Chitosan–Polyethylene Glycol Inspired Polyelectrolyte Complex Hydrogel Templates Favoring NEO-Tissue Formation for Cardiac Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiochemical Characterization
2.1.1. ATR-IR Spectroscopy
2.1.2. Contact Angle
2.1.3. Water Profile
2.1.4. Cross-Sectional Pore Morphometry
2.1.5. Water Transition Status
2.2. Release Profile
2.3. Conductance
2.4. Biodegradation
2.5. Mechanical Characterization
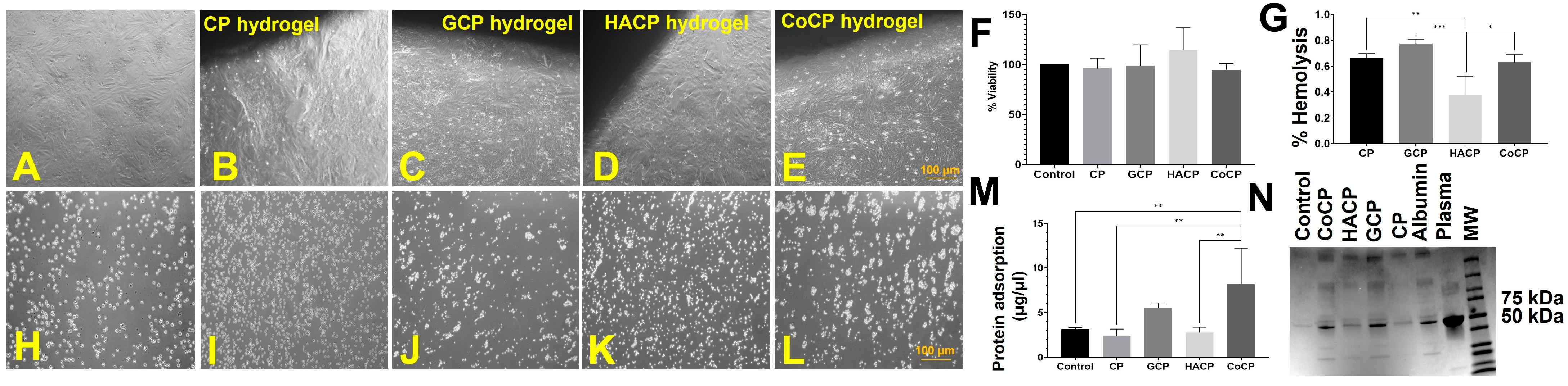
2.6. Cytocompatibility
2.7. Hemocompatibility
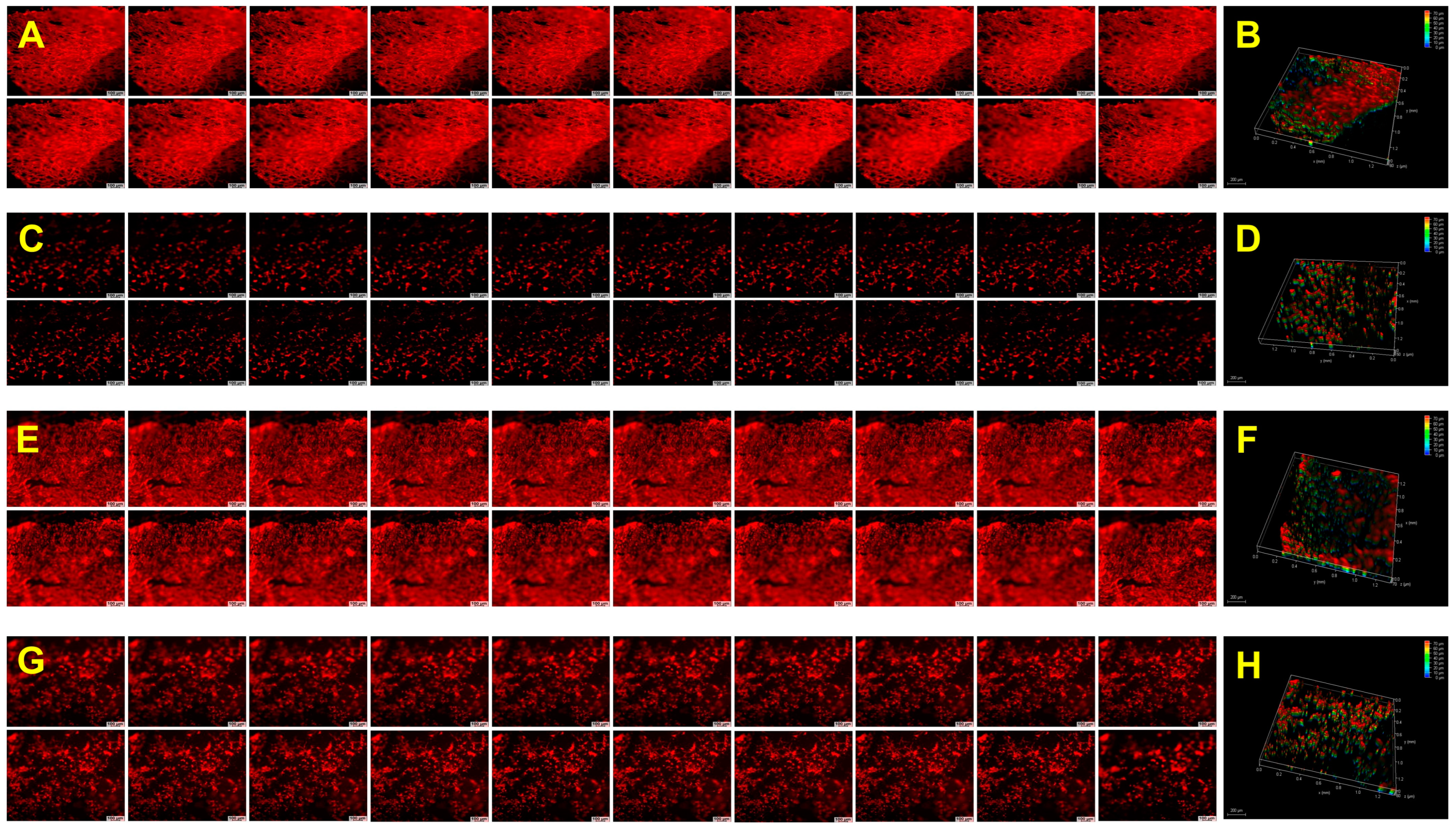
2.8. Biological Performance
2.9. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chitosan-PEG-Based Polyelectrolyte Hydrogels
4.3. Physiochemical Characterization
4.3.1. Attenuated Total Reflection Infrared Spectroscopy (ATR-IR)
4.3.2. Contact Angle
4.3.3. Water Profiling
4.3.4. Scanning Electron Microscopy (SEM)
4.3.5. Thermal Evaluation
4.4. Release Kinetics
4.5. Electrical Conductivity
4.6. Biodegradation
4.7. Mechanical Characterization
4.8. Cytocompatibility
4.8.1. Cell Culture and Maintenance
4.8.2. Direct Contact Assay
4.8.3. Test on Extract
4.9. Hemocompatibility
4.9.1. Hemolysis Assay Red Blood Cell Aggregation
4.9.2. Protein Adsorption
4.10. Biological Performance
4.10.1. Cell Spreading and Penetration
4.10.2. Ex Vivo Explant Culture and Neo-Tissue Formation
4.10.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Best, C.; Onwuka, E.; Pepper, V.; Sams, M.; Breuer, J.; Breuer, C. Cardiovascular Tissue Engineering: Preclinical Validation to Bedside Application. Physiology 2016, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A.I.P.M. Biomaterial-Driven in Situ Cardiovascular Tissue Engineering—A Multi-Disciplinary Perspective. npj Regen. Med. 2017, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Daebritz, S.; Martin, D.P.; Moran, A.M.; Kim, B.S.; Schoen, F.J.; Vacanti, J.P.; Mayer, J.E. Early In Vivo Experience with Tissue-Engineered Trileaflet Heart Valves. Circulation 2000, 102, Iii-22–Iii-29. [Google Scholar] [CrossRef]
- Bouten, C.V.C.; Cheng, C.; Vermue, I.M.; Gawlitta, D.; Passier, R. Cardiovascular Tissue Engineering and Regeneration: A Plead for Further Knowledge Convergence. Tissue Eng. Part A 2022, 28, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-G.; Surat’man, N.E.B.; Chang, J.J.; Ong, Z.L.; Li, B.; Fan, X.; Loh, X.J.; Li, Z. Polyelectrolyte Hydrogels for Tissue Engineering and Regenerative Medicine. Chem. Asian J. 2022, 17, e202200604. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Khan, A.; Jimenez, E. Chitosan Hydrogels Significantly Limit Left Ventricular Infarction and Remodeling and Preserve Myocardial Contractility. J. Surg. Res. 2016, 201, 490–497. [Google Scholar] [CrossRef]
- Deng, B.; Shen, L.; Wu, Y.; Shen, Y.; Ding, X.; Lu, S.; Jia, J.; Qian, J.; Ge, J. Delivery of Alginate-Chitosan Hydrogel Promotes Endogenous Repair and Preserves Cardiac Function in Rats with Myocardial Infarction. J. Biomed. Mater. Res. A 2015, 103, 907–918. [Google Scholar] [CrossRef]
- Fu, B.; Wang, X.; Chen, Z.; Jiang, N.; Guo, Z.; Zhang, Y.; Zhang, S.; Liu, X.; Liu, L. Improved Myocardial Performance in Infarcted Rat Heart by Injection of Disulfide-Cross-Linked Chitosan Hydrogels Loaded with Basic Fibroblast Growth Factor. J. Mater. Chem. B 2022, 10, 656–665. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A Chitosan–Glutathione Based Injectable Hydrogel for Suppression of Oxidative Stress Damage in Cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef]
- Dobner, S.; Bezuidenhout, D.; Govender, P.; Zilla, P.; Davies, N. A Synthetic Non-Degradable Polyethylene Glycol Hydrogel Retards Adverse Post-Infarct Left Ventricular Remodeling. J. Card. Fail. 2009, 15, 629–636. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Masumoto, H.; Jo, J.-I.; Yamazaki, K.; Ikeda, T.; Tabata, Y.; Minatoya, K. Sustained Release of Basic Fibroblast Growth Factor Using Gelatin Hydrogel Improved Left Ventricular Function through the Alteration of Collagen Subtype in a Rat Chronic Myocardial Infarction Model. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, L.M.; Lara, R.P.; Sani, E.S.; Xiao, C.; Roh, J.; Yu, X.; Ledesma, P.A.; Yu, C.H.; Annabi, N.; Ruskin, J.N. Gelatin Methacryloyl Bioadhesive Improves Survival and Reduces Scar Burden in a Mouse Model of Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014199. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Makhoul, G.; Duong, M.; Chiu, R.C.J.; Cecere, R. Hyaluronic Acid-Based Hydrogel Induces Neovascularization and Improves Cardiac Function in a Rat Model of Myocardial Infarction. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 767–772. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Tous, E.; Minakawa, M.; Morita, M.; Robb, J.D.; Koomalsingh, K.J.; Gorman, J.H.; Gorman, R.C.; Burdick, J.A. Injectable Hydrogel Properties Influence Infarct Expansion and Extent of Postinfarction Left Ventricular Remodeling in an Ovine Model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef]
- Martins, A.F.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Characterization of Polyelectrolytes Complexes Based on N,N,N-Trimethyl Chitosan/Heparin Prepared at Different pH Conditions. Carbohydr. Polym. 2011, 86, 1266–1272. [Google Scholar] [CrossRef]
- Acosta, B.B.; Advincula, R.C.; Grande-Tovar, C.D. Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules 2023, 28, 1920. [Google Scholar] [CrossRef]
- Buranachai, T.; Praphairaksit, N.; Muangsin, N. Chitosan/Polyethylene Glycol Beads Crosslinked with Tripolyphosphate and Glutaraldehyde for Gastrointestinal Drug Delivery. AAPS PharmSciTech 2010, 11, 1128–1137. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Cao, N.; Zhao, Y.; Chen, H.; Huang, J.; Yu, M.; Bao, Y.; Wang, D.; Cui, S. Poly(Ethylene Glycol) Becomes a Supra-Polyelectrolyte by Capturing Hydronium Ions in Water. Macromolecules 2022, 55, 4656–4664. [Google Scholar] [CrossRef]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded Gelatin Hydrogels for Extended Culture of Engineered Cardiac Tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, N.; Meng, H.-X.; Liu, H.-X.; Lu, Y.-Q.; Liu, C.-M.; Zhang, Z.-M.; Qu, K.-Y.; Huang, N.-P. Easy Applied Gelatin-Based Hydrogel System for Long-Term Functional Cardiomyocyte Culture and Myocardium Formation. ACS Biomater. Sci. Eng. 2019, 5, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chan, A.T.; Armstrong, P.A.; Luo, H.-C.; Higuchi, T.; Strehin, I.A.; Vakrou, S.; Lin, X.; Brown, S.N.; O’Rourke, B.; et al. Hyaluronic Acid-Human Blood Hydrogels for Stem Cell Transplantation. Biomaterials 2012, 33, 8026–8033. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Park, J.; Sutthiwanjampa, C.; Kim, H.; Bae, T.; Kim, W.; Choi, J.; Kim, M.; Kang, S.; Park, H. Surface Coating with Hyaluronic Acid-Gelatin-Crosslinked Hydrogel on Gelatin-Conjugated Poly(Dimethylsiloxane) for Implantable Medical Device-Induced Fibrosis. Pharmaceutics 2021, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Basara, G.; Ozcebe, S.G.; Ellis, B.W.; Zorlutuna, P. Tunable Human Myocardium Derived Decellularized Extracellular Matrix for 3D Bioprinting and Cardiac Tissue Engineering. Gels 2021, 7, 70. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Infiltration and sustenance of viability of cells by amphiphilic biosynthetic biodegradable hydrogels. J. Mater. Sci. Mater. Med. 2014, 25, 1953–1965. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Biosynthetic hydrogels—Studies on chemical and physical characteristics on long-term cellular response for tissue engineering. J. Biomed. Mater. Res. 2014, 107, 2238–2247. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Sharifisistani, M.; Khanmohammadi, M.; Badali, E.; Ghasemi, P.; Hassanzadeh, S.; Bahiraie, N.; Lotfibakhshaiesh, N.; Ai, J. Hyaluronic Acid/Gelatin Microcapsule Functionalized with Carbon Nanotube through Laccase-Catalyzed Crosslinking for Fabrication of Cardiac Microtissue. J. Biomed. Mater. Res. A 2022, 110, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Water and the Acute Biological Response to Surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Henry, S.M.; Anseth, K.S. Synthesis and Characterization of Photocrosslinkable, Degradable Poly(Vinyl Alcohol)-Based Tissue Engineering Scaffolds. Biomaterials 2002, 23, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99. [Google Scholar] [CrossRef]
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schünemann, M.; Bohn, S.; Stachs, O. Determination of Hydrogel Swelling Factors by Two Established and a Novel Non-Contact Continuous Method. J. Appl. Polym. Sci. 2021, 138, 50326. [Google Scholar] [CrossRef]
- Jacques, C.H.M.; Hopfenberg, H.B.; Stannett, V. Super Case II Transport of Organic Vapors in Glassy Polymers. In Permeability of Plastic Films and Coatings: To Gases, Vapors, and Liquids; Hopfenberg, H.B., Ed.; Polymer Science and Technology; Springer: Boston, MA, USA, 1974; pp. 73–86. ISBN 978-1-4684-2877-3. [Google Scholar]
- Das, R.; Dasgupta, C.; Karmakar, S. Time Scales of Fickian Diffusion and the Lifetime of Dynamic Heterogeneity. Front. Phys. 2020, 8, 210. [Google Scholar] [CrossRef]
- Si, R.; Gao, C.; Guo, R.; Lin, C.; Li, J.; Guo, W. Human Mesenchymal Stem Cells Encapsulated-Coacervated Photoluminescent Nanodots Layered Bioactive Chitosan/Collagen Hydrogel Matrices to Indorse Cardiac Healing after Acute Myocardial Infarction. J. Photochem. Photobiol. B 2020, 206, 111789. [Google Scholar] [CrossRef]
- Wu, T.; Liu, W. Functional Hydrogels for the Treatment of Myocardial Infarction. NPG Asia Mater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Wang, R.M.; Christman, K.L. Decellularized Myocardial Matrix Hydrogels: In Basic Research and Preclinical Studies. Adv. Drug Deliv. Rev. 2016, 96, 77–82. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.; Chen, S.; Gao, Y. α-Tocopherol Liposome Loaded Chitosan Hydrogel to Suppress Oxidative Stress Injury in Cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Mihic, A.; Cui, Z.; Wu, J.; Vlacic, G.; Miyagi, Y.; Li, S.-H.; Lu, S.; Sung, H.-W.; Weisel, R.D.; Li, R.-K. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015, 132, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, T.; Yu, Y.; Shi, K.; Bei, Z.; Qian, Y.; Qian, Z. An Injectable Conductive Hydrogel Restores Electrical Transmission at Myocardial Infarct Site to Preserve Cardiac Function and Enhance Repair. Bioact. Mater. 2022, 20, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Mashayekhan, S.; Baheiraei, N.; Pourjavadi, A. Biohybrid Oxidized Alginate/Myocardial Extracellular Matrix Injectable Hydrogels with Improved Electromechanical Properties for Cardiac Tissue Engineering. Int. J. Biol. Macromol. 2021, 180, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.; Wang, Z.; Zhang, Z.; Xia, Y.; Xue, H.; Liu, Y. Preparation of an Electrically Conductive Graphene Oxide/Chitosan Scaffold for Cardiac Tissue Engineering. Appl. Biochem. Biotechnol. 2019, 188, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Chacon, E.; Acosta, D.; Lemasters, J.J. 9—Primary Cultures of Cardiac Myocytes as In Vitro Models for Pharmacological and Toxicological Assessments. In In Vitro Methods in Pharmaceutical Research; Castell, J.V., Gómez-Lechón, M.J., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 209–223. ISBN 978-0-12-163390-5. [Google Scholar]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl Chitosan-Based Hydrogels Containing Fibroblast Growth Factors for Triggering Diabetic Wound Healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Semenova, M.V.; Osadchenko, S.V.; Mezhuev, Y.O.; Shtil’man, M.I.; Semenova, I.N. Synthesis of Hemocompatible Materials Based on Branched Polyvinyl Alcohol. Russ. J. Appl. Chem. 2016, 89, 1286–1291. [Google Scholar] [CrossRef]
- Amdursky, N.; Mazo, M.M.; Thomas, M.R.; Humphrey, E.J.; Puetzer, J.L.; St-Pierre, J.-P.; Skaalure, S.C.; Richardson, R.M.; Terracciano, C.M.; Stevens, M.M. Elastic Serum-Albumin Based Hydrogels: Mechanism of Formation and Application in Cardiac Tissue Engineering. J. Mater. Chem. B 2018, 6, 5604–5612. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Sabino, R.M.; Souza, P.R.; Muniz, E.C.; Popat, K.C.; Kipper, M.J.; Zola, R.S.; Martins, A.F. Chitosan/Gellan Gum Ratio Content into Blends Modulates the Scaffolding Capacity of Hydrogels on Bone Mesenchymal Stem Cells. Mater. Sci. Eng. C 2020, 106, 110258. [Google Scholar] [CrossRef] [PubMed]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J. Gelatin-Based Hydrogel Degradation and Tissue Interaction In Vivo: Insights from Multimodal Preclinical Imaging in Immunocompetent Nude Mice. Theranostics 2016, 6, 2114–2128. [Google Scholar] [CrossRef] [PubMed]
- Boucard, E.; Vidal, L.; Coulon, F.; Mota, C.; Hascoët, J.-Y.; Halary, F. The Degradation of Gelatin/Alginate/Fibrin Hydrogels Is Cell Type Dependent and Can Be Modulated by Targeting Fibrinolysis. Front. Bioeng. Biotechnol. 2022, 10, 920929. [Google Scholar] [CrossRef] [PubMed]
- Sedighim, S.; Chen, Y.; Xu, C.; Mohindra, R.; Liu, H.; Agrawal, D.K.; Thankam, F.G. Carboxymethyl Cellulose–Alginate Interpenetrating Hydroxy Ethyl Methacrylate Crosslinked Polyvinyl Alcohol Reinforced Hybrid Hydrogel Templates with Improved Biological Performance for Cardiac Tissue Engineering. Biotechnol. Bioeng. 2023, 120, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Finosh, G.T.; Jayabalan, M.; Vandana, S.; Raghu, K.G. Hybrid Alginate-Polyester Bimodal Network Hydrogel for Tissue Engineering—Influence of Structured Water on Long-Term Cellular Growth. Colloids Surf. B Biointerfaces 2015, 135, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Diaz, C.; Chandra, I.; Link, J.; Newton, J.; Dilisio, M.F.; Agrawal, D.K. Hybrid Interpenetrating Hydrogel Network Favoring the Bidirectional Migration of Tenocytes for Rotator Cuff Tendon Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 467–477. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J.; Sankar, V.; Gopal, R.K. Growth and Survival of Cells in Biosynthetic Poly Vinyl Alcohol–Alginate IPN Hydrogels for Cardiac Applications. Colloids Surf. B Biointerfaces 2013, 107, 137–145. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Alginate–polyester comacromer based hydrogels as physiochemically and biologically favorable entities for cardiac tissue engineering. J. Colloid Interface Sci. 2015, 457, 52–61. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Influence of Plasma Protein–Hydrogel Interaction Moderated by Absorption of Water on Long-Term Cell Viability in Amphiphilic Biosynthetic Hydrogels. RSC Adv. 2013, 3, 24509–24520. [Google Scholar] [CrossRef]
- Finosh, G.T.; Jayabalan, M. Hybrid Amphiphilic Bimodal Hydrogels Having Mechanical and Biological Recognition Characteristics for Cardiac Tissue Engineering. RSC Adv. 2015, 5, 38183–38201. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Alginate Based Hybrid Copolymer Hydrogels—Influence of Pore Morphology on Cell–Material Interaction. Carbohydr. Polym. 2014, 112, 235–244. [Google Scholar] [CrossRef] [PubMed]




| Parameters | CP | GCP | HACP | CoCP |
|---|---|---|---|---|
| ACA (°) (n = 10) | 29.38 ± 2.57 | 43.70 ± 4.60 | 44.401 ± 3.90 | 47.21 ± 7.20 |
| RCA (°) (n = 10) | 31.83 ± 2.42 | 46.55 ± 5.99 | 42.38 ± 7.10 | 49.05 ± 5.37 |
| Swelling Ratio (S) (n = 10) | 2.47 ± 0.12 | 4.48 ± 0.66 | 5.51 ± 1.14 | 9.27 ± 2.46 |
| Equilibrium Swelling Ratio (E) (n = 10) | 0.71 ± 0.01 | 0.79 ± 0.02 | 0.80 ± 0.03 | 0.81 ± 0.05 |
| % Swelling (%) (n = 10) | 247.3 ± 11.8 | 447.8 ± 65.62 | 551.2 ± 113.6 | 926.8 ± 245.9 |
| Equilibrium Water Content (EWC) (%) (n = 10) | 70.92 ± 0.94 | 79.12 ± 2.43 | 79.56 ± 3.30 | 80.65 ± 4.54 |
| Total Water Absorption Sites (TWAS) (n = 10) | 1.129 × 1021 ± 3.67 × 1019 | 1.520 × 1021 ± 2.85 × 1020 | 1.679 × 1021 ± 2.20 × 1020 | 1.433 × 1021 ± 4.53 × 1020 |
| Diffusional Exponent (n) (n = 10) | 0.0913 | −0.079 | −0.007 | −0.017 |
| Diffusion Constant (k) (n = 10) | 0.3542 | 0.8751 | 0.8475 | 1.0641 |
| Pore Length (µm) (n > 20) | 4.241 ± 0.181 | 13.089 ± 1.130 | 12.532 ± 1.301 | 15.881 ± 1.104 |
| Aspect Ratio (n > 20) | 1.256 ± 0.054 | 1.746 ± 0.165 | 1.728 ± 0.106 | 1.461 ± 0.047 |
| Enthalpy of Melting Endotherm (J/g) | 237.3 | 122.0 | 188.2 | 277.0 |
| Freezing Water Content (%) | 69.58 | 34.28 | 52.93 | 71.92 |
| Non-freezing Water Content (%) | 1.34 | 44.91 | 26.63 | 8.74 |
| Conductance (µS/cm) (n = 5) | 0.28 ± 0.01 | 0.69 ± 0.07 | 2.60 ± 1.23 | 0.44 ± 0.02 |
| Young’s Modulus (kPa) (n = 6) | 1141.0 ± 241.1 | 179.4 ± 51.5 | 368.4 ± 95.4 | 131.7 ± 16.7 |
| Tensile Stress at Failure (kPa) (n = 6) | 401.9 ± 54.2 | 39.22 ± 2.12 | 96.41 ± 18.81 | 47.22 ± 5.71 |
| Load at Failure (N) (n = 6) | 5.63 ± 0.75 | 0.94 ± 0.03 | 1.74 ± 0.31 | 1.34 ± 0.12 |
| Direct Contact—MTT assay (%Viability) (n = 5) | 71.88 ± 3.26 | 82.84 ± 2.82 | 92.44 ± 0.79 | 74.09 ± 1.43 |
| Test on Extract—MTT assay (% Cell) (n = 4) | 96.1 ± 5.2 | 98.8 ± 10.4 | 114.4 ± 11.1 | 94.9 ± 3.2 |
| % Hemolysis (n = 3) | 0.66 ± 0.02 | 0.76 ± 0.02 | 0.27 ± 0.08 | 0.63 ± 0.04 |
| Absorption of Total Plasma Protein (µg/µL) (n = 5) | 1.67 ± 0.51 | 5.48 ± 0.26 | 2.78 ± 0.26 | 8.19 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keklikian, A.; de Barros, N.R.; Rashad, A.; Chen, Y.; Tan, J.; Sheng, R.; Sun, D.; Liu, H.; Thankam, F.G. Chitosan–Polyethylene Glycol Inspired Polyelectrolyte Complex Hydrogel Templates Favoring NEO-Tissue Formation for Cardiac Tissue Engineering. Gels 2024, 10, 46. https://doi.org/10.3390/gels10010046
Keklikian A, de Barros NR, Rashad A, Chen Y, Tan J, Sheng R, Sun D, Liu H, Thankam FG. Chitosan–Polyethylene Glycol Inspired Polyelectrolyte Complex Hydrogel Templates Favoring NEO-Tissue Formation for Cardiac Tissue Engineering. Gels. 2024; 10(1):46. https://doi.org/10.3390/gels10010046
Chicago/Turabian StyleKeklikian, Angelo, Natan Roberto de Barros, Ahmad Rashad, Yiqing Chen, Jinrui Tan, Ruoyu Sheng, Dongwei Sun, Huinan Liu, and Finosh G. Thankam. 2024. "Chitosan–Polyethylene Glycol Inspired Polyelectrolyte Complex Hydrogel Templates Favoring NEO-Tissue Formation for Cardiac Tissue Engineering" Gels 10, no. 1: 46. https://doi.org/10.3390/gels10010046





