Transcriptome Analysis Revealed That Hydrogen Peroxide-Regulated Oxidative Phosphorylation Plays an Important Role in the Formation of Pleurotus ostreatus Cap Color
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Experiment with the Addition of Exogenous H2O2 or N,N′-dimethylthiourea (DMTU)
2.3. RNA Extraction, cDNA Library Construction, and RNA-seq
2.4. Analysis of DEGs
2.5. Bioinformatics Analysis
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Experiment with the Addition of Exogenous Oligomycin A or Valinomycin
2.8. Data Analysis
3. Results
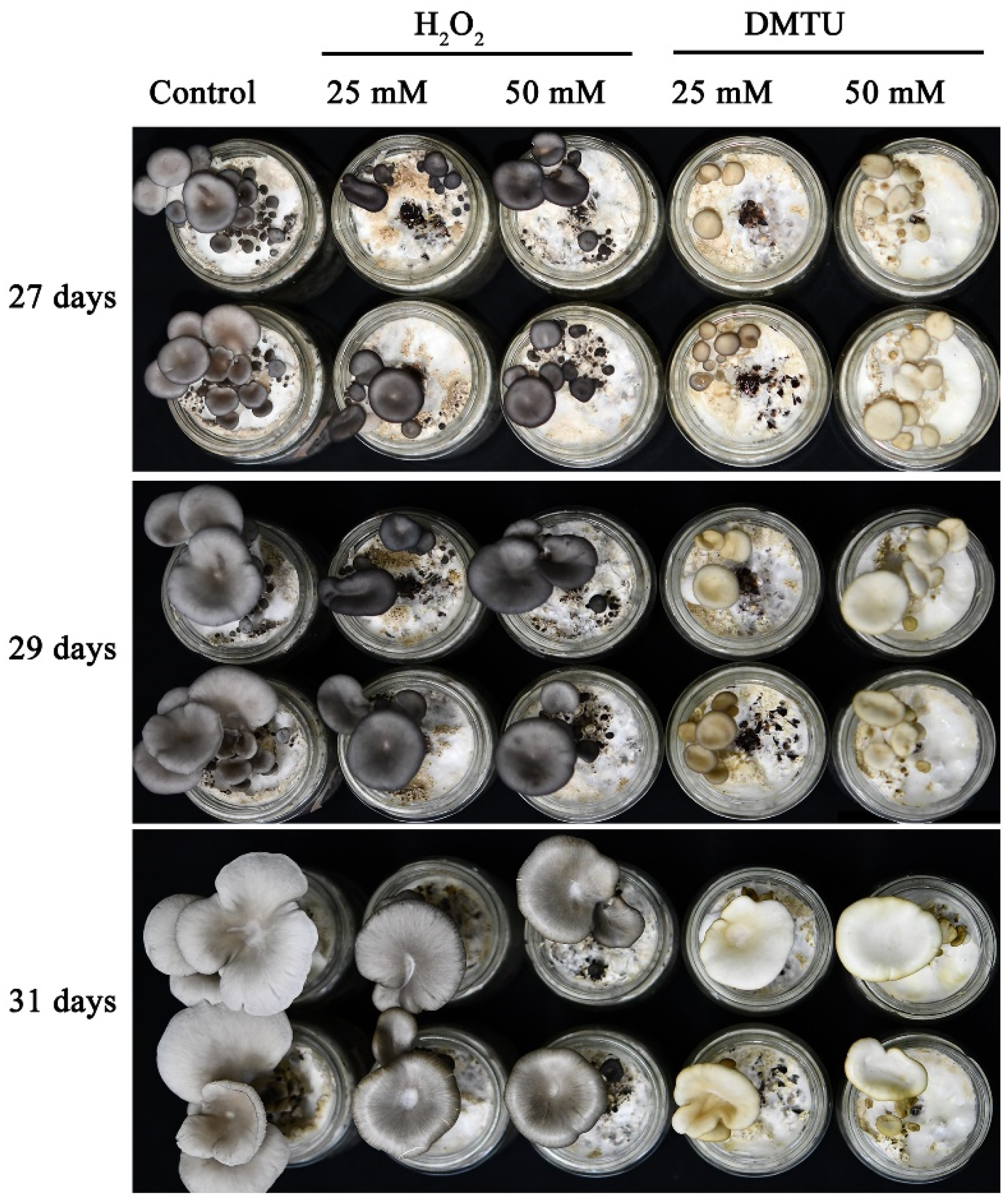
3.1. H2O2 Regulates Cap Color Formation in P. ostreatus
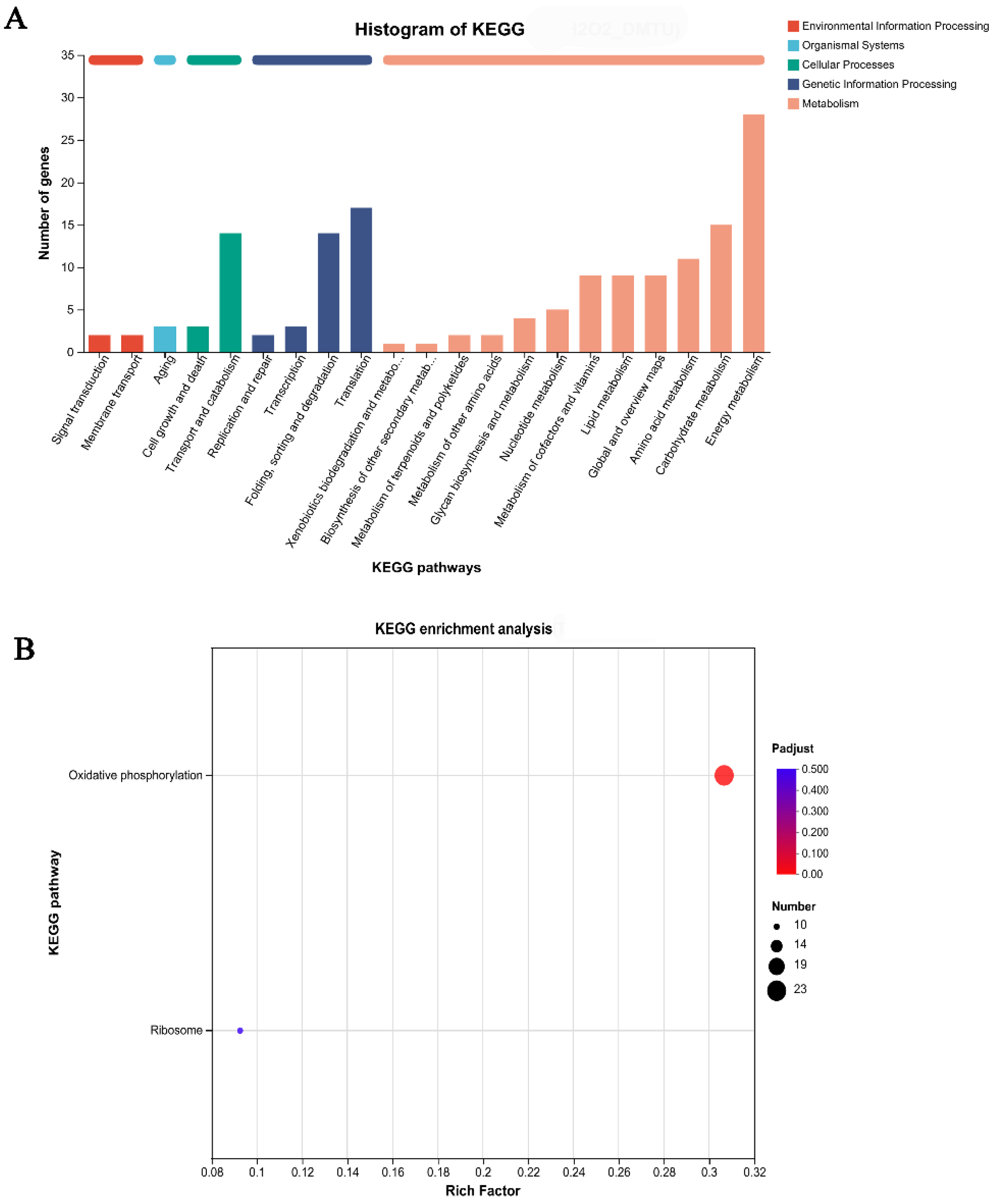
3.2. The Oxidative Phosphorylation Pathway Plays a Key Role in Cap Color Formation
3.3. H2O2 Regulates Cap Color Formation by Affecting the Respiratory Chain
3.4. ATP Synthesis Affects the Formation of P. ostreatus Cap Color
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Li, J.L.; Yang, L.Q.; Shi, F.; Ye, M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- Arun, G.; Eyini, M.; Gunasekaran, P. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J. Exp. Biol. 2015, 53, 380–387. [Google Scholar] [PubMed]
- Liu, X.; Hou, R.L.; Wang, D.T.; Mai, M.X.; Wu, X.P.; Zheng, M.F.; Fu, J. Comprehensive utilization of edible mushroom Auricularia auricula waste residue-extraction, physicochemical properties of melanin and its antioxidant activity. Food Sci. Nutr. 2019, 7, 3774–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.T.; Tang, C.H.; Zhao, Q.Y.; Jia, Y.X.; Qin, Y.C.; Junmin, Z. Melanin: A promising source of functional food ingredient. J. Funct. Foods 2023, 105, 105574. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.L.; Yang, L.Q.; Hou, G.H.; Ye, M. Hypolipidemic effect and protection ability of liver-kidney functions of melanin from Lachnum YM226 in high-fat diet fed mice. Food Funct. 2018, 9, 880–889. [Google Scholar] [CrossRef]
- Li, L.; Shi, F.; Li, J.L.; Huang, Q.L.; Xu, C.; Yang, L.Q.; Yang, Q.H.; Shaikh, F.; Ye, M. Immunoregulatory effect assessment of a novel melanin and its carboxymethyl derivative. Bioorg. Med. Chem. Lett. 2017, 27, 1831–1834. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Spiteller, P.; Arnold, N.; Spiteller, M.; Steglich, W. Lilacinone, a red aminobenzoquinone pigment from Lactarius lilacinus. Nat. Prod. 2023, 66, 1402–1403. [Google Scholar] [CrossRef]
- Sun, S.J.; Zhang, X.J.; Sun, S.W.; Zhang, L.Y.; Shan, S.K.; Zhu, H. Production of natural melanin by Auricularia auricula and study on its molecular structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Hou, R.L.; Liu, X.; Wu, X.P.; Zheng, M.F.; Fu, J.S. Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo. Food Sci. Hum. Well. 2021, 10, 514–522. [Google Scholar] [CrossRef]
- Weijn, A.; Bastiaan-Net, S.; Wichers, H.J.; Mes, J.J. Melanin biosynthesis pathway in Agaricus bisporus mushrooms. Fungal Genet. Biol. 2013, 55, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, L.F.; Tang, W.Q.; Wang, Y.Y.; Zhang, Q.; Wang, H.B.; Zhou, X.; Wu, H.F.; Guo, L.; Dou, M.J.; et al. Identifying a melanogenesis-related candidate gene by a high-quality genome assembly and population diversity analysis in Hypsizygus marmoreus. J. Genet. Genom. 2021, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M. Nutritional and medicinal importance of Pleurotus mushrooms: An overview. Food Rev. Int. 2012, 28, 313–329. [Google Scholar] [CrossRef]
- Kües, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000, 54, 141–152. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Freitas, M.M.S.; Oliveira, L.C.; Martins, L.H.S.; Almada-Vilhena, A.O.; Oliveira, R.M.; Julio, C.P.; Davi, D.S.B.B.; Raul, N.C.J. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Elramady, H.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Chemical composition and health attributes of agri-foods: A scientific overview on black foods. Sustainability 2023, 15, 3852. [Google Scholar] [CrossRef]
- Qing, Q.R.; Xiao, J.Y.; Yao, H. Opportunities, challenges and countermeasures of edible fungi industry development under the development background of big health industry. Edible Fungi China 2020, 39, 94–96. [Google Scholar] [CrossRef]
- Toledo, A.V.; Franco, M.E.E.; Lopez, S.M.Y.; Troncozo, M.I.; Saparrat, M.C.N.; Balatti, P.A. Melanins in fungi: Types, localization and putative biological roles. Physiol. Mol. Plant P 2017, 99, 2–6. [Google Scholar] [CrossRef]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and identification of pigments from oyster mushrooms with black, yellow and pink caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef]
- Guaraldo, M.M.D.S.; Pereira, T.M.; Santos, H.O.D.; Oliveira, T.L.D.; Pereira, W.V.S.; Pinho, E.V.D.R.V. Priming with sodium nitroprusside and hydrogen peroxide increases cotton seed tolerance to salinity and water deficit during seed germination and seedling development. Environ. Exp. Bot. 2023, 209, 1005294. [Google Scholar] [CrossRef]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Potikha, T.S.; Collins, C.C.; Johnson, D.I.; Delmer, D.P.; Levine, A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999, 119, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Xu, W.L.; Wang, P.; Gu, Z.X.; Zhang, H.Z.; Yang, R. UV-B-triggered H2O2 production mediates isoflavones synthesis in germinated soybean. Food Chem. X 2022, 14, 100331. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klusener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2022, 406, 731–734. [Google Scholar] [CrossRef]
- Rosei, M.A.; Blarzino, C.; Coccia, R.; Foppoli, C.; Cini, C. Production of melanin pigments by cytochrome c/H2O2 system. Int. J. Biochem. Cell Biol. 1998, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kemmerling, U.; Munoz, P.; Muller, M.; Sanchez, G.; Aylwin, M.L.; Klann, E.; Carrasco, M.A.; Hidalgo, C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium 2007, 41, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.J.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem. Biophys. Res. Commun. 2004, 32, 88–92. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, S.G. Induction of ATP synthase beta by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.S.; Li, C.Y.; Zhang, X.C.; Li, X.B.; Shi, L.; Ren, A.; Zhao, M.W. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, oxidative-stress resistance, and ganoderic acid biosynthesis regulation. Environ. Microbiol. 2014, 16, 1709–1728. [Google Scholar] [CrossRef]
- Shi, D.K.; Zhu, J.; Sun, Z.H.; Zhang, G.; Liu, R.; Zhang, T.J.; Wang, S.L.; Ren, A.; Zhao, M.W. Alternative oxidase impacts ganoderic acid biosynthesis by regulating intracellular ROS levels in Ganoderma lucidum. Microbiology 2017, 163, 1466–1476. [Google Scholar] [CrossRef]
- Hou, L.D.; Huang, C.Y.; Wu, X.L.; Zhang, J.X.; Zhao, M.R. Nitric oxide negatively regulates the rapid formation of Pleurotus ostreatus primordia by inhibiting the mitochondrial aco gene. J. Fungi 2022, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.D.; Wang, L.N.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; He, Q.; Zhang, L.J.; Zhang, J.X. Alternative oxidase gene induced by nitric oxide is involved in the regulation of ROS and enhances the resistance of Pleurotus ostreatus to heat stress. Microb. Cell Fact. 2021, 20, 137. [Google Scholar] [CrossRef]
- Wu, T.J.; Hu, C.C.; Xie, B.G.; Wei, S.L.; Zhang, L.; Zhu, Z.X.; Zhang, Z.Y.; Li, S.J. A putative transcription factor LFC1 negatively regulates development and yield of winter mushroom. Appl. Microbiol. Biotechnol. 2022, 104, 5827–5844. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Hu, C.C.; Xie, B.G.; Zhang, L.; Yan, S.; Wang, W.; Tao, Y.; Li, S. A single transcription factor (PDD1) determines development and yield of winter mushroom (Flammulina velutipes). Appl. Environ. Microbiol. 2019, 85, e01735-19. [Google Scholar] [CrossRef]
- Hou, L.D.; Yan, K.X.; Dong, S.; Liu, X.Y.; Chang, M.C.; Meng, J.L. Preliminary study on the mechanism of signal molecule H2O2 regulating the formation of Pleurotus ostreatus cap color. Acta Hortic. Sinica. 2023, 50, 1243–1254. [Google Scholar] [CrossRef]
- Qu, J.B.; Zhao, M.R.; Hsiang, T.; Feng, X.X.; Zhang, J.X.; Huang, C.Y. Identification and characterization of small noncoding RNAs in genome sequences of the edible fungus Pleurotus ostreatus. Biomed. Res. Int. 2016, 2016, 2503023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Chen, Q.; Wu, X.L.; Zhang, J.X.; Huang, C.Y. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr. Microbiol. 2014, 69, 611–616. [Google Scholar] [CrossRef]
- Yan, J.J.; Chekanova, J.; Liu, Y.Y.; Gan, B.C.; Long, Y.; Han, X.; Tong, Z.J.; Miao, J.; Lian, L.D.; Xie, B.G.; et al. Reactive oxygen species distribution involved in stipe gradient elongation in the mushroom Flammulina filiformis. Cells 2022, 11, 1896. [Google Scholar] [CrossRef]
- Desikan, R.; Mackerness, S.A.H.; Hancock, J.T.; Neill, S.J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001, 127, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yu, Y.; Tan, H.; Wang, B.; Peng, W.H.; Sun, Q. Metabolomics reveals dopa melanin involved in the enzymatic browning of the yellow cultivars of East Asian golden needle mushroom (Flammulina filiformis). Food Chem. 2022, 370, 131295. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, H.; Wang, B.; Peng, W.H.; Sun, Q.; Yu, Y. Integrated multi-omic analyses on yellow Flammulina filiformis cultivar reveal postharvest oxidative damage responses. Postharvest Biol. Tec. 2023, 195, 112111. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zhao, C.; Zhang, M.; Li, Y.Z.; Shen, Y.Y.; Guo, J.X. Transcriptome analysis around the onset of strawberry fruit ripening uncovers an important role of oxidative phosphorylation in ripening. Sci. Rep. 2017, 7, 41477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Luo, X.; Yang, X.Y.; Hopkins, D.L.; Mao, Y.; Zhang, Y.M. Understanding the development of color and color stability of dark cutting beef based on mitochondrial proteomics. Meat Sci. 2020, 163, 108046. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, R.; Ge, Q.; Taha, R.H.; Chen, K.Q. Complementary transcriptomic and proteomic analysis of bombyx mori middle silk glands reveals a predominant ribosome-biogenesis regulating network during silkworm yellow-cocoon color formation. J. Asia Pac. Entomol. 2021, 24, 260–270. [Google Scholar] [CrossRef]
- Li, Z.Z.; Li, Q.; Liu, S.K.; Han, Z.Q.; Kong, L.F.; Yu, H. Integrated analysis of coding genes and non-coding rnas associated with shell color in the pacific oyster (Crassostrea gigas). Mar. Biotechnol. 2021, 23, 417–429. [Google Scholar] [CrossRef]
- Li, L.; Sun, H.; Kitazawa, H.; Wang, X.Y. Effects of a high O2 dynamic-controlled atmosphere technology on the browning of postharvest white mushroom (Agaricus bisporus) in relation to energy metabolism. Food Sci. Technol. Int. 2017, 23, 385–395. [Google Scholar] [CrossRef]







| Gene ID | Gene Function |
|---|---|
| g10165 | ATP4 subunit B of the stator stalk of mitochondrial F1F0 ATP synthase |
| g2546 | Cytochrome bc1 complex subunit 7 |
| g7640 | Cytochrome c oxidase subunit 6 |
| g8839 | NdufA4 NADH dehydrogenase 1 ɑ subcomplex |
| g10989 | V-type proton ATPase 16 kDa proteolipid subunit 2 |
| g11401 | ATP17 subunit F of the F0 sector of mitochondrial F1F0 ATP synthase |
| g1602 | ATP3 γ subunit of the F1 sector of mitochondrial F1F0 ATP synthase |
| g11242 | ATP synthase F1 β subunit |
| g12225 | ATP synthase E chain-domain-containing protein |
| g12829 | ATP20 subunit g of the mitochondrial F1F0 ATP synthase |
| g3195 | Hypothetical protein |
| g9081 | Cytochrome c oxidase assembly protein cox15 |
| g9180 | ATP synthase F1 ɑ subunit |
| g12052 | Cytochrome c oxidase subunit vib |
| g13251 | Ubiquinol-cytochrome c reductase complex subunit 8 |
| g2860 | Ubiquinol-cytochrome-c reductase subunit 6 |
| g12390 | ATP synthase subunit 5 |
| g12499 | Cytochrome c oxidase subunit 4 |
| g8845 | ATP synthase d subunit |
| g2230 | Cytochrome c oxidase copper chaperone |
| g400 | Acyl carrier protein |
| g8493 | ATP18 subunit j of the mitochondrial F1F0 ATP synthase |
| g8872 | Hypothetical protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Yan, K.; Dong, S.; Guo, L.; Liu, J.; Wang, S.; Chang, M.; Meng, J. Transcriptome Analysis Revealed That Hydrogen Peroxide-Regulated Oxidative Phosphorylation Plays an Important Role in the Formation of Pleurotus ostreatus Cap Color. J. Fungi 2023, 9, 823. https://doi.org/10.3390/jof9080823
Hou L, Yan K, Dong S, Guo L, Liu J, Wang S, Chang M, Meng J. Transcriptome Analysis Revealed That Hydrogen Peroxide-Regulated Oxidative Phosphorylation Plays an Important Role in the Formation of Pleurotus ostreatus Cap Color. Journal of Fungi. 2023; 9(8):823. https://doi.org/10.3390/jof9080823
Chicago/Turabian StyleHou, Ludan, Kexing Yan, Shuai Dong, Lifeng Guo, Jingyu Liu, Shurong Wang, Mingchang Chang, and Junlong Meng. 2023. "Transcriptome Analysis Revealed That Hydrogen Peroxide-Regulated Oxidative Phosphorylation Plays an Important Role in the Formation of Pleurotus ostreatus Cap Color" Journal of Fungi 9, no. 8: 823. https://doi.org/10.3390/jof9080823




