Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19)
Abstract
1. Introduction
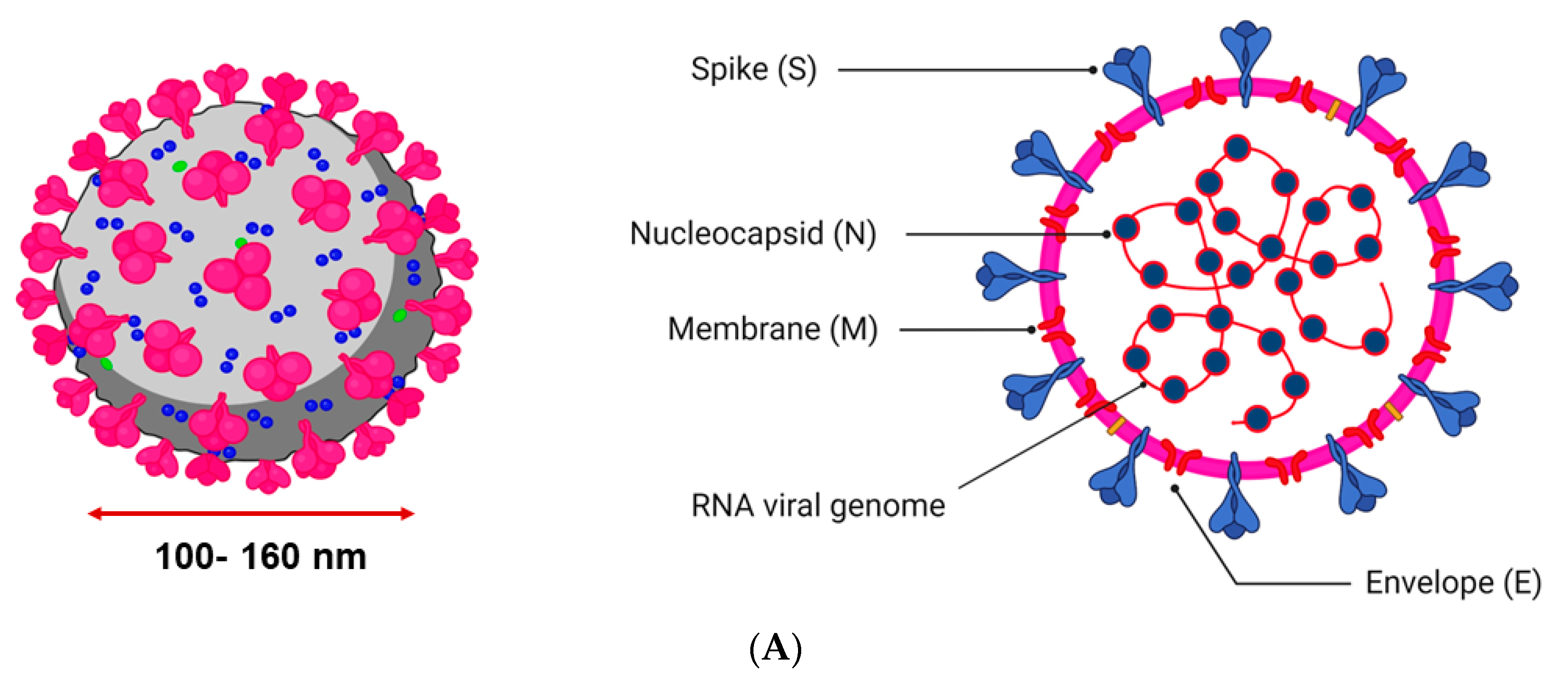
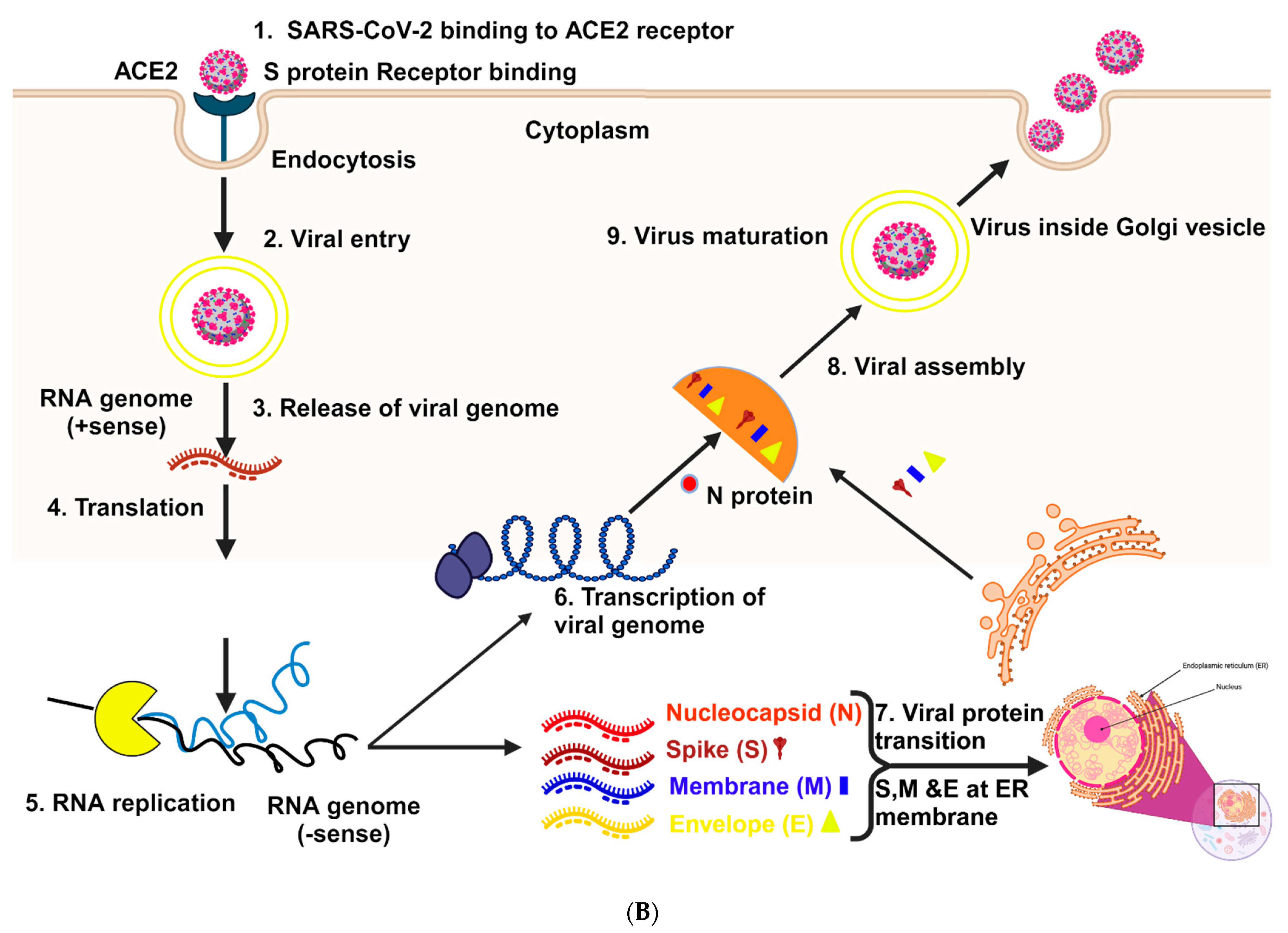
2. Structure and Mechanism of Infection
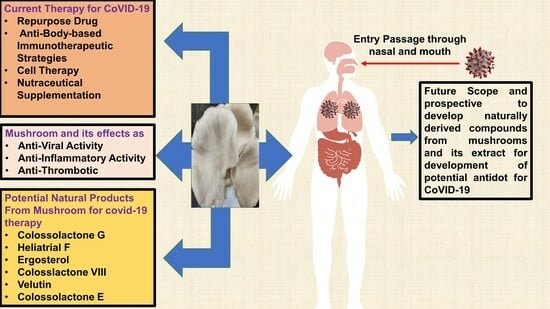
3. Current Therapy against COVID-19: The Key Issues and Challenges
3.1. Repurposed Drugs
3.2. Antibody-Based Immunotherapeutic Strategies
3.3. Cell Therapy
3.4. Nutraceutical Supplementation
4. Potential of Natural Products Derived from Mushrooms for the Treatment of SARS-CoV-2 Infection
4.1. Antiviral Activity of Mushroom-Derived Bioactive Compounds: Potential Therapeutics against SARS-CoV-2
4.2. Mushroom-Derived Anti-Inflammatory Compound: Potential Candidate to Reduce COVID-19-Associated Inflammation
4.2.1. Inflammation and Immune System
4.2.2. Lung Infection and Inflammation in COVID-19
4.2.3. Macrophages and Inflammation in COVID-19
4.3. Antithrombotic Effects of Bioactive Compounds of Edible Mushrooms
5. Future Scope and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Bang, T.H.; Ohnuki, K.; Sawai, T.; Sawai, K.; Shimizu, K. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci. Rep. 2015, 5, 13194. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. Science Forum: SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G.; Kadhom, M.; Hairunisa, N.; Yousif, E.; Mohammed, S.A. A review on COVID-19: Origin, spread, symptoms, treatment, and prevention. Biointerface Res. Appl. Chem. 2020, 10, 7234–7242. [Google Scholar]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 2022, 814, 146134. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates 2021, 59, 100794. [Google Scholar] [CrossRef]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021, 39, 3409–3418. [Google Scholar] [CrossRef]
- Gupta, M.K.; Vemula, S.; Donde, R.; Gouda, G.; Behera, L.; Vadde, R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 2021, 39, 2617–2627. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Carretta, D.M.; De Nitto, E.; Lovero, R. The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. J. Mol. Med. 2021, 99, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Rabie, A.M. Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication. ACS Omega 2022, 7, 2960–2969. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses 2015, 1282, 1–23. [Google Scholar]
- Hosseini, E.S.; Kashani, N.R.; Nikzad, H.; Azadbakht, J.; Bafrani, H.H.; Kashani, H.H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef]
- Senanayake, S.L. Drug repurposing strategies for COVID-19. Future Drug Discov. 2020, 2. [Google Scholar] [CrossRef]
- Molavi, Z.; Razi, S.; Mirmotalebisohi, S.A.; Adibi, A.; Sameni, M.; Karami, F.; Niazi, V.; Niknam, Z.; Aliashrafi, M.; Taheri, M. Identification of FDA approved drugs against SARS-CoV-2 RNA dependent RNA polymerase (RdRp) and 3-chymotrypsin-like protease (3CLpro), drug repurposing approach. Biomed. Pharmacother. 2021, 138, 111544. [Google Scholar] [CrossRef]
- Niknam, Z.; Jafari, A.; Golchin, A.; Danesh Pouya, F.; Nemati, M.; Rezaei-Tavirani, M.; Rasmi, Y. Potential therapeutic options for COVID-19: An update on current evidence. Eur. J. Med. Res. 2022, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chandele, A.; Sharma, A. Current status of therapeutic monoclonal antibodies against SARS-CoV-2. PLoS Pathog. 2021, 17, e1009885. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Debnath, B.; Debnath, P.; Debnath, S.; Zaki, M.E.; Masand, V.H. Identification of potential edible mushroom as SARS-CoV-2 main protease inhibitor using rational drug designing approach. Sci. Rep. 2022, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Rodríguez, Y.; Monsalve, D.M.; Acosta-Ampudia, Y.; Camacho, B.; Gallo, J.E.; Rojas-Villarraga, A.; Ramírez-Santana, C.; Díaz-Coronado, J.C.; Manrique, R. Convalescent plasma in COVID-19: Possible mechanisms of action. Autoimmun. Rev. 2020, 19, 102554. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Mansouri, F.; Azari, A.; Ranjbarvan, P.; Zarein, F.; Heidari, A.; Golchin, A. Stem cell therapy potency in personalizing severe COVID-19 treatment. Stem Cell Rev. Rep. 2021, 17, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, D.; Mori, H.; Chen, S. White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft. J. Nutr. Biochem. 2021, 89, 108580. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Buetti, N.; Houhou-Fidouh, N.; Patrier, J.; Abdel-Nabey, M.; Jaquet, P.; Presente, S.; Girard, T.; Sayagh, F.; Ruckly, S. HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. J. Crit. Care 2021, 25, 417. [Google Scholar] [CrossRef]
- Zhang, M.; Cheung, P.C.; Ooi, V.E.; Zhang, L. Evaluation of sulfated fungal β-glucans from the sclerotium of Pleurotus tuber-regium as a potential water-soluble anti-viral agent. Carbohydr. Res. 2004, 339, 2297–2301. [Google Scholar] [CrossRef]
- Masuda, Y.; Matsumoto, A.; Toida, T.; Oikawa, T.; Ito, K.; Nanba, H. Characterization and antitumor effect of a novel polysaccharide from Grifola frondosa. J. Agric. Food Chem. 2009, 57, 10143–10149. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals Under Investigation for COVID-19 Prevention and Treatment. Msystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Hetland, G.; Eide, D.M.; Tangen, J.M.; Haugen, M.H.; Mirlashari, M.R.; Paulsen, J.E. The Agaricus blazei-based mushroom extract, Andosan™, protects against intestinal tumorigenesis in the A/J Min/+ Mouse. PLoS ONE 2016, 11, e0167754. [Google Scholar] [CrossRef]
- Hetland, G.; Johnson, E.; Bernardshaw, S.V.; Grinde, B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scand. J. Immunol. 2021, 93, e12937. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, H.; Kato, H.; Kaneko, M.; Kumazawa, Y. Amelioration of skewed Th1/Th2 balance in tumor-bearing and asthma-induced mice by oral administration of Agaricus blazei extracts. Immunopharmacol. Immunotoxicol. 2008, 30, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Ellertsen, L.K.; Hetland, G. An extract of the medicinal mushroom Agaricus blazei Murill can protect against allergy. Clin. Mol. Allergy 2009, 7, 6. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. J. Deal. All Asp. Plant Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; EL-Ghwas, D.E.; Daba, G.M. Mushrooms as efficient enzymatic machinery. J. Biomed. Res. Environ. Sci. 2022, 3, 423–428. [Google Scholar] [CrossRef]
- Patel, Y.; Naraian, R.; Singh, V. Medicinal properties of Pleurotus species (oyster mushroom): A review. World J. Fungal Plant Biol. 2012, 3, 1–12. [Google Scholar]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Gu, C.-Q.; Li, J.-W.; Chao, F.-H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-α in HepG2 2.2. 15. Antivir. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Li, H.; Zhao, C.; Ma, H.; Zhao, X.; Wu, J.; Liu, K.; Shan, J.; Wang, Y. Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. Int. J. Biol. Macromol. 2016, 91, 248–257. [Google Scholar] [CrossRef]
- Meiers, J.; Siebs, E.; Zahorska, E.; Titz, A. Lectin antagonists in infection, immunity, and inflammation. Curr. Opin. Chem. Biol. 2019, 53, 51–67. [Google Scholar] [CrossRef]
- Rahaie, M.; Kazemi, S. Lectin-based biosensors: As powerful tools in bioanalytical applications. Biotechnology 2010, 9, 428–443. [Google Scholar] [CrossRef][Green Version]
- Faccin, L.C.; Benati, F.; Rincão, V.P.; Mantovani, M.S.; Soares, S.A.; Gonzaga, M.L.; Nozawa, C.; Carvalho Linhares, R.E. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007, 45, 24–28. [Google Scholar] [CrossRef]
- Nogusa, S.; Gerbino, J.; Ritz, B.W. Low-dose supplementation with active hexose correlated compound improves the immune response to acute influenza infection in C57BL/6 mice. Nutr. Res. 2009, 29, 139–143. [Google Scholar] [CrossRef]
- Song, A.-R.; Sun, X.-L.; Kong, C.; Zhao, C.; Qin, D.; Huang, F.; Yang, S. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch. Virol. 2014, 159, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Jülich, W.-D.; Witt, S. Ganoderma pfeifferi–a European relative of Ganoderma lucidum. Phytochemistry 2015, 114, 102–108. [Google Scholar] [CrossRef]
- Rincão, V.P.; Yamamoto, K.A.; Ricardo, N.M.; Soares, S.A.; Meirelles, L.D.; Nozawa, C.; Linhares, R.E. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Piraino, F.; Brandt, C.R. Isolation and partial characterization of an antiviral, RC-183, from the edible mushroom Rozites caperata. Antivir. Res. 1999, 43, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, X.; Zhang, L.; Zhao, N.; Yang, D.; Zhang, K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Hwang, K.-C.; Chiang, Y.-H.; Chou, P. The mushroom Agaricus blazei Murill extract normalizes liver function in patients with chronic hepatitis B. J. Altern. Complement. Med. 2008, 14, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Psurtseva, N.V.; Kosogova, T.A.; Mazurkova, N.A.; Khanin, V.A.; Vlasenko, V.A. Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from Altai Mountains (Russia). Int. J. Med. Mushrooms 2012, 14, 37–45. [Google Scholar] [CrossRef]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine storm in COVID-19—Immunopathological mechanisms, clinical considerations, and therapeutic approaches: The REPROGRAM consortium position paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Behrens, E.M. Weathering the storm: Improving therapeutic interventions for cytokine storm syndromes by targeting disease pathogenesis. Curr. Treat. Options Rheumatol. 2017, 3, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. 2020, 34, 327–331. [Google Scholar]
- Muta, T. Molecular basis for invertebrate innate immune recognition of (1→3)-β-D-glucan as a pathogen-associated molecular pattern. Curr. Pharm. Des. 2006, 12, 4155–4161. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, tumors, and immunity: An update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Park, Y.-M.; Won, J.-H.; Kim, Y.-H.; Choi, J.-W.; Park, H.-J.; Lee, K.-T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Clinckemalie, L.; Spans, L.; Dubois, V.; Laurent, M.; Helsen, C.; Joniau, S.; Claessens, F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. J. Mol. Endocrinol. 2013, 27, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, X.; Li, Q.; Xie, L.; Guo, T.; Su, T.; Tang, C.; Chang, X.; Liang, B.; Huang, D. Androgen receptor-activated enhancers simultaneously regulate oncogene TMPRSS2 and lncRNA PRCAT38 in prostate cancer. Cell 2019, 8, 864. [Google Scholar] [CrossRef]
- Becerra-Diaz, M.; Song, M.; Heller, N. Androgen and androgen receptors as regulators of monocyte and macrophage biology in the healthy and diseased lung. Front. Immunol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Baratchian, M.; McManus, J.M.; Berk, M.; Nakamura, F.; Mukhopadhyay, S.; Xu, W.; Erzurum, S.; Drazba, J.; Peterson, J.; Klein, E.A. Sex, androgens and regulation of pulmonary AR, TMPRSS2 and ACE2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, J.; Shi, P.; Chen, D.; Wang, S.; Wang, P.; Feng, X.; Zhang, L. Research status of the safety and efficacy of mesenchymal stem cells in the treatment of COVID-19-related pneumonia: A systematic review and meta-analysis. Stem Cells Dev. 2021, 30, 947–969. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Ha, D.; Yoshitake, R. White Button Mushroom (Agaricus bisporus) Interrupts Tissue AR-TMPRSS2 Expression and Attenuates Pro-inflammatory Cytokines in C57BL/6 Mice: Implication for COVID-19 Dietary Intervention. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuentea, A.; Moro, C.; Villares, A.; Guillamon, E.; Rostagno, M.A.; D’Arrigo, M.; Alfredo Martinez, J. Mushrooms as a source of anti-inflammatory agents. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2010, 9, 125–141. [Google Scholar] [CrossRef]
- Woo, C.W.; Man, R.Y.; Siow, Y.L.; Choy, P.C.; Wan, E.W.; Lau, C.S.J.M.; Biochemistry, C. Ganoderma lucidum inhibits inducible nitric oxide synthase expression in macrophages. Mol. Cell Biochem. 2005, 275, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.; Ajith, T.; Janardhanan, K. Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother. Res. 2004, 18, 43–46. [Google Scholar] [CrossRef]
- Jayasuriya, W.J.A.; Handunnetti, S.M.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Anti-Inflammatory Activity of Pleurotus ostreatus, a Culinary Medicinal Mushroom, in Wistar Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 6845383. [Google Scholar] [CrossRef]
- Li, H.; Lu, X.; Zhang, S.; Lu, M.; Liu, H. Anti-inflammatory activity of polysaccharide from Pholiota nameko. Biochemistry 2008, 73, 669–675. [Google Scholar] [CrossRef]
- Jeong, S.C.; Koyyalamudi, S.R.; Pang, G. Dietary intake of Agaricus bisporus white button mushroom accelerates salivary immunoglobulin A secretion in healthy volunteers. Nutrition 2012, 28, 527–531. [Google Scholar] [CrossRef]
- Kalita, B.; Saviola, A.J.; Samuel, S.P.; Mukherjee, A.K. State-of-the-art review-A review on snake venom-derived antithrombotics: Potential therapeutics for COVID-19-associated thrombosis? Int. J. Biol. Macromol. 2021, 192, 1040–1057. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Cavecchia, I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol. Dietol. 2020, 66, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Badalyan, S.M. Potential of mushroom bioactive molecules to develop healthcare biotech products. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; DMR Solan: Solan, India, 2014; pp. 373–378. [Google Scholar]
- Hajra, A.; Mathai, S.V.; Ball, S.; Bandyopadhyay, D.; Veyseh, M.; Chakraborty, S.; Lavie, C.J.; Aronow, W.S. Management of thrombotic complications in COVID-19: An update. Drugs 2020, 80, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.; Bonnin, P.; Stépanian, A.; Chousterman, B.G.; Le Gall, A.; Malissin, I.; Deye, N.; Siguret, V.; Mebazaa, A.; Mégarbane, B. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J. Am. Coll. Cardiol. 2020, 76, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Jilma, B.; Pabinger, I.; Ay, C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Hu, Y.; Konstantinides, S. Venous thromboembolism in COVID-19. J. Thromb. Haemost. 2020, 120, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Campia, U.; Hurwitz, S.; Snyder, J.E.; Rizzo, S.M.; Pfeferman, M.B.; Morrison, R.B.; Leiva, O.; Fanikos, J.; Nauffal, V. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, P.; Russo, V.; Carannante, N.; Imparato, M.; Rodolfi, S.; Cardillo, G.; Lodigiani, C. Clotting factors in COVID-19: Epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J. Clin. Med. 2020, 9, 1371. [Google Scholar] [CrossRef]
- Streiff, M.B.; Agnelli, G.; Connors, J.M.; Crowther, M.; Eichinger, S.; Lopes, R.; McBane, R.D.; Moll, S.; Ansell, J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J. Thromb. Thrombolysis 2016, 41, 32–67. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. J. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef]
- Veuthey, L.; Aliotta, A.; Bertaggia Calderara, D.; Pereira Portela, C.; Alberio, L. Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation—A Conceptual Review Summarizing Current Knowledge. Int. J. Mol. Sci. 2022, 23, 2536. [Google Scholar] [CrossRef]
- Choi, E.; Oh, J.; Sung, G.-H. Antithrombotic and antiplatelet effects of Cordyceps militaris. Mycobiology 2020, 48, 228–232. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, H.-W.; Kim, H.-H.; Lim, D.H.; Nam, G.S.; Shin, J.-H.; Kim, Y.-Y.; Kim, J.-L.; Lee, J.-J.; Kwon, H.-K. Cordycepin-enriched WIB801C from Cordyceps militaris inhibits ADP-induced [Ca2+] i mobilization and fibrinogen binding via phosphorylation of IP3R and VASP. Arch. Pharm. Res. 2015, 38, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, F.-Q.; Zhang, Q.; Wang, F.-Q.; Hu, Y.-J.; Xia, Z.-N. Natural products for antithrombosis. Evid. Based Complement. Altern. Med. 2015, 2015, 876426. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.W.; Jeong, S.C.; Lee, D.H.; Park, J.S.; Lee, J.S. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006, 27, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A.W. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar] [CrossRef]
- Linhult, M.; Binz, H.K.; Uhlén, M.; Hober, S. Mutational analysis of the interaction between albumin-binding domain from streptococcal protein G and human serum albumin. Protein Sci. 2002, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, D.-W.; Kim, S.; Kim, S.-J. Purification and partial characterization of a fibrinolytic enzyme from the fruiting body of the medicinal and edible mushroom Pleurotus ferulae. Prep. Biochem. Biotechnol. 2017, 47, 539–546. [Google Scholar] [CrossRef]
- Shin, H.H.; Choi, H.S. Purification and characterization of metalloproteases from Pleurotus sajor-caju. J. Microbiol. Biotechnol. 1999, 9, 675–678. [Google Scholar]
- Elkhateeb, W.A.; Daba, G.M.; Elnahas, M.O.; Thomas, P.W. Anticoagulant capacities of some medicinal mushrooms. ARC J. Pharma Sci. 2019, 5, 12–16. [Google Scholar]
- Kim, J.-H.; Kim, Y.S. A fibrinolytic metalloprotease from the fruiting bodies of an edible mushroom, Armillariella mellea. Biosci. Biotechnol. Biochem. 1999, 63, 2130–2136. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Cong, S.; Deng, Y.; Zheng, X. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.S. Characterization of a metalloenzyme from a wild mushroom, Tricholoma saponaceum. Biosci. Biotechnol. Biochem. 2001, 65, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Xu, H.; Xie, J.; Yi, Q.F.; Li, W.; Qiao, D.R.; Cao, Y.; Cao, Y. Thermostable sites and catalytic characterization of xylanase XYNB of Aspergillus niger SCTCC 400264. J. Microbiol. Biotechnol. 2014, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, H.-J.; Kim, S. Purification and antithrombotic activity of wulfase, a fibrinolytic enzyme from the fruit bodies of the edible and medicinal mushroom Sparassis crispa Wulf. ex. Fr. Appl. Biochem. Microbiol. 2016, 52, 608–614. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-Glucans from Fungi: Biological and Health-Promoting Potential in the COVID-19 Pandemic Era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- But, P.P.; He, Z.D.; Ma, S.C.; Chan, Y.M.; Shaw, P.C.; Ye, W.C.; Jiang, R.W. Antiviral constituents against respiratory viruses from Mikania micrantha. J. Nat. Prod. 2009, 72, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A Review of Chemical Composition and Bioactivity Studies of the Most Promising Species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech 2019, 9, 369. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Barbosa, B.V.R.; Lima, M.T.N.S.; Cardoso, P.G.; Contigli, C.; Pimenta, L.P.S.; Chemistry, M. Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorganic Med. Chem. 2021, 46, 116366. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef]
- Zhand, S.; Saghaeian Jazi, M.; Mohammadi, S.; Tarighati Rasekhi, R.; Rostamian, G.; Kalani, M.R.; Rostamian, A.; George, J.; Douglas, M.W. COVID-19: The immune responses and clinical therapy candidates. Int. J. Mol. Sci. 2020, 21, 5559. [Google Scholar] [CrossRef]
- Jesenak, M.; Urbancikova, I.; Banovcin, P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients 2017, 9, 779. [Google Scholar] [CrossRef]

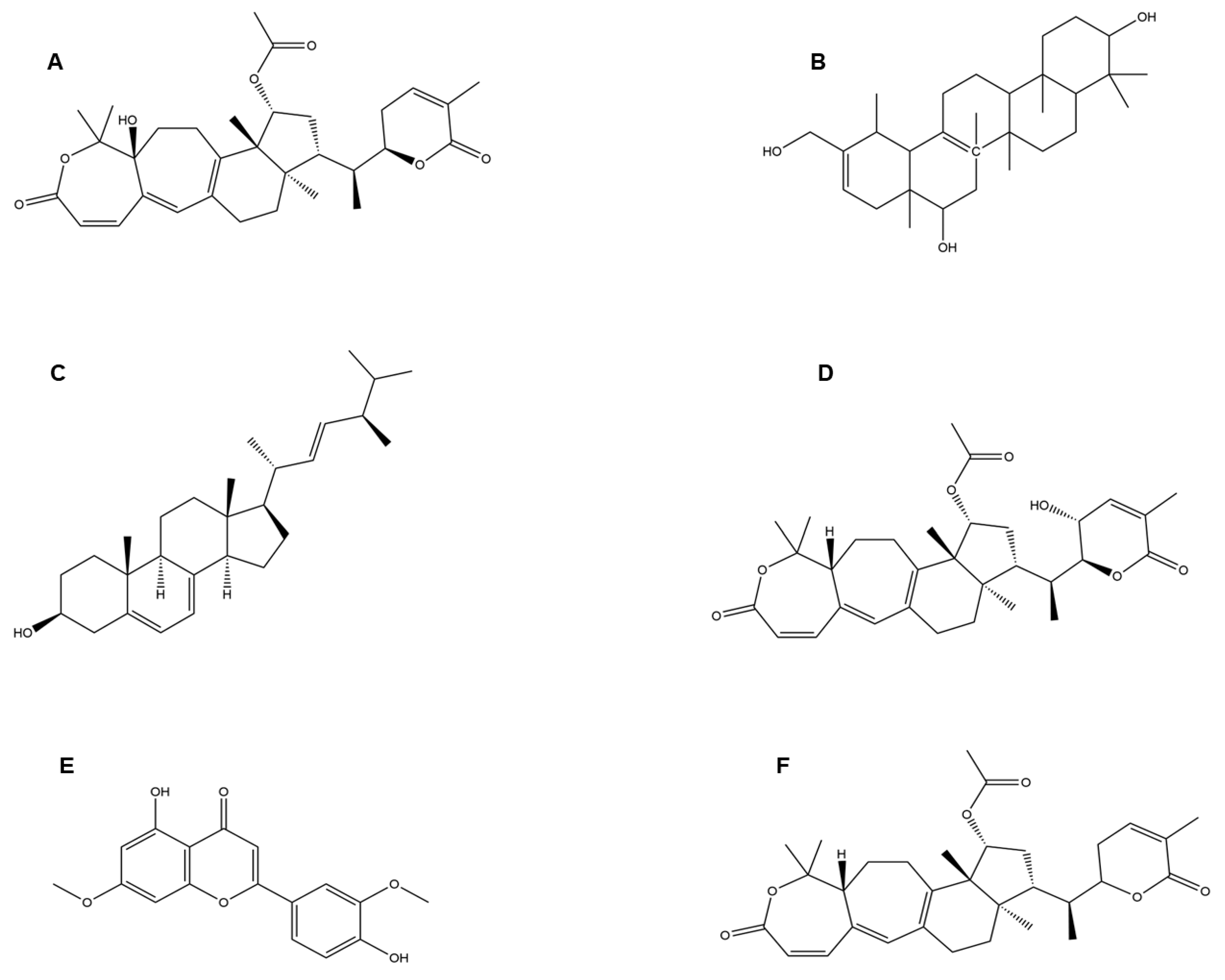

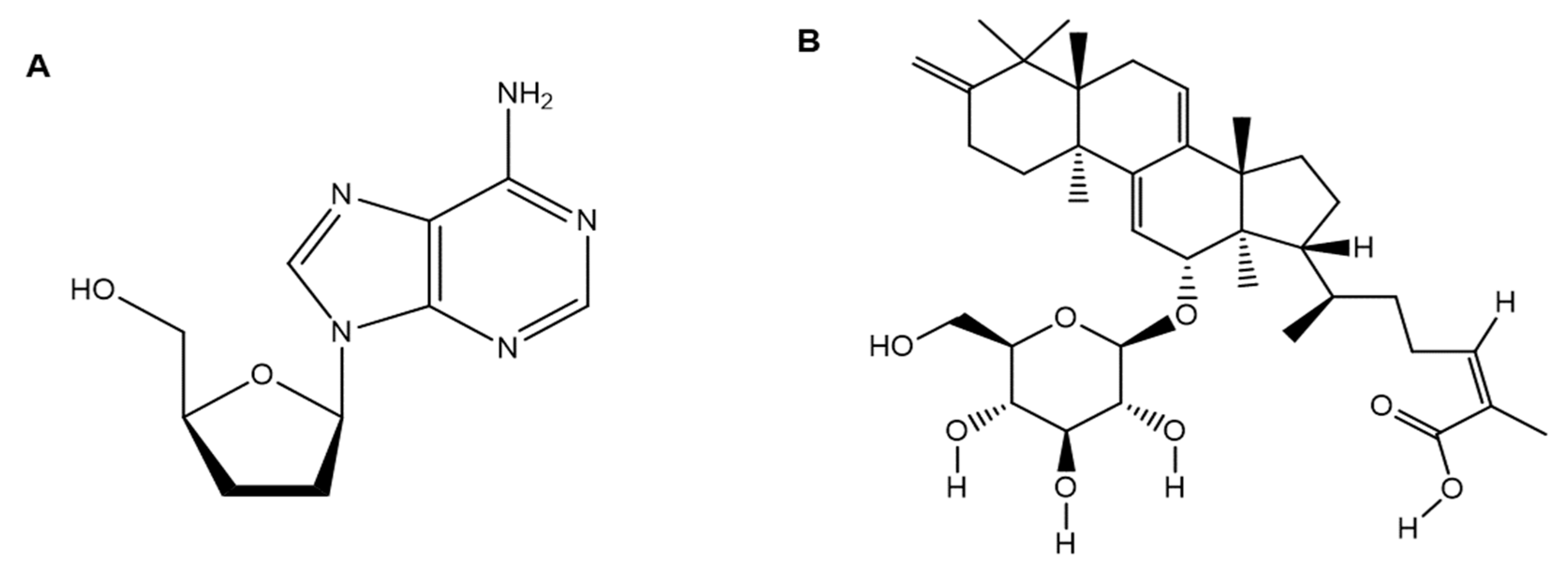
| Serial No. | Strain | Lineage |
|---|---|---|
| 1. | Alpha | B.1.1.7 |
| 2. | Beta | B.1.351 |
| 3. | Gamma | P.1 |
| 4. | Delta | B.1.617.7-3 |
| 5. | Epsilon | B.1.427-429 |
| 6. | Zeta | P.2 |
| 7. | Eta | B.1.525 |
| 8. | Theta | P.3 |
| 9. | Iota | B.1.526 |
| 10. | Kappa | B.1.617.1 |
| 11. | Lambda | C.37 |
| 12. | Omicron | B1.1.529 |
| 13. | Omicron | B.1.1.529 |
| Serial No. | Title | Conditions | Intervention | Location |
|---|---|---|---|---|
| 1. | RCT of Mushroom-Based Natural Product to Enhance Immune Response to COVID-19 Vaccination | COVID-19 Vaccination | Dietary supplement | University of California, San Diego, United States |
| 2. | Mushroom-Based Product for COVID-19 | COVID-19 | Drug: Fo Tv | University of California, Los Angeles Los Angeles, California, United StatesUniversity of California, San Diego San Diego, California, United States |
| 3. | COVID-19: Collecting Measurements of Renin–Angiotensin System Markers, such as Angiotensin-2 and Angiotensin 1–7 (Tomeka) | COVID-19 | Combination product: Tomeka® Drug: “Vernonia amygdalina” | Cliniques Universitaires de Kinshasa Kinshasa, Congo, The Democratic Republic of the Congo |
| 4. | Vitamin D3 Supplementation in Patients with Serum Values +/− 20 ng/mL | COVID-19 Influenza A Influenza B H1N1 Influenza | Dietary supplement: Vitamin D3 supplementation Dietary supplement: Diet and sun exposure | Hospital Clinica Nova de MonterreySan Nicolás De Los Garza, Nuevo León, Mexico |
| Serial No. | Mushroom Species (Common Name) | Functional Molecules | Function | Reference |
|---|---|---|---|---|
| 1. | Lentinus edodes (Shiitake mushroom) | β-Glucan | Most beta-glucans tested exhibited immunomodulatory activity by binding to receptors like dectin-1, toll-like receptors (TLRs), complement receptors type 3 (CR3), scavenger receptors (Src), and lactosylceramide receptors (LacCer) on immune cells. Thus, β-glucan can protect altered immune responses against various viral infections. | [116] |
| Lentinan (LNT) | Lentinan extracts reduced cytokine-induced NF-κB activation in human alveolar epithelial A549 cells and effectively attenuated pro-inflammatory cytokine production (TNF-α, IL-8, IL2, IL-6, IL-22) as well as TGF-β and IL-10. It attenuated oxidative stress-induced early apoptosis, and thus showed in vitro immunomodulatory and pulmonary cytoprotective effects that may also have positive relevance to candidate COVID-19 therapeutics targeting cytokine storm. | [71] | ||
| 2. | Ganoderma pfeifferi (Beeswax bracket) | Applanoxidic acid G Ergosta-7, 22-diene-3b-o l Lucidadiol Lucialdehyde B | Ganoderma triterpenoids inhibited influenza A H1N1 (A/WSN/33) infection by its antioxidant activity. It may modulate immune responses by binding to receptors like toll-like receptors (TLRs) and affecting the production of inflammatory cytokines. By doing so, Ergosta-7,22-diene-3b-ol could help regulate immune responses and reduce excessive inflammation associated with respiratory conditions. Through its anti-inflammatory and immunomodulatory activities, Lucidadiol has shown promising antiviral activities in preclinical and in vitro studies. This compound has shown antiviral activities against several viruses, including influenza A, HIV-1, HSV-1, HSV-2, HPV, HBV, and EV71. These antiviral effects are attributed to their ability to interfere with viral replication and entry into host cells. Specific antiviral mechanisms include inhibition of viral enzyme activity, interference with viral attachment to cellular receptors, and modulation of host immune responses to combat viral infection. | [2] [117] [118] [118] |
| 3. | Phellinus igniarius (Willow bracket mushroom) | Sesquiterpenoid | This compound has demonstrated anti-influenza activity by inhibiting the Neuraminidase (NA), a viral surface protein, and thus, can be used as a potent antiviral drug. | [2,51] |
| 4. | Agaricus bisporus (Button mushroom), Flammulina velutipes (Velvet foot; winter mushroom), Ganoderma lucidum (Lingzhi mushroom), Laetiporus sulphureus (Sulphur polypore), Lentinus lepideus (Scaly sawgill), Leucoagaricus leucothites (White dapperling), Macrocybe gigantean (Boro dhoodh chhatu), Pleurotus ostreatus (Oyster mushroom) | Colossolactone G Heliantriol F Ergosterol Colossolactone VIII Velutin | This compound has shown anti-viral activity against HIV and other viruses by inhibiting the surface proteins’ protease activity, such as HIV-1 protease. Heliantriol F exhibits characteristics as a potential inhibitor of the SARS-CoV-2 main protease (Mpro) and a rapid capturer of coronaviruses by strongly binding to the ACE2 receptor binding domain. Ergosterol shows anti-viral activity against a broad range of viruses by anti-inflammatory actions, reducing oxidative stress and immunomodulatory activities. This compound is reported to inhibit the SARS-CoV-2 main protease (Mpro) and rapidly captures coronaviruses by strongly binding to the ACE2 receptor binding domain, thereby preventing the entry of the virus into the respiratory tract. Velutin is shown to halt the virus’s protein synthesis and inhibit reverse transcriptase activities, thus restricting viral proliferation. | [119] [23] [120] [121] |
| 5. | Cordyceps militaris (Scarlet caterpillar club fungus) | Cordycepin | Cordycepin showed inhibitory affinities against the principal SARS-CoV-2 protein targets (e.g., SARS-CoV-2 spike (S) protein, main protease (Mpro) enzyme, and RNA-dependent RNA polymerase (RdRp) enzyme), and therefore, has therapeutic potential against SARS-CoV-2. | [2,14] |
| 6. | Oligoporus tephroleucus(Greyling bracket) | Oligoporin A | Oligoporin A shows antiplatelet activity by increasing the intracellular levels of both cAMP and cGMP in platelets and significantly repressing the collagen-induced ERK2 phosphorylation while diminishing the binding of fibrinogen to its cognate receptor, integrin IIb/IIIa, to exert its antiplatelet activity. Thus, this compound is a valuable candidate against COVID-induced thrombosis. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruah, P.; Patra, A.; Barge, S.; Khan, M.R.; Mukherjee, A.K. Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19). J. Fungi 2023, 9, 897. https://doi.org/10.3390/jof9090897
Baruah P, Patra A, Barge S, Khan MR, Mukherjee AK. Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19). Journal of Fungi. 2023; 9(9):897. https://doi.org/10.3390/jof9090897
Chicago/Turabian StyleBaruah, Paran, Aparup Patra, Sagar Barge, Mojibur R. Khan, and Ashis K. Mukherjee. 2023. "Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19)" Journal of Fungi 9, no. 9: 897. https://doi.org/10.3390/jof9090897
APA StyleBaruah, P., Patra, A., Barge, S., Khan, M. R., & Mukherjee, A. K. (2023). Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19). Journal of Fungi, 9(9), 897. https://doi.org/10.3390/jof9090897