A Sol–Gel Transition and Self-Healing Hydrogel Triggered via Photodimerization of Coumarin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation and Characterization of Coumarin-Containing Copolymer PAC
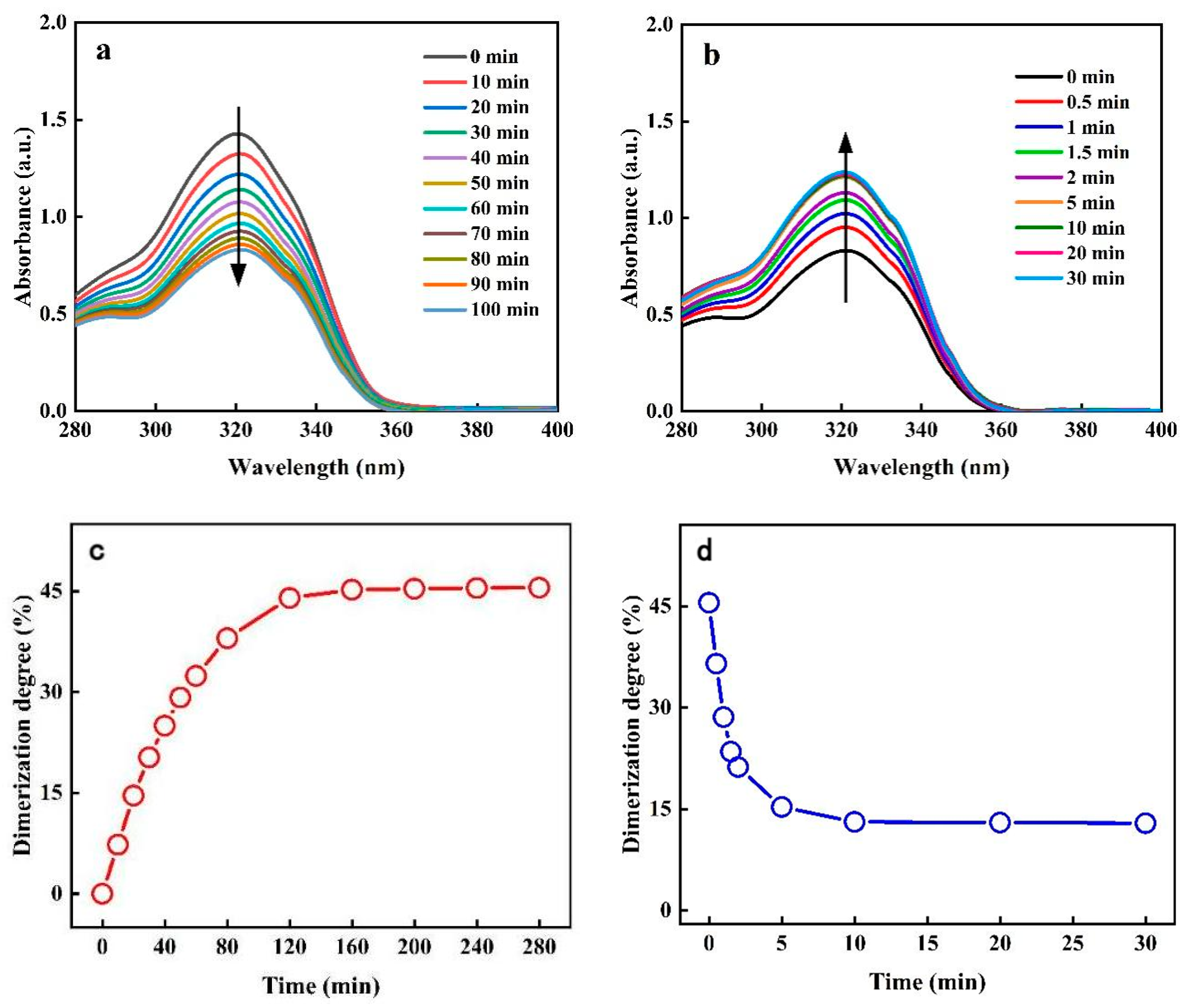
2.2. Photoresponsiveness of the Copolymer PAC
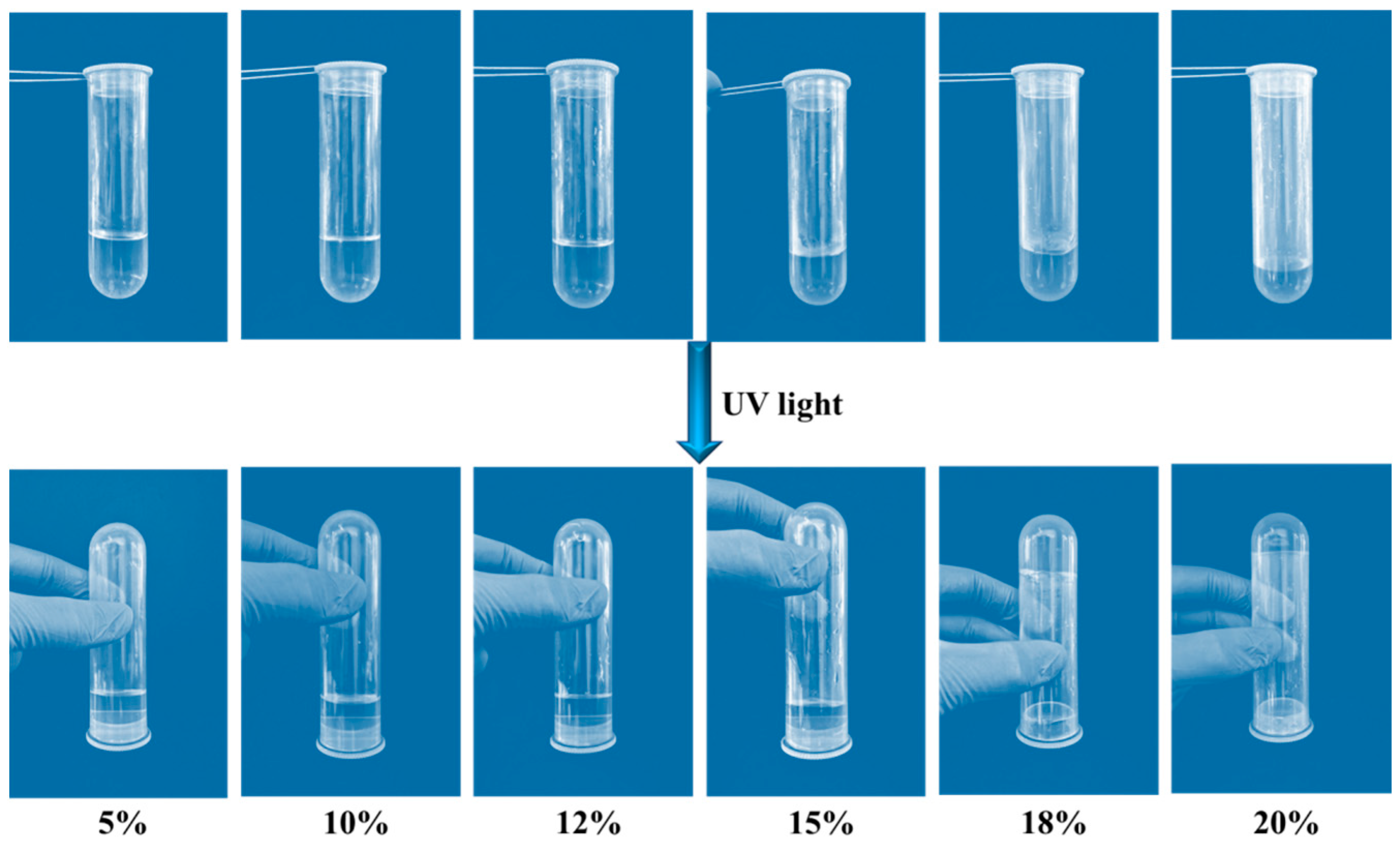
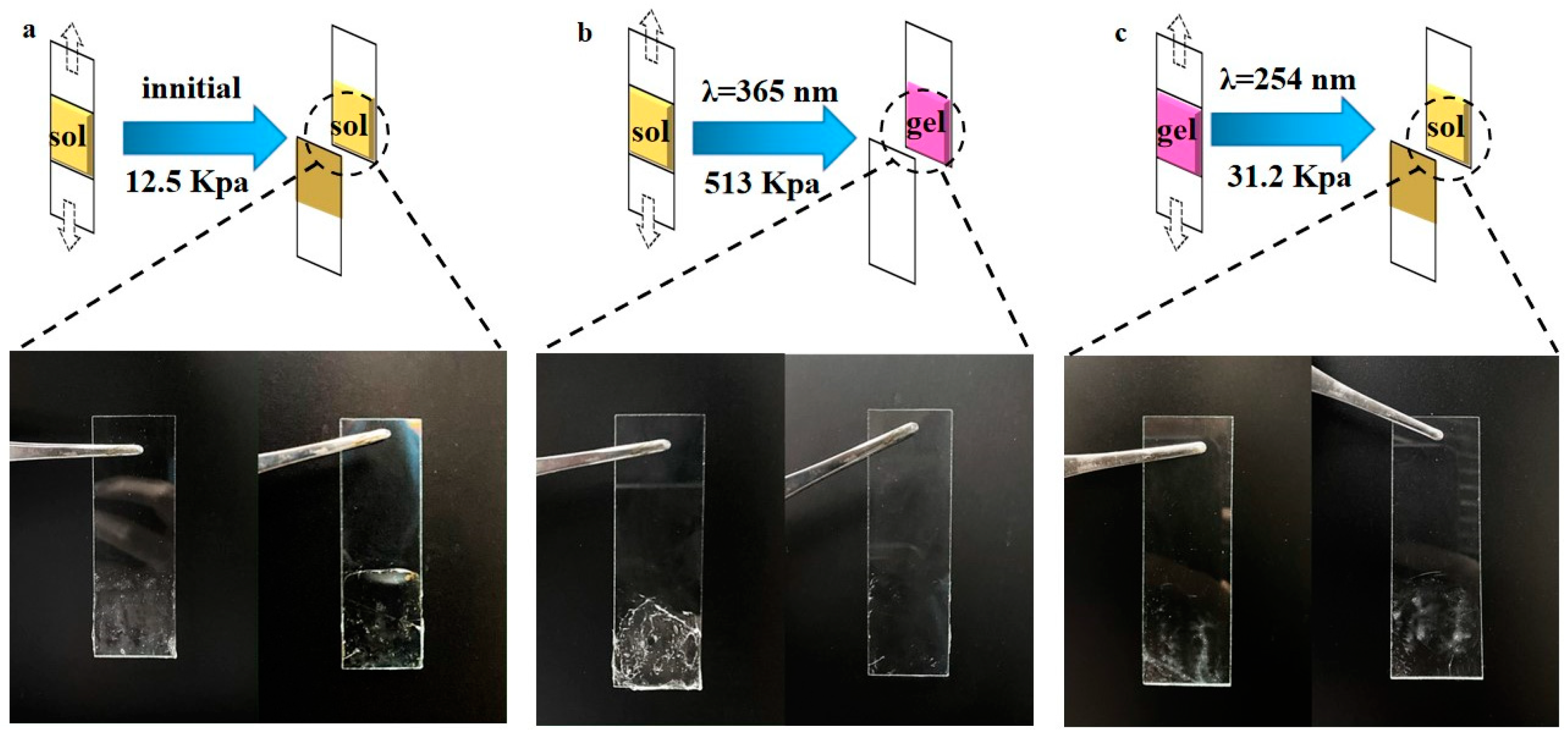
2.3. Reversible Sol–Gel Transition of PAC
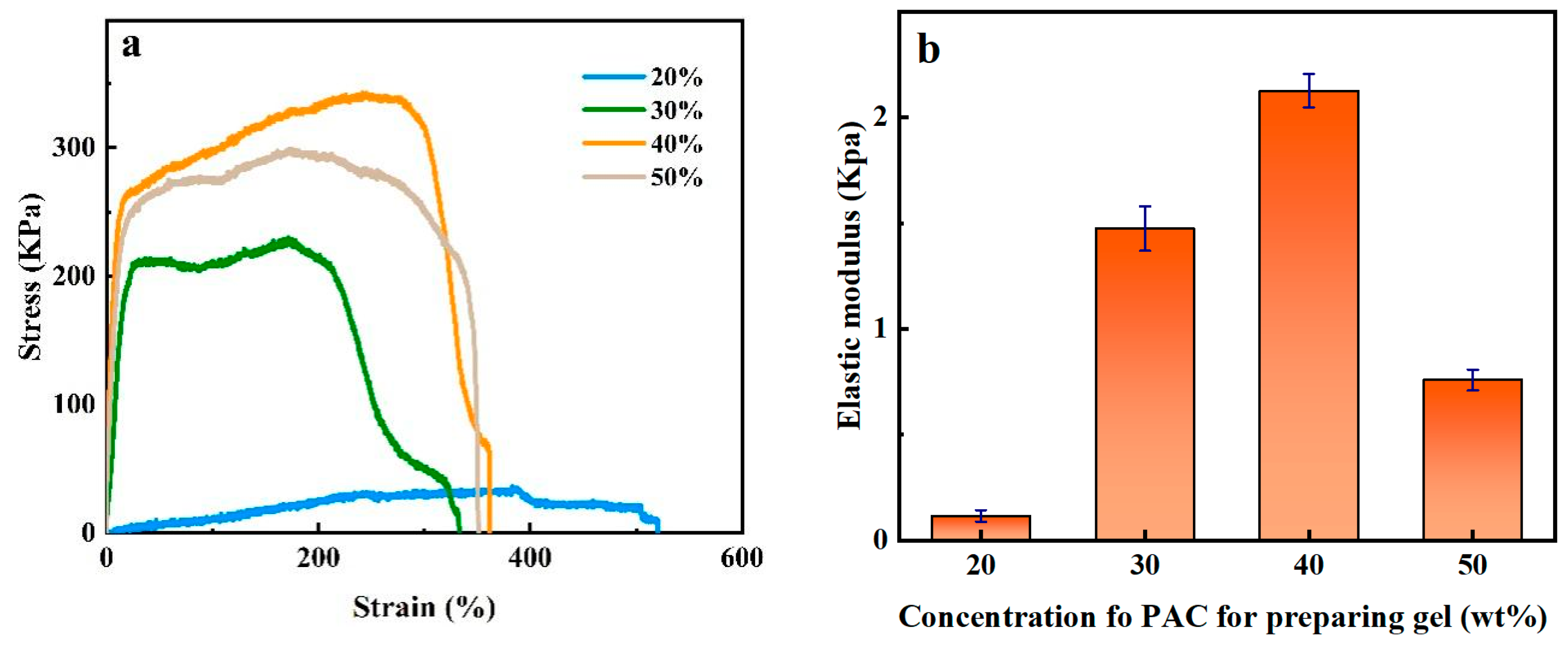
2.4. Mechanical Properties of PAC Gel
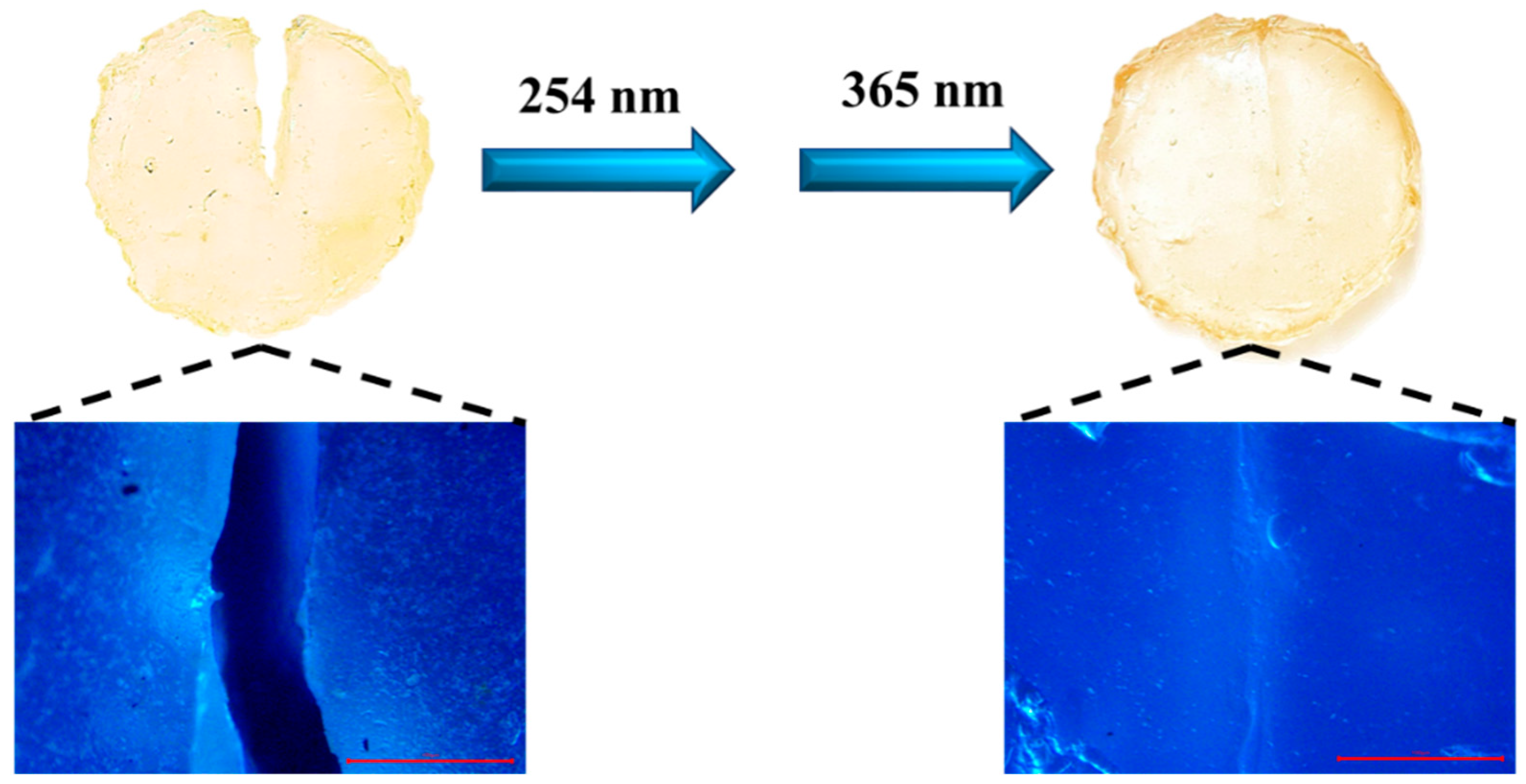
2.5. Self-Healing Performance of the PAC Gel
2.6. Photocontrolled Reversible Adhesion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of 7-(2-Hydroxyethoxy)-4-methylcoumarin
4.2. Synthesis of 7-(2-Acrylate ethoxy)-4-methylcoumarin
4.3. Synthesis of Coumarin Containing Copolymer PAC
4.4. Preparation of the PAC Gel
4.5. Characterization of the Sol–Gel Transition Behavior
4.6. Mechanical Performance Test
4.7. Characterization of the Self-Healing Property
4.8. Adherence Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, A.; Tanaka, M.; Matsumoto, H.; Ochi, K.; Moro-Oka, Y.; Kuwata, H.; Suganami, T. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 2017, 3, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Le, X.; Zhou, S.; Chen, T. Recent progress in smart polymeric gel-based information storage for anti-counterfeiting. Adv. Mater. 2022, 34, 2201262–2201273. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kawai, M.; Mitsumata, T. Anomalous Magnetorheological Response for Carrageenan Magnetic Hydrogels Prepared by Natural Cooling. Gels 2023, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y.; Qu, K.; Ma, X.; Zhang, S.; Ma, H.; Xu, W. Gel Smart Window with Controllable LCST by Adding Ethylene Glycol for Ice and Evaporation Resistance. J. Polym. Environ. 2023, 31, 423–431. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Peng, W.; Yu, X.; Chen, J. The Evaluation and Application of Smart Gel for Deepwater Loss-Circulation Control. Processes 2023, 11, 1890. [Google Scholar] [CrossRef]
- Bai, Y.; Ban, C.; He, S.; Zhao, J.; Zhang, H. Temperature-responsive self-lubricating hydrogel from dynamic Diels-Alder crosslinking for reservoir in-depth profile control. J. Mol. Liq. 2021, 323, 114595–114604. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Reyes-Palacios, G.A.; García-Montejano, L.A.; Antonio-Carmona, I.D.; Martínez-Gutiérrez, H. Electroactive polyacrylamide/chitosan/polypyrrole hydrogel for captopril release controlled by electricity. J. Vinyl Addit. Technol. 2021, 27, 679–690. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186–100221. [Google Scholar] [CrossRef]
- Jindřich, K. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 185–192. [Google Scholar]
- Pan, S.; Zhu, C.; Wu, Y.; Tao, L. Chitosan-Based Self-Healing Hydrogel: From Fabrication to Biomedical Application. Polymers 2023, 15, 3768. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Kim, Y.; Ahn, H.; Ahn, H.; Moon, H. Non-volatile, phase-transition smart gels visually indicating in situ thermal status for sensing applications. Nanoscale 2019, 11, 16733–16742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Wei, Z.; Zhang, B.; Weng, X. Advances and progress in self-healing hydrogel and its application in regenerative medicine. Materials 2023, 6, 1215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Shi, C.; Liu, Y.; Zhu, Y.; Song, Y. Self-healing hydrogel with multiple adhesion as sensors for winter sports. J. Colloid Interface Sci. 2023, 629, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Dolatsshahi-Pirouz, A. Self-healing hydrogels: The next paradigm shift in tissue engineering? Adv. Sci. 2019, 6, 1801664–1801711. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; An, H.; Li, X.; Zhou, R.; Qin, J.; Tian, Y.; Deng, K. Self-Healable Polymer Gels with Multi-Responsiveness of Gel-Sol-Gel Transition and Degradability. Polym. Chem. 2017, 8, 1263–1271. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Yang, S.; Yang, S.; Li, Y.; Dong, R.; Chen, Y.; Guo, X. Facile In Situ Chemical Cross-Linking Gel Polymer Electrolyte, which Confines the Shuttle Effect with High Ionic Conductivity and Li-Ion Transference Number for Quasi-Solid-State Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2021, 13, 44497–44508. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Guo, B. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef]
- Wang, X.; Bian, G.; Zhang, M.; Li, Z.; Li, X.; Qin, J. Self-healable hydrogels with cross-linking induced thermo-responsiveness and multi-triggered gel-sol-gel transition. Polym. Chem. 2017, 18, 2872–2880. [Google Scholar] [CrossRef]
- Liu, J.; Du, X.; Wang, C.; Li, Q.; Chen, S. Construction of triple non-covalent interaction-based ultra-strong self-healing polymeric gels via frontal polymerization. J. Mater. Chem. C 2020, 8, 14083–14091. [Google Scholar] [CrossRef]
- Tsuge, A.; Suehara, S.; Takemori, Y.; Nakano, M.; Araki, K. Formation of Organogel In Situ Based on a Dynamic Imine Bond. Chem. Lett. 2021, 50, 1091–1094. [Google Scholar] [CrossRef]
- Bennett, C.W.; Collins, J.; Xiao, Z.; Klinger, D.; Connal, L.A. Scalable and Versatile Synthesis of Oxime-Based Hormone Dimers and Gels for Sustained Release. Chem. Asian J. 2017, 12, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, X.; Fu, X.; Hu, J.; Lang, X.; Liu, X.; Qin, J. Self-healable hydrogels with NaHCO3 degradability and a reversible gel-sol-gel transition from phenolic ester containing polymers. RSC Adv. 2017, 7, 31212–31220. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, H.; Chen, F. Modified lignin: Preparation and use in reversible gel via Diels-Alder reaction. Int. J. Biol. Macromol. 2018, 107, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Karen, A.; Simon, N.J.; Bobak, M.; Marym, R.; Mohammady, G.M.; Whitesides, S.P. Disulfide-Based Diblock Copolymer Worm Gels: A Wholly-Synthetic Thermoreversible 3D Matrix for Sheet-Based Cultures. Biomacromolecules 2015, 16, 3952–3958. [Google Scholar]
- Pan, S.; Zhang, N.; He, X.; Fang, Z.; Wu, Y.; Wei, Y.; Tao, L. Poly (vinyl alcohol) Modified via the Hantzsch Reaction for Biosafe Antioxidant Self-Healing Hydrogel. ACS Macro Lett. 2023, 12, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Li, Z.; Gao, T.; Liu, Y.; Liu, X.; Liu, F. Hydrophobic Association Hydrogel. Prog. Chem. 2017, 29, 757–765. [Google Scholar]
- Wang, J.; Qin, W.; Lei, N.; Chen, X. Lamellar hydrogel fabricated by host-guest interaction between α-cyclodextrin and amphiphilic phytosterol ethoxylates. Colloids Surf. A 2019, 570, 462–470. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, M.; Hu, X.; Lu, W.; Wang, M.; Li, T.; Zhao, Y. A Novel Double-Network, Self-Healing Hydrogel Based on Hydrogen Bonding and Hydrophobic Effect. Macromol. Chem. Phys. 2020, 221, 1900320–1900333. [Google Scholar]
- Li, G.; Yan, Q.; Xia, H.; Zhao, Y. Therapeutic-Ultrasound-Triggered Shape Memory of a Melamine-Enhanced Poly(vinyl alcohol) Physical Hydrogel. ACS Appl. Mater. Interfaces 2015, 7, 12067–12073. [Google Scholar] [CrossRef]
- Liu, S.; Tian, L.; Mao, H.; Ning, W.; Shang, P.; Wu, J.; Shi, X. Micellization and sol-gel transition of novel thermo- and pH-responsive ABC triblock copolymer synthesized by RAFT. J. Polym. Res. 2018, 25, 264–275. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, C.D.; Liu, C.; Zong, L.; Gu, H.; Wang, C.; Jian, X. Self-healing, pH-sensitive and shape memory hydrogels based on acylhydrazone and hydrogen bonds. Eur. Polym. J. 2022, 162, 110838. [Google Scholar] [CrossRef]
- Collins, J.; Nadgorny, M.; Xiao, Z.; Connal, L.A. Doubly dynamic self-healing materials based on oxime click chemistry and boronic acids. Macromol. Rapid Commun. 2017, 38, 1600760–1600766. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Hu, Y.; Zhou, W.; Xiao, L. Self-healing polyurethane based on disulfide bond and hydrogen bond. Polym. Adv. Technol. 2018, 29, 463–469. [Google Scholar] [CrossRef]
- Bonetti, L.; Nardo, L.D.; Farè, S. Thermo-responsive methylcellulose hydrogels: From design to applications as smart biomaterials. Tissue Eng. B Rev. 2021, 27, 486–513. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Wang, J.; Li, T.; Chen, J.; Duan, X.; Guo, B. Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos. B Eng. 2023, 266, 110985–110989. [Google Scholar] [CrossRef]
- Wang, M.; Nie, C.; Liu, J.; Wu, S. Organic–Inorganic semi-interpenetrating networks with orthogonal light-and magnetic-responsiveness for smart photonic gels. Nat. Commun. 2023, 14, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Mrinalini, M.; Prasanthkumar, S. Recent advances on stimuli-responsive smart materials and their applications. ChemPlusChem 2019, 84, 1103–1121. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Yu, M.; Hou, M.; Gong, G.; Tan, H.; Li, N.; Xu, J. NIR light-induced rapid self-healing hydrogel toward multifunctional applications in sensing. Nano Energy 2023, 107, 108119–108125. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhao, J. Fabrication of dual-functional cellulose nanocrystals/fluorinated polyacrylate containing coumarin derivatives by RAFT-assisted Pickering emulsion polymerization for self-healing application. Appl. Surf. Sci. 2023, 614, 156180–156185. [Google Scholar] [CrossRef]
- Safavi-Mirmahalleh, S.A.; Golshan, M.; Gheitarani, B.; Hosseini, M.S.; Salami-Kalajahi, M.S. A review on applications of coumarin and its derivatives in preparation of photo-responsive polymers. Eur. Polym. J. 2023, 198, 112430–112440. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Hao, T.; Zhang, N.; Li, M.; Wang, X.; Wei, W.; Zhao, J. Fluorogenic Reactions in Chemical Biology: Seeing Chemistry in Cells. Chem. Biomed. Imaging 2023, 1, 590–619. [Google Scholar] [CrossRef]
- Alemdar, M.; Tuncaboylu, D.C.; Batu, H.K.; Temel, B.A. Pluronic based injectable smart gels with coumarin functional amphiphilic copolymers. Eur. Polym. J. 2022, 177, 111378–111390. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Ahmadi, Z.; Gupta, K.C.; Kumar, P. Polyethylenimine-polyacrylic acid nanocomposites: Type of bonding does influence the gene transfer efficacy and cytotoxicity. Colloid Surface B 2016, 140, 117–120. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Wang, W.; Liu, X.; Chen, Y.; Tian, S.; Fu, P. A Sol–Gel Transition and Self-Healing Hydrogel Triggered via Photodimerization of Coumarin. Gels 2024, 10, 21. https://doi.org/10.3390/gels10010021
Ye Y, Wang W, Liu X, Chen Y, Tian S, Fu P. A Sol–Gel Transition and Self-Healing Hydrogel Triggered via Photodimerization of Coumarin. Gels. 2024; 10(1):21. https://doi.org/10.3390/gels10010021
Chicago/Turabian StyleYe, Yong, Wenkai Wang, Xin Liu, Yong Chen, Shenghui Tian, and Peng Fu. 2024. "A Sol–Gel Transition and Self-Healing Hydrogel Triggered via Photodimerization of Coumarin" Gels 10, no. 1: 21. https://doi.org/10.3390/gels10010021





