo, L.; Kasitanon, N.; Chen, D.Y.; Chen, S.C.; Yamaoka, K.; Oku, K.; L’i, M.T.; Zamora, L. The Asia-Pacific League of Associations for Rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. 2021, 3, e517–e531. [Google Scholar] [CrossRef]
- Chen, S.C.; Sorrell, T.C.; Chang, C.C.; Paige, E.K.; Bryant, P.A.; Slavin, M.A. Consensus guidelines for the treatment of yeast infections in the haematology, oncology and intensive care setting, 2014. Intern. Med. J. 2014, 44, 1315–1332. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Nikiphorou, E.; Dey, M.; Zhao, S.S.; Courvoisier, D.S.; Arnaud, L.; Atzeni, F.; Behrens, G.M.; Bijlsma, J.W.; Bohm, P.; et al. 2022 EULAR recommendations for screening and prophylaxis of chronic and opportunistic infections in adults with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2022, 82, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Reinwald, M.; Silva, J.T.; Mueller, N.J.; Fortun, J.; Garzoni, C.; de Fijter, J.W.; Fernandez-Ruiz, M.; Grossi, P.; Aguado, J.M. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (Intracellular signaling pathways: Tyrosine kinase and mTOR inhibitors). Clin. Microbiol. Infect. 2018, 24 (Suppl. 2), S53–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopeit, M.; Schmidt-Hieber, M.; Sprute, R.; Buchheidt, D.; Hentrich, M.; Karthaus, M.; Penack, O.; Ruhnke, M.; Weissinger, F.; Cornely, O.A.; et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kameda, H.; Fujii, T.; Nakajima, A.; Koike, R.; Sagawa, A.; Kanbe, K.; Tomita, T.; Harigai, M.; Suzuki, Y.; Japan College of Rheumatology Subcommittee on the Guideline for the Use of Methotrexate in Patients with Rheumatoid Arthritis. Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Rua-Figueroa Fernandez de Larrinoa, I.; Carreira, P.E.; Brito Garcia, N.; Diaz Del Campo Fontecha, P.; Pego Reigosa, J.M.; Gomez Puerta, J.A.; Ortega-Castro, R.; Tejera Segura, B.; Aguado Garcia, J.M.; Torre-Cisneros, J.; et al. Recommendations for prevention of infection in systemic autoimmune rheumatic diseases. Reumatol. Clin. 2022, 18, 317–330. [Google Scholar] [CrossRef]
- Proudfoot, R.; Phillips, B.; Wilne, S. Guidelines for the prophylaxis of Pneumocystis jirovecii pneumonia (PJP) in children with solid tumors. J. Pediatr. Hematol. Oncol. 2017, 39, 194–202. [Google Scholar] [CrossRef]
- Tieu, J.; Smith, R.; Basu, N.; Brogan, P.; D’Cruz, D.; Dhaun, N.; Flossmann, O.; Harper, L.; Jones, R.B.; Lanyon, P.C.; et al. Rituximab for maintenance of remission in ANCA-associated vasculitis: Expert consensus guidelines. Rheumatology 2020, 59, e24–e32. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]

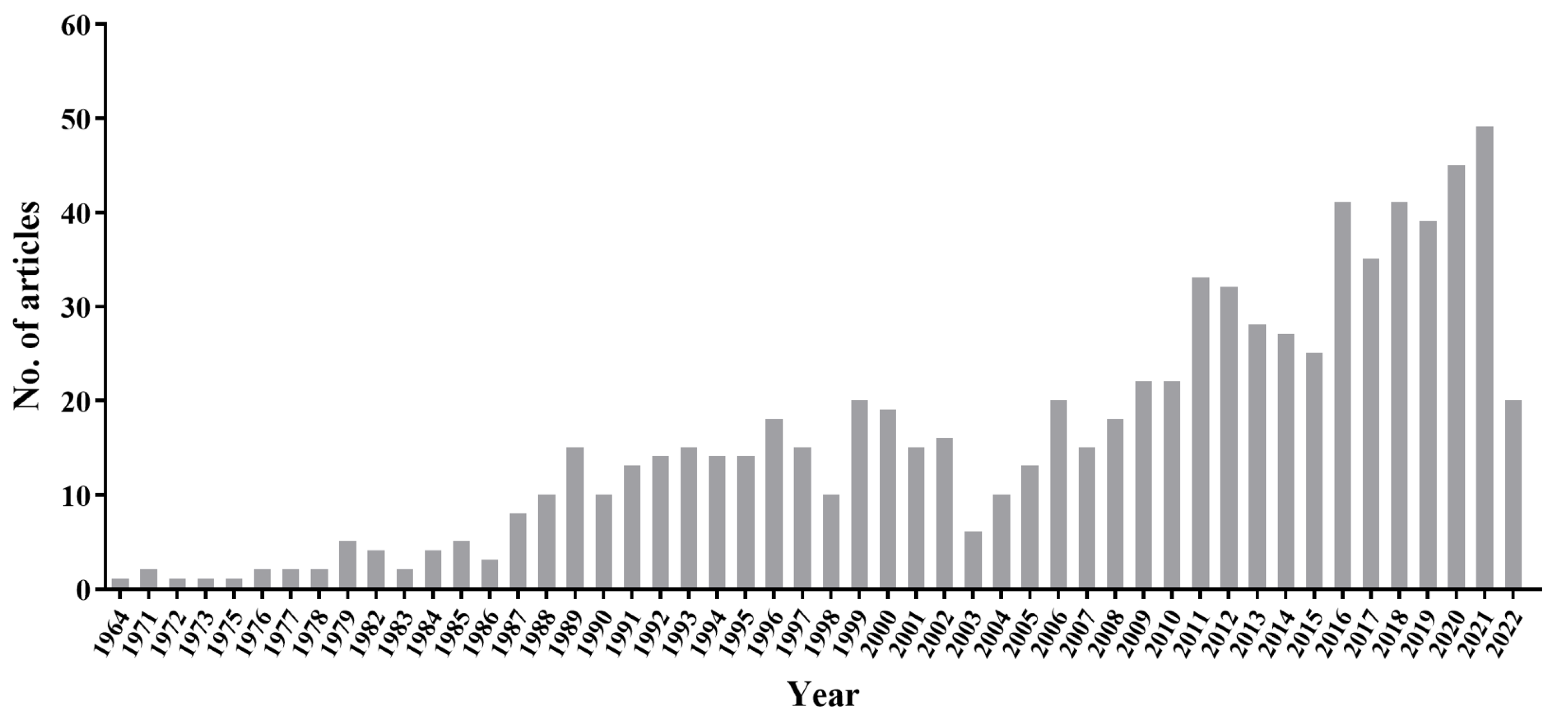
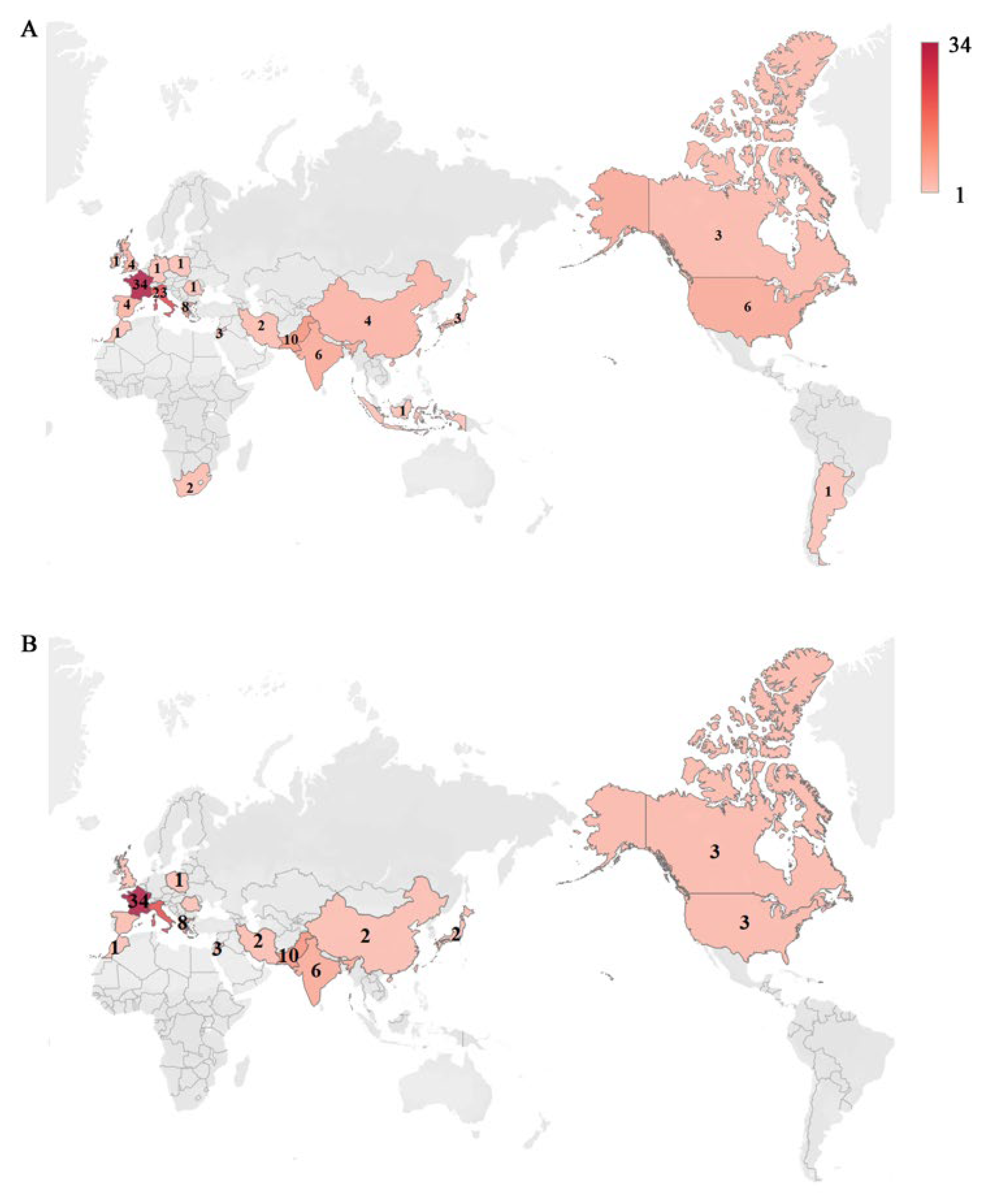

| Immunodeficiency Category (according to the Classification by the Inborn Errors of Immunity Committee 2022 [220]) | Genetic Defects |
|---|---|
| |
| T-B+ SCID | IL2RG, JAK3, ADA |
| CID generally less profound than SCID | CARD11, CD40, CD40LG, DOCK8, IKBKB, IKZF1, IL21R, MALT1, RFXANK, ZAP70 |
| |
| DNA repair defects other than those in Category I | DNMT3B, ZBTB24 |
| Hyper IgE syndromes (HIES) | STAT3 |
| Defects of vitamin B12 and folate metabolism | MTHFD1, SLC46A1, TCN2 |
| Anhidrotic ectodermodysplasia with immunodeficiency | IKBKB, IKBKG |
| Calcium channel defects | ORAI1 |
| Other combined immunodeficiencies with syndromic features | IKZF3, KMT2A, SKIV2L, SP110 |
| |
| Agammaglobulinemia | BTK |
| Common variable immune deficiency | NFKB1 |
| |
| Regulatory T cell defects | CTLA4 |
| |
| Defects of motility | CFTR |
| Defects of respiratory burst | CYBB, G6PD |
| Other non-lymphoid defects | GATA2 |
| |
| Predisposition to severe viral infection | NOS2 |
| Predisposition to mucocutaneous candidiasis | IL17RA, STAT1 |
| Other inborn errors of immunity related to leukocytes | IRF4 |
| IFIH1, IL36RN, TNFRSF1A |
| C7 |
| RTEL1, TERC, TP53 |
| First Author [Reference] | Year of Report | Country | No. of Patients | Transplanted Organs | No. of P. jirovecii Strains/Clusters |
|---|---|---|---|---|---|
| Olsson [312] | 2001 | Sweden | 10 | Kidney | 3 |
| Hocker [313] | 2005 | Germany | 7 | Kidney | 5 |
| de Boer [314] | 2007 | Netherlands | 8 | Kidney | >5 |
| Schmoldt [315] | 2008 | Germany | 16 | Kidney | 1 |
| Yazaki [316] | 2009 | Japan | 27 | Kidney | 1 |
| Arichi [317] | 2009 | Japan | 9 | Kidney | 5 |
| Gianella [318] | 2010 | Switzerland | 20 | Kidney | 1 |
| Wynckel [319] | 2011 | France | 17 | Kidney | 2 |
| Thomas [321] | 2011 | UK | 21 * 11 * | Kidney Kidney | >5 >5 |
| Pliquett [322] | 2012 | Germany | 17 | Kidney | 3 |
| Brunot [323] | 2012 | France | 7 | Kidney | 1 |
| Rostvet [326] | 2013 | Denmark | 29 | Kidney, liver | 3 |
| Debourgogne [327] | 2014 | France | 13 | Kidney | 2 |
| Gits-Muselli [329] | 2015 | France | 6 | Kidney | 1 |
| Desoubeaux [330] | 2016 | France | 4 | Liver | 1 |
| Mulpuru [331] | 2016 | Canada | 10 | Kidney | 1 |
| Urabe [332] | 2016 | Japan | 8 | Kidney | 1 |
| Inkster [333] | 2017 | UK | 24 | Kidney | 2 |
| Vindrios [107] | 2017 | France | 7 | Heart | 1 |
| Robin [334] | 2017 | France | 12 | Stem cell | 5 |
| Frealle [335] | 2017 | France | 5 | Kidney | 2 |
| Alanio [336] | 2017 | France | 13 | Kidney | 1 |
| Belgium | 5 | Kidney | 1 | ||
| UK | 2 | Kidney | 1 | ||
| Wintenberger [337] and Charpentier [338] | 2017 | France | 12 | Lung, kidney, heart, liver | 9 |
| Nevez [339] | 2018 | France | 22 | Kidney | 1 |
| Ricci [340] | 2018 | Brazil | 17 | Kidney | 5 |
| Veronese [341] | 2018 | Italy | 6 | Heart | 2 |
| Szydlowicz [342] | 2019 | Poland | 8 | Kidney | >5 |
| Hosseini-Moghaddam [343] | 2020 | France | 10 | Heart, kidney, liver | 1 |
| Azar [58] | 2022 | USA | 19 | Kidney | 5 |
| Countries | Release Years | Immunocompromised Conditions | References |
|---|---|---|---|
| Asia | 2021 | Systemic lupus erythematosus | [368] |
| Australia | 2014 | Haematological malignancies and stem cell transplantation | [369] |
| Europe | 2016 | Haematological malignancies and stem cell transplantation | [52] |
| Europe | 2022 | Autoimmune inflammatory rheumatic diseases | [370] |
| Europe | 2018 | Relapsed or refractory lymphocytic leukemia patients | [371] |
| Germany | 2021 | Haematopoietic stem cell transplantation, solid tumors, and autoimmune disorders | [372] |
| Japan | 2019 | Use of methotrexate in rheumatoid arthritis | [373] |
| Spain | 2022 | Autoimmune rheumatic diseases | [374] |
| UK | 2017 | Solid tumors in children | [375] |
| UK | 2020 | Anti-neutrophil cytoplasm antibody-associated vasculitis patients receiving Rituximab for maintenance | [376] |
| USA | 2019 | Solid organ transplantation | [111] |
| USA | 2018 | Cancers | [377] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Kong, X.; Ma, L. Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection. J. Fungi 2023, 9, 812. https://doi.org/10.3390/jof9080812
Xue T, Kong X, Ma L. Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection. Journal of Fungi. 2023; 9(8):812. https://doi.org/10.3390/jof9080812
Chicago/Turabian StyleXue, Ting, Xiaomei Kong, and Liang Ma. 2023. "Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection" Journal of Fungi 9, no. 8: 812. https://doi.org/10.3390/jof9080812
APA StyleXue, T., Kong, X., & Ma, L. (2023). Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection. Journal of Fungi, 9(8), 812. https://doi.org/10.3390/jof9080812






